Introduction
It has been reported that approximately 366 million
individuals worldwide were affected by diabetes in 2011 and the
numbers are estimated to reach 522 million by 2030 (1). With the increase in diabetes cases,
complications associated with diabetes, such as large-vessel
obstruction (including coronary artery diseases, atherosclerosis
and peripheral vascular diseases) and microvascular pathologies
(including retinopathy, nephropathy and neuropathy) are also
expected to increase (2). Insulin
resistance is known to play a vital role in diabetes and its
complications (3,4).
Glucagon-like peptide-1 (GLP-1), an anorexigenic
hormone, secreted by intestinal L-cells, has several physiological
functions, such as lowering blood glucose levels and increasing
insulin secretion (5–7). Liraglutide, marketed under the brand
name, Victoza, is a long-acting GLP-1 agonist developed by Novo
Nordisk (Bagsvaerd, Denmark) for the treatment of type 2 diabetes
(8). The product was approved by
the European Medicines Agency (EMA) on July 3, 2009 and by the US
Food and Drug Administration (FDA) on January 25, 2010. It has been
reported that liraglutide activates the GLP-1 receptor and exerts
several physiological effects, such as decreasing blood glucose
levels, improving lipid metabolism and reducing blood pressure and
endothelial dysfunction (9,10).
However, the mechanisms behind its anti-diabetic effects remain
unknown.
Studies have confirmed that important insulin
signaling molecules, such as insulin receptor (InsR),
phosphatidylinositide 3-kinases (PI3Ks) and glucose transporter
(GLUT), play a vital role in glucose metabolism and insulin
resistance (11,12). In particular, GLUT4 is one of the
predominant GLUTs in cells, promoting glucose trafficking and
uptake. The concentration of glucose increases abnormally with
anomalies in GLUT4 expression (13,14). KK/Upj-Ay/J (KKAy) mice can
spontaneously become obese, hyperglycemic and hypertriglyceridemic,
and are thus used as a polygenic type 2 diabetes model (15). In the present study, we used
diabetic KKAy mice to investigate the effects and the mechanisms of
action of liraglutide on glucose metabolism and insulin
resistance.
Materials and methods
Animals, grouping and treatment
Twelve male KKAy mice (11–13 weeks old) and 6
C57BL/6J (C57) mice (same age) were obtained from the Chinese
Academy of Medical Sciences (Beijing, China) and were kept
individually in plastic cages covered with wood shavings at a
temperature of 20–25°C and a humidity of 45–55% with illumination
for 12 h in a specific pathogen-free (SPF) environment. The KKAy
mice were fed high-fat chow and the C57 mice were fed ordinary
animal chow. All the mice were allowed free access to water. The
present study was approved by the Ethics Committee on the Use of
Experimental Animals of Xi’an Jiaotong University, Xi’an,
China.
After the mice were allowed to acclimatize for 1
week, fasting blood glucose (FBG) levels in the KKAy mice were
measured. The mice with FBG values >16.7 mmol/l were considered
diabetic and randomly divided into 2 experimental groups: the
liraglutide group [n=6, subcutaneous (s.c.) liraglutide injection
250 μg/kg/day; obtained from Novo Nordisk] and the model
group (n=6, s.c. equivalent volume of normal saline). The C57 mice
were considered non-diabetic and used as the control group (n=6,
s.c. equivalent volume of normal saline). Drugs were administered
to the mice between 16:30 and 17:00 p.m. each day. During the
6-week treatment period, FBG levels (6-h fast) and body weights of
all the animals were measured weekly using a glucose meter (Roche
Diagnostics GmbH, Mannheim, Germany) and a laboratory electronic
scale. At the end of the experiment, blood samples from the KKAy
mice were obtained through the venous plexus behind the eyeball
following anesthesia by 10% chloral hydrate. The samples were
placed into Eppendorf tubes and centrifuged at 1,368 ×g for 10 min
at room temperature and the plasma was collected and stored at
−80°C until analysis. The mice were then sacrificed. Tissues were
separated into 3 specimens with one immediately stored in Eppendorf
tubes frozen in liquid nitrogen and the second immersed into
stationary liquid and observed under an electron microscope and
then rapidly placed in a refrigerator at 4°C until analysis. The
third was fixed in 10% formalin for immunohistochemical
examination.
Oral glucose tolerance test (OGTT)
The OGTT was performed on the 7th week of the
experimental period. After 6 h of fasting, a glucose solution of 2
g/kg was orally administered, and blood glucose was measured at 0,
30, 60 and 120 min, after obtaining blood from an injection into
the tail vein, using a glucose meter and the area under the curve
(AUC) was calculated for FBG during the OGTT.
Insulin tolerance test (ITT)
After 6 weeks of treatment, an ITT was performed. A
subcutaneous injection of 4 U/kg insulin (Jiangsu Wanbang
Biochemistry Medicine Co., Xuzhou, China) was administered after 6
h of fasting and blood glucose levels were measured at 0, 40 and 90
min using a glucose meter. The AUC was calculated for FBG during
the ITT.
Serum insulin detection and insulin
resistance index calculation
Serum insulin levels were measured by enzyme-linked
immunosorbent assay (ELISA) using a mouse insulin ELISA kit
(R&D System, Inc., Minneapolis, MN, USA). The homeostasis model
assessment of insulin resistance (HOMA-IR) and the insulin
sensitivity index (ISI) were calculated according to the following
formula: HOMA-IR = FBG (mmol/l) × FINS (μU/ml)/22.5; ISI =
1/[FBG (mmol/l) × FINS (μU/ml)], where FINS represents the
fasting insulin levels, as previously described (16).
