Introduction
Phospholipase A2 (PLA2) catalyzes the
hydrolysis of membrane phospholipids, the products of which can be
transformed into potent inflammatory lipid mediators,
platelet-activating factor and eicosanoids, which include
prostaglandins, thromboxanes, leukotrienes and lipoxins. Multiple
forms of PLA2s have been identified in mammalian tissues, including
10 secretory PLA2s (sPLA2s; group IB, IIA, IIC, IID, IIE, IIF, III,
V, X and XIIA), six Ca2+-dependent PLA2s (group IV) and
six Ca2+-independent PLA2s (groups VI) (1,2).
Group IV cytosolic PLA2 (cPLA2)-α is known to
release arachidonic acid (AA) from phospholipids as the first step
for the biosynthesis of eicosanoids. Increasing data suggest that
sPLA2 can play a role in eicosanoid production. Expression of group
IIA sPLA2 (IIAPLA2) in HEK293 or mouse mesangial cells induces AA
in a cPLA2-α-dependent manner (3,4).
Previous studies showed that the addition of IIAPLA2 or VPLA2 to
mammalian cells leads to the generation of eicosanoids in a
cPLA2-dependent or -independent manner (5–7).
Since sPLA2 is a secretory enzyme and accumulated in inflammatory
exudates, it has been proposed that sPLA2 is secreted to
extracellular medium upon stimulation and acts on target cells.
However, IIAPLA2 may be scarcely able to release AA from plasma
membrane without internalization or priming by proinflammatory
cytokines, because IIAPLA2 as a highly basic protein revealed poor
interfacial binding to phosphatidycholine (PC), a major
phospholipid on the outer leaflet of the plasma membrane in
mammalian cells. Consistent with this speculation, previous studies
have demonstrated that IIAPLA2 was internalized and promoted AA
release and prostaglandin E2 production in rat
fibroblast or stable HEK cells expressing mouse IIAPLA2 when the
cells were treated with inflammatory cytokines (8,9).
Moreover, sPLA2, including IIAPLA2, human group V sPLA2 (hVPLA2)
and gXPLA2 can act transcellularly to a different extent to
generate AA in distal cells via the paracrine mechanism (10–12). Thus, endocytosis and trafficking
of sPLA2 plays an essential regulatory role in the physiological
function of sPLA2, including inflammation and atherosclerosis.
However, the mechanisms by which sPLA2 is internalized in mammalian
cells and the spatio-temperal mobilization of internalized sPLA2 is
regulated are poorly understood, although basic sPLA2s such as
IIAPLA2 and hVPLA2 are shown to bind to heparan sulfate
proteoglycan (HSPG) and to be colocalized with caveolin (4,13).
In the present study, we investigated the molecular
mechanism for the internalization of hVPLA2 in CHO and HEK293
cells. To the best of our knowledge, in the present study, we
showed for the first time that hVPLA2 is associated with lipid
rafts partly by binding to heparan sulfate and internalized in a
flotillin-dependent pathway. Flotillin-mediated endocytosis led to
trafficking of some hVPLA2 to non-lysosomal vesicles in addition to
lysosomal compartments and then to Golgi apparatus. Moreover, the
association of hVPLA2 with lipid rafts caused the attenuation of AA
release from plasma membrane. These data therefore suggested that
the association of hVPLA2 with lipid rafts may be important in
protecting the plasma membrane from excessive degradation and
increasing AA release and eicosanoid production coupled with the AA
release from intracellular targets.
Materials and methods
Materials
Antibodies against syndecan-1, syndecan-4,
caveolin-1 and FITC-conjugated antibody against GM130 were
purchased from BD Transduction Laboratories (San Diego, CA, USA).
Antibodies specific for flotillin-1 and human lysosomal-associated
membrane protein (Lamp)-2 were purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). Antibodies recognizing
rat Lamp-2 were purchased from Zymed Laboratories (South San
Francisco, CA, USA). Monoclonal antibody specific to hVPLA2 was
prepared as described previously (6). Methy-β-cyclodextrin (MβCD), filipin
III, fumonitin B1, chlorpromazine HCl, brefeldin A and all other
reagents were of an analytical grade and were obtained from
Sigma-Aldrich (St. Louis, MO, USA). Dulbecco’s modified Eagle’s
medium (DMEM), Ham, Hank’s balanced salt solution (HBSS) and fetal
bovine serum (FBS) were from Life Technologies (Carlsbad, CA, USA).
HEK293, human embryonic kidney cells and CHO-K1, Chinese hamster
ovary cells were purchased from the American Type Culture
Collection (Manassas, VA, USA). Fatty acid-free bovine serum
albumin (BSA) was purchased from Bayer, Inc. (Kankakee, IL, USA).
Recombinant hVPLA2 and its mutants were expressed and purified to
homogeneity, as described in a previous study (14). We used recombinant hVPLA2-W79A
instead of wild-type and designated hVPLA2-W79A as hVPLA2
throughout the present study, since wild-type purification of
W79A-hVPLA2 was much easier than that of wild-type and hVPLA2-W79A
was demonstrated to be essentially the same as wild-type hVPLA2
(15).
Cell culture
CHO and HEK293 cells were grown in Ham and DMEM,
respectively, supplemented with 10% FBS, 100 U/ml penicillin G and
100 μg/ml streptomycin sulfate in a humidified atmosphere of 95%
air and 5% CO2 at 37°C. Cell viability after chemical
treatment was determined by 0.4% trypan blue staining.
Determining internalization rates
HEK293 cells were plated in a 60-mm dish and
cultivated until cells reached subconfluence. The cells were washed
with HBSS and incubated in Krebs-HEPES buffer (KHB; 140 mM NaCl, 4
mM KCl, 1 mM CaCl2, 1 mM Na2HPO4,
1 mM MgCl2, 10 mM HEPES, pH 7.4, 11.7 mM glucose, 0.2%
BSA) containing 150 nM hVPLA2 for 10 min at 4°C and transferred to
37°C. Incubation was quenched by the addition of ice-cold PBS at
appropriate times. PLA2 bound to the surface was recovered by
washing with PBS containing 0.8 M NaCl. Cells were lysed by
incubation with lysis buffer (20 mM Tris-HCl, 50 mM NaCl, 1 mM
EDTA, pH 7.4) containing 0.4% Nonidet P-40, 1 mM NaF, protease
inhibitor mix 20 min on ice. After brief sonication, the cell
lysates were centrifuged at 10,000 × g for 3 min. Activities of
PLA2 bound to the surface, internalized and remained in medium were
assayed, respectively, by measuring the initial rate of
[14C]SAPC (Amersham Biosciences Ltd., Little Chalfont,
Buckinghamshire, UK) hydrolysis as described previously (14). The rate of internalization was
determined by fitting the data to a single-phase exponential
curve.
siRNA knockdown
The following RNA duplexes with overhanging dTs for
human flotillin-1 and scrambled, non-specific control were prepared
from IDT as described previously (16): human flotillin-1 (sense),
5′-UGAGGCCAUGGUGGUCUCC dTdT-3′, scrambled control,
5′-UAGCUCGUGGGGCAGUCC dTdT-3′. To assess the effect of siRNA on the
endocytosis of hVPLA2, HEK293 cells were transfected with 200 nM of
the RNA duplex using Oligofectamine (Invitrogen, Carlsbad, CA, USA)
as per the manufacturer’s instructions. Two days after
transfection, 150 nM hVPLA2 was added to the cells. The cells were
incubated at 4°C for 10 min and 37°C for 30 min, and the amount of
internalized hVPLA2 was determined as described earlier.
AA release assay
Cells were plated onto 35-mm dish and cultivated for
two days. Radiolabeling of HEK293 or CHO cells was achieved by
incubating the cells with 0.5 μCi/ml [3H] AA (American
Radiolabeled Chemicals, St. Louis, MO, USA.) for 12 h at 37°C. MβCD
was treated for 90 min. After washing the cells twice with DMEM
containing 0.2% BSA, followed by washing with DMEM, the cells were
treated with 150 nM prediluted hVPLA2 in the medium at the
specified times. After quenching of the reaction by adding 2 ml of
ice-cold DMEM, the cells were separated immediately by
centrifugation. The radioactivity of the pellet and supernatant,
respectively, was measured by liquid scintillation counter.
