Introduction
Glioblastoma is one of the most malignant human
tumors (1), and despite
aggressive surgical resection and radiotherapy, the median survival
for patients with glioma does not normally exceed one year
(2–4). Glioblastoma multiforme (GBM) is able
to avoid immunosurveillance and the tumor cells proliferate
extensively with strikingly high rates due to its intrinsic
properties. The success of chemotherapy in patients with GBM is
hampered by the issue of drug resistance, and the need for the
discovery of more effective agents and therapeutic regimens to
treat GBM is becoming increasingly urgent (5). Numerous plant-derived compounds,
such as betulinic (6) and asiatic
acids (7) have been reported to
be potential anti-glioma agents, although most of these compounds
are no longer considered as possible treatments for human glioma.
This is due either to their modification in the liver or to their
inability to pass through the blood-brain barrier.
Traditional Chinese medicinal herbs are widely known
to be effective in the treatment of a number of diseases. For
example, arteannuin and dihydroartemisinin tablets purified from
sweet wormwood have been used to cure malaria. Examples of
plant-based therapeutic anticancer drugs include camptothecin from
Camptotheca acuminata, etoposide from Podophyllum
peltatum, vincristine from Catharanthus roseus and
paclitaxel from Taxus (yew) (8,9).
Saponin B was first isolated from Anemone
taipaiensis, a ferine plant of the Qinling mountains of
southern Shaanxi province, China, that is distributed in the hill
country of the fertile slopes or rocky grasslands at altitudes
between 2,900 and 3,700 m. Wang et al isolated eight
saponins from Anemone taipaiensis (10). In this study, we first
investigated the effects of saponin B on the human U87MG GBM cell
line, as an in vitro model to explore the effects of saponin
B on GBM cell growth and apoptosis. Apoptosis is a physiological,
energy-requiring process that is characterized by the formation of
apoptotic bodies inside cells and seems to be genetically
programmed (11). It is widely
accepted that apoptosis is preferred to necrosis as a mechanism of
tumor cell killing, since apoptosis does not promote inflammatory
processes (12,13). The demonstration of apoptotic
capacity would therefore make this substance of interest as a
potential anticancer reagent.
Materials and methods
Cell culture
The following three cell lines were obtained from
the American Type Culture Collection (ATCC): ECV304 (human
umbilical vein endothelial cells), U87MG and U251MG (GBM cells).
Human GBM cells used in this study were grown as a monolayer
culture in Dulbecco’s modified Eagle’s medium (DMEM; Gibco-BRL,
Carlsbad, CA, USA) supplemented with 10% fetal bovine serum
(Gibco-BRL) and antibiotics in a humidified atmosphere containing
5% CO2 at 37ºC.
Drug sample
Saponin B was obtained from the Department of
Pharmacology of Xijing Hospital, Fourth Military Medical
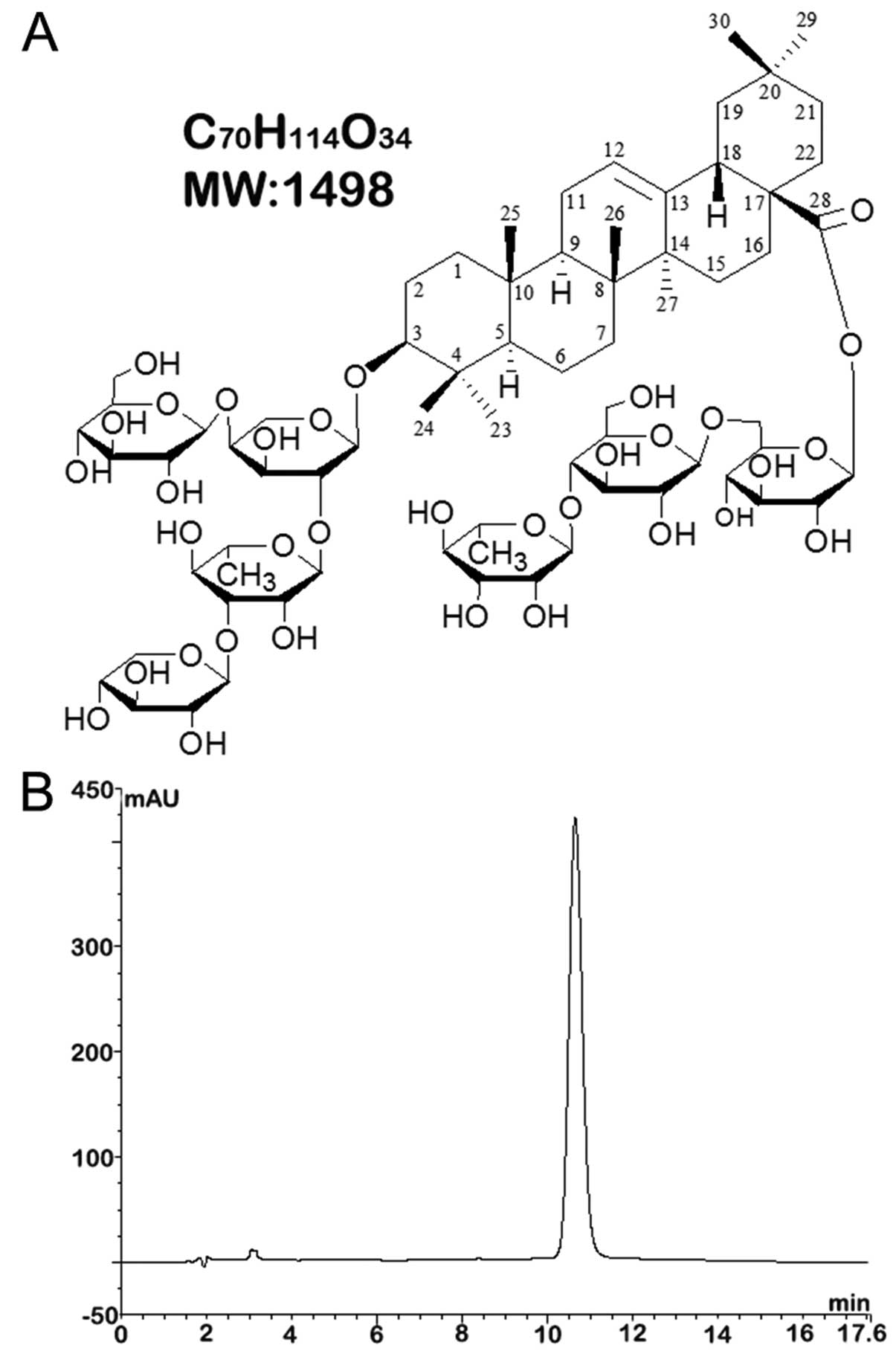
University, Xi’an, China and established as
3β-O-{β-D-xylopyranosyl-(1→3)-α-L-rhamnopyranosyl-(1→2)-[β-D-gluco-
pyranosyl-(1→4)]-α-L-arabinopyranosyl} oleanolic acid
28-O-{α-L-rhamnopyranosyl-(1→4)-β-D-glucopyranosyl-(1→6)-β-D-glucopyranoside};
the molecular formula was
C70H114O34 (molecular weight,
1,498). The molecular structure of saponin B is shown in Fig. 1A. The purity of the sample was
