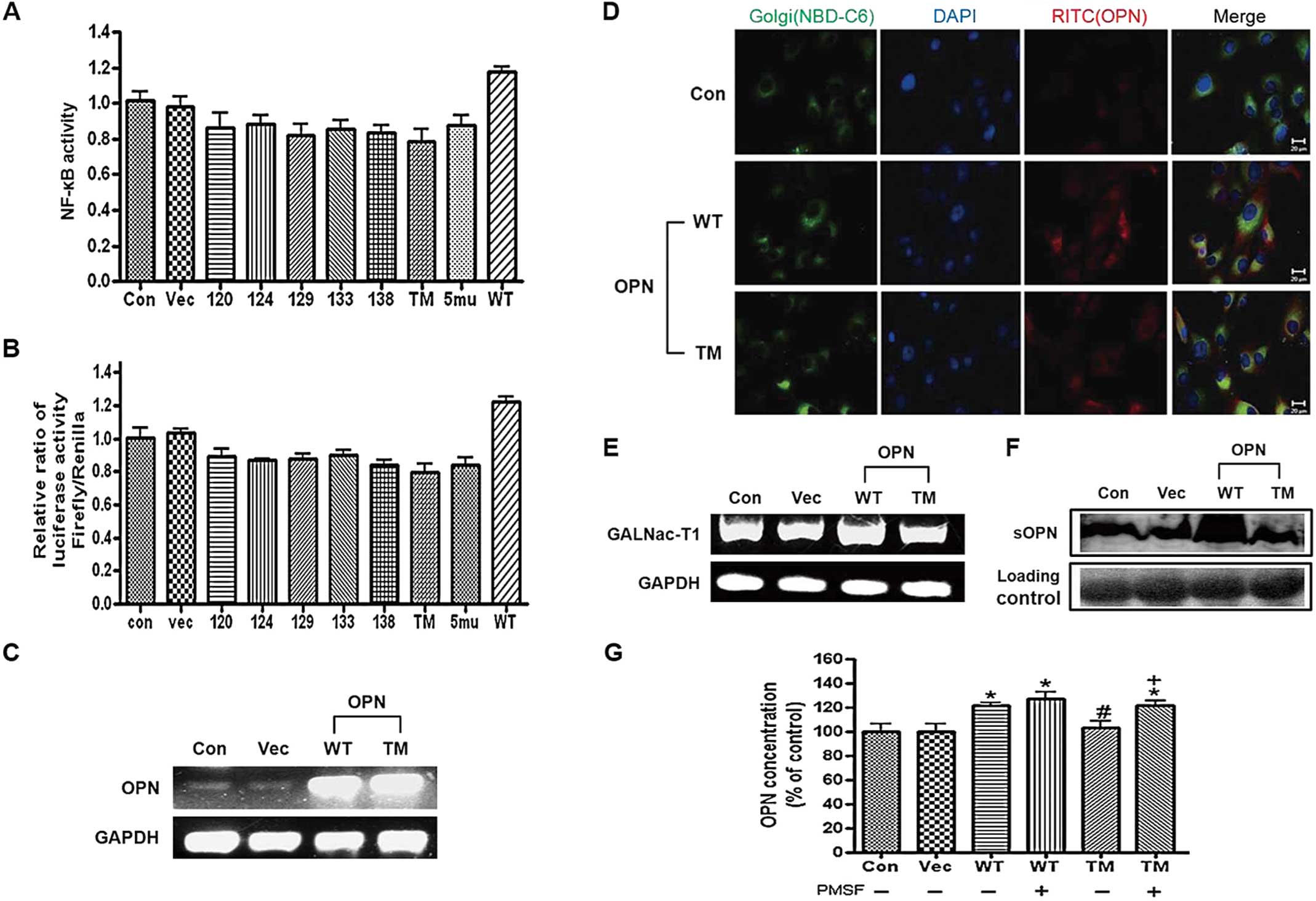
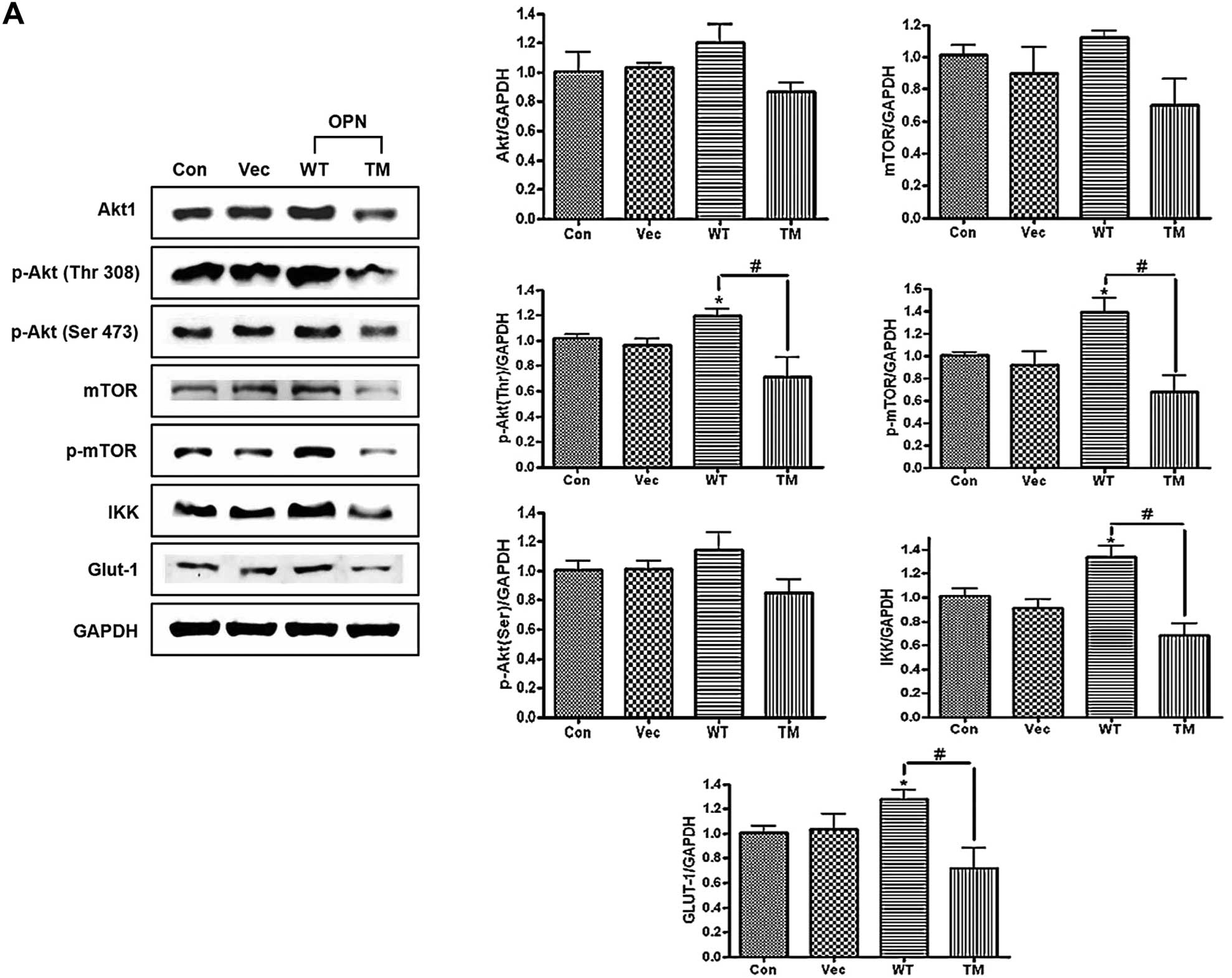
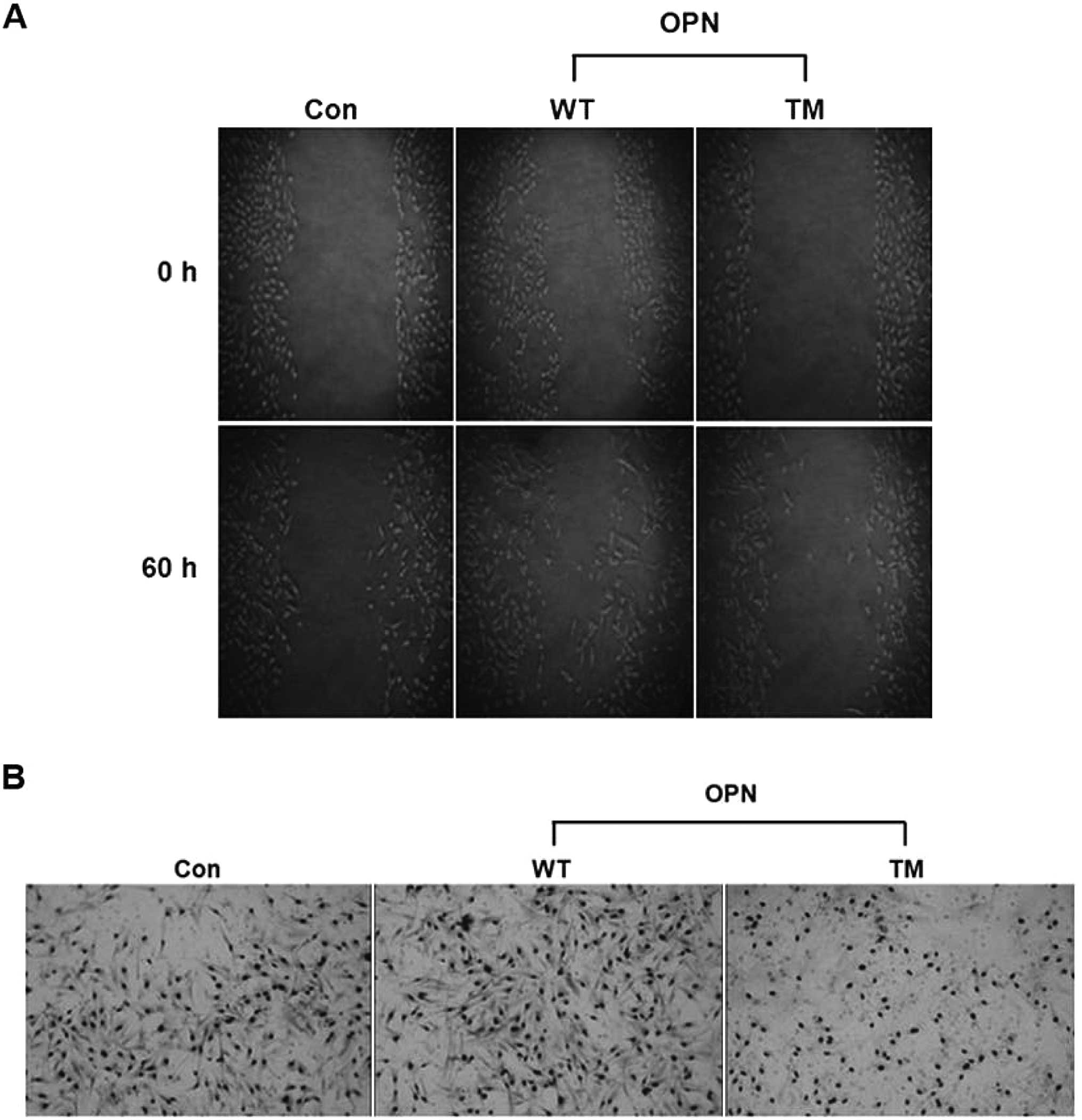