Detergent solubilization and OptiPrep
gradient fractionation
The HEK293 or CHO cells were plated in 100-mm dishes
and cultivated in a monolayer for 2 days. The cells were treated
with 100 nM wild-type hVPLA2, hVPLA2-W79A, or
hVPLA2-W79A/R100E/K101E for 30 min at 10°C and washed twice in
ice-cold HBSS and once in ice-cold hypotonic solution (42 mM KCl, 5
mM MgCl2, 10 mM HEPES, pH 6.5). The cells were incubated
in the hypotonic solution, supplemented with a Protease Inhibitor
Mix (Roche Diagnostics, Indianapolis, IN, USA) for 15 min on ice
and lysed by passing through 25-gauge needle. Following the
addition of sucrose to make a final 0.25 M, cell lysates were
sonicated briefly. The lysates were centrifuged at 300 × g for 10
min at 4°C and resulting supernatants were centrifuged at 10,000 ×
g for 10 min at 4°C. The membrane pellets were resuspended in 0.6
ml of 0.5% Triton X-100 or 1% Brij-58 (Pierce Chemical Co.,
Rockford, IL, USA) in 20 mM NaCl/5 mM EDTA 50 mM Tris, pH 7.4 (TSE)
containing a protease inhibitor mix and 0.25 M sucrose and
incubated for 30 min at 4°C. The solublilized lysates were mixed
with 1.2 ml of cold 60% OptiPrep (Life Technologies) for 10 min and
overlaid successively with 2 ml of 35, 30, 25, 20 and 0% OptiPrep
in TSE. After centrifugation at 52,000 × g in a Sorvall TH-641
rotor (Thermo Scientific, Asheville, NC, USA) for 1.5 h at 4°C, six
fractions were collected from the top of the gradient. The
fractions were TCA precipitated and analyzed by western blot
analysis.
Internalization of hVPLA2 and western
blot analysis
HEK293 and CHO cells were treated with 100 nM of
hVPLA2 or hVPLA2-W79A/R100E/K101E for the indicated period and the
incubation was quenched by adding a solution of ice-cold 0.8 M NaCl
in DMEM or Ham. After washing with the same solution, the pellet
was collected by scraping and centrifugation, then lysed in 100 μl
of lysis buffer (20 mM Tris-HCl, 50 mM NaCl, 5 mM EDTA, pH 7.4)
containing 0.5% Nonidet P-40, 1 mM NaF, protease inhibitor mix and
0.1% deoxycholic acid. After incubation for 20 min on ice, the cell
lysates were sonicated briefly and centrifuged at 12,000 × g for 2
min. The supernatants were analyzed for protein concentration by
BCA (Pierce Chemical Co.). Equal amounts of the samples were
subjected to 16% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE). The electrotransfer of proteins from
the gels to polyvinylidene fluoride (PVDF) membrane was achieved
using a semidry system (400 mA, 120 min). The membrane was blocked
with 2% BSA for 60 min, then incubated with 1 μg/ml of the
anti-hVPLA2 monoclonal antibody 3G1 diluted in Tris-buffered saline
plus 0.05% Tween-20 (TBST) overnight. The membranes were washed
three times for 20 min with TBST. Goat anti-mouse IgG conjugated
with horseradish peroxidase was diluted 3,000-fold in TBST and
incubated with the PVDF membrane for 60 min. The membrane was
washed three times with TBST and analyzed with an ECL
chemiluminescence system (Amersham Biosciences).
Antibody-induced patching,
immunofluorescence and confocal microscopy
For the patching experiments, CHO cells were plated
on cover glass and incubated at 37°C with 5% CO2 for two
days. The cells were incubated with hVPLA2 (150 nM) diluted in
culture media containing 20 mM HEPES for 20 min at 12°C. After
washing with cold HBSS three times, the cells were incubated with
mouse anti-hVPLA2 (5 μg/ml) diluted in HBSS containing 2% BSA for
30 min at 12°C. For the clustering of syndecan, the cells were
treated with rat anti-syndecan-4 (1/25) or anti-syndecan-1 (1/25)
for 10 min at 12°C, which was dialyzed against PBS and diluted in
the Ham containing 20 mM HEPES. For further patching, the cells
were incubated with Alexa568-conjugated anti-mouse IgG (1/100) or
Alexa488-conjugated anti-rat IgG (1/100) diluted in HBSS containing
2% BSA for 30 min at 12°C. Control cells were treated with HBSS-2%
BSA and non-immune serum. After washing with HBSS six times, the
cells were fixed with 4% formaldehyde for 4 min followed by fixing
in −20°C methanol for 1 min. After washing with PBS three times,
copatching of GM1 was revealed by incubation of Alexa488-cholera
toxin B subunit (Alexa488-CT, 15 μg/ml; Life Technologies) or
Alexa568-CT (15 μg/ml) for 30 min under gentle rocking. After
washing five times with PBS, the slide was mounted with
Fluoromount-G (Southern Biotech Associates, Birmingham, AL, USA).
For immunofluorescence experiments, the cells were plated onto a
sterile cover glass. The cells were treated with 100 nM of hVPLA2
in culture media at a 37°C in a 5% CO2 humidified
incubator. At the specific time-point, the cells were washed twice
with cold PBS and were then fixed at room temperature with 3.6%
paraformaldehyde in PBS for 10 min. After fixation, the cells were
washed three times with PBS and placed in a blocking solution (10%
normal goat serum and 100 μM goat IgG in PBS) at room temperature
for 1 h. The cells were then permeabilized with PBS containing 0.1%
Triton X-100 and 2% BSA for 1 h at room temperature, washed four
times with PBS and incubated with the monoclonal antibodies raised
against hVPLA2 and rabbit anti-human caveolin-1 or goat anti-human
flotillin-1 (Santa Cruz Biotechnology, Inc.) or rabbit anti-rat
Lamp-2, respectively, in the presence of 2% BSA. After overnight
incubation at 4°C or 1 h at room temperature, the antibodies were
removed and the cells were washed six times with PBS. A secondary
antibody, Alexa488 donkey anti-goat antibody or Alexa488 donkey
anti-rabbit antibody (both from Molecular Probes) diluted in PBS
containing 2% BSA, was applied for 1 h at room temperature followed
by washing and incubation with another secondary antibody, Alexa568
donkey anti-mouse antibody (Molecular Probes) diluted in PBS
containing 2% BSA for 1 h at room temperature. Golgi apparatus was
stained by incubation with FITC-conjugated anti-GM130 for 1 h.
After washing six times with PBS, the slide was mounted with
Fluoromount-G (Southern Biotech Associates). Imaging was performed
using a LSM 5 PASCAL confocal microscope.
Statistical analysis
Results are expressed as mean ± SEM. Statistical
significances were analyzed by one-way ANOVA followed by paired
t-test using Prism (GradPad Software, La Jolla, CA, USA). P<0.05
was considered statistically significant.