assessed by high-performance liquid chromatography (HPLC) as more
than 95% (Fig. 1B). The reagent
was placed in dimethyl sulfoxide (DMSO) and shaken until the powder
dissolved, then diluted with the culture solution (DMEM). The stock
solutions were stored at 4ºC and the volume of DMSO was maintained
at <0.5% so as not to affect cell growth. The final
concentration of the stock solution was confirmed at 500 μg/ml.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
Following the treatments, cell viability was
examined by MTT assay. The U87MG and U251MG cells were seeded at a
density of 3×104 cells/well into 96-well plates prior to
drug treatment. Saponin B was added to the medium and the cells
were continuously treated for 72 h at different concentrations
(1.3–42.7 μmol/l), and then MTT assay was performed as previously
described by Mickisch et al (14). The controls cells were incubated
in control medium containing 0.1% DMSO.
Cell cycle analysis
In order to analyze the effect of saponin B on the
cell cycle distribution of U87MG glioblastoma cells, flow cytometry
was used to elucidate the mechanisms behind the inhibition of
proliferation. The U87MG cells were trypsinized, counted,
centrifuged and fixed in ethanol at certain time points after the
treatment. The cells were then washed twice in phosphate-buffered
saline (PBS) and centrifuged. The pellets were resuspended in a
solution of ribonuclease (0.02 mg/ml, Sigma, St. Louis, MO, USA)
and propidium iodide (PI; 0.02 mg/ml, Sigma), and incubated at 4ºC
for 30 min. The fluorescence of approximately 10,000–20,000 stained
cells was measured. The results were expressed as a plot of
fluorescence intensity vs. cell number as previously described
(15).
Cellular and nuclear morphology
We determined the residual cell viability and number
of apoptotic U87MG glioblastoma cells following treatment with
saponin B at various concentrations [inhibitory concentration
(IC)25, IC50 and IC75] for 8 and
24 h. Apoptosis was analyzed after staining the cells with Hoechst
33342 (Sigma) at a final concentration of 1.5 μM for 10 min and
cell morphology was determined using a fluorescence microscope
(Zeiss Axiovert 200; Carl Zeiss, Oberkochen, Germany). The cells
were analyzed at ×40 magnification and documented using a
charge-coupled device (CCD) camera (Zeiss Axiocam MR; Carl Zeiss).
We determined the number of apoptotic cells based on characteristic
morphological features. The percentage of apoptotic cells was
calculated from three separate experiments (16,17).
Annexin V/PI staining
Fluorophore-labeled Annexin V [a protein that
exhibits nanomolar affinity for phosphatidylserine (PS)] binding to
externalized PS has been extensively employed as a reliable marker
of apoptosis. The appearance of PS on the extracellular cell
membranes was therefore evaluated with Annexin V/PI staining
(18). The U87MG glioblastoma
cells (1×106) treated as described above were washed
twice in PBS, and then trypsinized with 0.13 g/l trypsin in
PBS-EDTA (ethylenediaminetetraacetic acid). We then terminated the
trypsin reaction with culture medium containing 10% fetal bovine
serum. The cells were collected, centrifuged (5 min, 800 × g, 4ºC),
washed twice in ice-cold PBS and resuspended in 1X binding buffer
(10 mM HEPES/NaOH, pH 7.4, 140 mM NaCl, 2.5 mM CaCl2).