Liver and skeletal muscle biochemical
index analysis
Liver and skeletal muscle glycogen levels were
measured using the anthracenone method. Skeletal muscle pyruvate
kinase (PK) levels were measured by colorimetric assay and
hexokinase (HK) levels were measured using the glucose 6 phosphate
dehydrogenase coupling colorimetric method. The measurements of
such indexes were performed using commercial kits (Nanjing
Jiancheng Bioengineering Institute, Nanjing, China).
Transmission electron microscopy
For electron microscopy, portions of the splenic
region of each pancreas were minced into 1-mm cubes, fixed with
2.5% glutaraldehyde and 2% osmic acid and then dehydrated and
embedded in epoxy resin. Ultrathin sections were collected onto
200-mesh copper grids and double stained with uranyl acetate and
lead acetate, and each section was observed under a Hitachi H-7650
Transmission Electron Microscope (Hitachi, Tokyo, Japan).
RNA isolation and real-time PCR
Total RNA was isolated from mouse liver tissues
using TriPure RNA isolation reagent (Roche, Basel, Switzerland),
and 2 μg of RNA were reverse-transcribed using the Prime
Script™ RT Master Mix (Perfect Real Time) (Takara Bio, Inc., Tokyo,
Japan). Quantitative RT-PCR was performed using SYBR®
Premix Ex Taq™ II (Perfect Real-Time; Takara Bio, Inc.). PCR
reactions were performed in 96-well plates in an iQ5 Real-Time PCR
Detection System (Bio-Rad Laboratories, Hercules, CA, USA).
All of the primers and probes for RT-PCR were
obtained from Takara Bio, Inc. Murine InsR was amplified using the
primers InsR-F (5′-GCC GCT CCT ATG CTC TGG TAT C-3′) and InsR-R
(5′-AGT TGC CTC AGG TTC TGG TTG TC-3′); murine PI3K was amplified
using the primers PI3K regulatory subunit 1 (Pik3r1)-F (5′-GCT CCT
GGA AGC CAT TGA GAA-3′) and Pik3r1-R (5′-CGT CGA TCA TCT CCA AGT
CCA C-3′); murine GLUT2 was amplified using the primers solute
carrier family 2 (facilitated glucose transporter), member 2
(Slc2a2)-F (5′-GGC ATC AGC CAG CCT GTG TA-3′) and Slc2a2-R (5′-CAT
GCC AAT CAT CCC GGT TAG-3′); murine GLUT4 was amplified using the
primers solute carrier family 2 (facilitated glucose transporter),
member 4 (Slc2a4)-F (5′-TCT TAT TGC AGC GCC TGA GTC-3′) and
Slc2a4-R (5′-GCC AAG CAC AGC TGA GAA TAC A-3′); and murine GAPDH
was amplified using the primers GAPDH-F (5′-TGT GTC CGT CGT GGA TCT
GA-3′) and GAPDH-R (5′-TTG CTG TTG AAG TCG CAG GAG-3′). GAPDH
served as the endogenous control. The results were normalized to
GAPDH. Efficiencies of RT-PCR for the target gene and the
endogenous control were approximately equal. The −ΔCT expresses the
difference between the number of cycles (CT) of the target genes
and the endogenous control. The results were expressed as
2−ΔΔCt, and express the x-fold increase of gene
expression compared with the control group. The standard curve and
data analysis were produced using Bio-Rad iQ5 software (Bio-Rad
Laboratories).
Immunohistochemistry (IHC) in liver
tissue and skeletal muscle
IHC was performed to examine the expression of
GLUT4. Liver tissue and skeletal muscle from each group were fixed
in 10% formalin and embedded in paraffin. The sections were
deparaffinized and rehydrated and then immersed in citrate buffer
and heated in a microwave oven for antigen retrieval. After cooling
to room temperature, the sections were rinsed and incubated with 3%
H2O2 solution to block endogenous enzymes.
Normal goat serum was incubated to block non-specific binding after
rinsing with PBS. Primary antibody to GLUT4 (1:200; Abcam,
Cambridge, MA, USA) was then added, followed by overnight
incubation at 4°C. The sections were incubated with the appropriate
secondary antibody and horseradish-peroxidase. DAB substrate buffer
(Beijing Zhongshan Golden Bridge Biotechnology Co., Beijing, China)
was then added to achieve coloration. The specimens were
subsequently dehydrated with ethanol, cleared with xylene and then
mounted on glass cover slips. Brown colored sites were quantified
at a final magnification of ×400 using a microscope by a
pathologist and photographed using an Olympus DP71 digital camera
(Olympus, Tokyo, Japan).
Statistical analysis
Values are presented as the means ± standard error
of the mean (SEM). Differences between the C57 mice and KKAy mice
were tested for significance with an independent sample t-test and
ANOVA. All statistical analyses were performed using SPSS v13.0
software and the data in the figures were analyzed using GraphPad
Prism 5.0 software. Parameters with P-values <0.05 were
considered to indicate statistically significant differences.