Results
Association of hVPLA2 with
detergent-resistant membranes (DRMs)
Two different methods can be applied for studies on
the cellular trafficking or action mechanism of PLA2. One method
involves monitoring endogenous protein by indirect
immunohistochemistry or by the heterologous expression of the
protein fused with fluorescent protein. The other method is based
on the detection of proteins added exogenously. Limitations of the
first method on the internalization mechanism of sPLA2 include: i)
detection of the specific isoform of PLA2 is not feasible due to
lack of availability of isoform-specific antibodies; ii) secreted
PLA2 level is usually beyond the detection limit of conventional
visualization. Even if detection of re-internalized PLA2 is allowed
or even if it is visualized by expression of the protein as a
fluorescent fusion protein, it is difficult to obtain clear data
due to strong interference from the high level of the PLA2 stored
endogenously. Therefore, in the present study, we studied the
internalization mechanism by use of the exogenous addition of
purified hVPLA2 to the cells that express endogenous sPLA2 in very
low levels and detected hVPLA2 by a specific monoclonal antibody
against hVPLA2 as described previously (14). To address the manner in which
hVPLA2 is internalized, we determined first the internalization
kinetics in HEK293 cells by quantification of internalized hVPLA2
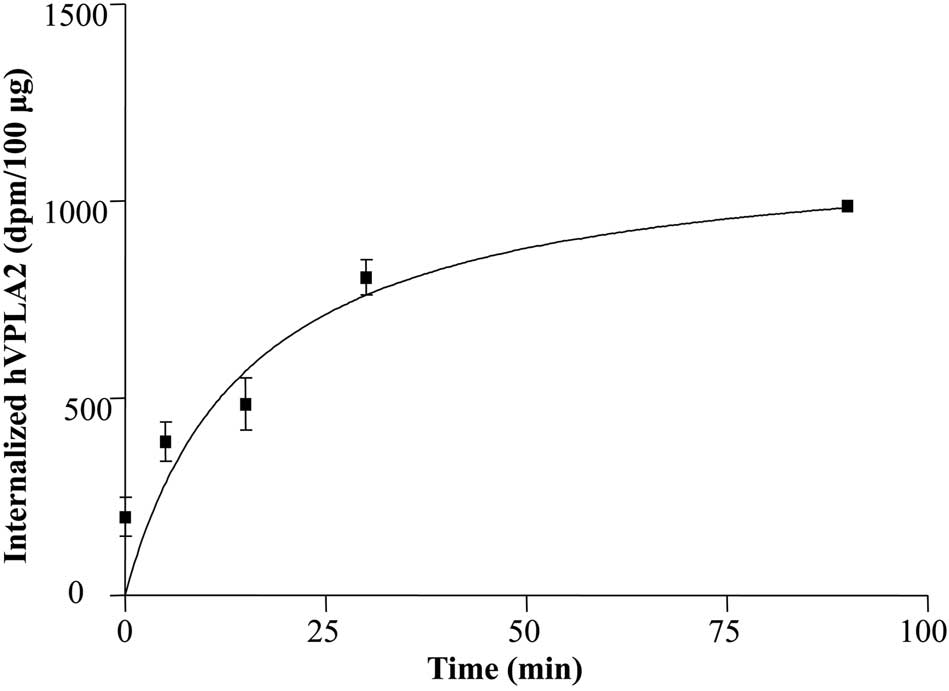
based on its activity. The half-life of internalization was ~16 min
and the plateau level was attained in 70 min (Fig. 1), suggesting that internalization
may occur in a clathrin-independent manner, as clathrin-coated
pitch-mediated endocytosis was known to occur within a few minutes.
Determination of internalization kinetics using wild-type hVPLA2
showed the essentially same result as that of hVPLA2 (unpublished
data). Insolubility to Triton X-100 extraction is a practical
criterion for the detection of lipid rafts association with
protein. To determine whether hVPLA2 is associated with lipid rafts
when hVPLA2 is bound to plasma membrane or internalized,
hVPLA2-treated CHO and HEK293 cell lysates were solubilized in 0.5%
Triton X-100 and floated onto OptiPrep density gradient. In CHO
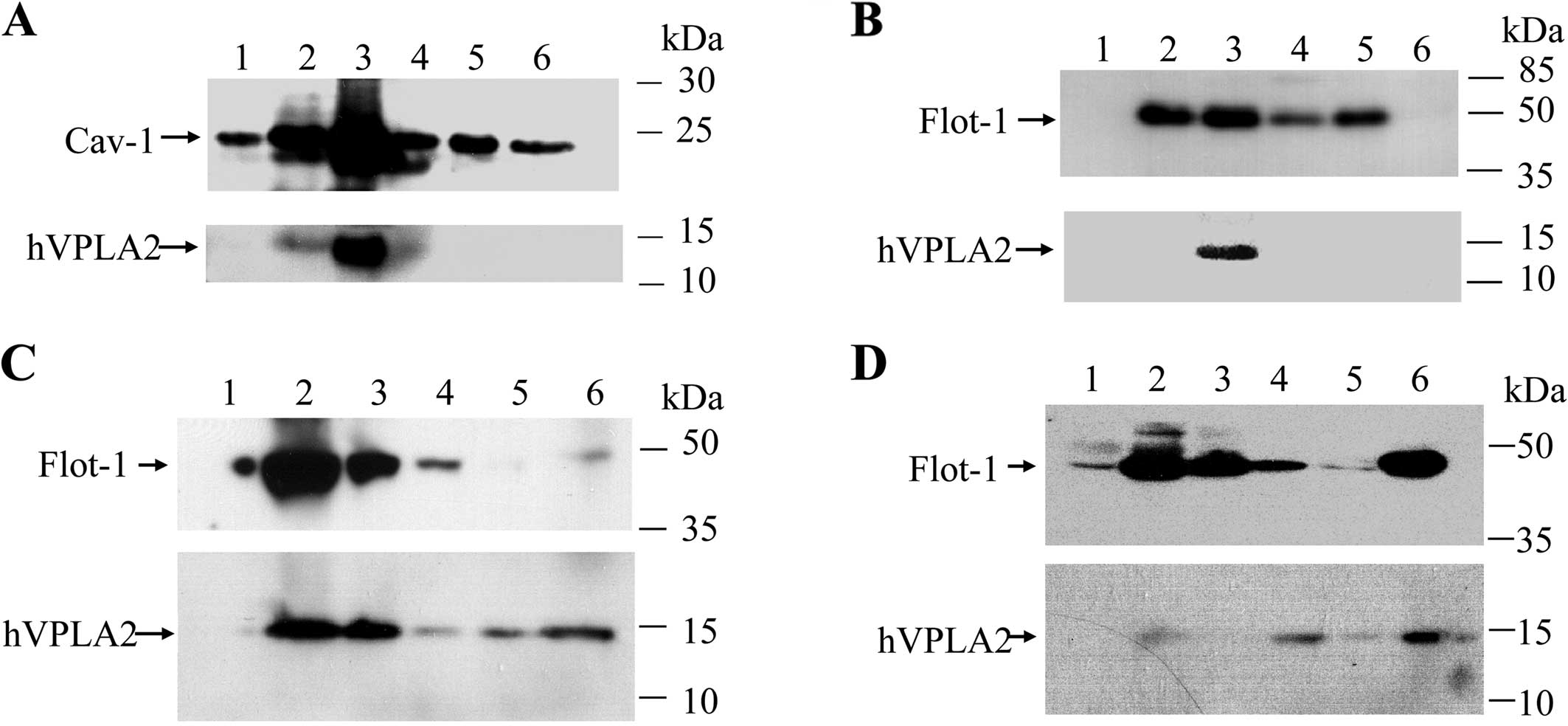
cells, hVPLA2 was enriched in the low-density membrane fractions
containing well-known lipid raft marker, 22-kDa caveolin-1
(Fig. 2A). As the expression
levels of caveolin-1 are reported to be low in HEK293 cells, we
used 48-kDa flotillin-1 as a lipid raft marker in HEK293 cells.
Distribution of flotillin-1 in HEK293 cells showed essentially the
same pattern as that of CHO cells (Fig. 2B). When Brij-58 was used to
solubilize membrane proteins, both hVPLA2 and flotillin-1 were
predominantly partitioned into low-density fractions, whereas
hVPLA2 was partitioned more broadly (Fig. 2C). To determine whether
hVPLA2-enriched fractions were DRMs, HEK293 cells were treated with
MβCD for the depletion of cholesterol and DRMs were isolated from
the cell lysates. Most of the hVPLA2 was solubilized by Brij-58 and
detected in non-raft fractions along with partial redistribution of
flotillin-1 to the non-raft fractions (Fig. 2D, lane 4–6). These data showed
that hVPLA2 is associated with cholesterol-sensitive DRMs in both
HEK293 and CHO cells.