Subsequently, 5 μl of Annexin V-FITC (fluorescein isothiocyanate;
BD Pharmingen, San Jose, CA, USA) and 10 μl of PI (50 μg/ml) were
added to the 100 μl of cell suspension and incubated for 15 min at
room temperature in the dark. Finally, 400 μl of binding buffer
were added to the samples, which were kept ice cold until they were
analyzed on a FACSCalibur (Becton Dickinson, Franklin Lakes, NJ,
USA) flow cytometer. Ten thousand cells were analyzed per sample:
viable cells (FITC−PI−), apoptotic cells
(FITC+/PI− and
FITC+/PI+) and necrotic cells
(FITC−/PI+) as previously described (19–22).
Analysis of DNA fragmentation
After being treated with saponin B for the
pre-determined periods of time, the cells were collected, washed
and pelleted by centrifugation, and 200 μl binding/lysis buffer (20
ml nucleic acid binding, 6 M guanidine-HCL, 10 mM urea, 10 mM
tris-HCL, 20% Triton X-100, pH 4.4) were added to 2×106
cells in a volume of 200 μl and mixed immediately. The cells were
then incubated for 10 min at 25ºC, 100 μl isopropanol were added
and the sample was vortexed. We combined a filter tube and a
collection tube and pipetted the sample. In addition, the
suspension was centrifuged for 1 min at 8,000 rpm in a standard
tabletop centrifuge. We discarded the flow through and again
combined the filter tube and the used collection tube. We added 500
μl washing buffer to the upper reservoir and centrifuged for 1 min
at 8,000 rpm. We then discarded the flow through and again combined
the filter tube and the used collection tube. We added 500 μl
washing buffer to the upper reservoir and centrifuged for 1 min at
8,000 rpm, finally centrifuging for 10 sec at the maximum speed,
13,000 rpm, to remove the residual washing buffer. We discarded the
collection tube and inserted the filter tube in a clean 1.5 ml cup
or tube, then used 200 μl of pre-warmed (70ºC) elution buffer. We
added elution buffer to the filter tube and centrifuged for 1 min
at 8,000 rpm. Finally, following the manufacturer’s instructions,
the samples were loaded on 1% agarose gel, separated by
electrophoresis (50 V, 1.5 h) and visualized by staining with
ethidium bromide (Roche, Cat. no. 11835246001, Basel, Switzerland)
as previously described (23).
Western blot analysis
The activation of caspases followed by their
cleavage is well known to be an important phenomenon for the
induction of apoptosis (24). The
Bcl-2 family, including Bcl-2, Bcl-xL, Bax and Bad, regulates
various steps of apoptosis. Bax and Bad promote programmed cell
death, whereas Bcl-2 and Bcl-xL block cell death (25,26) and the involvement of the
Bcl-2 gene in apoptosis has been reported by several studies
on anticancer drugs (27,28). To identify the molecular
mechanisms involved in the enhanced apoptosis observed in the U87MG
cells treated with saponin B, we performed immunoblot analysis to
detect caspase activation using specific antibodies. The cells were
treated with saponin B, incubated for 8 and 24 h, and then
collected, lysed in lysis buffer [150 mM NaCl, 1% NP-40, 0.5%
sodium deoxycholate, 0.1% SDS, 50 mM Tris-HCl, pH 8.0, 10 mM EDTA
and 1 mM phenylmethanesulfonylfluoride (PMSF); Sigma] for 30 min,
and the cells were homogenized at 4ºC. For each sample, 30 μg of
protein were loaded onto a 12.5% SDS-polyacrylamide gel,
electrophoresed, and transferred onto a nitrocellulose membrane
(0.22 μm, Schleicher & Schuell Protran; Schleicher &
Schuell BioScience GmbH, Dassel, Germany). The membranes were
blocked for 1 h at room temperature with a blocking buffer
[Tris-buffered saline containing 0.1% Tween-20 (w/v); Sigma] and 5%
non-fat milk (w/v). Primary antibodies (applied for 1 h at room
temperature, or overnight at 4ºC) were anti-Bcl-2 (rabbit
monoclonal Ab-1, Oncogene Science, Cambridge, MA, USA), anti-Bax
(rabbit polyclonal N-20, Santa Cruz Biotechnology, Santa Cruz, CA,
USA), anti-caspase-3 (rabbit, purity >95%), anti-Fas-l (rabbit,
purity >95%), anti-actin (goat monoclonal C-2, Santa Cruz
Biotechnology). All antibodies were diluted 1:1000, except for
anti-actin (1:300).
