Introduction
Hantaviruses are enveloped RNA viruses with a
diameter varying from 70 to 350 nm (1), which form a genus within the
Bunyaviridae family. The viral genomes are tri-segmented,
negative sense RNA. Hantavirus genome segments are designated as
large (L), medium (M) and small (S). The L segment encodes an RNA
polymerase. The M segment encodes an envelope glycoprotein
precursor cleaved to form 2 surface glycoproteins, Gn and Gc. The S
segment encodes the nucleocapsid protein (NP) (2). To date, more than 23 hantavirus
species have been identified (3).
Hantavirus infection is manifested as 2 different
forms of severe febrile diseases, hemorrhagic fever with renal
syndrome (HFRS) and hantavirus pulmonary syndrome (HPS) (4). Hantaan virus (HTNV), Seoul virus
(SEOV), Puumala virus (PUUV) and Dobrava virus (DOBV) cause HFRS;
Sin Nombre virus (SNV), New York virus (NYV), Black Creek Canal
virus (BCCV), Bayou virus (BAYV), Laguna Negra virus (LNV) and
Andes virus (ANDV) cause HPS (5).
Human infection by hantaviruses can result in profound morbidity
and mortality; the HFRS mortality rate varies from 0.1 to 10%,
while HPS has a mortality rate of 40 to 50% (6). Hantavirus infection can also cause
potentially long-term cardiovascular consequences (7). More than 90% of HFRS infections
occur in Asian countries and the most severe forms of HFRS are
mainly caused by HTNV infection (8). In China, inactivated vaccines have
been produced by using rodent brain and cell cultures (9). Although the protective efficacy of
several inactivated vaccines has been confirmed, there are still
unresolved issues concerning their production and human use. The
viral yield obtained from virus-infected cell cultures is low and
hantaviruses require high-level safety conditions for handling. In
terms of safety and yield, recombinant vaccines should be able to
overcome these disadvantages.
Both humoral and cellular immunity play important
roles in virus clearance during hantavirus infection. The 2
glycoproteins, Gn and Gc, are presumed to be the major elements
involved in the induction of neutralizing antibodies during
hantavirus infection. Monoclonal antibodies (mAbs) directed against
Gn and Gc have displayed virus-neutralizing activity in
vitro as well as in vivo (10). Cellular immunity is believed to be
associated with the hantavirus NP (11). Although anti-NP antibodies do not
display neutralizing activities, studies have shown that
immunization with NP induces a protective immune response which can
increase the survival of mice following challenge with a lethal
dose of hantavirus (12). It has
been suggested that the protection afforded by anti-NP antibodies
is mediated by the induction of antibody-dependent cytotoxic T
cells. Therefore, utilization of both the glycoprotein and NP
components for vaccine development may prove to be a promising
approach. The N-terminus of the NP contains major hantavirus
antigenic epitopes, in particular in the 0.7-kb fragment of the S
segment (13). Mice immunized
with the GnS0.7 fusion protein (Gn of the M segment and a 0.7-kb
fragment of the S segment) and the GcS0.7 (Gc of the M segment and
a 0.7-kb fragment of the S segment) elicit anti-NP,
anti-glycoprotein and neutralizing antibodies. In addition, mice
immunized with these fusion proteins also elicit better cellular
immune responses than mice immunized with the unfused proteins
(14,15).
In the vaccine development process, improving
antigen expression is the most important aspect that should be
considered. Antigen expression can be improved at both the
transcriptional and post-transcriptional levels. The human
cytomegalovirus (CMV) immediate-early enhancer/promoter is the most
commonly used promoter in DNA vaccines; however, the activity of
this promoter varies among different tissues and cell lines and
gene expression driven by the CMV promoter has been shown to be
downregulated in the presence of interferon (IFN)-γ (16). The hybrid CMV enhancer/chicken
β-actin (CAG) promoter shows higher antigen expression than the CMV
promoter and therefore, this promoter has been universally adopted
in DNA vaccine research. The woodchuck hepatitis virus (WHV)
contains a post-transcriptional regulatory element (WPRE) (17), which increases the stability and
extranuclear transport of mRNA to the cytoplasm, resulting in
enhanced protein production. Several studies have shown that WPRE
increases transgenic expression from a variety of viral vectors
(18–20). An earlier vaccination study using
DNA vaccines that include either CAG or WPRE demonstrated that
compared with conventional CMV vectors, the vaccines including CAG
or WPRE increased immune responses to increased antigen expression
(21).
In previous studies, transgenic expression from
adenoviral vectors has been optimized by the incorporation of CAG
and WPRE (22). With the
optimization of promoter and transcriptional regulatory elements in
adenoviral vectors, the expression of the fusion protein encoded by
the chimeric gene, GnS0.7, was promoted. Humoral and cellular
immunity was also enhanced in mice immunized with optimized
adenoviral vectors containing GnS0.7. In this study, we promoted
the expression of the fusion protein, GcS0.7, by using the strong
CAG promoter and the powerful post-transcriptional response
element, WPRE, either together or individually. The effects of
these 2 elements were compared by cloning the fusion protein and
elements into the reconstructed adenoviral vectors and packaging
recombinant adenoviruses. The immunogenicity of recombinant
adenoviruses incorporating CAG or WPRE or both was also examined by
the immunization of C57BL/6 mice. Based on the results of this
study, we expect to find an effective means of amplifying the
immune response which elicits protective immunity against HTNV.
Materials and methods
Antibodies, cells and culture medium
The human embryonic kidney (HEK) 293 cell line (ATCC
No. CRL-1573; ATCC, Manassas, VA, USA) and B16 murine melanoma
cells (B16F10, ATCC no. CRL-6475) were maintained in Dulbecco’s
modified Eagle’s medium (DMEM; Gibco, Grand Island, NY, USA)
supplemented with 10% fetal bovine serum (FBS; HyClone, Logan, UT,
USA). Vero E6 cells (Vero C1008, ATCC no. CRL-1586) were maintained
in RPMI-1640 (Invitrogen, Carlsbad, CA, USA) supplemented with 10%
fetal calf serum (FCS; Gibco). All cells were incubated at 37ºC in
5% CO2. The HTNV strain 76–118 was used in this study.
mAb 1A8 (HTNV NP-specific), mAb 3G1 (with a high neutralizing
activity against HTNV) (23),
HFRS patient serum, Sp2/0 ascites and purified NP were all provided
by our laboratory. Purified HTNV glycoprotein was purchased from
the Lanzhou Institute of Biological Products, Lanzhou, China.
