Introduction
Human amniotic mesenchymal cells (hAMCs), which are
derived from amniotic membranes, are an attractive stem cell source
in the field of regenerative medicine (1). hAMCs have weak immunogenicity due to
their negligible expression of human leukocyte antigen (HLA) class
II molecules, low expression levels of HLA class I molecules
(2), and high expression levels
of immunosuppressive factors, including interleukin-1 receptor
antagonist (IL-1ra), IL-10 and collagen XVIII (3,4).
There are relatively fewer ethical constraints in using hAMCs,
since hAMCs are obtained from the amnion, which is discarded after
childbirth. Since the amniotic membrane is derived from the inner
cell mass of the blastocyst and is of fetal origin, it is expected
that amniotic cells contain pluripotent stem cells. Amniotic
epithelial and mesenchymal cells express POU domain class 5
transcription factor 1 (Oct-3/4, ES cell makers), nestin and
musashi (neural stem cell markers) (5), suggesting that the cells derived
from the amniotic membranes indeed contain undifferentiated
cells.
Several methods have been used to induce the
differentiation of hAMCs into endothelial cells. When hAMCs were
cultured in a medium appropriate for endothelial cell culture
(EGM-2™ medium) containing hydrocortisone, human epidermal growth
factor (hEGF), fetal bovine serum (FBS), vascular endothelial
growth factor (VEGF), human fibroblast growth factor-basic
(hFGF-B), the cells changed in morphology from fibroblast-like
shape to endothelial cell-like shape, and they also developed the
ability to take up acetylated low-density lipoprotein (LDL) and
form endothelial-like networks in the Matrigel™ assay (6). VEGF has also been shown to induce
hAMCs to differentiate into endothelial cells. When the cells were
cultured on Matrigel™, spontaneous differentiation of hAMCs into
endothelial cells was also detected. VEGF was shown to enhance the
expression of fms-like tyrosine kinase (Flt)-1 and kinase domain
region (KDR) in hAMCs, and to also induce the expression of
endothelial cell-specific markers such as intercellular adhesion
molecule (ICAM)-1, CD34 and von Willebrand factor (vWF) (7). Human amniotic fluid-derived cells
have also been used as a source of mesenchymal stem cells. The
cells acquired endothelial cell characteristics when cultured in
EGM-2 medium or under a shear force created by setting the culture
dish on an orbital shaker, and to produce angiogenic factors such
as VEGF, placental growth factor (PGF) and hepatocyte growth factor
(HGF) when cultured under hypoxic conditions (5% O2)
(8).
Angiogenesis is induced by hypoxia in several
situations, such as in the development of the retinal circulation
in premature infants (9), wound
repair (10,11), and tumor angiogenesis (12,13). The hypoxia-inducible factor (HIF)
system plays an important role in the regulation of angiogenesis
under hypoxic conditions. In the presence of hypoxia, HIF
upregulates VEGF transcription (14,15), as well as the expression of two
VEGF receptors, Flt-1 (16) and
KDR (17), which increase the
biological activity of secreted VEGF.
Cells that are negatively stained by Hoechst 33342
(DNA-binding fluorescent dye), i.e., cells with the highest efflux
capacity for the dye, are known to be a very small and homogeneous
population of highly primitive cells, known as side population (SP)
cells (18–21). SP cells found in a number of
species, including mice, monkeys and humans, and isolated from
several organs, including the bone marrow, skeletal muscle and
liver (18–21), have demonstrated the potential for
differentiation into cell types beyond their organ of origin
(22). SP cells have also been
isolated from hAMCs (23). hAM-SP
cells express Oct-3/4 and have the potential to differentiate into
multiple lineages, including several organ- or tissue-specific
cells including neurons, osteoblasts, chondrocytes, and adipocytes,
as found for the other type of mesenchymal stem cells (23).
The aim of the present study was to clarify whether
hAM-SP cells, which can be regarded as an undifferentiated stem
cell fraction of AMCs, were effectively induced to differentiate
into cells of endothelial lineage by hypoxia. Therefore, we
cultured hAM-SP cells in an endothelial induction medium containing
VEGF in a hypoxic (1% O2) or a normoxic (20%
O2) environment.
Materials and methods
Preparation of human amniotic mesenchymal
side population cells
The Institutional Ethics Committee approved all
protocols (Kitasato University, School of Allied Health Sciences,
no. 2009–015). The protocol for the preparation of amniotic
mesenchymal SP cells has previously been described (23). Briefly, after informed consent was
obtained from a pregnant woman scheduled for caesarean section, the
amniotic membrane was separated from the post-partum placenta. The
human amniotic membrane consists of two cell layers, the epithelial
layer and mesenchymal layer, with the basement membrane between the
two. To prepare AMCs, the amniotic membrane was first treated with
trypsin to remove the amniotic epithelial cells, and then the
remnant layer was treated with an enzyme mixture (0.1% papain, 1
mg/ml collagenase, 0.01% DNase, and 0.1% dispase) to dissolve the
mesenchymal layer and disperse cells. The AMCs were stained with
Hoechst 33342 and the SP cells were sorted using a cell sorter
(EPICS Altra; Beckman Coulter, Fullerton, CA, USA).