Results
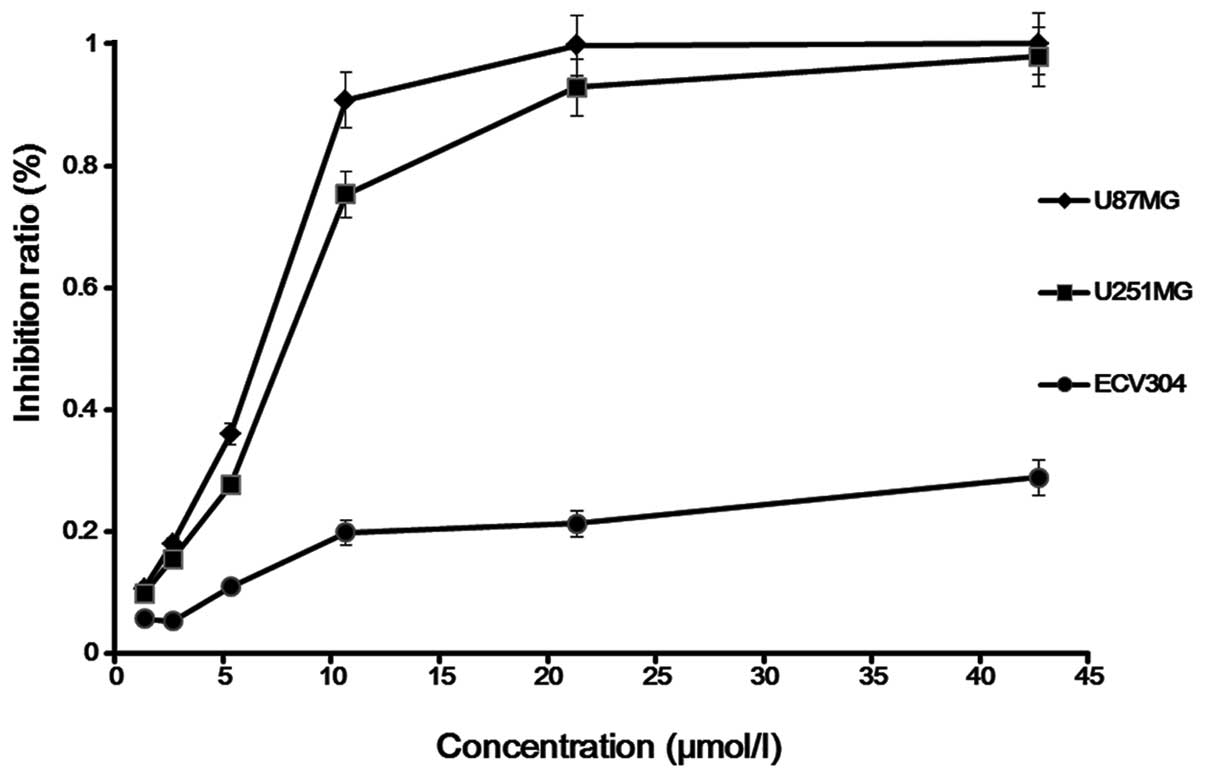
Cytostatic effect
Saponin B at doses ranging from 1.67 to 13.35 μmol/l
exerted a significant anti-proliferative effect on the human
glioblastoma cell line, as shown by time course analysis, and also
exerted a strong dose-dependent cytostatic effect on human glioma
cells. For the U87MG cells, the IC25, IC50
and IC75 of saponin B at 72 h were found to be
IC25, 5.2 μmol/l; IC50, 6.7 μmol/l; and
IC75, 8.7 μmol/l. For the U251MG cells, these
concentrations were IC25, 6.2 μmol/l; IC50,
7.9 μmol/l; and IC75, 10.5 μmol/l. In order to clarify
the mechanisms behind the inhibition of cell growth, we selected
the U87MG cells as the subject for the following experiments
(Fig. 2).
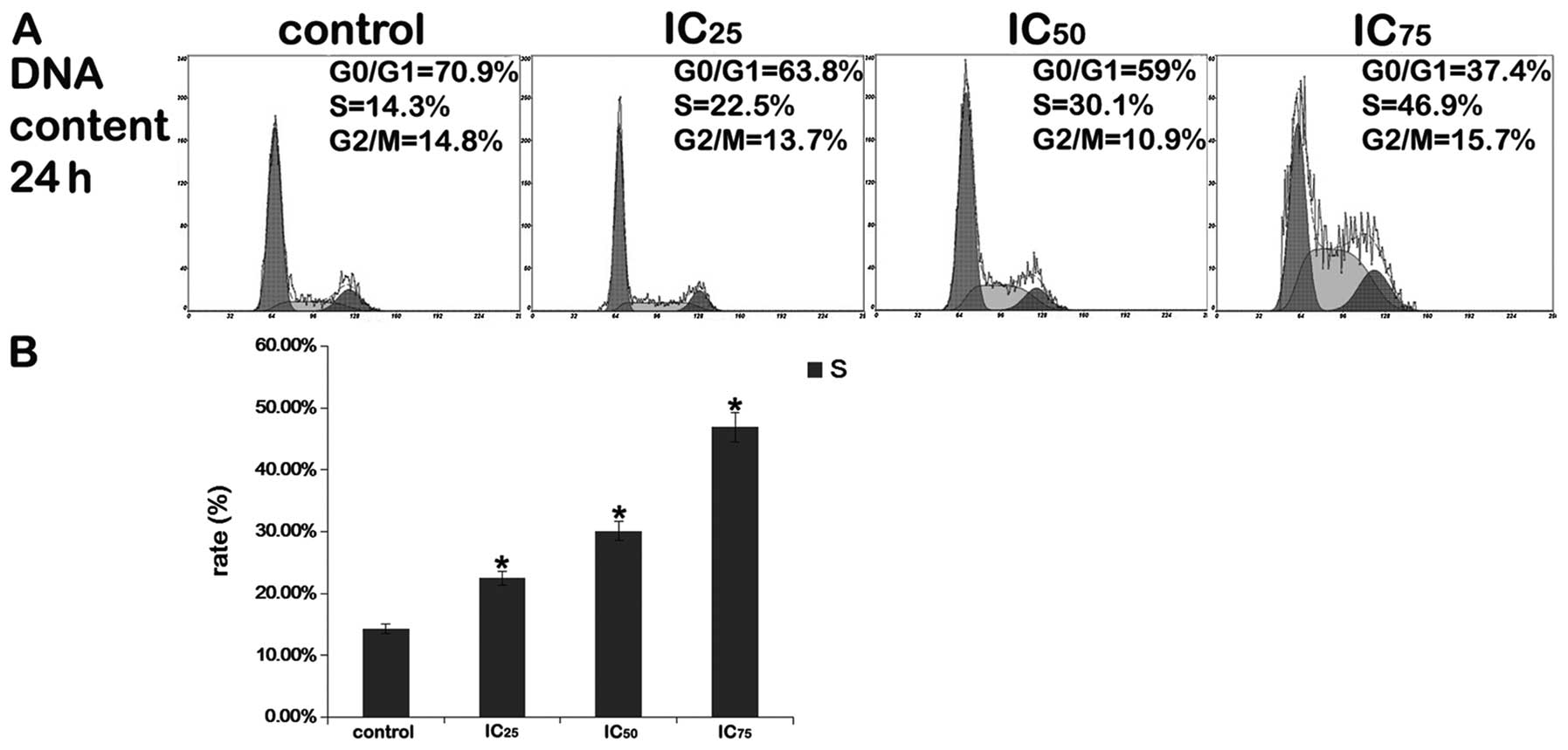
Cell cycle analysis
After the cells were exposed to various
concentrations of saponin B (IC25, IC50 or
IC75) for 24 h, flow cytometric analysis of the
PI-stained cells revealed that substantial numbers of cells