Copatching of GM1 rafts by cross-linking
of hVPLA2
Antibodies or multivalent toxins against membrane
components cause lateral cross-linking of the components on the
cell membrane by which recruitment of lipid rafts associated with
the patched components can be induced around the crossed-linked
patch. Thus, antibody-induced copatching of proteins provided a
common tool for analyzing lipid rafts association with proteins
(17–20). To address the association of
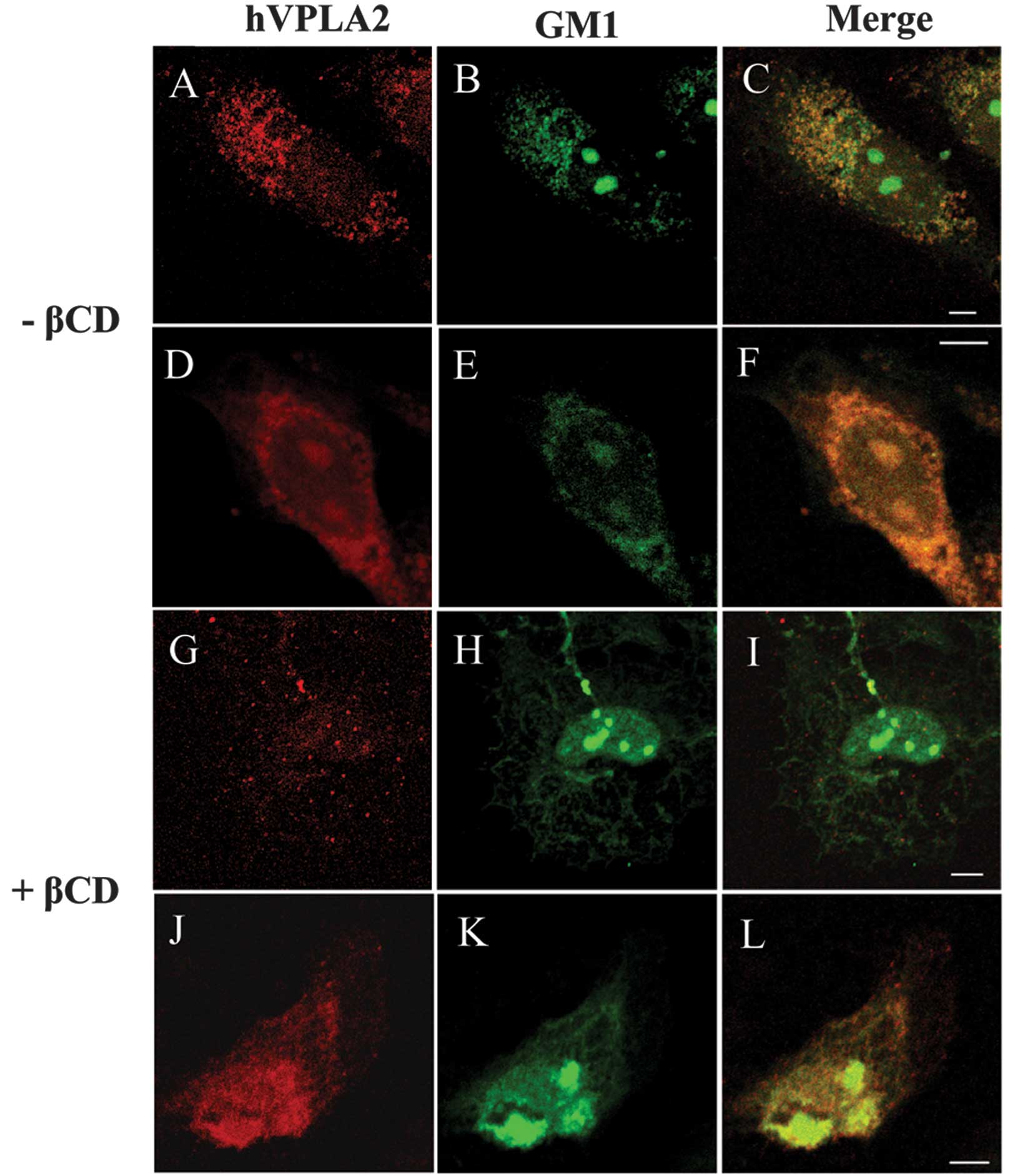
hVPLA2 with lipid rafts, CHO cells were treated with hVPLA2 at 12°C
to minimize the metabolic activities of cells and patching was
induced by anti-hVPLA2 and secondary antibody in living cells.
Ganglioside GM1 was visualized by staining with CT conjugated with
Alexa488. Confocal image analysis revealed that ganglioside
GM1-containing rafts were copatched well with the patches of
cross-linked hVPLA2, and thus most of the GM1 rafts were
colocalized with the patched hVPLA2 (Fig. 3A–C). In the non-patched control
cells, GM1 immunostaining showed an even distribution on the cell
surface but not on the punctate pattern (Fig. 3D–F). After inducing copatching by
cross-linking of hVPLA2, spots of GM1-containing patches (~200–400
nm) were larger than those of the punctate spot of the non-patched
control (Fig. 3C and F). By
contrast, in the patched cells pretreated with MβCD, the
hVPLA2-patch was clearly segregated from the GM1 rafts (Fig. 3I). Depletion of cholesterol by
MβCD disrupted the punctate immunostaining of GM1 and hVPLA2 in the
non-patched control cells (Fig. 3J
and K).
Heparin binding site of hVPLA2 is
involved in the association with lipid rafts
Findings of previous studies have demonstrated that
hVPLA2 binds to HSPG (13,21)
which mediates the association of HSPG-binding proteins such as
prion and eosinophil cationic protein with lipid rafts (22,23). To determine whether heparan
sulfate binding is involved in the association of hVPLA2 with lipid
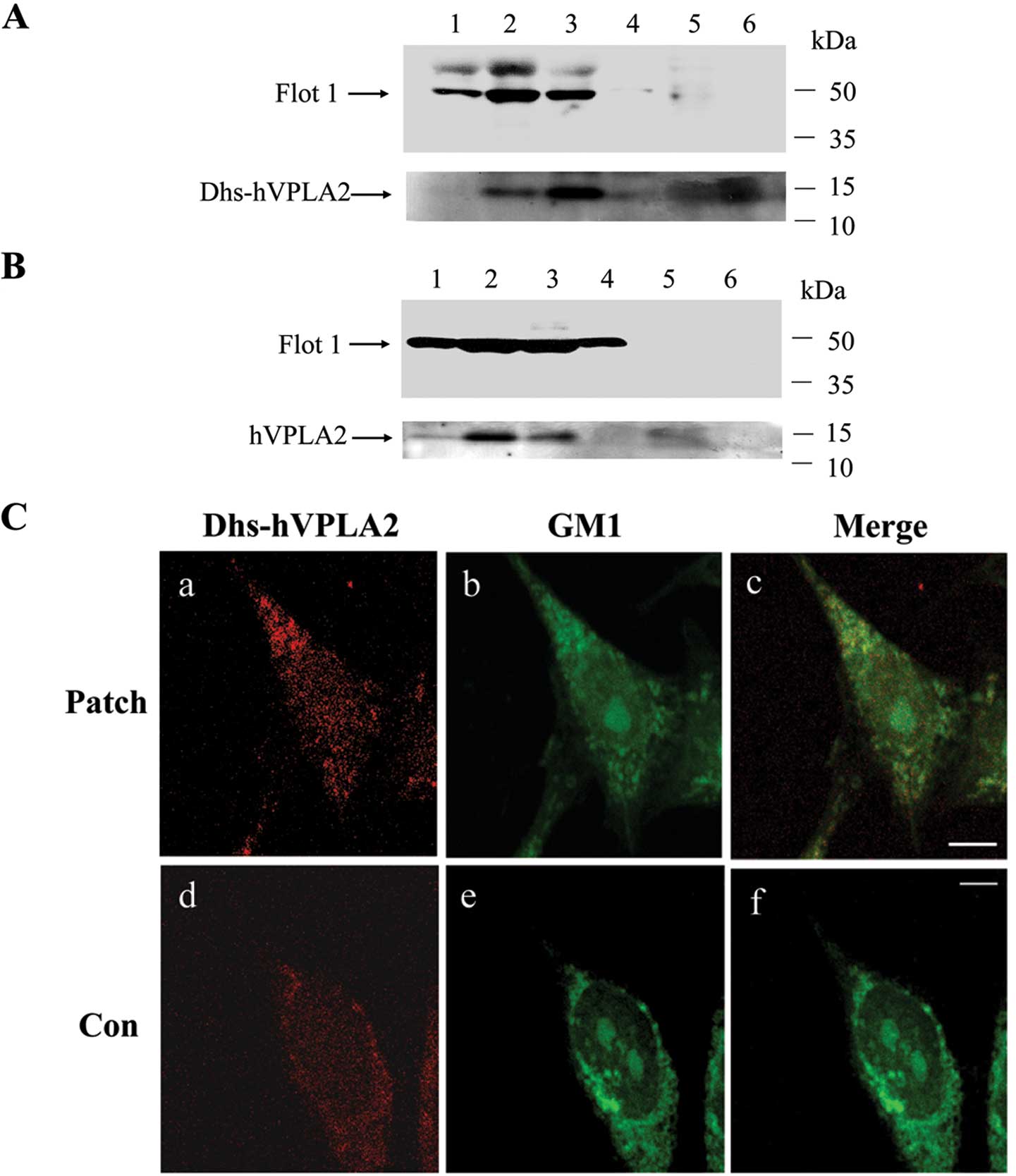
rafts, we examined whether hVPLA2-W79A/R100E/K101E, a mutant hVPLA2
with deficiency in heparan sulfate binding (Dhs-hVPLA2) was capable
of associating with lipid rafts. HEK293 cells were treated with
hVPLA2 or Dhs-hVPLA2, subjected to solubilization in Brij-58 and
floated onto OptiPrep gradient. Approximately half of Dhs-hVPLA2 in
lipid raft fractions was shifted into non-raft fractions (Fig. 4A), while some hVPLA2 remained in
lipid raft fractions (Fig. 4B),
suggesting that the association of hVPLA2 with lipid rafts was