The sorted SP cells in the hAMCs (hAM-SP cells) were
cultured in Dulbecco’s modified Eagle’s medium nutrient mixture
F-12 Ham (DMEM/F12) containing 5% FBS (both were from
Sigma-Aldrich, St. Louis, MO, USA), 10 ng/ml human leukemia
inhibitory factor (hLIF; Chemicon-Merck Millipore, Billerica, MA,
USA), 10 ng/ml hFGF-B (PeproTech, Inc., Rocky Hill, NJ, USA) and 10
ng/ml platelet-derived growth factor-BB (PDGF-BB; PeproTech, Inc.),
on a type I collagen-coated dish (Iwaki, Chiba, Japan) in a 5%
CO2 environment at 37°C. The cells were cultured until
they reached 90% confluence and were recovered with 0.1% trypsin
(Sigma-Aldrich)-ethylenediaminetetraacetic acid (EDTA; Gibco-Life
Technologies, Carlsbad, CA, USA), and sub-cultured at a density of
104 cells/cm2.
Endothelial differentiation culture
The hAM-SP cells at the fourth or fifth passages
cultured in cell growth medium were rinsed with phosphate-buffered
saline (PBS) without Ca2+ and Mg2+ and then
cultured in endothelial induction medium consisting of DMEM/F12
supplemented with 2% FBS and 50 ng/ml VEGF (PeproTech, Inc.) for 1
or 2 weeks in a hypoxic (1% O2) or normoxic (20%
O2) environment, on a type I collagen-coated dish. As
control, the hAM-SP cells were cultured in normal medium consisting
of DMEM/F12 supplemented with 2% FBS in the absence of VEGF for 1
or 2 weeks. The endothelial induction medium and normal medium were
replenished every two days. The endothelial markers after
differentiation of the hAM-SP cells were evaluated by real-time PCR
and fluorescence immunostaining.
Real-time PCR
To examine the expression of endothelial
cell-specific mRNA, we performed a quantitative reverse
transcription-polymerase chain reaction (qRT-PCR) assay.
RNAprotect® Cell Reagent (Qiagen, Hilden, Germany) was
used to stabilize the RNA of the cultivated cells and total RNA was
extracted from the cells using RNeasy Plus Mini kit (Qiagen). The
mRNA was transcribed into cDNA using the iScript™ cDNA Synthesis
kit (Bio-Rad, Hercules, CA, USA). PCR was carried out by mixing 1
μl of cDNA template, each primer (Table I) and SYBR-Green (Bio-Rad) in a
volume of 20 μl. The samples were amplified in a thermocycler. Each
sample was analyzed in triplicate by the Chromo4 system (Bio-Rad).
Amplification data were obtained using the software of Opticon
Monitor (Bio-Rad). The expression levels were quantified relative
to the expression level of GAPDH.
 | Table IPrimer sets used for RT-PCR analysis
of the endothelial differentiation. |
Table I
Primer sets used for RT-PCR analysis
of the endothelial differentiation.
| Forward
primers | Reverse
primers |
|---|
| GAPDH | 5′-GGCC TCCA AGGA
GTAA GACC-3′ | 5′-AGGG GTCT ACAT
GGCA ACTG-3′ |
| KDR | 5′-AGCC AGCT CTGG
ATTT GTGG A-3′ | 5′-CATG CCCT TAGC
CACT TGGA A-3′ |
| Flt-1 | 5′-GCGC TTCA CCTG
GACT GACA-3′ | 5′-GAAA CTGG GCCT
GCTG ACAT C-3′ |
| VCAM-1 | 5′-ATTG ACTT GCAG
CACC ACAG-3′ | 5′-ATCT CCAG CCTG
TCAA ATGG-3′ |
| vWF | 5′-AGAT GTTT GCCT
ACGG CTTG-3′ | 5′-CAGC CTGT GACC
CTCT TCTC-3′ |
Immunochemistry
hAM-SP cells subjected to induction were fixed and
incubated for 1 h with diluted primary antibodies specific for the
endothelial cells. The primary antibodies used as endothelial
markers were: anti-human vascular cell adhesion molecule (VCAM)-1
mouse IgG (1:100; Immunotech, Beckman Coulter, Inc.), anti-human
KDR mouse IgG (1:100; Sigma-Aldrich), anti-human vascular
endothelial (VE)-cadherin mouse IgG (1:100; R&D Systems, Inc.,
Minneapolis, MN, USA), and anti-human vWF mouse IgG (1:50; Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA). The secondary
antibodies were Alexa Fluor 488-conjugated anti-mouse goat IgG
(1:500; Molecular Probes-Life Technologies). The immunostained
cells were analyzed by confocal laser scanning microscopy (CLSM)
(IX70; Olympus Corporation, Tokyo, Japan).
Expression of the Oct-3/4 protein was evaluated by
fluorescent immunostaining following induction with VEGF under
hypoxic conditions for 1 or 2 weeks. The primary antibody used was
anti-human Oct-3/4 rabbit IgG (1:200; Santa Cruz Biotechnology,
Inc.), and the secondary antibody was Alexa 488-conjugated
anti-rabbit goat IgG (1:500; Molecular Probes). Immunostained cells
were analyzed by confocal laser microscopy using a CLSM. Each RGB
image was separated into red, green and blue by color deconvolution
with the ImageJ 1.40 software (National Institutes of Health,
Bethesda, MD, USA), and the numbers of pixels in green color
(endothelial markers) and red color (Oct-3/4) were calculated.