accumulated in the S phase of the cell cycle, and that the
percentage of cells in the G0/G1 phase decreased, whereas the
number of cells in the S and G2/M phases of the cell cycle
increased. As shown in Fig. 3,
saponin B (IC75) significantly increased S phase arrest.
These results demonstrate that one mechanism by which saponin B
inhibits the growth of U87MG cells is through the induction of S
phase cell cycle arrest in a dose-dependent manner (Fig. 3).
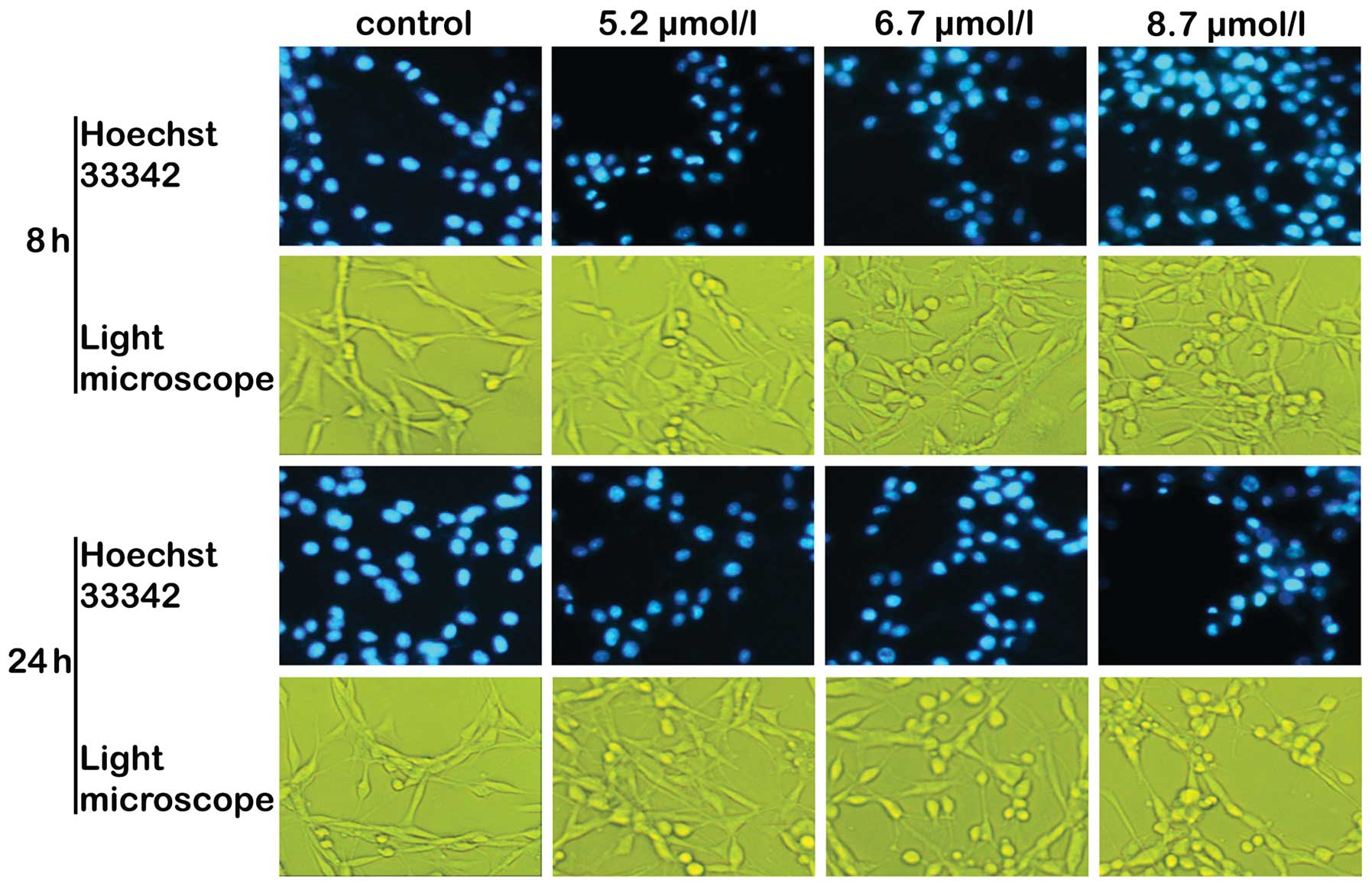
Cellular and nuclear morphology
Chromatin condensation and nuclear fragmentation,
typical of the induction of apoptosis, were observed under a
fluorescence microscope using Hoechst 33342 staining. Saponin B
induced the characteristic morphological features of apoptosis,
including cell shrinkage with condensation of the cytoplasm,
membrane blebbing and the formation of apoptotic bodies (Fig. 4). Under a light microscope, with
increasing concentrations of saponin B, the cells were increasingly
found in rounded form (Fig. 4).