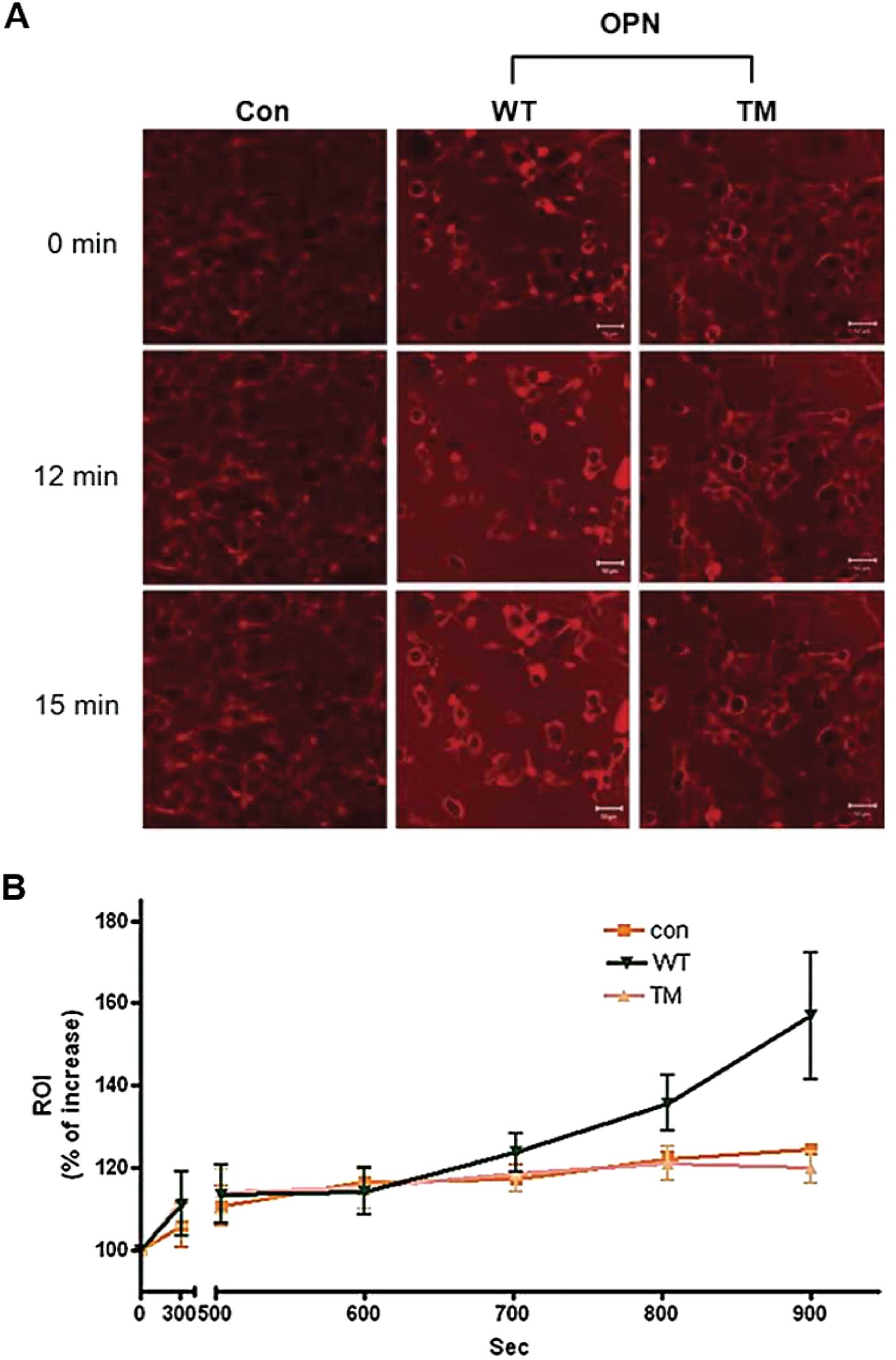
|
1
|
Donati V, Boldrini L, Dell’Omodarme M, et
al: Osteopontin expression and prognostic significance in non-small
cell lung cancer. Clin Cancer Res. 11:6459–6465. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ahmed M, Behera R, Chakraborty G, et al:
Osteopontin: a potentially important therapeutic target in cancer.