Microarray processing
All experiments were performed using commercially
available microarrays for humans (Human Genome U133 Plus 2.0 Array,
Affymetrix, Santa Clara, CA, USA). The hAM-SP cells cultivated with
VEGF under hypoxic (1% O2) conditions for 2 or 0 weeks
were removed from the culture dishes using trypsin and washed with
PBS. Total RNA was isolated from the cells using an RNeasy Mini
kit® (Qiagen). The isolation and purification of the
total RNA were carried out according to the manufacturer’s protocol
(Qiagen). The quality and amount of starting RNA were confirmed by
agarose gel electrophoresis in addition to the ratio of absorbance
(1.9< A260 nm/A280 nm <2.0). Total RNA
was used to prepare a biotinylated target cRNA according to the
manufacturer’s recommendation (Affymetrix). Briefly, 1 μg of mRNA
was used to generate the first-strand cDNA using a T7-linked
oligo(dT) primer. After second-strand synthesis, in vitro
transcription was performed using a synthetic biotinylated
nucleotide analog (biotinylated uridine-triphosphate), which
yielded an ~50–100-fold amplification of RNA. The cRNA was
fragmented prior to overnight hybridization. The arrays were then
washed, stained with streptavidin-phycoerythrin, and scanned
(GeneChip Scanner 3000®; Affymetrix).
After scanning, the array images were visually
inspected to confirm the scanner alignment and the absence of
significant bubbles or scratches on the chip surface. The 3′/5′
ratios for GAPDH were 1.34 and 1.63, which were within the
acceptable limits; BioB spike controls were also present on all the
chips, with BioC, BioD and Cre being present in increasing
intensities. BioB, BioC, BioD and Cre are genes from Escherichia
coli or bacteriophage P1 and were added before hybridization to
check the hybridization quality. Background intensities were 93.8
and 102.6 and noise factors were 5.08 and 5.12, which were
sufficiently low.
Statistical analysis
All data are presented as the means + SD.
Bonferroni’s post hoc test was used for multiple-group comparisons.
P-values <0.05 were considered to indicate statistically
significant differences.
Results
For evaluation of the endothelial differentiation
potency, the gene expression of endothelial markers such as KDR
(24), Flt-1 (25), VCAM (26) and vWF (27) was evaluated by real-time PCR.
While the expression of KDR, VCAM and vWF did not change after 1
week of culture with VEGF under hypoxic conditions, the expression
of Flt-1 increased (Fig. 1).
 | Figure 1Gene expression of endothelial cell
markers evaluated by real-time PCR after 1 week of induction with
VEGF. (A) KDR, (B) Flt-1, (C) VCAM, (D) vWF. Cells were induced by
VEGF under normoxic or hypoxic conditions. The expression levels
relative to the GAPDH expression level were compared. While no
changes in the expression of KDR, VCAM or vWF were observed after
induction with VEGF under hypoxic conditions, the expression of
Flt-1 increased. Data shown are the means + SD (n=5);
(**P<0.01). VEGF, vascular endothelial growth factor;
KDR, kinase domain region; Flt-1, fms-like tyrosine kinase-1; VCAM,
vascular cell adhesion molecule; vWF, von Willebrand factor. |
After 2 weeks of induction with VEGF under hypoxic
conditions (Fig. 2), the hAM-SP
cells showed enhanced expression of KDR, Flt-1, VCAM and vWF. The
cells cultivated only under hypoxic conditions in the absence of
VEGF or the cells induced with VEGF under normoxic conditions
showed no significant changes in the expression of genes as
compared to those in the control (cultivated without VEGF under
normoxic conditions). On the other hand, the cells induced with
VEGF under hypoxic conditions showed significantly increased
expression levels of KDR, VCAM, Flt-1 and vWF as compared to the
cells induced with VEGF under normoxic conditions as well as to the
cells cultured only under hypoxic conditions in the absence of VEGF
or the cells induced with VEGF under normoxic conditions (Fig. 2). Thus, culture under hypoxic
conditions enhanced the cellular differentiation by VEGF into the
endothelial lineage.