mediated partially through the heparin binding of hVPLA2. In line
with this interpretation, confocal image analysis revelaed that
cross-linking of Dhs-hVPLA2 was able to recruit GM1 rafts to the
mutant hVPLA2 patches, although the mutant hVPLA2 yielded a weaker
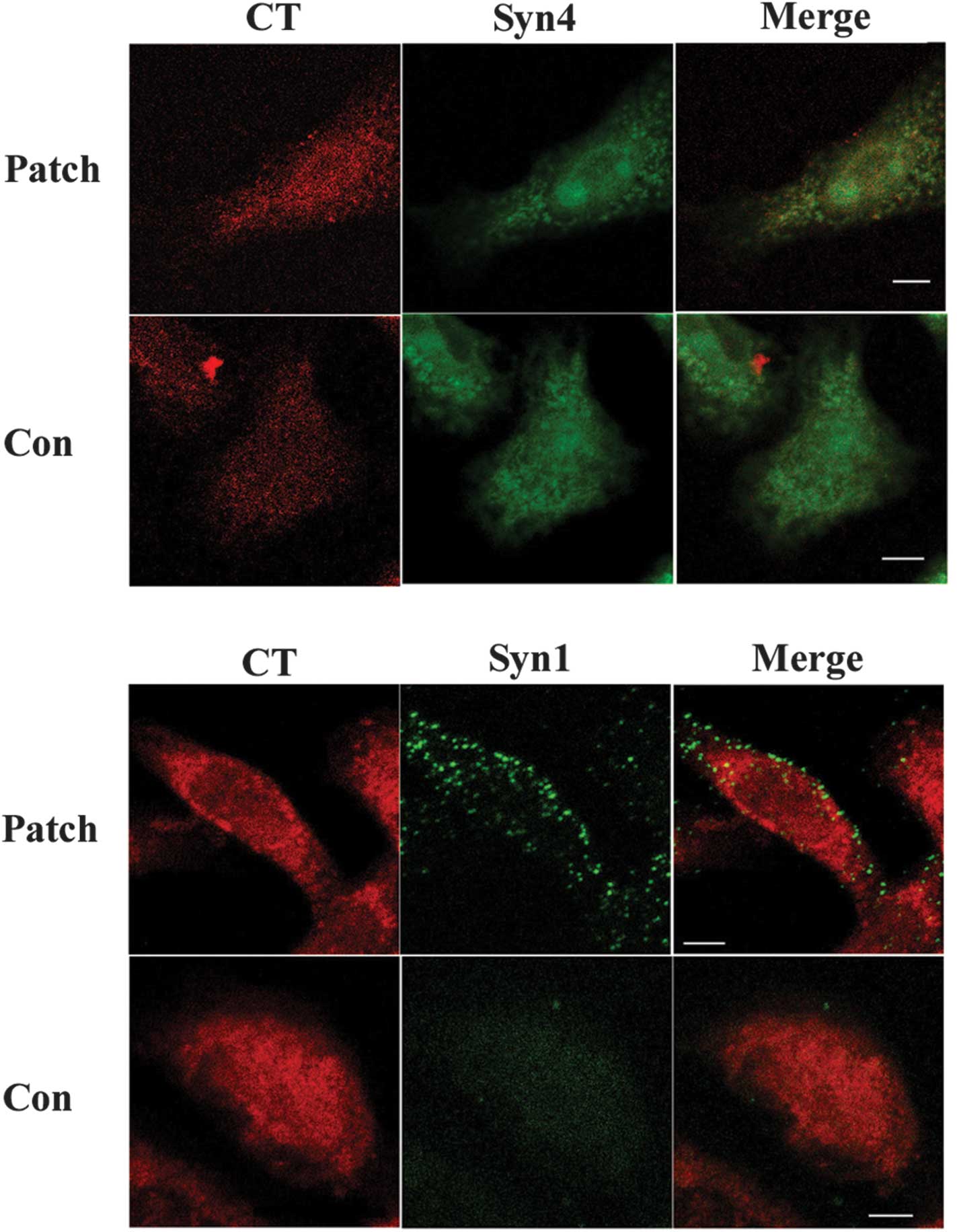
GM1 immunostaining compared with that of hVPLA2 (Fig. 3C). To clarify whether HSPG is
associated with lipid rafts, we performed a copatching experiment
in CHO cells with antibody against syndecan, a major subfamily of
HSPG. Immunofluorescence staining indicated that syndecan-4 patches
were extensively copatched with GM1 rafts, while sydecan-1 patches
were clearly separated with GM1 rafts (Fig. 5). These data suggested that since
syndecan-4 was associated with lipid rafts in CHO cells, it may be
involved in the association of hVPLA2 with lipid rafts.
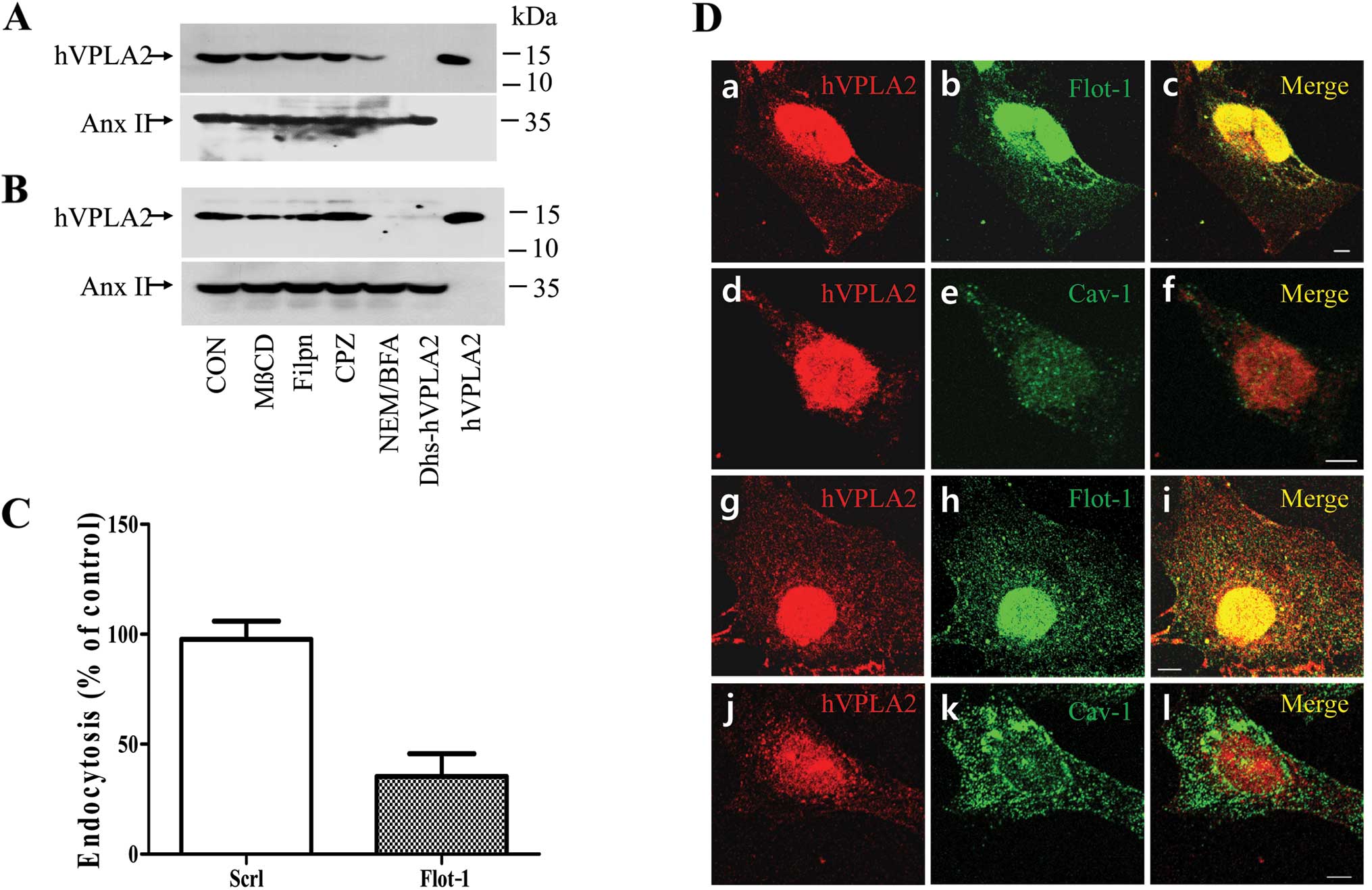
Flotillin-dependent endocytosis of hVPLA2
in CHO and HEK293 cells
To determine how hVPLA2 is internalized, HEK293 or
CHO cells were pretreated with endocytic inhibitors and
internalization of hVPLA2 was detected by western blotting. Cell
viability was examined subsequent to chemical treatment. As HEK293
cells exhibited sensitivity to the inhibitors, we optimized the
conditions to maintain cell viability >90%. Internalization of
hVPLA2 was suppressed partially by MβCD and marginally by filipin,
a cholesterol sequestration reagent (Fig. 6). However, chlorpromazine, an
inhibitor for the endocytosis mediated by clathrin-coated pit had
no effect on the internalization of hVPLA2. Pretreatment with
N-ethylmaleimide (NEM) and brefeldin A, an inhibitor for the
N-ethylmaleimide-sensitive factor (NSF) and a Golgi and endosome
traffic disrupting drug, respectively, inhibited endocytosis. Along
with the association of hVPLA2 with lipid rafts, these data
suggested that hVPLA2 is likely internalized in lipid
raft-dependent but not clathrin-dependent pathways. To examine
whether lipid rafts were involved in the endocytosis of hVPLA2, we
detected hVPLA2 in endocytic vesicles by double immunostaining with
flotillin or caveolin. HEK293 or CHO cells were fixed immediately
after the endocytosis of hVPLA2 was allowed to occur. To detect the
low amount of internalized hVPLA2, we used a relatively high
concentration (2 μg/ml) of anti-hVPLA2 and secondary antibody
(1/300 dilution), which resulted in non-specific immunostaining
inside the nucleus but not cytoplasmic staining (unpublished data).