Expert Opin Ther Targets. 15:1113–1126. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Weber GF: The metastasis gene osteopontin:
a candidate target for cancer therapy. Biochim Biophys Acta.
1552:61–85. 2001.PubMed/NCBI
|
|
4
|
Shevde LA, Das S, Clark DW and Samant RS:
Osteopontin: an effector and an effect of tumor metastasis. Curr
Mol Med. 10:71–81. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chellaiah MA, Biswas RS, Rittling SR,
Denhardt DT and Hruska KA: Rho-dependent Rho kinase activation
increases CD44 surface expression and bone resorption in
osteoclasts. J Biol Chem. 278:29086–29097. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Christensen B, Kazanecki CC, Petersen TE,
Rittling SR, Denhardt DT and Sørensen ES: Cell type-specific
post-translational modifications of mouse osteopontin are
associated with different adhesive properties. J Biol Chem.
282:19463–19472. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fukuda M: Roles of cell surface
carbohydrates in cell adhesion; in particular those for sialyl
Lewis x and polysialic acid. Seikagaku. 72:269–283. 2000.(In
Japanese).
|
|
8
|
López-Ferrer A, Barranco C and de Bolós C:
Differences in the O-glycosylation patterns between lung squamous
cell carcinoma and adenocarcinoma. Am J Clin Pathol. 118:749–755.
2002.PubMed/NCBI
|
|
9
|
Christensen B, Nielsen MS, Haselmann KF,
Petersen TE and Sørensen ES: Post-translationally modified residues
of native human osteopontin are located in clusters: identification
of 36 phosphorylation and five O-glycosylation sites and their
biological implications. Biochem J. 390:285–292. 2005. View Article : Google Scholar
|
|
10
|
Keykhosravani M, Doherty-Kirby A, Zhang C,
Brewer D, Goldberg HA, Hunter GK and Lajoie G: Comprehensive
identification of post-translational modifications of rat bone
osteopontin by mass spectrometry. Biochemistry. 44:6990–7003. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Miwa HE, Gerken TA, Jamison O and Tabak
LA: Isoform-specific O-glycosylation of osteopontin and bone
sialoprotein by polypeptide N-acetylgalactosaminyltransferase-1. J
Biol Chem. 285:1208–1219. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aggarwal BB, Vijayalekshmi RV and Sung B:
Targeting inflammatory pathways for prevention and therapy of
cancer: short-term friend, long-term foe. Clin Cancer Res.
15:425–430. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Aggarwal BB and Sung B: NF-κB in cancer: a
matter of life and death. Cancer Discov. 1:469–471. 2011.
|
|
14
|
Chaturvedi MM, Sung B, Yadav VR, Kannappan
R and Aggarwal BB: NF-κB addiction and its role in cancer: ‘one
size does not fit all’. Oncogene. 30:1615–1630. 2011.