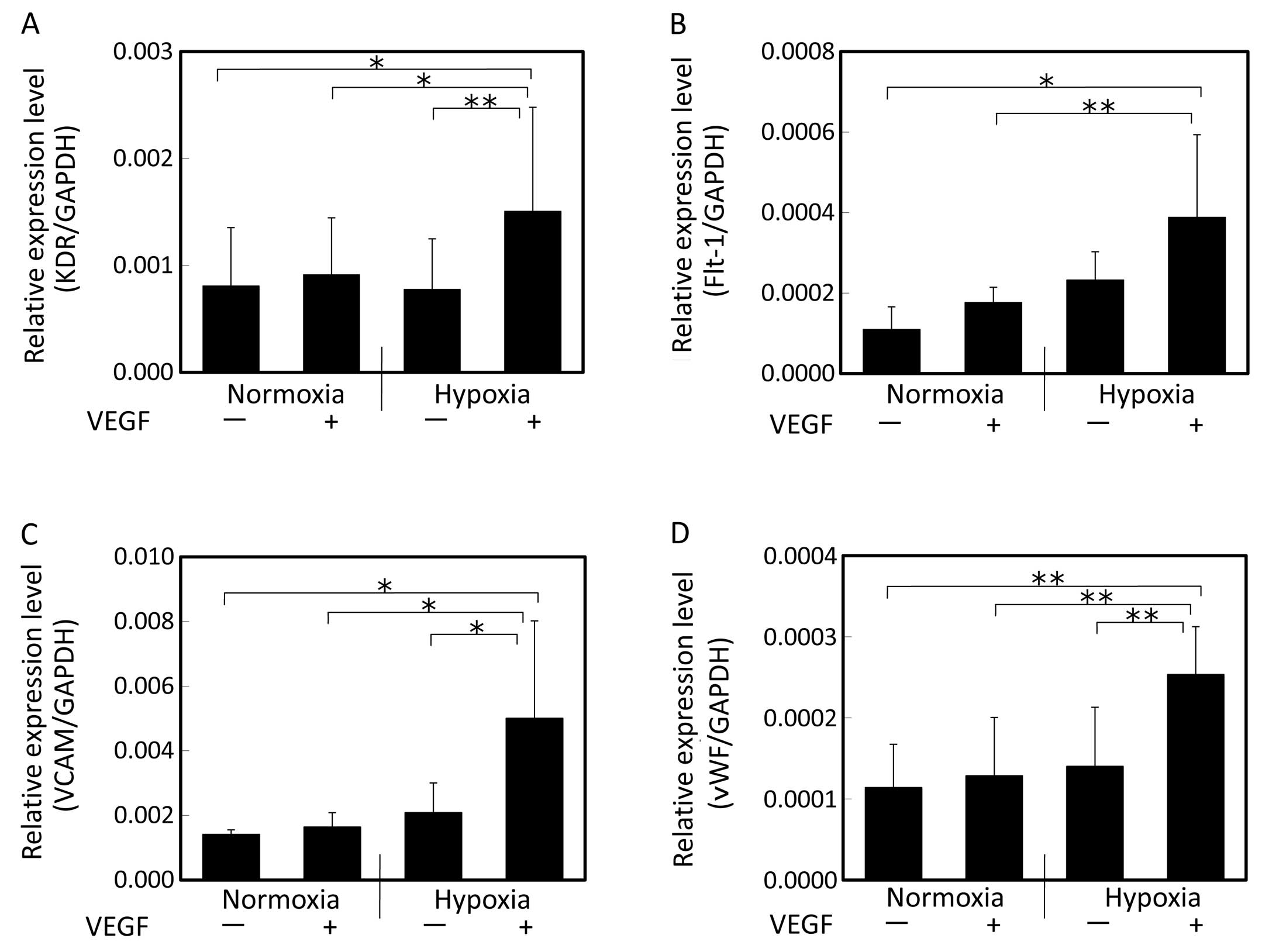 | Figure 2Gene expression of endothelial cell
markers evaluated by real-time PCR after 2 weeks of induction with
VEGF. (A) KDR, (B) Flt-1, (C) VCAM, (D) vWF. Cells were induced by
VEGF under normoxic or hypoxic conditions. The expression levels
relative to the GAPDH expression level were compared. Following
cultivation under hypoxic conditions in the presence of VEGF,
significant increase in the expression levels of KDR, Flt-1, VCAM
and vWF was observed. Data shown are the means + SD (n=5);
(*P<0.05 and **P<0.01). VEGF, vascular
endothelial growth factor; KDR, kinase domain region; Flt-1,
fms-like tyrosine kinase-1; VCAM, vascular cell adhesion molecule;
vWF, von Willebrand factor. |
Immunocytochemical assay for endothelial markers
such as VCAM, KDR, vWF and VE-cadherin (28) was performed following induction of
hAM-SP cells with VEGF under normoxic/hypoxic conditions for 2
weeks. Positive staining for KDR and VE-cadherin proteins was
observed following induction with VEGF under both normoxic and
hypoxic conditions, while staining for the VCAM and vWF proteins
was negative (Fig. 3A). Human
pulmonary artery endothelial cells (HPAECs) were used as positive
control, and showed positive staining for all markers (data not
shown). The number of pixels in green color was calculated with the
ImageJ software (Fig. 3B). KDR
was significantly induced by VEGF under both normoxic and hypoxic
conditions as compared with that in the control conditions
(P<0.01). VE-cadherin expression was increased by VEGF under
normoxic conditions as compared with that in the control conditions
(P<0.05), and was induced even more significantly under hypoxic
condition as compared with that in normoxic conditions (P<0.01).
To evaluate if any undifferentiated cells remained after the
induction, immunocytochemical staining for Oct-3/4 was carried out
after induction with VEGF under hypoxic conditions for 1 or 2 weeks
(Fig. 4). Strong expression of
Oct-3/4, which is a marker gene of undifferentiated cells (29,30), was observed in the hAM-SP cells
prior to the induction. While some expression of Oct-3/4 protein
was still observed after 1 week of induction with VEGF under
hypoxic conditions, the red staining for this protein disappeared
almost entirely after 2 weeks of induction. The expression of
Oct-3/4 protein decreased significantly with the induction
time.