Results
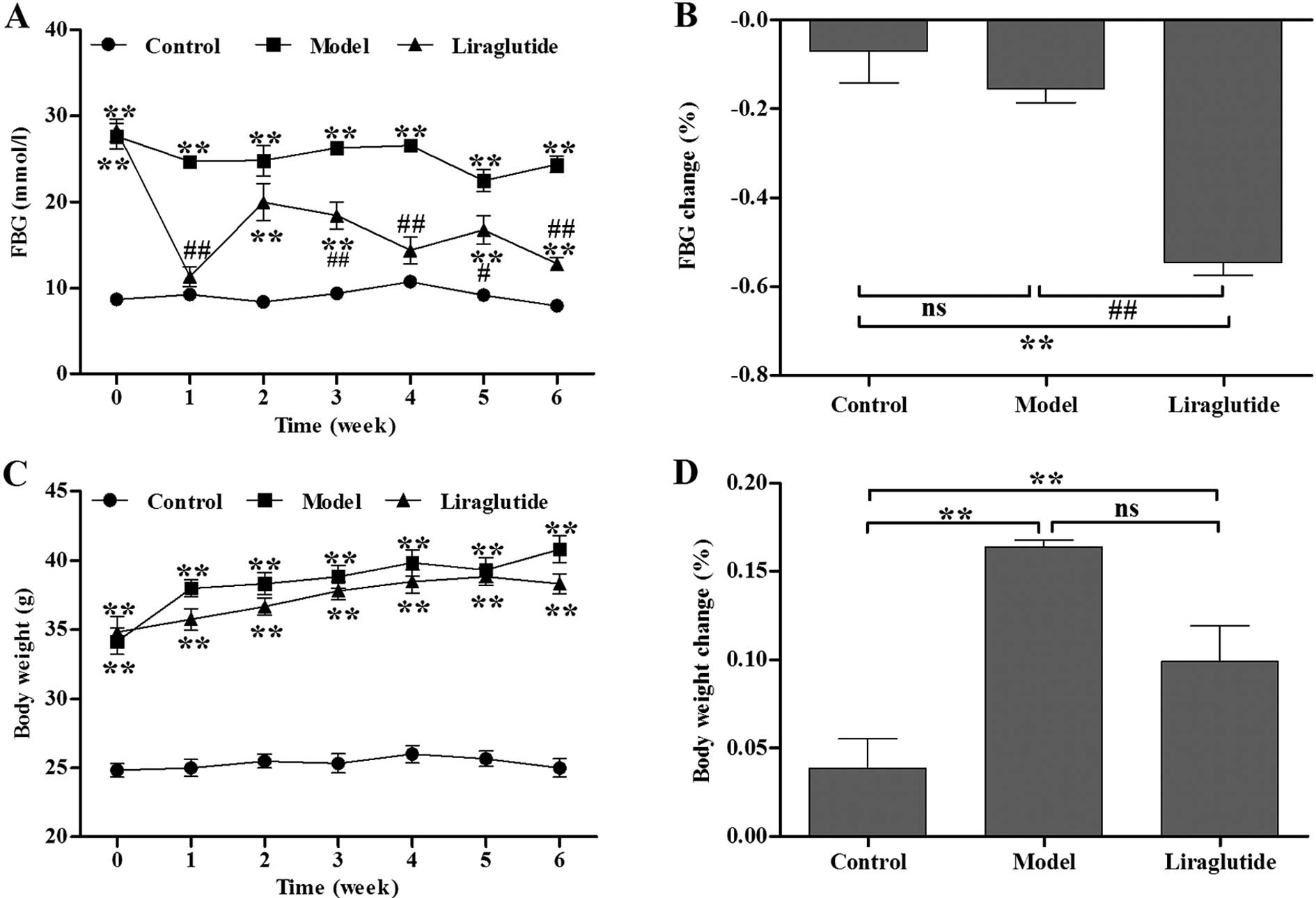
Liraglutide decreases FBG levels and body
weight in KKAy mice
Initially, baseline FBG levels in the diabetic KKAy
mice were significantly higher than those in the C57 mice (Fig. 1A). Following 1 week of injections,
the liraglutide-treated KKAy mice demonstrated a significant
decrease in FBG levels compared with the normal saline-treated KKAy
mice (Fig. 1A). During the 6
weeks of treatment, the blood glucose levels in the liraglutide
group had a decreasing tendency. The decreasing percentage of FBG
in the liragutide-treated KKAy mice was significantly higher than
the normal saline-treated KKAy mice after the treatment period
ended (P<0.01, vs. model group) (Fig. 1B). There was no statistically
significant difference in body weight between the
liraglutide-treated and normal saline-treated KKAy mice before and
during the treatment period (P>0.05) (Fig. 1C and D).
Liraglutide improves the glucose
tolerance of KKAy mice
The OGTT results revealed that the blood glucose
levels in all groups peaked at 30 min after oral glucose loading
and then decreased. We observed that the blood glucose levels in
the liraglutide group increased and decreased (Fig. 2A) much more promptly in a
persistent state compared with the control and model group, whereas
the levels of blood glucose in the model group remained at high
levels before and after the glucose solution loading. The AUC in
the liraglutide group was significantly lower than that in the
model group (P<0.01) (Fig.
2B). The AUC in the liraglutide group was significantly higher
than that in the control group (P<0.05) (Fig. 2B). These results demonstrate that
liraglutide improves glucose tolerance in KKAy mice.
Liraglutide improves insulin tolerance in
KKAy mice
In all the groups, FBG levels rapidly decreased
within 40 min after the insulin injection. In the control group and
liraglutide group, the FBG levels continued to decrease (Fig. 2C). However, the blood glucose
levels in the model group began to gradullay increase after 40 min.