|
|
15
|
Ruggero D and Pandolfi PP: Does the
ribosome translate cancer? Nat Rev Cancer. 3:179–192. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Abraham RT and Eng CH: Mammalian target of
rapamycin as a therapeutic target in oncology. Expert Opin Ther
Targets. 12:209–222. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hagner PR, Schneider A and Gartenhaus RB:
Targeting the translational machinery as a novel treatment strategy
for hematologic malignancies. Blood. 115:2127–2135. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim YK, Kwon JT, Choi JY, et al:
Suppression of tumor growth in xenograft model mice by programmed
cell death 4 gene delivery using folate-PEG-baculovirus. Cancer
Gene Ther. 17:751–760. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Minai-Tehrani A, Jiang HL, Kim YK, et al:
Suppression of tumor growth in xenograft model mice by small
interfering RNA targeting osteopontin delivery using biocompatible
poly(amino ester). Int J Pharm. 431:197–203. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tartakoff AM: Perturbation of vesicular
traffic with the carboxylic ionophore monensin. Cell. 32:1026–1028.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shijubo N, Uede T, Kon S, et al: Vascular
endothelial growth factor and osteopontin in stage I lung
adenocarcinoma. Am J Respir Crit Care Med. 160:1269–1273. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Philip S, Bulbule A and Kundu GC:
Osteopontin stimulates tumor growth and activation of promatrix
metalloproteinase-2 through nuclear factor-kappa B-mediated
induction of membrane type 1 matrix metalloproteinase in murine
melanoma cells. J Biol Chem. 276:44926–44935. 2001. View Article : Google Scholar
|
|
23
|
Jain S, Chakraborty G and Kundu GC: The
crucial role of cyclooxygenase-2 in osteopontin-induced protein
kinase C alpha/c-Src/IkappaB kinase alpha/beta-dependent prostate
tumor progression and angiogenesis. Cancer Res. 66:6638–6648. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rodrigues LR, Teixeira JA, Schmitt FL,
Paulsson M and Lindmark-Mänsson H: The role of osteopontin in tumor
progression and metastasis in breast cancer. Cancer Epidemiol
Biomarkers Prev. 16:1087–1097. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xue YH, Zhang XF, Dong QZ, et al: Thrombin
is a therapeutic target for metastatic osteopontin-positive
hepatocellular carcinoma. Hepatology. 52:2012–2022. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chang VT, Crispin M, Aricescu AR, et al:
Glycoprotein structural genomics: solving the glycosylation
problem. Structure. 15:267–273. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Patsos G, Robbe-Masselot C, Klein A, et
al: O-glycan regulation of apoptosis and proliferation in
colorectal cancer cell lines. Biochem Soc Trans. 35:1372–1374.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Varki A: Glycan-based interactions
involving vertebrate sialic-acid-recognizing proteins. Nature.
446:1023–1029. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ten Hagen KG, Tetaert D, Hagen FK, et al:
Characterization of a UDP-GalNAc: polypeptide
N-acetylgalactosaminyltransferase that displays glycopeptide
N-acetylgalactosaminyltransferase activity. J Biol Chem.
274:27867–27874. 1999.PubMed/NCBI
|
|
30
|
Perrine CL, Ganguli A, Wu P, et al:
Glycopeptide-preferring polypeptide GalNAc transferase 10 (ppGalNAc
T10), involved in mucin-type O-glycosylation, has a unique
GalNAc-O-Ser/Thr-binding site in its catalytic domain not found in
ppGalNAc T1 or T2. J Biol Chem. 284:20387–20397. 2009. View Article : Google Scholar
|
|
31
|
Han I and Kudlow JE: Reduced O
glycosylation of Sp1 is associated with increased proteasome
susceptibility. Mol Cell Biol. 17:2550–2558. 1997.PubMed/NCBI
|
|
32
|
Lin YH and Yang-Yen HF: The
osteopontin-CD44 survival signal involves activation of the
phosphatidylinositol 3-kinase/Akt signaling pathway. J Biol Chem.
276:46024–46030. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mann M and Jensen ON: Proteomic analysis
of post-translational modifications. Nat Biotechnol. 21:255–261.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kazanecki CC, Uzwiak DJ and Denhardt DT:
Control of osteopontin signaling and function by post-translational
phosphorylation and protein folding. J Cell Biochem. 102:912–924.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jackson SP and Tjian R: O-glycosylation of
eukaryotic transcription factors: implications for mechanisms of
transcriptional regulation. Cell. 55:125–133. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gasbarri A, Del Prete F, Girnita L,
Martegani MP, Natali PG and Bartolazzi A: CD44s adhesive function
spontaneous and PMA-inducible CD44 cleavage are regulated at
post-translational level in cells of melanocytic lineage. Melanoma
Res. 13:325–337. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Solá RJ and Griebenow K: Effects of
glycosylation on the stability of protein pharmaceuticals. J Pharm
Sci. 98:1223–1245. 2009.
|
|
38
|
Dai J, Peng L, Fan K, et al: Osteopontin
induces angiogenesis through activation of PI3K/AKT and ERK1/2 in
endothelial cells. Oncogene. 28:3412–3422. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Iacoangeli A, Lin Y, Morley EJ, et al:
BC200 RNA in invasive and preinvasive breast cancer.