We also analyzed gene expression by microarray
analysis and focused on the gene expression of stem cell markers
(31) and neural stem cell
markers. The gene expression of stem cell markers, such as Nanog
homeobox (NANOG) (32,33), Oct-3/4 (29,30) and growth differentiation factor 3
(GDF3) (34), was decreased by
less than half in the cells cultivated with VEGF under hypoxic
conditions for 2 weeks as compared to those cultivated for 0 weeks
(prior to induction). The gene expression of Kruppel-like factor 4
(KLF4) (35), v-myc
myelocytomatosis viral oncogene homolog (MYC) (36), sex determining region Y (SRY)-box
2 (SOX2) (37), RNA exonuclease 1
homolog (REX1) (38), fibroblast
growth factor 4 (FGF4) (39),
telomerase reverse transcriptase (TERT) (40) slightly decreased (or remained the
same) following induction with VEGF under hypoxic conditions for 2
weeks. The neural stem cell markers such as nestin (41) and musashi (42) were decreased after induction with
VEGF under hypoxic conditions for 2 weeks (Table II).
 | Table IIGene expression of stem cell markers
and neural stem cell markers by microarray analysis. |
Table II
Gene expression of stem cell markers
and neural stem cell markers by microarray analysis.
| Gene | Detection | Hypoxia VEGF(+) 2/0
week | Author/(ref.) |
|---|
| Stem cell
markers |
| Kruppel-like
factor 4 (gut) (KLF4) | P/P | 0.98 | Li et al
(35) |
| v-myc
myelocytomatosis viral oncogene homolog (MYC) | P/P | 0.88 | Cartwright et
al (36) |
| Nanog homeobox
(NANOG) | A/A | 0.08 | Chambers et
al (32)
Mitsui et al (33) |
| POU domain class 5
transcription factor 1 (Oct-3/4) | A/A | 0.47 | Nichols et
al (29)
Niwa et al (30) |
| Sex determining
region Y (SRY)-box 2 (SOX2) | A/A | 1.00 | Avilion et
al (37) |
| RNA exonuclease 1
homolog (REX1) | A/A | 0.83 | Ben-Shushan et
al (38) |
| Growth
differentiation factor 3 (GDF3) | A/A | 0.24 | Levine and
Brivanlou (34) |
| Fibroblast growth
factor 4 (FGF4) | A/A | 0.81 | Yuan et al
(39) |
| Telomerase reverse
transcriptase (TERT) | P/P | 0.67 | Yang et al
(40) |
| Neural stem cell
markers |
| Nestin (NES) | P/A | 0.09 | Park et al
(41) |
| Musashi homolog 1
(MSI1) | A/A | 0.71 | Kaneko et al
(42) |
To confirm the involvement of HIF in the induction
under hypoxic conditions, we selected downstream genes of HIF-1.
The results revealed that the gene expression of VEGF-A (15), Flt-1 (16), erythropoietin (EPO) (43), enolase (ENO)-1 (44), adrenomedullin (ADM) (45) and Egl nine homolog (EGLN)-3
(46), which are known to be
upregulated by HIF-1, were increased by more than 2-fold in the
cells cultivated with VEGF under hypoxic conditions for 2 weeks as
compared to those cultivated with VEGF under normoxic conditions
(Table III).
 | Table IIIHypoxia-inducible factor-1 target
genes upregulated by hypoxic culture. |
Table III
Hypoxia-inducible factor-1 target
genes upregulated by hypoxic culture.
| Gene | Detection | Hypoxia VEGF(+)2/0
week | Author/(ref.) |
|---|
| Vascular
endothelial growth factor A (VEGF-A) | P/P | 2.0 | Forsythe et
al (15) |
| Fms-like tyrosine
kinase-1 (flt-1) | P/P | 2.7 | Gerber et al
(16) |
| Erythropoietin
(EPO) | A/A | 6.6 | Wang and Semenza
(43) |
| Enolase 1
(ENO-1) | P/P | 2.0 | Semenza et
al (44) |
| Adrenomedullin
(ADM) | P/P | 6.3 | Nguyen and Claycomb
(45) |
| EGL nine homolog 3
(EGLN-3) | P/P | 17.9 | Pescador et
al (46) |
Discussion
To clarify whether hAM-SP cells can be effectively
induced to differentiate into endothelial lineage cells by hypoxia,
hAM-SP cells were cultured in endothelial cell induction medium
containing VEGF under hypoxic (1% O2) or normoxic (20%
O2) conditions. Our data revealed that under hypoxic
conditions: i) the gene expression of endothelial lineage markers
such as KDR, Flt-1, VCAM and vWF was induced; ii) the expression of
endothelial marker proteins including KDR and VE-cadherin was
induced; and iii) expression of the HIF target genes was
upregulated in the hAM-SP cells. Cultivation in the presence of
VEGF under hypoxic conditions for 2 weeks enhanced the expression
of endothelial lineage markers. While the expression of Oct-3/4 was
still observed after 1 week of induction with VEGF under hypoxic
conditions, it disappeared almost completely after 2 weeks. These
results suggest that induction of hAM-SP cells for 1 week was
inadequate to induce differentiation of the cells into endothelial
cells, while induction for 2 weeks led to pronounced
differentiation of the cells into the endothelial lineage. However,
protein expression of VCAM and vWF could not be detected under
these conditions. Therefore, the characteristics of hAM-SP cells
following induction by VEGF under hypoxic condition for 2 weeks
were not entirely identical to those of endothelial cells, and it
is suggested that extended culture time would have created more
mature endothelial cells, since enhanced mRNA expression of vWF and
VCAM was observed.