Female C57BL/6 mice (6–8 weeks old) were purchased from the Animal
Center of the Fourth Military Medical University, and housed in
ventilated cages. All animal experiments were conducted in
accordance with the procedures described in the Guide for the Care
and Use of Laboratory Animals (NIH Publications no. 80–23, revised
1978).
Construction of modified vectors and
recombinant adenoviruses
The chimeric gene, GcS0.7, the recombinant plasmid,
GcS0.7-pShuttle, and the recombinant adenovirus containing GcS0.7
(rAd-GcS0.7-pShuttle) were constructed as previously described by
Professor Fang-Lin Zhang of our laboratory (24). The CAG (GenBank accession no.
EF186086.1) sequence was synthesized with appropriate restriction
enzyme sites and inserted into the GcS0.7-pShuttle at the
MfeI and NheI restriction sites, while the
synthesized WPRE (GenBank accession no. AX823860.1) fragment was
inserted at the KpnI and AflII sites. The
reconstructed vectors were designated as GcS0.7-WPRE, GcS0.7-pCAG
and GcS0.7-pCAG-WPRE. The expression units were cloned into the
PI-SceI and I-CeuI sites of the pAdeno cosmid that
contained the genome of adenovirus type 5 without the E1 and E3
regions. The positive recombinant adenoviral DNA was linearized by
PacI and transfected into early-passage HEK 293 cells using
Lipofectamine 2000 according to the manufacturer’s instructions.
The recombinant adenoviruses designated as rAd-GcS0.7-WPRE,
rAd-GcS0.7-pCAG and rAd-GcS0.7-pCAG-WPRE were amplified and
purified using the ViraBind™ Adenovirus Purification kit (Cell
Biolabs, Inc., San Diego, CA, USA), and their titers were
determined using the Adeno-X™ Rapid Titer kit (Clontech, Mountain
View, CA, USA). The adenovirus Lac-Z and rAd-GcS0.7-pShuttle were
prepared in the same way. The final preparations were stored at
−80ºC.
Identification and comparison of GcS0.7
expressed in different recombinant adenoviruses
The GcS0.7 fusion protein of different recombinant
adenoviruses was identified by immunofluorescence assay (IFA). The
HEK 293 cells were plated in 24-well plates at a density of
2×105 cells/well−1 24 h before infection and
were persistently infected with recombinant adenoviruses at a
multiplicity of infection (MOI) of 100 pfu/cell for 4 h. Following
infection, fresh medium was added and the infected cells were
incubated at 37ºC in 5% CO2 for 48 h. By aspirating the
medium, the cells were allowed to dry in the hood for 5 min and
fixed by very gently adding ice-cold 100% methanol followed by
incubation at −20ºC for 10 min. mAb 1A8 and FITC-labeled goat
anti-mouse IgG antibody were used as the detecting antibodies.
After thorough washing, the infected cells were observed under a
fluorescence microscope. To determine which recombinant
adenoviruses had the highest expression level of the fusion
protein, GcS0.7, the HEK 293 cells were persistently infected 24 h
post-plating in 6-well plates at a MOI of 100 pfu/cell for 4 h.
After a further 48 h of incubation, the cells were harvested for
western blot analysis, and detected with mAb 1A8 and the IRDye 800
anti-mouse IgG antibody. All experiments were repeated, and similar
results were obtained each time.
Immunization of mice
Female C57BL/6 mice were randomly divided into 7
groups, including 4 experimental groups and 3 control groups; each
groups comprised 5 mice. The experimental groups were immunized
with 0.5 ml 108 pfu/ml recombinant adenovirus per mouse,
while the control groups were immunized with 0.5 ml physiological
saline, 0.5 ml 108 pfu/ml Adeno-X-Lac-Z or 10 μl HFRS
inactivated vaccine per mouse. All the immunizations were
administered 3 times at 2-week-intervals. Mouse sera were collected
individually via tail vein puncture at 2 and 4 weeks from the first
day of immunization or by retro-orbital plexus puncture 10 days
after the final immunization. Additionally, splenocytes were
isolated for subsequent tests.
Antibody detection
In the present study, 3 types of antibodies were
detected, including the anti-NP, anti-glycoprotein and neutralizing
antibody against HTNV. Indirect enzyme-linked immunosorbent assays
(ELISA) were used to detect NP and glycoprotein-specific
antibodies. Purified NP or glycoprotein was used as the coating
antigen, HRP-labeled goat anti-mouse antibody was used as the
detection antibody and OPD was used as the substrate. The
colorimetric reaction was terminated by the addition of 2 M
H2SO4, and the optical density (OD) at 490 nm
was determined using a standard ELISA plate reader. The antibody
titers were defined as the reciprocal of the serum dilution with
the highest positive response. Neutralizing antibodies were
detected by cell microculture neutralization tests. The heat
inactivated sera were serially diluted 2-fold from 1:5 in RPMI-1640
containing 2% FCS, and combined with an equal volume of 100
TCID50 (median tissue culture infective dose) HTNV
(76–118 strain). Following incubation at 37ºC for 90 min, 100
μl/cell of the mixture was applied to monolayers of Vero E6 cells
followed by further incubation at 37ºC for 9 to 11 days in a 5%
CO2 incubator. Thereafter, the cells were lysed by 3
consecutive freeze-thaw cycles. The presence of HTNV antigen in the
cell lysates was detected by sandwich ELISA with mAb 1A8 used as a
coating antibody, and HRP-conjugated 1A8 used as the detection
antibody. The mAb 3G1 and Sp2/0 ascites were used as the positive
and negative controls, respectively. The absorbance was measured at
490 nm using a standard ELISA plate reader. The neutralizing
antibody titer was defined as the maximum dilution of serum that
inhibited HTNV infection in 50% of the cells.