The AUC in the liraglutide group was significantly lower than that
in the model group (P<0.05) (Fig.
2D) and higher than that in the control group (P<0.05)
(Fig. 2D). These results indicate
that liraglutide ameliorates insulin tolerance in KKAy mice.
Liraglutide improves serum biochemical
parameters in KKAy mice
Following treatment with liraglutide for 6 weeks,
the insulin levels in the liraglutide group were significantly
higher than those in the control and model group (P<0.01)
(Fig. 2E). Fig. 2F illustrates the FBG levels of the
3 groups at the end of the experiment. HOMA-IR in the liraglutide
group was significantly decreased compared with the model group
(P<0.05), but was still higher compared with the control group
(P<0.05) (Fig. 2G). Likewise,
the ISI in the liraglutide group significantly improved compared
with the model group (P<0.05), but was still higher compared
with the control group (P<0.05) (Fig. 2H).
Liraglutide influences the glucometabolic
biochemical index in KKAy mice
As indicated by the content of hepatic glycogen,
liraglutide significantly increased glucose metabolism in the liver
compared with the normal saline-treated KKAy mice (P<0.05)
(Fig. 3A). As indicated by the
levels of skeletal muscle glycogen, following treatment with
liraglutide, glucose metabolism in skeletal muscle improved;
however, no difference was observed between the model group and the
liraglutide group (P>0.05) (Fig.
3B). As indicated by the skeletal muscle PK and HK levels,
liraglutide significantly increased PK and HK activity compared
with the model group (P<0.05) (Fig. 3C and D). In the normal C57 mice,
skeletal muscle PK and HK levels were significantly higher than
those of the KKAy mice (P<0.05) (Fig. 3C and D).
Effects of liraglutide on ultrastructure
of β cells
Ultrastructural changes were observed in the
pancreatic β cells of the KKAy mice compared with the C57 mice
(Fig. 4). The number of secretory
granules, which presumably contain insulin, in the β cells from the
normal saline-treated KKAy mice was found to be markedly lower than
that in the control C57 mice. Enlargement of the mitochondria,
destruction of mitochondrial cristae, development of the Golgi
apparatus and an increase in the amount of the rough endoplasmic
reticulum (RER) in the electron microscopic images of pancreatic β
cells from the normal saline-treated KKAy mice were observed
compared with the C57 mice. The number of secretory granules
increased in the β cells from the liraglutide-treated KKAy mice;
the morphology of the mitochondria, the Golgi apparatus and the RER
had a more normal appearance, comparable to that in C57 mice.
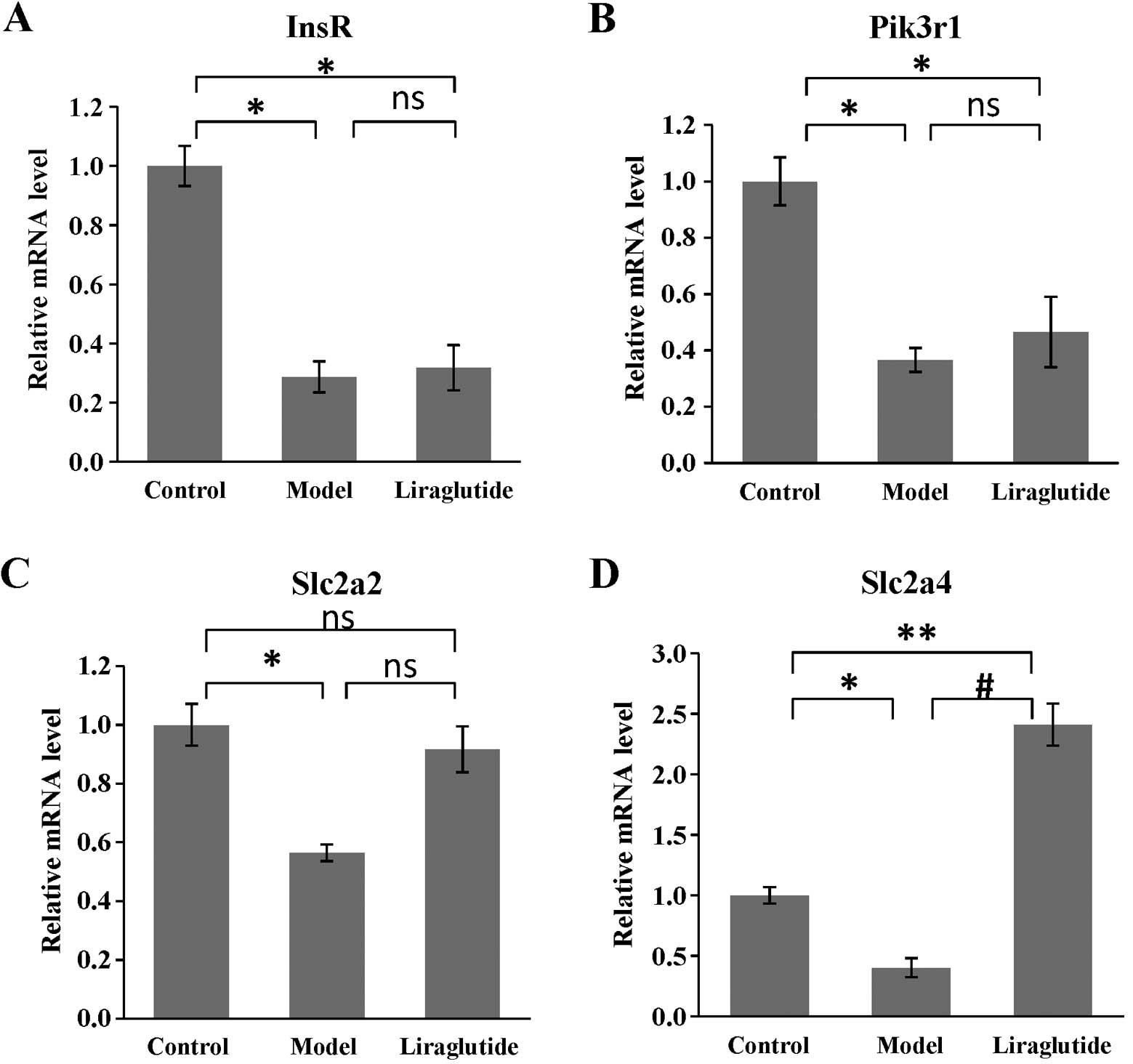
Effects of liraglutide on gene expression
in KKAy mice
As shown in Fig.