Confocal analysis of double immunostaining revealed extensive
colocalization of hVPLA2 with flotillin-1 in the cytoplasmic and
perinuclear region in both HEK293 and CHO cells (Fig. 8A–C and G–I). However, hVPLA2
immunostaining was clearly separated from that of caveolin-1 with
partial colocalization only in the perinuclear region (Fig. 8D–E and J–L). These data suggest
that hVPLA2 associated with lipid rafts on the outside membrane is
internalized in vesicles containing flotillin-1 but not caveolin-1.
Observation of punctate immunostaining showing that sizes of
hVPLA2-positive spots were slightly smaller than those of
caveolin-1-positive ones (Fig. 6D-a,
g, e and k), suggested that hVPLA2 is likely internalized in
vesicles that are different from that resembling a caveolae (50–100
nm). To obtain direct evidence that flotillin-1 mediated the
internalization of hVPLA2, we determined the effect of flotillin-1
knockdown on hVPLA2 endocytosis by transfection of siRNA-specific
flotillin-1 in HEK293 cells. Knockdown of flotillin-1 markedly
inhibited hVPLA2 endocytosis (Fig.
6C). Internalization efficiency of hVPLA2 was 36% in
flotillin-1 knockdown cells, which was normalized against the
amount of internalization in the control cells transfected with
scrambled siRNA. Considering transfection was regarded as efficient
at 90%, the inhibition of hVPLA2 endocytosis by flotillin-1
knockdown was ~72%.
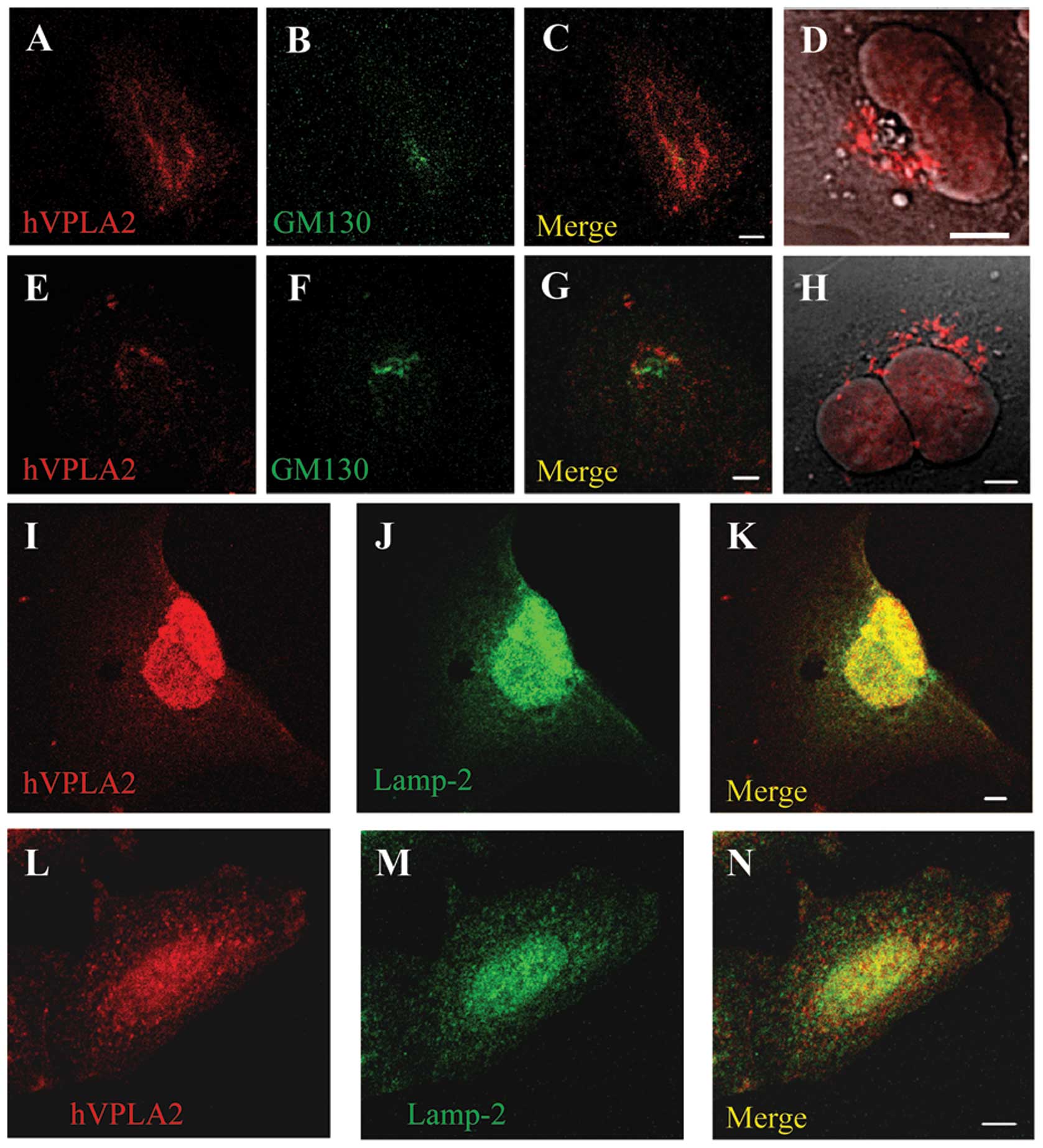
Localization of internalized hVPLA2 into
late endocytic compartments and Golgi in HEK293 and CHO cells
To determine the destiny of internalized hVPLA2, the
cells were fixed at specific times after treatment with hVPLA2,
immunostained and analyzed by confocal microscopy. For the double
immunostaining of hVPLA2 and GM130, a low concentration of
anti-hVPLA2 (1 μg/ml) and secondary antibody (1/1,000 dilution)
were used to minimize non-specific staining. Following a 10-min
incubation, internalized hVPLA2 moved partially to the late
endocytic compartment containing Lamp-2 as shown by colocalization
with Lamp-2 in addition to perinuclear localization (Fig. 7I–N). After 40 min, the
colocalization of hVPLA2 around GM130, a cis-Golgi marker was
observed. Then, after 90 min, the internalized hVPLA2 was
accumulated in large vesicular form around Golgi apparatus as shown
in the DIC image (Fig. 7D and H),
suggesting that some of the internalized hVPLA2 was not destined to
degradation via lysosome but was instead recycled via Golgi.