Detection of IFN-γ secretion by T
cells
The enzyme-linked immunospot (ELISPOT) assay was
used to determine the amount of T cells capable of responding to
IFN-γ stimulus. Mice were sacrificed and orbital blood samples were
collected 10 days after the final booster immunization. Spleen
cells were purified in lymphocyte separation medium. Freshly
isolated splenocytes (1×106 cells in 100 μl) were added
into each well of pre-coated IFN-γ plates (Mabtech AB, Stockholm,
Sweden), and stimulated with purified HTNV glycoprotein antigen (10
μg/ml), or the positive stimulator, concanavalin A (ConA; 4 μg/ml).
Splenocytes incubated with 100 μl 2% FCS DMEM were used as the
negative or background controls. These plates were incubated at
37ºC for 18 h. Cytokine ELISPOT assays were carried out according
to the manufacturer’s instructions. Spots were counted using an
ELISPOT reader system (Cellular Technology Ltd., Hong Kong, China),
and the results were expressed as the mean number of specific IFN-γ
spot-forming cells per 1×106 splenocytes.
Cytotoxicity assay
The cytotoxicity of GcS0.7-specific cytotoxic T
lymphocytes (CTLs) was determined using the CytoTox 96™
Non-Radioactive Cytotoxicity Assay kit (Promega, Madison, WI, USA)
in accordance with the instructions of the manufacturer.
Splenocytes from mice immunized with recombinant adenoviruses or
sham-inoculated mice were prepared as previously described and used
as effector cells, while B16 cells transfected with GcS0.7-pCDNA3.1
and screened by G418 were used as the target cells, with normal
splenocytes as the negative control. The target B16-GcS0.7 or B16
cells were plated at 1×104 cells/well in 96-well
U-bottomed plates and effector splenocytes were then added at
effector:target (E:T) ratios of 20:1, 10:1 and 5:1 in a final
volume of 100 μl. Following 4 h of incubation at 37ºC and 5%
CO2, the cytotoxicity assay plates were centrifuged at
250 × g for 5 min, and 50 μl aliquots from all wells were
transferred to fresh 96-well flat-bottom plates, and an equal
volume of reconstituted substrate mix was added to each well. The
plates were incubated in the dark at room temperature for 30 min.
Subsequently, 50 μl of the stop solution were added, and the
absorbance values at 490 nm were measured. The percentage
cytotoxicity was calculated according to the following formula: %
cytotoxicity = [(E - St - Se)/(M - St)] ×100 [E, effector-target
co-culture cells lactate dehydrogenase (LDH) release; St, target
cell spontaneous LDH release; Se, effector cell spontaneous LDH
release; M, target cell maximum LDH release].
Statistical analysis
Statistical analysis was performed using GraphPad
Prism software version 5.0. One-way ANOVA was used to determine