5, the expression of all the selected target genes (InsR,
Pik3r1, Slc2a2, Slc2a4) in the insulin pathway was decreased in
KKAy mouse livers, compared with those in the C57 mice (P<0.05
or P<0.01). Following the administration of liraglutide for 6
weeks, the expression of Slc2a4 (coding GLUT4) increased
significantly compared with that in the model group (KKAy mice
treated with normal saline; P<0.01) (Fig. 5D). Although the other genes showed
no difference in expression between the model group and liraglutide
group, the expression of Slc2a2 (coding GLUT2) was slightly
increased, which was not statistically different compared with that
in the C57 mice (P>0.05) (Fig.
5C).
IHC in liver tissue and skeletal
muscle
The positive signal of GLUT4 was presented as brown
(Fig. 6). We used Image-Pro Plus
6.0 software to analyze the IHC images and found that following
liraglutide administration, the expression of GLUT4 in liver tissue
and skeletal muscle increased significantly compared with the model
group (P<0.01) (Fig. 6). These
results demonstrate that liraglutide upregulates the expression of
GLUT4 in liver tissue and skeletal muscle in diabetic KKAy
mice.
Discussion
Liraglutide, a long-lasting GLP-1 analogue, has been
used for the treatment of patients with type 2 diabetes mellitus
since 2009. The degree of sequence identity between liraglutide and
native GLP-1 is high, at 97%. Liraglutide is suitable for quaque
die (q. d.; one a day) dosing via subcutaneous injection with
an average half-life (t1/2) of 11.6 to 12.8 h, without
regard to meals (17). The
activation of the GLP-1 receptor on β cells results in insulin
production and exocytosis in a glucose-dependent manner (i.e., only
during hyperglycemia), which minimizes the risk of hypoglycemia.
However, the mechanisms behind its effects on insulin resistance
and glycometabolism remain unknown. In our study, we used a
spontaneous diabetic animal model (KKAy mice) to investigate the
mechanisms behind the anti-diabetic effects of liraglutide. Our
experiments confirmed that liraglutide decreases FBG levels,
increases insulin secretion, improves insulin sensitivity and
ameliorates insulin resistance in KKAy mice. Moreover, liraglutide
increased glycogen in the liver and skeletal muscle and activated
HK and PK. Furthermore, liraglutide improved β cell morphology,
increased insulin secretion, enhanced the gene expression of GLUT4
in the liver and upregulated the expression of GLUT4 in liver
tissue and skeletal muscle in diabetic KKAy mice.
Several studies have reported that GLP-1 receptor
agonists (e.g., exenatide and liraglutide) stimulate the production
and secretion of insulin from pancreatic β cells, reduce plasma
glucose levels and reduce gastric emptying in patients with type 2
diabetes mellitus and rodents, such as Zucker Diabetic Fatty (ZDF)
rats, Otsuka-Long-Evans-Tokushima Fatty (OLETF) rats, db/db mice,
ob/ob mice, Swiss TO mice and β cell-specific glucokinase-deficient
Gck(−/−) mice (18–20). KKAy mice recapitulate the
characteristics of human type 2 diabetes mellitus, such as insulin
resistance, hyperglycemia and obesity (15), as a spontaneous type 2 diabetic
model. Our present results demonstrate that treatment with
liraglutide reduces FBG levels, lowers the AUC following OGTT and
ITT, lowers the HOMA-IR index, increases insulin levels,
ameliorates glucose tolerance and insulin sensitivity in diabetic
KKAy mice, which are consistent with the results from the
abovementioned studies (18–20).
It is well known that glucose homeostasis disorder
and hyperglycemia and diabetes are closely related. Glycogen, HK
and PK are important glycometabolism indexes that correlate with
glucose distribution and uptake (21). It has been reported that glycogen
synthesis disorder promotes the occurrence of hyperglycemia and
diabetes (22,23). HK and PK are the key enzymes of
glycolysis. HK catalyzes glucose to generate glucose 6 phosphate in
the first step in glycolysis. Activated HK promotes glycogen
synthesis and glycolysis (24,25). PK catalyzes the conversion of
phosphoenolpyruvate to pyruvic acid and the phosphorylation of
adenosine diphosphate (ADP) to adenosine triphosphate (ATP)
(26,27). Sun et al (28) and Ye et al (29) found that the increased expression
of PK M2 (one of the PK isoforms) stimulates glycolysis. Shirakawa
et al (30) reported that
liraglutide increases the glycogen content in neonatal Gck(−/−)
mouse livers. Our results firstly demonstrated that liraglutide
increases skeletal muscle HK and PK activity. We also demonstrated
that liraglutide increased liver and skeletal muscle glycogen
content in KKAy mice.