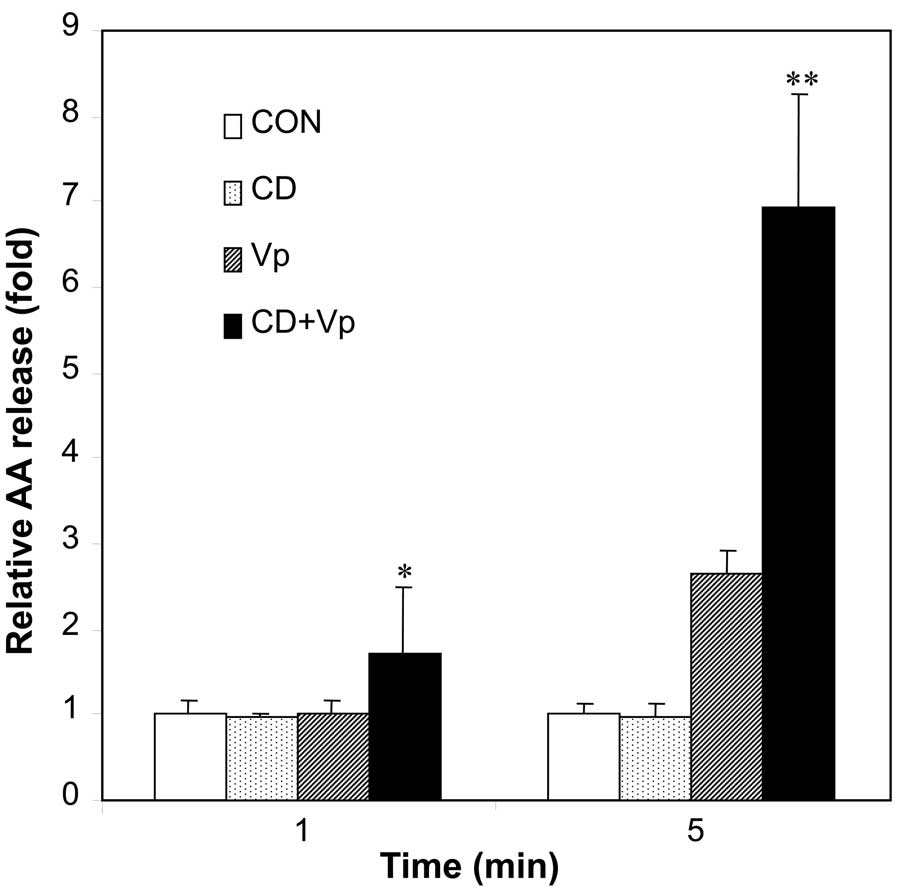
Effect of lipid rafts on the action mode
of hVPLA2 in HEK293 cells
To examine the importance of lipid rafts association
in hVPLA2 action on target cells, we examined the effect of
disruption of hVPLA2-containing lipid rafts on AA release from the
plasma membrane. Our previous result (14) shows that exogenous hVPLA2
treatment generated AA release inside cells for ~5 min in the
HEK293 cells. Initial AA release before any significant generation
of AA release from inside the membrane can represent AA release
from the hVPLA2 action on the plasma membrane. Therefore,
steady-state level of AA release was measured at 1 and 5 min with
or without MβCD pretreatment. Disruption of association of hVPLA2
with the rafts by MβCD pretreatment enhanced AA release
significantly to 1.8- and 2.4-fold 1 and 5 min after hVPLA2
incubation, respectively (Fig.
8). Thus, the association of hVPLA2 with lipid rafts resulted
in a reduction of AA release from the plasma membrane.
Discussion
Despite extensive studies on sPLA2, its mode of
action and internalization mechanisms remain largely unknown. In
the present study, we have shown that hVPLA2 is associated with
lipid rafts and internalized in a flotillin-dependent pathway. The
association of hVPLA2 with lipid rafts was shown by
cholesterol-sensitive enrichment of hVPLA2 in low-density DRM
fraction and copatching of GM1 rafts through hVPLA2 cross-linking
in a cholesterol-sensitive manner (Figs. 2 and 3). As Dhs-hVPLA2, a mutant hVPLA2
deficient in binding to heparan sulfate, distributed dispersedly in
both low-density and high-density fraction, binding to HSPG may be
necessary for the association of hVPLA2 with lipid rafts (Figs. 4 and 5). In addition, cross-linking of
Dhs-hVPLA2 may recruit GM1 rafts less efficiently than that of
hVPLA2, even though residual copatching of GM1 was induced
(Figs. 3C and 4C). We then determined whether HSPG is
associated with lipid rafts to mediate recruiting hVPLA2 to lipid
rafts. Copatching experiment with antibody against syndecan, a
major family of HSPG showed that lipid rafts containing syndecan-4
but not syndecan-1 recruited with GM1 rafts efficiently.
Immunostaining in unpatched control cells showed a punctate pattern
of syndecan-1 (Fig. 5),
indicating that syndecan-1 may be associated with lipid rafts,
although syndecan-1 did not recruit GM1 rafts. This interpretation
is supported by a recent finding that syndecan-1 and −4 are
specifically associated with sphingomyelin-enriched lipid rafts in
parathyroid cell (24). Taken
together, these data suggested that additional interaction other
than heparan sulfates with molecules residing in lipid rafts may be
necessary for association of hVPLA2 with lipid rafts.
Internalization of hVPLA2 was shown to occur in a
flotillin-dependent mechanism by flotillin-1 knockdown and
endocytic inhibitors. Internalization of hVPLA2 was determined
based on the enzyme activity of hVPLA2 and immunocytochemistry
using a specific antibody against hVPLA2. Internalization kinetics
showed that the half-life of internalization was 16 min and the
plateau was attained in 70 min. As some of the internalized hVPLA2
was destined to late endosome or lysosome within which hVPLA2 lost
enzymatic activity (Fig. 7),
determination of the internalization rate based on enzyme activity
would be underestimated and may represent the sum of complex
kinetics including internalization, trafficking, and degradation.
Rough estimation of internalization rate, and relatively rapid
internalization (t1/2 =16 min) suggested that the
internalization rate is consistent with nonclathrin endocytosis.
Consistent with these data, chlorpromazine, an inhibitor of
clathrin-mediated endocytosis did not inhibit hVPLA2
internalization, whereas, MβCD, a specific cholesterol-depletion
drug, attenuated hVPLA2 internalization. However, filipin, a
cholesterol-sequestrating drug that suppresses caveolin-mediated
endocytosis was slightly inhibited, suggesting that hVPLA2 was
internalized in a non-caveolin endocytic route. Results of the
flotillin knockdown experiment revealed that flotillin-1 is
responsible for hVPLA2 endocytosis. In addition, internalized
hVPLA2 was extensively colocalized with flotillin-1 in the punctate
structures, although not with caveolin-1. Unlike our result, murine
VPLA2 tagged with GFP was shown to be localized in
caveolin-2-containing granules in LPS-stimulated P388D1
macrophage-like cells (13). This
difference in the localization of VPLA2 may reflect the difference
in cell type. In addition, VPLA2-GFP expressed in secreting
granules in P388D1 cells can interfere with imaging of reuptake of
secreted VPLA2-GFP. These data have shown that hVPLA2 is
internalized in a flotillin-dependent pathway in HEK293 cells.
Previous studies have shown that cationic molecules including
polyamines, polypeptide and polyplexes enter cells through HSPG and
are endocytosed in a flotillin-dependent route in BS-C-1 cells
(25,26), which is consistent with our
result. However, contrary to our result showing that hVPLA2 was
trafficked not only to the lysosomal compartments, but also to
Golgi, the internalized cationic molecules bound to HSPG were
trafficked predominantly to late endosome within 120 min, but not
to Golgi apparatus. Different cell type or additional interaction
of hVPLA2 with lipid rafts other than the interaction through
heparan sulfate, which may control interaction with trafficking
adaptors, may contribute to this difference in trafficking routes.
The trafficking of internalized hVPLA2 to non-lysosomal vesicles
and later to Golgi (Fig. 7) shows
that some of the hVPLA2 is delivered to other targets instead of
degradation. Consistent with this result, it is reported that AA is
generated from inside the membrane following treatment with
exogenous hVPLA2 (10,14).