statistically significant differences among the experimental
groups. Student’s t-tests were used to determine significant
differences between experimental and control groups. A value of
P<0.05 was considered to indicate a statistically significant
difference.
Results
Identification of modified vectors and
recombinant adenoviral DNA containing GcS0.7
In this study, we modified the pShuttle vector with
the chimeric gene, GcS0.7 (GcS0.7-pShuttle), as follows: i)
replacement of conventional human cytomegalovirus immediate-early
promoter/enhancer (CMV) of pShuttle with the hybrid CMV
enhancer/chicken β-actin promoter (CAG); ii) replacement of the
enhancer/promoter in conjunction with the mRNA post-transcriptional
regulatory element of the woodchuck hepatitis virus (WPRE); iii)
single insertion of WPRE upstream of the 3 pShuttle stop codons
(Fig. 1). The accuracy of cloning
in each of these constructs and packaging of recombinant
adenoviruses was confirmed by restriction enzyme (Fig. 2) and polymerase chain reaction
(PCR) analysis.
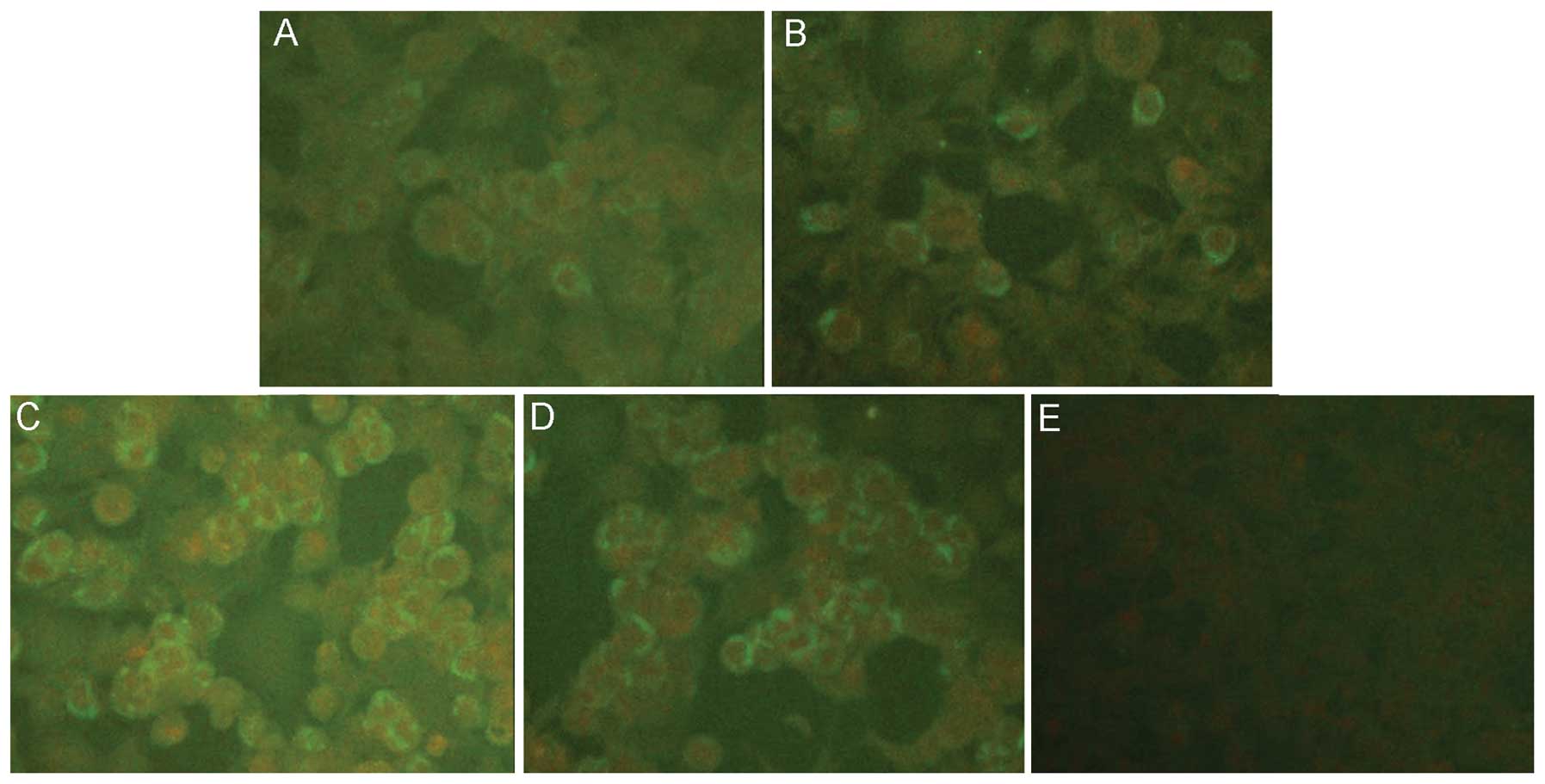
Immunofluorescence assay detecting the
target proteins expressed by recombinant adenoviruses
After purification, the recombinant adenoviruses
containing the chimeric gene, GcS0.7, were concentrated to
1010 pfu/ml. HEK 293 cells infected with the recombinant
adenoviruses were examined by IFA using mAb 1A8 (Fig. 3). Positive fluorescence was
observed in the cells 48 h after infection. The most intense
fluorescence due to the expression of the target protein, GcS0.7,
was observed in the cells infected with the vector containing the
CAG promoter and with the CAG promoter in conjunction with WPRE.
The fluorescence due to the expression of GcS0.7 from the vector
incorporating only WPRE was less intense than the other 2
vectors.
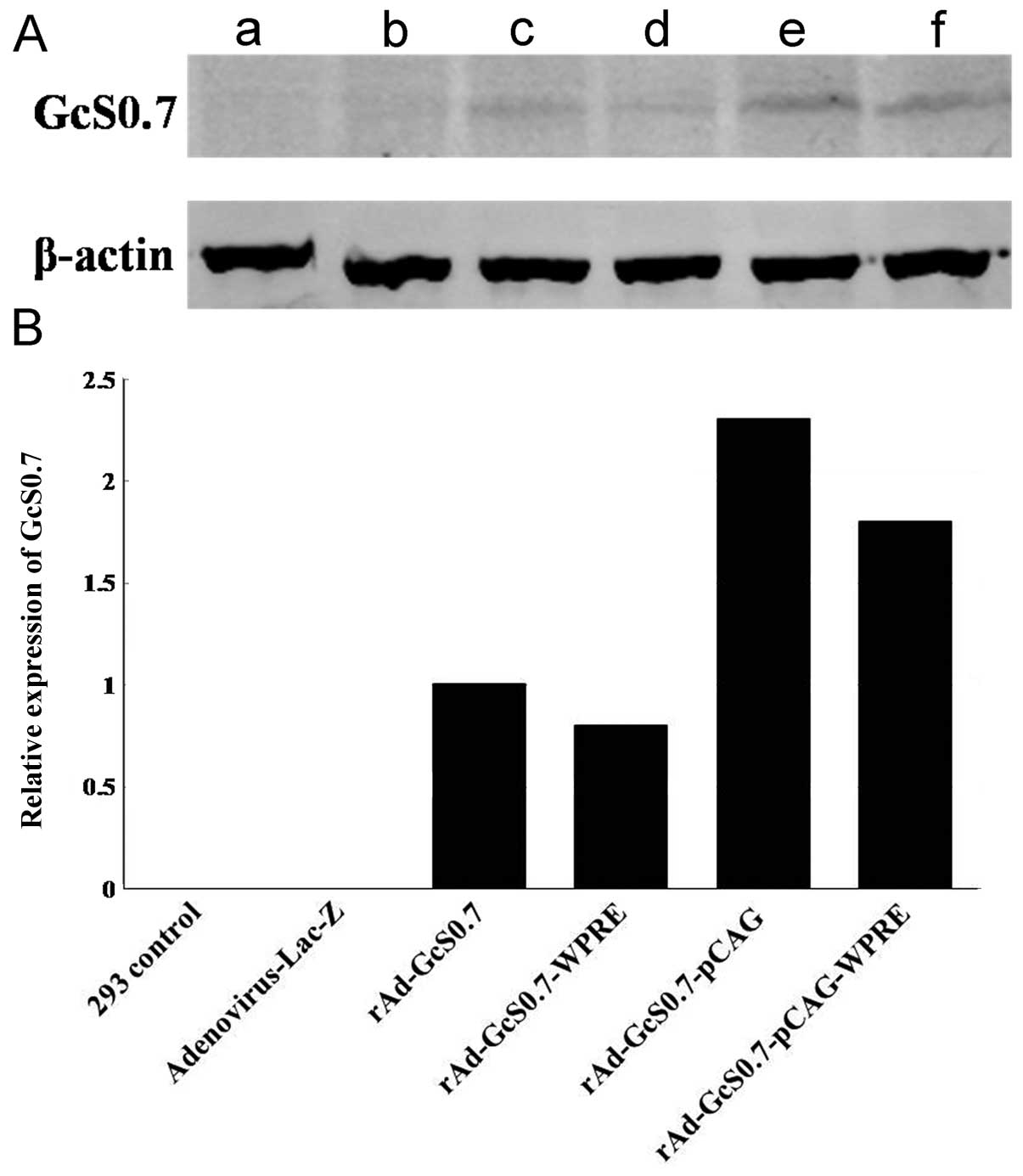
Comparison of the fusion proteins
expressed by recombinant adenoviruses
Western blot analysis was performed to investigate
the fusion protein expression levels. Equal amounts of GcS0.7
extracted from the HEK 293 cells infected for 48 h were separated
on a 12% SDS-polyacrylamide gel and analyzed by western blot
analysis using mAb 1A8. The proteins extracted from the
non-infected HEK 293 cells and from adenovirus-Lac-Z-infected cells
were used as the controls. A specific protein band of approximately
80 kDa was bound by mAb 1A8, which was consistent with the
molecular weight of the GcS0.7 fusion protein. The western blot
analysis results further demonstrated that the expression
efficiency of the CAG hybrid promoter/enhancer was greater than
that of the other groups: the expression of GcS0.7 from
rAd-GcS0.7-pCAG was 2.3-fold greater than that from the unmodified
rAd-GcS0.7 vector (Fig. 4).