Islet β cell dysfunction and insulin resistance are
the main risk factors correlated with type 2 diabetes (31,32). Islet β cell dysfunction induces
insulin secretion disorders and hyperglycemia (33,34). The study by Emamaullee et
al (35) confirmed that
liraglutide improves β cell function by reducing apoptosis. Sturis
et al (36) treated ZDF
rats with liraglutide for 6 weeks and found that liraglutide
induced islet β cell proliferation, thus inducing
anti-hyperglycemic effects. The study by Knudsen (37) demonstrated that liraglutide
modulates the progressive loss of β cell function that drives the
continous deterioration in glycaemic control in patients with type
2 diabetes. These findings are in accordance with our electron
microscopy observations that liraglutide normalized the state and
number of mitochondria, the Golgi apparatus and the RER, and
increased the number of secretory granules in islet β cells. The
serum content of insulin was also increased by liraglutide in KKAy
mice, which suggests that liraglutide improves β cell morphology
and function.
Diabetes mellitus is part of the insulin resistance
syndrome and insulin resistance leads to a decrease in insulin
sensitivity. Insulin secretion abnormalities and insulin
sensitivity disorders in the target organs lead to blood glucose
abnormalities (38,39). Insulin is secreted by islets β
cells as a response mechanism for counteracting the increasing
excess amounts of glucose in the blood. After insulin enters the
bloodstream, it binds to the α-subunit of the InsR on the cell
membrane, which triggers the tyrosine kinase activity of the
β-subunit that is attached to the α-subunit. The InsR substrate
family, such as IRS-1 and IRS-2, is phosphorylated and combines
with the regulatory subunit of PI3K and passes insulin signaling to
the protein kinase B (Akt) molecule, leading to the induction of a
variety of insulin bioactivities, thus improving insulin resistance
(11,40–43). GLUTs are a wide group of membrane
proteins, one of the downstream substrates of Akt, which facilitate
the transport of glucose over the plasma membrane. GLUT2 is the
principal transporter for the transfer of glucose between the liver
and blood, and renal glucose reabsorption. GLUT4 is frequently
expressed in skeletal muscle, cardiac muscle and adipose tissue,
the major tissues of the body that respond to insulin. GLUT4 is a
major mediator of glucose removal from the circulation and
functions as a key regulator of whole-body glucose homeostasis
(44). It has been confirmed that
increases in GLUT4 protein expression improve glucose homeostasis
in dilated cardiomyopathy animals (45). Studies had been found that insulin
resistance is related to a decrease in GLUT4 expression (46). It has been reported that
liraglutide increases insulin secretion and target organ
sensitivity to insulin, thus improving the insulin resistance state
(18,47). We obtained similar results,
showing that liraglutide improves the glucose tolerance and insulin
tolerance, decreases the value of HOMA-IR and exerts a potent
protective effect on insulin resistance. However, to our knowledge,
there are no reports to date as to whether liraglutide influences
the gene expression of the insulin pathway in KKAy mice. Our
real-time PCR results revealed that the gene expression of InsR,
PI3K, GLUT2 and GLUT4 was downregulated in the diabetic KKAy mice,
compared with the control C57 mice. In addition, to our knowledge,
we demonstrate for the first time that liraglutide significantly
increases the gene expression of GLUT4 in KKAy mouse livers. We
also found that liraglutide significantly increased the expression
of GLUT4 in KKAy mouse liver tissue and skeletal muscle, which was
consistent with the real time-PCR results. Thus, the upregulation
of GLUT4 may be one of the mechanisms involved in the effects of
liraglutide in increasing insulin sensitivity and improving insulin
resistance.
In conclusion, the results from the present study
suggest that liraglutide ameliorates glycometabolism by increasing
glycogenesis production and glycolysis, improving β cell
dysfunction and increasing insulin secretion, ameliorating insulin
sensitivity and reducing insulin resistance, which may be
associated with the upregulation of GLUT4. Thus, liraglutide exerts
potent anti-diabetic effects. Our data provide the molecular basis
for further investigation into the mechanisms by which liraglutide
moderates glucose metabolism.
Acknowledgements
The present study was supported by grants from the
National Natural Science Youth Foundation of China (no. 30901808)
and the Technology and Innovation Project of Shaanxi Province (no.
2011KTCL03-20).
Abbreviations:
|
FBG
|
fasting blood glucose
|
|
AUC
|
area under the curve
|
|
OGTT
|
oral glucose tolerance test
|
|
ITT
|
insulin tolerance test
|
|
HOMA-IR
|
homeostasis model assessment of
insulin resistance
|
|
ISI
|
insulin sensitivity index
|
|
HK
|
hexokinase
|
|
PK
|
pyruvate kinase
|
|
GLP-1
|
glucagon-like peptide-1
|
|
InsR
|
insulin receptor
|
|
PI3K
|
phosphatidylinositide 3-kinase
|
|
GLUT
|
glucose transporter
|
|
KKAy
|
KK/Upj-Ay/J
|
|
C57
|
C57BL/6J
|
References
|
1
|
Wild S, Roglic G, Green A, Sicree R and
King H: Global prevalence of diabetes: estimates for the year 2000
and projections for 2030. Diabetes Care. 27:1047–1053.