Three different types of changes were observed: i) weak and
irregularly shaped marginal chromatin condensation; ii) highly
condensed nuclear chromatin that was inverted on one side; iii)
relatively compact and irregularly shaped marginal chromatin
condensation, characteristics of apoptosis (29,30).
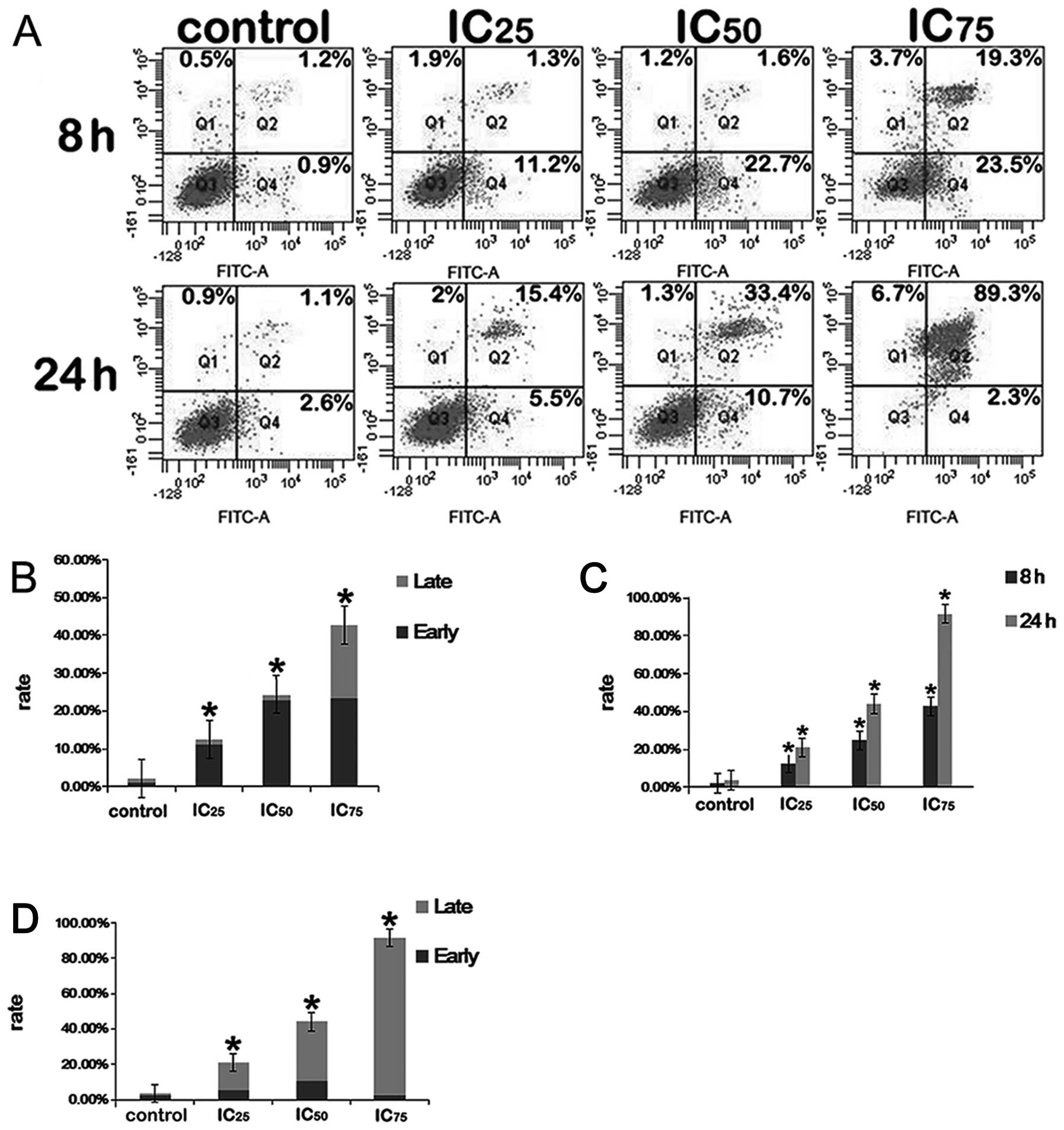
Annexin V-PI dual staining
In the present study, we investigated the effects of
saponin B on the apoptosis of U87MG glioblastoma cells.