Specific humoral immune responses
elicited by recombinant adenoviruses
The geometric mean titer (GMT) values (glycoprotein
and NP-specific) of mice immunized with rAd-GcS0.7-pCAG were the
highest among all recombinant adenovirus groups; (69.6 and 139.3,
respectively). The GMT (glycoprotein and NP-specific) values of
mice immunized with the HFRS inactivated vaccine were 160 and 640,
respectively (Tables I and
II). As shown in Table III, the recombinant adenovirus
generated immune sera against the 220
 | Table IHTNV glycoprotein-specific antibody
titers detected in the serum of immunized mice. |
Table I
HTNV glycoprotein-specific antibody
titers detected in the serum of immunized mice.
| Mouse no. |
rAd-GcS0.7-pShuttle | rAd-GcS0.7-WPRE | rAd-GcS0.7-pCAG | rAd-GcS0.7-pW | Adeno-Lac-Z | Vaccine |
|---|
| 1 | 20 | 40 | 80 | 40 | 5 | 160 |
| 2 | 20 | 20 | 40 | 80 | 10 | 160 |
| 3 | 40 | 10 | 80 | 40 | 10 | 320 |
| 4 | 10 | 40 | 40 | 20 | 5 | 80 |
| 5 | 10 | 20 | 160 | 80 | 5 | 160 |
 | Table IIHTNV nucleocapsid protein-specific
antibody titers detected in the serum of immunized mice. |
Table II
HTNV nucleocapsid protein-specific
antibody titers detected in the serum of immunized mice.
| Mouse no. |
rAd-GcS0.7-pShuttle |
rAd-GcS0.7-WPRE |
rAd-GcS0.7-pCAG | rAd-GcS0.7-pW | Adeno-Lac-Z | Vaccine |
|---|
| 1 | 80 | 40 | 320 | 160 | 10 | 640 |
| 2 | 40 | 80 | 160 | 80 | 5 | 640 |
| 3 | 40 | 20 | 80 | 160 | 5 | 640 |
| 4 | 20 | 40 | 160 | 40 | 2 | 640 |
| 5 | 40 | 40 | 80 | 80 | 5 | 640 |
 | Table IIINeutralizing antibody titers detected
in the serum of immunized mice. |
Table III
Neutralizing antibody titers detected
in the serum of immunized mice.
| Mouse no. |
rAd-GcS0.7-pShuttle |
rAd-GcS0.7-WPRE |
rAd-GcS0.7-pCAG | rAd-GcS0.7-pW | Adeno-Lac-Z | Vaccine |
|---|
| 1 | 10 | 10 | 40 | 20 | - | 20 |
| 2 | 20 | 20 | 20 | 40 | - | 10 |
| 3 | 10 | 20 | 20 | 20 | 5 | 10 |
| 4 | 20 | 20 | 40 | 10 | - | 20 |
| 5 | - | 20 | 20 | 20 | - | 20 |
220HTNV strain 76–118 showed neutralizing
titers ranging from 10 to 40, of which the titer values of
rAd-GcS0.7-pCAG were higher than those of the others
Similar levels of neutralizing antibodies were
detected in the inactivated vaccine immunized group, while the sera
from the control and Adeno-Lac-Z groups showed no obvious
neutralizing activity. These results suggest that immunization with
recombinant adenoviruses carrying GcS0.7 elicits neutralizing
antibodies as efficiently as the inactivated vaccine.