hAMCs have been reported to be induced by EGM-2,
which contain several growth factors, including VEGF; however, the
differentiated cells did not express mature endothelial cell
markers, such as vWF and VE-cadherin (6). Also, VEGF receptors 1 (Flt-1) and 2
(KDR) were basally expressed in hAMCs, and enhanced expression of
endothelial-specific markers such as Flt-1 KDR and ICAM-1 was
observed following exposure to VEGF (7). These results indicate that while
hAMCs may have the potential to differentiate into endothelial
cells, they are a heterogeneous population of cells. hAM-SP cells
have a larger population of undifferentiated cells, while the hAMCs
contain these cells only at the rate of 0.1–0.2%. In the present
study conducted using hAM-SP cells, low expression levels of KDR
and Flt-1 in the hAM-SP cells and a high expression level of
Oct-3/4 were observed prior to induction. The gene expression of
KDR, Flt-1 and vWF, as well as the protein expressions of
VE-cadherin increased following induction with VEGF under hypoxic
conditions. hAM-SP cells include several stem cells in a more
pluripotent state than non-SP cells, and are, therefore, preferable
as the source of cells for use in the field of regenerative
medicine.
Bone marrow-derived mesenchymal stem cells (BM-MSCs)
have also been used as candidate cells in the field of regenerative
medicine. Reduced oxygen tension in the physiological range (4–7%)
(47) has been shown to enhance
the proliferation of these cells. Attention has been focused on the
effects of hypoxia on the differentiation of pluripotent cells into
various mesenchymal lineages. Although the exact outcome depended
on the oxygen tension, time in culture, and use/non-use of hypoxic
preculture, the beneficial effects appeared more often on
osteogenic, chondrogenic and adipogenic differentiation (48). Although the target cells of these
studies were not endothelial cells, they did show that hypoxia
clearly plays an important role in the differentiation of
mesenchymal stem cells. Hypoxia was also found to be an important
factor inducing the differentiation of hAM-SP cells into the
endothelial lineage.
It is well-known that VEGF transcription is
upregulated under hypoxic conditions by the effects of HIF-1. HIF-1
also upregulates two VEGF receptors, Flt-1 (16) and KDR (17), which increase the biological
activities of secreted VEGF (49). To confirm the involvement of HIF
in the effective induction of hAM-SP cells under hypoxic
conditions, we analyzed the changes in the expression of downstream
genes of HIF-1. Our results revealed that the gene expression of
VEGF-A, Flt-1, EPO, ENO-1, ADM and EGLN-3, which are known to be
upregulated by HIF, (15,16,43–46) increased following induction with
VEGF under hypoxic conditions for 2 weeks. These results indicate
that under hypoxic conditions, the HIF system is activated, which
enhances the expressions of VEGF and VEGF receptors leading to the
differentiation into the endothelial lineage.
The hypoxic environment (3–5% of oxygen) is
physiologically normal for embryonic stem cells and its
pluripotency is regulated by the family of HIFs (50). From the microarray data and
Oct-3/4 staining, stem cell markers are gradually decreased by
induction of VEGF under hypoxic conditions. These results indicate
that hypoxic conditions would also contribute to maintaining an
undifferentiated state of hAM-SP cells and maximizing the
differentiation into vascular endothelial cells by VGEF by
suppressing the differentiation into the other types of cells.
In conclusion, the hAM-SP cells cultivated in the
presence of VEGF under hypoxic conditions differentiated into the
vascular endothelial lineage, possibly due to upregulation of the
gene expression associated with angiogenesis through activation of
the HIF system.
References
|
1
|
Sakuragawa N, Yoshikawa H and Sasaki M:
Amniotic tissue transplantation: clinical and biochemical
evaluations for some lysosomal storage diseases. Brain Dev.
14:7–11. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wolbank S, Peterbauer A, Fahrner M, et al:
Dose-dependent immunomodulatory effect of human stem cells from
amniotic membrane: a comparison with human mesenchymal stem cells
from adipose tissue. Tissue Eng. 13:1173–1183. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hori J, Wang M, Kamiya K, Takahashi H and
Sakuragawa N: Immunological characteristics of amniotic epithelium.