Fluorophore-labeled Annexin V (a protein that exhibits nanomolar
affinity for OS) binding to externalized PS has been extensively
employed as a reliable marker of apoptosis (31). To establish whether such processes
were induced due to treatment with saponin B, the U87MG cells were
exposed to various reagent concentrations (5.2, 6.7 and 8.7 μmol/l)
for 8 and 24 h. As shown in Fig.
5, the untreated control cells showed few or no apoptotic
cells, whereas apoptosis was markedly induced by saponin B compared
with the untreated controls.
DNA fragmentation
As shown in Fig.
6B, the untreated control group showed few or no apoptotic
cells, whereas apoptosis was markedly induced by saponin B. To
further confirm that saponin B induced apoptosis, a DNA
fragmentation assay was performed. After the U87MG cells were
treated for 24 h with saponin B, cellular DNA revealed a
distinctive ladder pattern of DNA cleavage in the apoptotic U87MG
cells.
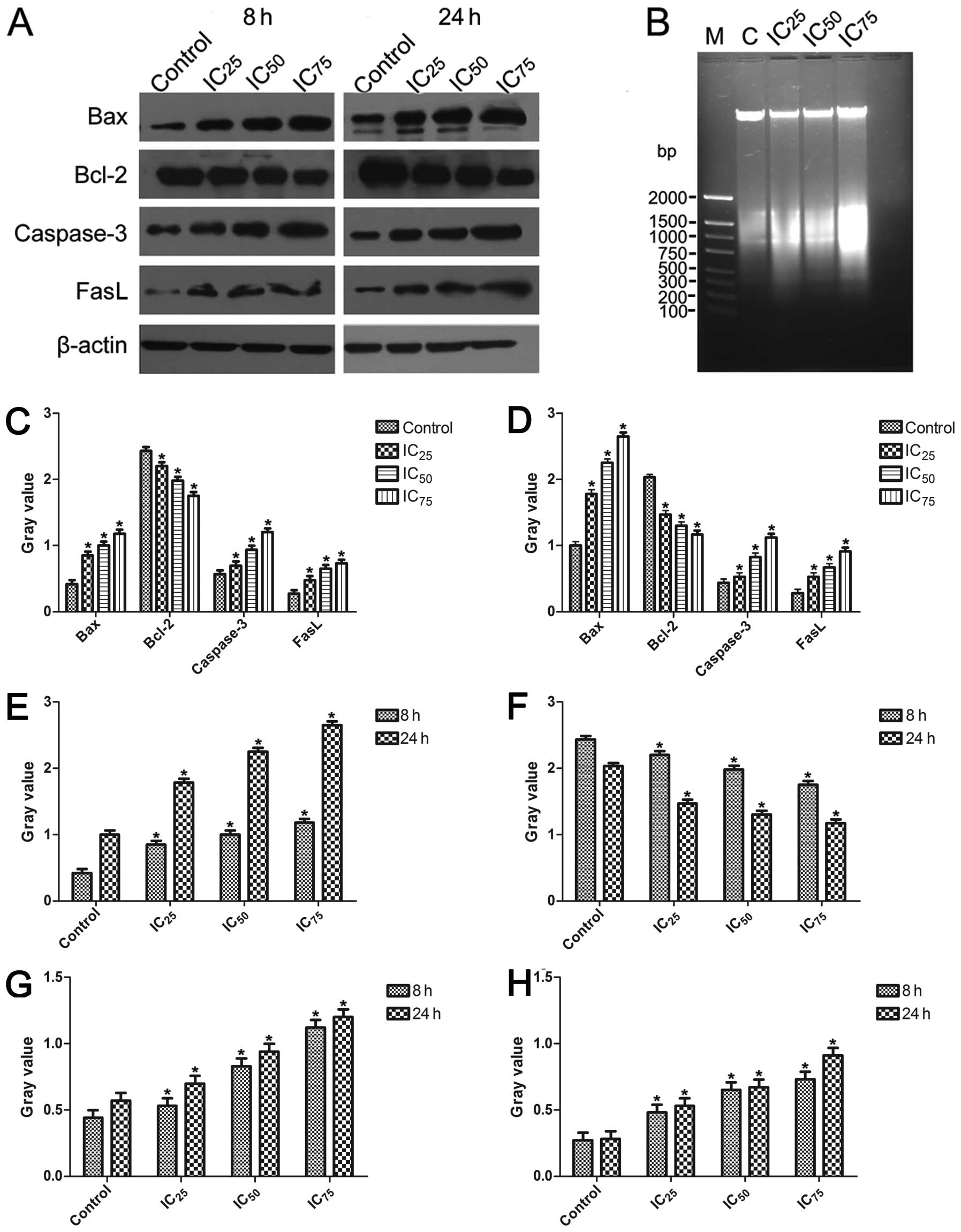 | Figure 6(A) Western blot analsyis was used to
examine the activation of the intrinsic pathway of apoptosis in
U87MG glioblastoma cells. (B) Approximately 1×106 U87MG
cells were treated and cultured for 24 h with various
concentrations of saponin B. Isolated DNA was analyzed using 1%
agarose gel electrophoresis. (C) After the U87MG cells were treated
for 8 h, the ratios for Bax, Bcl-2, caspase-3 and Fas-l were
examined at different concentrations. All data are presented as the
means ± SE of triplicate experiments relative to the control,
*P<0.05, Dunnett’s t-test. (D) After the U87MG cells
were treated for 24 h, the ratios for Bax, Bcl-2, caspase-3 and
Fas-l were examined at different concentrations.