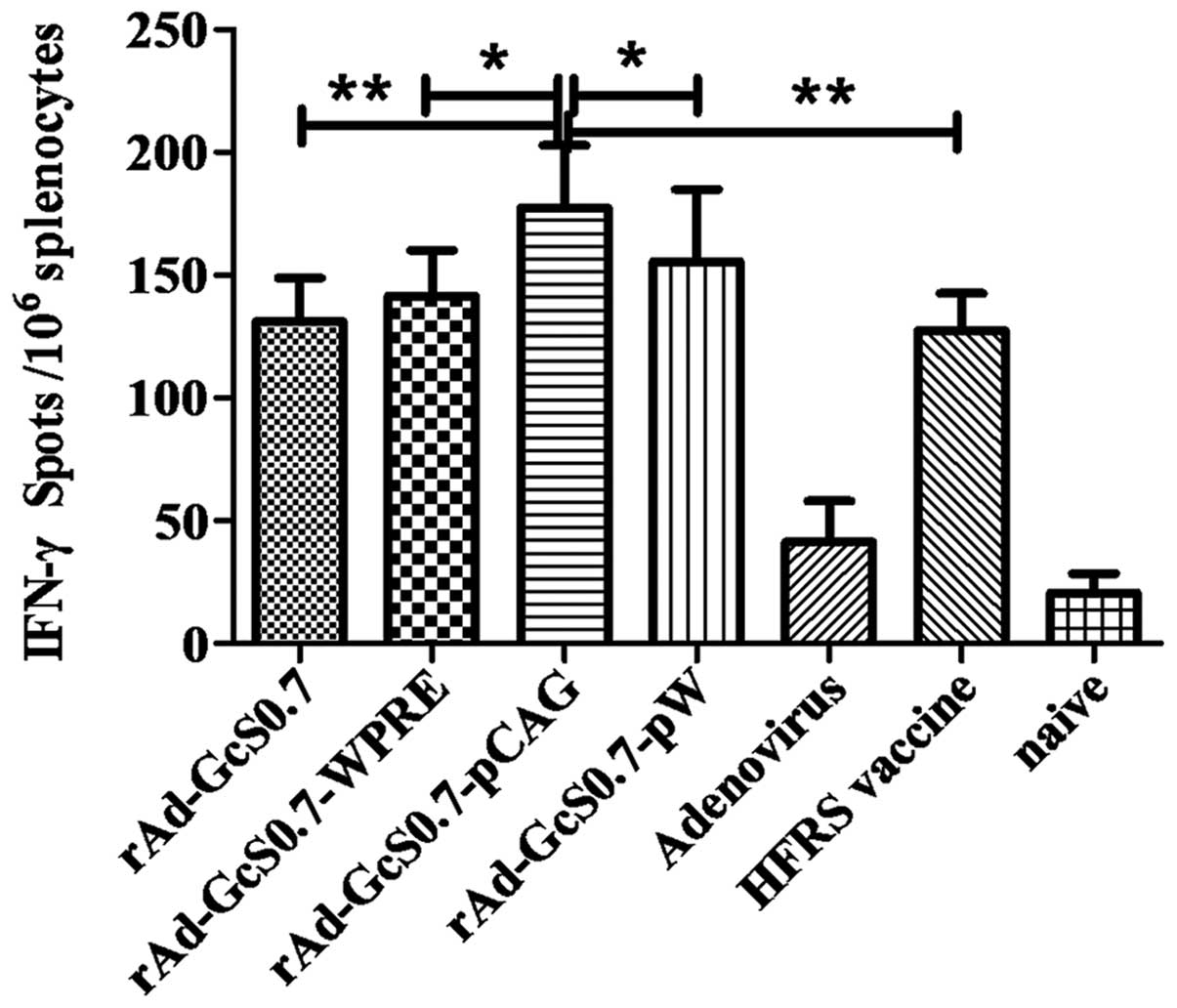
Cellular immune responses elicited by
recombinant adenoviruses
The frequency of splenic CD8+ T cells
secreting IFN-γ from the immunized mice was detected by ELISPOT
assay. As shown in Fig. 5,
adenovirus-Lac-Z and the naïve control induced negligible IFN-γ
responses. By contrast, all recombinant adenoviruses expressing the
fusion protein induced an effective IFN-γ response, and among
these, the group immunized with rAd-GcS0.7-pCAG had a significantly
higher response compared to the other groups. The specific T
cell-mediated cytotoxicity of vaccination-activated splenocytes was
detected using the CytoTox 96 Non-Radioactive Cytotoxicity assay,
whereby the release of LDH from attacked HTNV GcS0.7-expressing B16
cells is measured. As shown in Fig.
6, splenocytes from the mice immunized with recombinant
adenoviruses containing GcS0.7 exhibited variable levels of
specific cytotoxicity against the B16-GcS0.7 cells (P<0.05), and
the cytotoxicity was enhanced with the E/T ratio, which was the
most significant at the ratio of 100:1. Among all the experimental
groups, splenocytes from the mice immunized with rAd-GcS0.7-pCAG
showed higher specific cytotoxicity compared to the other groups at
E/T ratios of 100:1, 50:1 and 20:1 (P<0.05). The cytotoxicity
from the rAd-GcS0.7-pCAG immunized mouse spleens was even greater
than that from the mice immunized with the inactivated vaccine at
E/T ratios of 100:1 and 50:1 (P<0.05). By contrast, the
non-specific cytotoxicity against the B16-GcS0.7 cells of the
control mice immunized with adenovirus-Lac-Z or NC was very weak at
E/T ratios of 100:1, 50:1 and 20:1.
Discussion
Hantaviruses are distributed worldwide and cause
serious human diseases. Until now, rodent brain and cell
culture-derived inactivated hantavirus vaccines have been used in
South Korea and China (25).
Although the inactivated vaccines have demonstrated promising
protective effects, there are still unresolved issues concerning
their production and human use: the titers of virus-neutralizing
antibodies were found to be low and to possess low immunogenicity
(26). Furthermore, the
preparation of inactivated vaccines requires high-level safety
conditions. Thus, there is still a need to improve these vaccines
to generate stronger and more long-lasting immune responses.
In hantavirus infections, both humoral and cellular
immunity are involved in protection. As enveloped viruses,
hantavirus Gn and Gc glycoproteins are able to induce
virus-neutralizing antibodies which are sufficient for protection
against virus challenge in animal models (27). The Gc glycoprotein of hantaviruses
and of other members of the Bunyaviridae family directs the
viral fusion activity and is therefore classified as a class II
viral fusion protein (28). Thus,
antibodies against Gc may play a significant role in preventing
viral entry into the cell. The role of CTLs in the elimination of
hantavirus-infected cells was also observed in vivo and
in vitro. The CTL response was directed against the NP.
Previous studies have indicated that recombinant
vaccines based on adenoviral vectors expressing both glycoprotein
and NP may be able to overcome the defects of conventional
hantavirus vaccines. With the help of the expression-regulating
elements, CAG and WPRE, the expression of the recombinant
adenovirus chimeric gene, GnS0.7, elicited high-level immune
responses of both the humoral and cellular type. Mice immunized
with rAd-GnS0.7-pCAG demonstrated the highest immunity of all the
groups (22).