Cornea. 25(Suppl 1): S53–S58. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lefebvre S, Adrian F, Moreau P, et al:
Modulation of HLA-G expression in human thymic and amniotic
epithelial cells. Hum Immunol. 61:1095–1101. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sakuragawa N, Kakinuma K, Kikuchi A, et
al: Human amnion mesenchyme cells express phenotypes of neuroglial
progenitor cells. J Neurosci Res. 78:208–214. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Konig JM, Huppertz B, Desoye G, et al:
Amnion-derived mesenchymal stromal cells show angiogenic properties
but resist differentiation into mature endothelial cells. Stem
Cells Dev. 21:1309–1320. 2012. View Article : Google Scholar
|
|
7
|
Alviano F, Fossati V, Marchionni C, et al:
Term amniotic membrane is a high throughput source for multipotent
mesenchymal stem cells with the ability to differentiate into
endothelial cells in vitro. BMC Dev Biol. 7:112007.PubMed/NCBI
|
|
8
|
Zhang P, Baxter J, Vinod K, Tulenko TN and
Di Muzio PJ: Endothelial differentiation of amniotic fluid-derived
stem cells: synergism of biochemical and shear force stimuli. Stem
Cells Dev. 18:1299–1308. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ashton N, Ward B and Serpell G: Effect of
oxygen on developing retinal vessels with particular reference to
the problem of retrolental fibroplasia. Br J Ophthalmol.
38:397–432. 1954. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Knighton DR, Silver IA and Hunt TK:
Regulation of wound-healing angiogenesis-effect of oxygen gradients
and inspired oxygen concentration. Surgery. 90:262–270.
1981.PubMed/NCBI
|
|
11
|
Knighton DR, Hunt TK, Scheuenstuhl H,
Halliday BJ, Werb Z and Banda MJ: Oxygen tension regulates the
expression of angiogenesis factor by macrophages. Science.
221:1283–1285. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thomlinson RH and Gray LH: The
histological structure of some human lung cancers and the possible
implications for radiotherapy. Br J Cancer. 9:539–549. 1955.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Folkman J, Merler E, Abernathy C and
Williams G: Isolation of a tumor factor responsible for
angiogenesis. J Exp Med. 133:275–288. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu Y, Cox SR, Morita T and Kourembanas S:
Hypoxia regulates vascular endothelial growth factor gene
expression in endothelial cells. Identification of a 5′ enhancer.
Circ Res. 77:638–643. 1995.
|
|
15
|
Forsythe JA, Jiang BH, Iyer NV, et al:
Activation of vascular endothelial growth factor gene transcription
by hypoxia-inducible factor 1. Mol Cell Biol. 16:4604–4613.
1996.PubMed/NCBI
|
|
16
|
Gerber HP, Condorelli F, Park J and
Ferrara N: Differential transcriptional regulation of the two
vascular endothelial growth factor receptor genes. Flt-1, but not
Flk-1/KDR, is up-regulated by hypoxia. J Biol Chem.
272:23659–23667. 1997. View Article : Google Scholar
|
|
17
|
Waltenberger J, Mayr U, Pentz S and
Hombach V: Functional upregulation of the vascular endothelial
growth factor receptor KDR by hypoxia. Circulation. 94:1647–1654.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Goodell MA, Rosenzweig M, Kim H, et al:
Dye efflux studies suggest that hematopoietic stem cells expressing
low or undetectable levels of CD34 antigen exist in multiple
species. Nat Med. 3:1337–1345. 1997. View Article : Google Scholar
|
|
19
|
Welm BE, Tepera SB, Venezia T, Graubert
TA, Rosen JM and Goodell MA: Sca-1(pos) cells in the mouse mammary
gland represent an enriched progenitor cell population. Dev Biol.
245:42–56. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wulf GG, Luo KL, Jackson KA, Brenner MK
and Goodell MA: Cells of the hepatic side population contribute to
liver regeneration and can be replenished with bone marrow stem
cells. Haematologica. 88:368–378. 2003.PubMed/NCBI
|
|
21
|
Uchida N, Fujisaki T, Eaves AC and Eaves
CJ: Transplantable hematopoietic stem cells in human fetal liver
have a CD34(+) side population (SP)phenotype. J Clin Invest.
108:1071–1077. 2001.
|
|
22
|
Gussoni E, Soneoka Y, Strickland CD, et
al: Dystrophin expression in the mdx mouse restored by stem cell
transplantation. Nature. 401:390–394. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kobayashi M, Yakuwa T, Sasaki K, et al:
Multilineage potential of side population cells from human amnion
mesenchymal layer. Cell Transplant. 17:291–301. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yamaguchi TP, Dumont DJ, Conlon RA,
Breitman ML and Rossant J: flk-1, an flt-related receptor tyrosine
kinase is an early marker for endothelial cell precursors.
Development. 118:489–498. 1993.PubMed/NCBI
|
|
25
|
Shibuya M, Seetharam L, Ishii Y, et al:
Possible involvement of VEGF-FLT tyrosine kinase receptor system in
normal and tumor angiogenesis. Princess Takamatsu Symp. 24:162–170.
1994.PubMed/NCBI
|
|
26
|
Lobb R, Chi-Rosso G, Leone D, et al:
Expression and functional characterization of a soluble form of
vascular cell adhesion molecule 1. Biochem Biophys Res Commun.