To get insight on the role of lipid rafts in the
regulation of hVPLA2 action, we determined AA release from the
plasma membrane in the presence or absence of MβCD. Disruption of
the association with lipid rafts hVPLA2 by MβCD pretreatment
increased AA release from the plasma membrane up to 2.45-fold
compared with absence of MβCD pretreatment. These data can be
interpreted in different ways. One is that limited diffusion and
clustering of hVPLA2 in lipids rafts enriched in cholesterol and
glycosphingolipids have limited access to PC, a major substrate
outside the plasma membrane. Another reason involves reduced
residence time in the plasma membrane by relatively rapid
internalization of hVPLA2 associated with lipid rafts to
intracellular targets. When CHO cells were treated with MβCD to
disrupt lipid rafts and treated with hVPLA2 (150 nM), extensive
conversion of PC (>15%) into the lysoPC in plasma membrane
occurred, resulting in cell death (unpublished data). The
localization of some of the internalized hVPLA2 in Lamp 2-negative
punctate structures and later around the Golgi complex indicated
that internalized hVPLA2 is capable of inducing AA release from the
intracellular target sites instead of degradation inside the
lysosomal compartments. Therefore, these data suggest that the
association of hVPLA2 with lipid rafts may play a role in
protecting plasma membrane from excessive degradation and
increasing AA release and eicosanoid production coupled with
released AA from intracellular targets.
Acknowledgements
The present study was supported by National Research
Foundation of Korea Grant funded by the Korean Government
(2010-0005848) and by the Priority Research Centers Program through
the National Research Foundation of Korea (NRF) funded by the
Ministry of Education, Science and Technology
(NRF-2009-0094071).
References
|
1
|
Schaloske RH and Dennis EA: The
phospholipase A2 superfamily and its group numbering
system. Biochim Biophys Acta. 1761:1246–1259. 2006.PubMed/NCBI
|
|
2
|
Lambeau G and Gelb MH: Biochemistry and
physiology of mammalian secreted phospholipases A2. Annu
Rev Biochem. 77:495–520. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Han WK, Sapirstein A, Hung CC,
Alessandrini A and Bonventre JV: Cross-talk between cytosolic
phospholipase A2 alpha (cPLA2 alpha) and
secretory phospholipase A2 (sPLA2) in
hydrogen peroxide-induced arachidonic acid release in murine
mesangial cells: sPLA2 regulates cPLA2 alpha
activity that is responsible for arachidonic acid release. J Biol
Chem. 278:24153–24163. 2003.PubMed/NCBI
|
|
4
|
Murakami M, Kambe T, Shimbara S, Yamamoto
S, Kuwata H and Kudo I: Functional association of type IIA
secretory phospholipase A(2) with the
glycosylphosphatidylinositol-anchored heparan sulfate proteoglycan
in the cyclooxygenase-2-mediated delayed prostanoid-biosynthetic
pathway. J Biol Chem. 274:29927–29936. 1999. View Article : Google Scholar
|
|
5
|
Fleisch JH, Armstrong CT, Roman CR, et al:
Recombinant human secretory phospholipase A2 released thromboxane
from guinea pig bronchoalveolar lavage cells: in vitro and ex vivo
evaluation of a novel secretory phospholipase A2 inhibitor. J
Pharmacol Exp Ther. 278:252–257. 1996.
|
|
6
|
Kim YJ, Kim KP, Han SK, et al: Group V
phospholipase A2 induces leukotriene biosynthesis in
human neutrophils through the activation of group IVA phospholipase
A2. J Biol Chem. 277:36479–36488. 2002.PubMed/NCBI
|
|
7
|
Muñoz NM, Kim YJ, Meliton AY, et al: Human
group V phospholipase A2 induces group IVA phospholipase
A2-independent cysteinyl leukotriene synthesis in human
eosinophils. J Biol Chem. 278:38813–38820. 2003.
|
|
8
|
Bezzine S, Koduri RS, Valentin E, et al:
Exogenously added human group X secreted phospholipase A(2) but not
the group IB, IIA, and V enzymes efficiently release arachidonic
acid from adherent mammalian cells. J Biol Chem. 275:3179–3191.
2000. View Article : Google Scholar
|
|
9
|
Murakami M, Koduri RS, Enomoto A, et al:
Distinct arachidonate-releasing functions of mammalian secreted
phospholipase A2s in human embryonic kidney 293 and rat mastocytoma
RBL-2H3 cells through heparan sulfate shuttling and external plasma
membrane mechanisms. J Biol Chem. 276:10083–10096. 2001. View Article : Google Scholar
|
|
10
|
Wijewickrama GT, Kim JH, Kim YJ, et al:
Systematic evaluation of transcellular activities of secretory
phospholipases A2. High activity of group V
phospholipases A2 to induce eicosanoid biosynthesis in
neighboring inflammatory cells. J Biol Chem. 281:10935–10944.
2006.PubMed/NCBI
|
|
11
|
Reddy ST and Herschman HR: Transcellular
prostaglandin production following mast cell activation is mediated
by proximal secretory phospholipase A2 and distal
prostaglandin synthase 1. J Biol Chem. 271:186–191. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Murakami M, Kambe T, Shimbara S and Kudo
I: Functional coupling between various phospholipase A2s
and cyclooxygenases in immediate and delayed prostanoid
biosynthetic pathways. J Biol Chem. 274:3103–3115. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Balboa MA, Shirai Y, Gaietta G, Ellisman
MH, Balsinde J and Dennis EA: Localization of group V phospholipase
A2 in caveolin-enriched granules in activated
P388D1 macrophage-like cells. J Biol Chem.
278:48059–48065. 2003.PubMed/NCBI
|
|
14
|
Kim YJ, Kim KP, Rhee HJ, et al:
Internalized group V secretory phospholipase A2 acts on
the perinuclear membranes. J Biol Chem. 277:9358–9365. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Han SK, Kim KP, Koduri R, et al: Roles of
Trp31 in high membrane binding and proinflammatory activity of
human group V phospholipase A2. J Biol Chem.
274:11881–11888. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Glebov OO, Bright NA and Nichols BJ:
Flotillin-1 defines a clathrin-independent endocytic pathway in
mammalian cells. Nat Cell Biol. 8:46–54. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Harder T, Scheiffele P, Verkade P and
Simons K: Lipid domain structure of the plasma membrane revealed by
patching of membrane components. J Cell Biol. 141:929–942. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Janes PW, Ley SC and Magee AI: Aggregation
of lipid rafts accompanies signaling via the T cell antigen
receptor. J Cell Biol. 147:447–461. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ringerike T, Blystad FD, Levy FO, Madshus
IH and Stang E: Cholesterol is important in control of EGF receptor
kinase activity but EGF receptors are not concentrated in caveolae.
J Cell Sci. 115:1331–1340. 2002.PubMed/NCBI
|
|
20
|
Roepstorff K, Thomsen P, Sandvig K and van
Deurs B: Sequestration of epidermal growth factor receptors in
non-caveolar lipid rafts inhibits ligand binding. J Biol Chem.
277:18954–18960. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim KP, Rafter JD, Bittova L, et al:
Mechanism of human group V phospholipase A2
(PLA2)-induced leukotriene biosynthesis in human
neutrophils. A potential role of heparan sulfate binding in
PLA2 internalization and degradation. J Biol Chem.
276:11126–11134. 2001.PubMed/NCBI
|
|
22
|
Fan TC, Chang HT, Chen IW, Wang HY and
Chang MD: A heparan sulfate-facilitated and raft-dependent
macropinocytosis of eosinophil cationic protein. Traffic.
8:1778–1795. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Taylor DR, Whitehouse IJ and Hooper NM:
Glypican-1 mediates both prion protein lipid raft association and
disease isoform formation. PLoS Pathog. 5:e10006662009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Podyma-Inoue KA, Hara-Yokoyama M,
Shinomura T, Kimura T and Yanagishita M: Syndecans reside in
sphingomyelin-enriched low-density fractions of the plasma membrane
isolated from a parathyroid cell line. PloS One. 7:e323512012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Payne CK, Jones SA, Chen C and Zhuang X:
Internalization and trafficking of cell surface proteoglycans and
proteoglycan-binding ligands. Traffic. 8:389–401. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vercauteren D, Piest M, van der Aa LJ, et
al: Flotillin-dependent endocytosis and a phagocytosis-like
mechanism for cellular internalization of disulfide-based
poly(amido amine)/DNA polyplexes. Biomaterials. 32:3072–3084. 2011.
View Article : Google Scholar
|