The effective expression of protein antigens is the
basic requirement in vaccine development. With their many
advantages over other vectors, adenoviral vectors were used in this
study. Recombinant adenoviruses containing the chimeric gene,
GcS0.7, were constructed with the CAG promoter and WPRE used as
substitutes for the original CMV promoter in the commercial
adenoviral vector to increase the expression of the fusion protein,
GcS0.7. The fusion protein, GcS0.7, was detected in all recombinant
adenovirus groups by western blot analysis and IFA, with both the
CAG promoter and WPRE elements shown to act separately to boost the
expression level of the fusion protein compared with the
conventional CMV promoter-based viral vector. Recombinant
adenoviruses containing the CAG promoter showed the highest
expression level and a synergistic effect of the 2 elements was not
observed in our study. An earlier vaccination study using DNA
vaccines that include either CAG or WPRE demonstrated that compared
with conventional CMV vectors, the vaccines including CAG or WPRE
increased immune responses to increased antigen expression
(21). The WPRE has been shown to
enhance transgenic expression driven from a series of different
promoters in various vector and cell types at the
post-transcriptional level (10,18). However, certain studies have shown
conflicting, even opposing effects of the WPRE due to differences
between cell lines and promoters (29). In our previous study, the
combination of the CAG promoter/enhancer and WPRE did not produce
the highest expression levels of the fusion protein among all
groups in HEK 293 cells (22).
The data obtained in this study were consistent with this
phenomenon. The CAG and WPRE did not show a synergistic effect,
which may be attributed to the inhibitory effect of WPRE on the CAG
promoter activity during transcription.
To further evaluate the correlation of the immune
responses with the fusion protein expression level, we investigated
both the humoral and cellular immune responses in C57BL/6 mice
immunized with recombinant adenoviruses or inactivated vaccine. The
antibody titers against the glycoprotein and NP of mice immunized
with rAd-GcS0.7-pCAG were the highest among all experimental
groups. Antibody titers of the mice immunized with the HFRS
inactivated vaccine were higher than those generated by any of the
recombinant viral vectors. Neutralizing antibodies are key
effectors involved in protection against hantavirus infection. Our
results revealed that immunization with recombinant adenoviruses
carrying GcS0.7 elicited equivalent neutralizing antibody responses
compared with the inactivated vaccine, thus indicating that
recombinant adenoviruses can elicit the same protection as
inactivated vaccines. The elevated antigen expression leading to
improved humoral immunity through incorporation of the CAG promoter
into the adenovirus expression system has also been confirmed by
other studies. Richardson et al demonstrated the improved
expression of Ebola glycoprotein from adenoviral vectors by using
the CAG promoter and showed that immunization of mice with the
improved recombinant adenovirus resulted in enhanced B cell immune
responses (30).
As cellular immune responses are also important in
limiting viral infection and replication, the hantavirus-specific
cellular immune responses were further assessed in vitro.
During hantavirus infection of humans there is a mixed Th1/Th2
response, where the Th1 type response is induced mainly by NP and
the Th2 response is induced by the Gn/Gc glycoproteins (10). The significant role of NP-induced
CTL in the protection against viral infection is well established,
and to further evaluate the cell-mediated immune responses we
focused on the Th1 type response in this study. The
antigen-specific production of IFN-γ has been promoted as a
quantitative marker of Th1-type protective cell-mediated immune
responses. Additionally, IFN-γ is a very important cytokine for the
maturation of natural killer (NK) cells, which contribute to innate
immune responses against viruses. During hantavirus infection, when
kidney failure and pneumonia are 2 main symptoms, an increased
number of NK cells in the kidneys or lung tissue would accelerate
hantavirus clearance. In this study, ELISPOT assay analysis of the
IFN-γ production by hantavirus-specific CTLs showed that
rAd-GcS0.7-pCAG stimulated higher IFN-γ release after immunization
compared with that detected in the other experimental groups and
the vaccine control. Although the IFN-γ ELISPOT assay is widely
adopted for the measurement of IFN-γ secretion to demonstrate
specific CTL reactivity, this method is limited in that IFN-γ can
also be released by non-cytotoxic cells involved in innate and
adaptive immune responses (31).
To overcome this deficiency, the CytoTox 96 Non-Radioactive
Cytotoxicity assay was used to measure the frequency specific
target cell lysis by CTLs in order to assess the T cell functional
activity. All the recombinant adenoviruses used in this study were
shown to induce specific cytotoxic effects on the target cells,
with the strongest effects induced by rAd-GcS0.7-pCAG in comparison
with the other experimental groups and even the vaccine control at
E/T ratios of 100:1 and 50:1 (P<0.05). This may be attributed to
the high expression level of NP, which elicits greatest
antibody-dependent cytotoxic T cell responses among the hantavirus
structural proteins.
In this study, we investigated the immunity induced
by the hantavirus fusion antigen and the effects of the CAG
promoter and the WPRE regulatory element on protein expression. The
results revealed that the recombinant adenovirus containing the
chimeric gene, GcS0.7, induced effective humoral and cellular
immune responses. With the help of the powerful hybrid CAG
promoter, immunity was promoted to a level at which the cellular
immunity surpassed even that of the inactivated vaccine. However,
the protective effects of the recombinant virus still need further
validation in vivo. The results of this study indicate that
this strategy may enhance Th1 type cellular immune responses and
may thus be suitable for the future prevention of HFRS.
Acknowledgements
The present study was supported by grants from the
National Key Basic Research Program (973 Program) (no.
2012CB518905), the National High Technology Research and
Development Program (863 Program) (no. 2006AA02A225) and the
Natural Science Foundation of China (nos. 81001344 and
31070810).
References
|
1
|
Hepojoki J, Strandin T, Lankinen H and
Vaheri A: Hantavirus structure - molecular interactions behind the
scene. J Gen Virol. 93:1631–1644. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Plyusnin A, Vapalahti O and Vaheri A:
Hantaviruses: genome structure, expression and evolution. J Gen
Virol. 77:2677–2687. 1996. View Article : Google Scholar
|
|
3
|
Charrel RN, Coutard B, Baronti C, et al:
Arenaviruses and hantaviruses: from epidemiology and genomics to
antivirals. Antiviral Res. 90:102–114. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hart CA and Bennett M: Hantavirus
infections: epidemiology and pathogenesis. Microbes Infect.