2004.PubMed/NCBI
|
|
2
|
Geraldes P and King GL: Activation of
protein kinase C isoforms and its impact on diabetic complications.
Circ Res. 106:1319–1331. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Abdin AA, Baalash AA and Hamooda HE:
Effects of rosiglitazone and aspirin on experimental model of
induced type 2 diabetes in rats: focus on insulin resistance and
inflammatory markers. J Diabetes Complications. 24:168–178. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hayden MR and Sowers JR: Treating
hypertension while protecting the vulnerable islet in the
cardiometabolic syndrome. J Am Soc Hypertens. 2:239–266. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li L, Miao Z, Liu R, Yang M, Liu H and
Yang G: Liraglutide prevents hypoadiponectinemia-induced insulin
resistance and alterations of gene expression involved in glucose
and lipid metabolism. Mol Med. 17:1168–1178. 2011.PubMed/NCBI
|
|
6
|
Mundil D, Cameron-Vendrig A and Husain M:
GLP-1 receptor agonists: a clinical perspective on cardiovascular
effects. Diab Vasc Dis Res. 9:95–108. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Senda M, Ogawa S, Nako K, Okamura M,
Sakamoto T and Ito S: The glucagon-like peptide-1 analog
liraglutide suppresses ghrelin and controls diabetes in a patient
with Prader-Willi syndrome. Endocr J. 59:889–894. 2012. View Article : Google Scholar
|
|
8
|
Hunter K and Hölscher C: Drugs developed
to treat diabetes, liraglutide and lixisenatide, cross the blood
brain barrier and enhance neurogenesis. BMC Neurosci. 13:332012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kesavadev J, Shankar A, Krishnan G and
Jothydev S: Liraglutide therapy beyond glycemic control: an
observational study in Indian patients with type 2 diabetes in real
world setting. Int J Gen Med. 5:317–322. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fujishima Y, Maeda N, Inoue K, et al:
Efficacy of liraglutide, a glucagon-like peptide-1 (GLP-1)
analogue, on body weight, eating behavior, and glycemic control, in
Japanese obese type 2 diabetes. Cardiovasc Diabetol. 11:1072012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fukushima T, Arai T, Ariga-Nedachi M, et
al: Insulin receptor substrates form high-molecular-mass complexes
that modulate their availability to insulin/insulin-like growth
factor-I receptor tyrosine kinases. Biochem Biophys Res Commun.
404:767–773. 2011. View Article : Google Scholar
|
|
12
|
Zhu Y, Pereira RO, O’Neill BT, et al:
Cardiac PI3K-Akt impairs insulin-stimulated glucose uptake
independent of mTORC1 and GLUT4 translocation. Mol Endocrinol.
27:172–184. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen S, Wasserman DH, MacKintosh C and
Sakamoto K: Mice with AS160/TBC1D4-Thr649Ala knockin mutation are
glucose intolerant with reduced insulin sensitivity and altered
GLUT4 trafficking. Cell Metab. 13:68–79. 2011. View Article : Google Scholar
|
|
14
|
Munkonda MN, Lapointe M, Miegueu P, et al:
Recombinant acylation stimulating protein administration to
C3−/− mice increases insulin resistance via adipocyte
inflammatory mechanisms. PLoS One. 7:e468832012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wei X, Wang D, Yang Y, et al:
Cyanidin-3-O-β-glucoside improves obesity and triglyceride
metabolism in KK-Ay mice by regulating lipoprotein lipase activity.
J Sci Food Agric. 91:1006–1013. 2011.
|
|
16
|
Li PP, Shan S, Chen YT, et al: The
PPARalpha/gamma dual agonist chiglitazar improves insulin
resistance and dyslipidemia in MSG obese rats. Br J Pharmacol.
148:610–618. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Drucker DJ: The biology of incretin
hormones. Cell Metab. 3:153–165. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Porter DW, Kerr BD, Flatt PR, Holscher C
and Gault VA: Four weeks administration of Liraglutide improves
memory and learning as well as glycaemic control in mice with high
fat dietary-induced obesity and insulin resistance. Diabetes Obes
Metab. 12:891–899. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gault VA, Kerr BD, Harriott P and Flatt
PR: Administration of an acylated GLP-1 and GIP preparation
provides added beneficial glucose-lowering and insulinotropic
actions over single incretins in mice with type 2 diabetes and
obesity. Clin Sci (Lond). 121:107–117. 2011. View Article : Google Scholar
|
|
20
|
Shimoda M, Kanda Y, Hamamoto S, et al: The
human glucagon-like peptide-1 analogue liraglutide preserves
pancreatic beta cells via regulation of cell kinetics and
suppression of oxidative and endoplasmic reticulum stress in a
mouse model of diabetes. Diabetologia. 54:1098–1108. 2011.
View Article : Google Scholar
|
|
21
|
Magnoni LJ, Vraskou Y, Palstra AP and
Planas JV: AMP-activated protein kinase plays an important
evolutionary conserved role in the regulation of glucose metabolism
in fish skeletal muscle cells. PLoS One. 7:e312192012. View Article : Google Scholar
|
|
22
|
Oosterveer MH, Mataki C, Yamamoto H, et
al: LRH-1-dependent glucose sensing determines intermediary
metabolism in liver. J Clin Invest. 122:2817–2826. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ros S, García-Rocha M, Calbó J and
Guinovart JJ: Restoration of hepatic glycogen deposition reduces
hyperglycaemia, hyperphagia and gluconeogenic enzymes in a
streptozotocin-induced model of diabetes in rats. Diabetologia.