Carcinogenesis. 25:2125–2133. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bader AG and Vogt PK: An essential role
for protein synthesis in oncogenic cellular transformation.
Oncogene. 23:3145–3150. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kaiser C, Dobrikova EY, Bradrick SS,
Shveygert M, Herbert JT and Gromeier M: Activation of
cap-independent translation by variant eukaryotic initiation factor
4G in vivo. RNA. 14:2170–2182. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Beretta L, Gingras AC, Svitkin YV, Hall MN
and Sonenberg N: Rapamycin blocks the phosphorylation of 4E-BP1 and
inhibits cap-dependent initiation of translation. EMBO J.
15:658–664. 1996.PubMed/NCBI
|
|
43
|
Marcotrigiano J, Gingras AC, Sonenberg N
and Burley SK: Cap-dependent translation initiation in eukaryotes
is regulated by a molecular mimic of elF4G. Mol Cell. 3:707–716.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Herbert TP, Kilhams GR, Batty IH and Proud
CG: Distinct signalling pathways mediate insulin and phorbol
ester-stimulated eukaryotic initiation factor 4F assembly and
protein synthesis in HEK 293 cells. J Biol Chem. 275:11249–11256.
2000. View Article : Google Scholar
|
|
45
|
Haghighat A, Mader S, Pause A and
Sonenberg N: Repression of cap-dependent translation by 4E-binding
protein 1: competition with p220 for binding to eukaryotic
initiation factor-4E. EMBO J. 14:5701–5709. 1995.PubMed/NCBI
|
|
46
|
Brunn GJ, Hudson CC, Sekulić A, et al:
Phosphorylation of the translational repressor PHAS-I by the
mammalian target of rapamycin. Science. 277:99–101. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chen YJ, Wei YY, Chen HT, et al:
Osteopontin increases migration and MMP-9 up-regulation via
alphavbeta3 integrin, FAK, ERK, and NF-kappaB-dependent pathway in
human chondrosarcoma cells. J Cell Physiol. 221:98–108. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kumar V, Behera R, Lohite K, Karnik S and
Kundu GC: p38 kinase is crucial for osteopontin-induced furin
expression that supports cervical cancer progression. Cancer Res.
70:10381–10391. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chang MS, Lee WS, Chen BC, Sheu JR and Lin
CH: YC-1-induced cyclooxygenase-2 expression is mediated by
cGMP-dependent activations of Ras, phosphoinositide-3-OH-kinase,
Akt, and nuclear factor-kappaB in human pulmonary epithelial cells.
Mol Pharmacol. 66:561–571. 2004.
|
|
50
|
Younes M, Lechago LV, Somoano JR, Mosharaf
M and Lechago J: Wide expression of the human erythrocyte glucose
transporter Glut1 in human cancers. Cancer Res. 56:1164–1167.
1996.PubMed/NCBI
|
|
51
|
Chang SH, Chung YS, Hwang SK, et al:
Lentiviral vector-mediated shRNA against AIMP2-DX2 suppresses lung
cancer cell growth through blocking glucose uptake. Mol Cells.
33:553–562. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sommermann TG, Mack HI and Cahir-McFarland
E: Autophagy prolongs survival after NFκB inhibition in B-cell
lymphomas. Autophagy. 8:265–267. 2012.PubMed/NCBI
|
|
53
|
Park J, Lee HY, Cho MH and Park SB:
Development of a cy3-labeled glucose bioprobe and its application
in bioimaging and screening for anticancer agents. Angew Chem Int
Ed Engl. 46:2018–2022. 2007. View Article : Google Scholar : PubMed/NCBI
|