1:1229–1237. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Clement JP: Hantavirus. Antiviral Res.
57:121–127. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kruger DH, Schonrich G and Klempa B: Human
pathogenic hantaviruses and prevention of infection. Hum Vaccin.
7:685–693. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Simmons JH and Riley LK: Hantaviruses: an
overview. Comp Med. 52:97–110. 2002.
|
|
8
|
Kariwa H, Yoshimatsu K and Arikawa J:
Hantavirus infection in East Asia. Comp Immunol Microbiol Infect
Dis. 30:341–356. 2007. View Article : Google Scholar
|
|
9
|
Schmaljohn C: Vaccines for hantaviruses.
Vaccine. 27(Suppl 4): D61–D64. 2009. View Article : Google Scholar
|
|
10
|
Khaiboullina SF and St Jeor SC: Hantavirus
immunology. Viral Immunol. 15:609–625. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Van Epps HL, Schmaljohn CS and Ennis FA:
Human memory cytotoxic T-lymphocyte (CTL) responses to Hantaan
virus infection: identification of virus-specific and
cross-reactive CD8(+) CTL epitopes on nucleocapsid protein. J
Virol. 73:5301–5308. 1999.PubMed/NCBI
|
|
12
|
Yoshimatsu K, Yoo YC, Yoshida R, Ishihara
C, Azuma I and Arikawa J: Protective immunity of Hantaan virus
nucleocapsid and envelope protein studied using
baculovirus-expressed proteins. Arch Virol. 130:365–376. 1993.
View Article : Google Scholar
|
|
13
|
Xue X, Xu Z and Ma W: Expression of
truncated HTNV nucleoprotein and analysis of antigenic epitope.
Virol Sin. 15:220–225. 2000.
|
|
14
|
Zhang FL, Wu XA, Luo W, et al: The
expression and genetic immunization of chimeric fragment of Hantaan
virus M and S segments. Biochem Biophys Res Commun. 354:858–863.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Luo W, Zhang F, Yan Y, et al:
Immunological properties of a fusion protein containing
nucleocapsid protein and glycoprotein Gn of Hantaan virus. Acta
Virol. 52:243–249. 2008.PubMed/NCBI
|
|
16
|
Gribaudo G, Ravaglia S, Caliendo A, et al:
Interferons inhibit onset of murine cytomegalovirus immediate-early
gene transcription. Virology. 197:303–311. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Donello JE, Loeb JE and Hope TJ: Woodchuck
hepatitis virus contains a tripartite posttranscriptional
regulatory element. J Virol. 72:5085–5092. 1998.PubMed/NCBI
|
|
18
|
Real G, Monteiro F, Burger C and Alves PM:
Improvement of lentiviral transfer vectors using cis-acting
regulatory elements for increased gene expression. Appl Microbiol
Biotechnol. 91:1581–1591. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu ZL, Mizuguchi H, Mayumi T and Hayakawa
T: Woodchuck hepatitis virus post-transcriptional regulation
element enhances transgene expression from adenovirus vectors.
Biochim Biophys Acta. 1621:266–271. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zufferey R, Donello JE, Trono D and Hope
TJ: Woodchuck hepatitis virus posttranscriptional regulatory
element enhances expression of transgenes delivered by retroviral
vectors. J Virol. 73:2886–2892. 1999.
|
|
21
|
Garg S, Oran AE, Hon H and Jacob J: The
hybrid cytomegalovirus enhancer/chicken beta-actin promoter along
with woodchuck hepatitis virus posttranscriptional regulatory
element enhances the protective efficacy of DNA vaccines. J
Immunol. 173:550–558. 2004. View Article : Google Scholar
|
|
22
|
Li PY, Yu L, Wu XA, et al: Modification of
the adenoviral transfer vector enhances expression of the
Hantavirus fusion protein GnS0.7 and induces a strong immune
response in C57BL/6 mice. J Virol Methods. 179:90–96. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu Z, Wei L, Wang L, Wang H and Jiang S:
The in vitro and in vivo protective activity of monoclonal
antibodies directed against Hantaan virus: potential application
for immunotherapy and passive immunization. Biochem Biophys Res
Commun. 298:552–558. 2002. View Article : Google Scholar
|
|
24
|
Zhang F, Liu Y, Yu L, et al: Construction
and identification of recombinant adenovirus containing chimeric
gene G2S0.7 of Hantaan virus. J Fourth Mil Med Univ. 25:1057–1060.
2004.
|
|
25
|
Kruger DH, Ulrich R and Lundkvist AA:
Hantavirus infections and their prevention. Microbes Infect.
3:1129–1144. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hooper JW and Li D: Vaccines against
hantaviruses. Curr Top Microbiol Immunol. 256:171–191.
2001.PubMed/NCBI
|
|
27
|
Schonrich G, Rang A, Lutteke N, Raftery
MJ, Charbonnel N and Ulrich RG: Hantavirus-induced immunity in
rodent reservoirs and humans. Immunol Rev. 225:163–189. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tischler ND, Gonzalez A, Perez-Acle T,
Rosemblatt M and Valenzuela PD: Hantavirus Gc glycoprotein:
evidence for a class II fusion protein. J Gen Virol. 86:2937–2947.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Klein R, Ruttkowski B, Knapp E, Salmons B,
Gunzburg WH and Hohenadl C: WPRE-mediated enhancement of gene
expression is promoter and cell line specific. Gene. 372:153–161.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Richardson JS, Yao MK, Tran KN, et al:
Enhanced protection against Ebola virus mediated by an improved
adenovirus-based vaccine. PLoS One. 4:e53082009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lehmann PV and Zhang W: Unique strengths
of ELISPOT for T cell diagnostics. Methods Mol Biol. 792:3–23.
2012. View Article : Google Scholar : PubMed/NCBI
|