54:2639–2648. 2011. View Article : Google Scholar
|
|
24
|
John S, Weiss JN and Ribalet B:
Subcellular localization of hexokinases I and II directs the
metabolic fate of glucose. PLoS One. 6:e176742011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sun L, Shukair S, Naik TJ, Moazed F and
Ardehali H: Glucose phosphorylation and mitochondrial binding are
required for the protective effects of hexokinases I and II. Mol
Cell Biol. 28:1007–1017. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Holyoak T, Zhang B, Deng J, Tang Q,
Prasannan CB and Fenton AW: Energetic coupling between an
oxidizable cysteine and the phosphorylatable N-terminus of human
liver pyruvate kinase. Biochemistry. 52:466–476. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cortés-Cros M, Hemmerlin C, Ferretti S, et
al: M2 isoform of pyruvate kinase is dispensable for tumor
maintenance and growth. Proc Natl Acad Sci USA. 110:489–494.
2013.PubMed/NCBI
|
|
28
|
Sun Q, Chen X, Ma J, et al: Mammalian
target of rapamycin up-regulation of pyruvate kinase isoenzyme type
M2 is critical for aerobic glycolysis and tumor growth. Proc Natl
Acad Sci USA. 108:4129–4134. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ye J, Mancuso A, Tong X, et al: Pyruvate
kinase M2 promotes de novo serine synthesis to sustain mTORC1
activity and cell proliferation. Proc Natl Acad Sci USA.
109:6904–6909. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shirakawa J, Tanami R, Togashi Y, et al:
Effects of liraglutide on beta-cell-specific glucokinase-deficient
neonatal mice. Endocrinology. 153:3066–3075. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Janghorbani M and Amini M: Incidence of
metabolic syndrome and its risk factors among type 2 diabetes
clinic attenders in Isfahan, Iran. Endokrynol Pol. 63:372–380.
2012.
|
|
32
|
Ohshima K, Mogi M, Jing F, et al: Direct
angiotensin II type 2 receptor stimulation ameliorates insulin
resistance in type 2 diabetes mice with PPARγ activation. PLoS One.
7:e483872012.PubMed/NCBI
|
|
33
|
Rosengren AH, Braun M, Mahdi T, et al:
Reduced insulin exocytosis in human pancreatic β-cells with gene
variants linked to type 2 diabetes. Diabetes. 61:1726–1733.
2012.
|
|
34
|
Tersey SA, Nishiki Y, Templin AT, et al:
Islet β-cell endoplasmic reticulum stress precedes the onset of
type 1 diabetes in the nonobese diabetic mouse model. Diabetes.
61:818–827. 2012.
|
|
35
|
Emamaullee JA, Merani S, Toso C, et al:
Porcine marginal mass islet autografts resist metabolic failure
over time and are enhanced by early treatment with liraglutide.
Endocrinology. 150:2145–2152. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sturis J, Gotfredsen CF, Rømer J, et al:
GLP-1 derivative liraglutide in rats with beta-cell deficiencies:
influence of metabolic state on beta-cell mass dynamics. Br J
Pharmacol. 140:123–132. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Knudsen LB: Liraglutide: the therapeutic
promise from animal models. Int J Clin Pract. 167:4–11. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kawamori R: Insulin resistance seen in
non-insulin dependent diabetes mellitus and hypertension. Hypertens
Res. 19(Suppl 1): S61–S64. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tomimoto S, Ojika T, Shintani N, et al:
Markedly reduced white adipose tissue and increased insulin
sensitivity in adcyap1-deficient mice. J Pharmacol Sci. 107:41–48.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Taniguchi CM, Emanuelli B and Kahn CR:
Critical nodes in signalling pathways: insights into insulin
action. Nat Rev Mol Cell Biol. 7:85–96. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Inokuchi J: Membrane microdomains and
insulin resistance. Febs Lett. 584:1864–1871. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chang YC and Chuang LM: The role of
oxidative stress in the pathogenesis of type 2 diabetes: from
molecular mechanism to clinical implication. Am J Transl Res.
2:316–331. 2010.PubMed/NCBI
|
|
43
|
Muniyappa R, Montagnani M, Koh KK and Quon
MJ: Cardiovascular actions of insulin. Endocr Rev. 28:463–491.
2007. View Article : Google Scholar
|
|
44
|
Lee JO, Song YH, Kim MW, et al: A
sub-1-volt nanoelectromechanical switching device. Nat Nanotechnol.
8:36–40. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Giannocco G, Oliveira KC, Crajoinas RO, et
al: Dipeptidyl peptidase IV inhibition upregulates GLUT4
translocation and expression in heart and skeletal muscle of
spontaneously hypertensive rats. Eur J Pharmacol. 698:74–86. 2013.
View Article : Google Scholar
|
|
46
|
Nieto-Vazquez I, Fernández-Veledo S, de
Alvaro C and Lorenzo M: Dual role of interleukin-6 in regulating
insulin sensitivity in murine skeletal muscle. Diabetes.
57:3211–3221. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang WY, Lee JJ, Kim IS, Kim Y, Park JS
and Myung CS: 7-O-methylaromadendrin stimulates glucose uptake and
improves insulin resistance in vitro. Biol Pharm Bull.
33:1494–1499. 2010. View Article : Google Scholar : PubMed/NCBI
|