178:1498–1504. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jones TR, Kao KJ, Pizzo SV and Bigner DD:
Endothelial cell surface expression and binding of factor VIII/von
Willebrand factor. Am J Pathol. 103:304–308. 1981.PubMed/NCBI
|
|
28
|
Lampugnani MG, Resnati M, Raiteri M, et
al: A novel endothelial-specific membrane protein is a marker of
cell-cell contacts. J Cell Biol. 118:1511–1522. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nichols J, Zevnik B, Anastassiadis K, et
al: Formation of pluripotent stem cells in the mammalian embryo
depends on the POU transcription factor Oct4. Cell. 95:379–391.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Niwa H, Miyazaki J and Smith AG:
Quantitative expression of Oct-3/4 defines differentiation,
dedifferentiation or self-renewal of ES cells. Nat Genet.
24:372–376. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Imamura M, Miura K, Iwabuchi K, et al:
Transcriptional repression and DNA hypermethylation of a small set
of ES cell marker genes in male germline stem cells. BMC Dev Biol.
6:342006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chambers I, Colby D, Robertson M, et al:
Functional expression cloning of Nanog, a pluripotency sustaining
factor in embryonic stem cells. Cell. 113:643–655. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mitsui K, Tokuzawa Y, Itoh H, et al: The
homeoprotein Nanog is required for maintenance of pluripotency in
mouse epiblast and ES cells. Cell. 113:631–642. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Levine AJ and Brivanlou AH: GDF3, a BMP
inhibitor, regulates cell fate in stem cells and early embryos.
Development. 133:209–216. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li Y, McClintick J, Zhong L, Edenberg HJ,
Yoder MC and Chan RJ: Murine embryonic stem cell differentiation is
promoted by SOCS-3 and inhibited by the zinc finger transcription
factor Klf4. Blood. 105:635–637. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cartwright P, McLean C, Sheppard A, Rivett
D, Jones K and Dalton S: LIF/STAT3 controls ES cell self-renewal
and pluripotency by a Myc-dependent mechanism. Development.
132:885–896. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Avilion AA, Nicolis SK, Pevny LH, Perez L,
Vivian N and Lovell-Badge R: Multipotent cell lineages in early
mouse development depend on SOX2 function. Genes Dev. 17:126–140.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ben-Shushan E, Thompson JR, Gudas LJ and
Bergman Y: Rex-1, a gene encoding a transcription factor expressed
in the early embryo, is regulated via Oct-3/4 and Oct-6 binding to
an octamer site and a novel protein, Rox-1, binding to an adjacent
site. Mol Cell Biol. 18:1866–1878. 1998.PubMed/NCBI
|
|
39
|
Yuan H, Corbi N, Basilico C and Dailey L:
Developmental-specific activity of the FGF-4 enhancer requires the
synergistic action of Sox2 and Oct-3. Genes Dev. 9:2635–2645. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yang C, Przyborski S, Cooke MJ, et al: A
key role for telomerase reverse transcriptase unit in modulating
human embryonic stem cell proliferation, cell cycle dynamics, and
in vitro differentiation. Stem Cells. 26:850–863. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Park D, Xiang AP, Mao FF, et al: Nestin is
required for the proper self-renewal of neural stem cells. Stem
Cells. 28:2162–2171. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kaneko Y, Sakakibara S, Imai T, et al:
Musashi1: an evolutio- nally conserved marker for CNS progenitor
cells including neural stem cells. Dev Neurosci. 22:139–153. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang GL and Semenza GL: Characterization
of hypoxia-inducible factor 1 and regulation of DNA binding
activity by hypoxia. J Biol Chem. 268:21513–21518. 1993.PubMed/NCBI
|
|
44
|
Semenza GL, Roth PH, Fang HM and Wang GL:
Transcriptional regulation of genes encoding glycolytic enzymes by
hypoxia-inducible factor 1. J Biol Chem. 269:23757–23763.
1994.PubMed/NCBI
|
|
45
|
Nguyen SV and Claycomb WC: Hypoxia
regulates the expression of the adrenomedullin and HIF-1 genes in
cultured HL-1 cardiomyocytes. Biochem Biophys Res Commun.
265:382–386. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Pescador N, Cuevas Y, Naranjo S, et al:
Identification of a functional hypoxia-responsive element that
regulates the expression of the egl nine homologue 3 (egln3/phd3)
gene. Biochem J. 390:189–197. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lennon DP, Edmison JM and Caplan AI:
Cultivation of rat marrow-derived mesenchymal stem cells in reduced
oxygen tension: Effects on in vitro and in vivo
osteochondrogenesis. J Cell Physiol. 187:345–355. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Das R, Jahr H, van Osch GJ and Farrell E:
The role of hypoxia in bone marrow-derived mesenchymal stem cells:
considerations for regenerative medicine approaches. Tissue Eng
Part B Rev. 16:159–168. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Pugh CW and Ratcliffe PJ: Regulation of
angiogenesis by hypoxia: role of the HIF system. Nat Med.
9:677–684. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Szablowska-Gadomska I, Zayat V and
Buzanska L: Influence of low oxygen tensions on expression of
pluripotency genes in stem cells. Acta Neurobiol Exp. 71:86–93.
2011.PubMed/NCBI
|


