*P<0.05, Dunnett’s t-test. (E) Bar graph represents
the relative expression of the Bax ratio calculated from each
group. *P<0.05, paired-samples t-test. (F) Bar graph
represents the relative expression of the Bcl-2 ratio calculated
from each group. *P<0.05, paired samples t-test. (G)
Bar graph represents the relative expression of the caspase-3 ratio
calculated from each group. *P<0.05, paired samples
t-test. (H) Bar graph represents the relative expression of the
Fas-l ratio calculated from each group. *P<0.05,
paired samples t-test. |
Western blot analysis
The Bax, caspase-3 and Fas-l proteins were
upregulated in the treated groups following exposure to saponin B,
and the expression levels of a typical pro-survival protein (Bcl-2)
were downregulated by saponin B (Fig.
6A, C and D). β-actin expression was monitored to ensure that
an equal amount of protein was loaded in each lane. Monitoring
revealed the expression of pro-apoptotic proteins (caspase-3, Bax
and Fas-l) in a dose-dependent manner, and time-dependence was
shown by comparison between the 8 and 24 h observations (Fig. 6E, G and H). The decreased
expression of Bcl-2 was observed (Fig. 6F).
Discussion
GBM, which is categorized amongst the tumors with
the greatest angiogenic ability and occurs more frequently than
other types of primary tumors of the central nervous system, is a
highly vascularized and invasive malignant tumor with poor clinical
outcomes. Even after optimal aggressive surgery, radiation and
chemotherapy treatments, the mean reported survival rate is less
than one year (32). Traditional
Chinese medicinal herbs are widely known to be effective in the
treatment of several diseases. It has been reported that among the
155 small molecular antitumor drugs developed from the 1940s to
June 2006, 47% are natural compounds or are directly derived from
natural compounds (33); thus,
the development of chemopreventive agents derived from Chinese
medicinal herbs is an important area of cancer research.
The present study demonstrates the potent and
selective cytostatic effects of saponin B in a panel of U87MG GBM
cell lines. We demonstrate that saponin B exerts a strong time- and
dose-dependent growth inhibitory effect on highly malignant human
glioma cell lines, an effect that was confirmed by an MTT assay.
The antitumor effects of saponin B may result from multiple
mechanisms of action, such as interfering with cell cycle
progression and inducing apoptosis.
Mammalian cells have evolved a complex defense
network to maintain genomic integrity by inhibiting the fixation of
permanent damage. Cell cycle checkpoints prevent cells with damaged
genomes from undergoing DNA replication or mitosis (34). The control of the cell cycle
progression in cancer cells is a potentially effective strategy for
controling tumor growth (35). In
the study by Zhou et al, using flow cytometric analysis of
DNA in U87MG cells, the authors demonstrated that novaeguinoside II
induced the prominent appearance of an S phase peak in the cell
cycle (36). Therefore, in this
study, we analyzed the effects of saponin B on the cell cycle
distribution of U87MG glioblastoma cells in order to elucidate the
mechanisms behind the inhibition of proliferation. Our data
indicated that exposure to saponin B resulted in an increased S
phase arrest in the GBM cells, which suggests that DNA synthesis
was inhibited by saponin B.
Following exposure to saponin B, apoptotic cells
aggregated, shrank and detached from the surface of the culture
flask. Hoechst 33342 staining of the nuclei revealed fragmented
nuclei in the treated cells. The classical alterations of apoptosis
were more distinct in the 24 h-treated group than those of the 8
h-treated group. These results were consistent with the changes
observed under a light microscope. This observation confirmed the
data obtained by Annexin V/PI assay.
When the U87MG cells were exposed to 5.2, 6.7 and
8.7 μmol/l saponin B for 8 and 24 h, the apoptotic ratio was
proportional to the concentration and treatment time. Annexin V/PI
assay suggested that PS externalization was apparent at the higher
drug concentrations. A significant induction of apoptosis following
treatment with saponin B demonstrated its anticancer effects on GBM
cell lines. The results were consistent with the biochemical
characteristics of apoptosis that PS externalization is an early
and sensitive event during the cascade of apoptosis (37). Taken together, our data
demonstrate that the potent effects of saponin B on apoptosis and
cell cycle progression indicate that saponin B is able to trigger
different signal transduction pathways and to exert effects on two
key processes in glioma cells.
Treatment of the GBM cells with saponin B triggered
an intrinsic caspase cascade due to an increase in the Bax:Bcl-2
ratio. Bax and Bcl-2 have been reported to play a dominating role
in determining whether cells die or survive (38). The Bcl-2 family members are
characterized by containing at least one of four Bcl-2 homology
domains (BH1–BH4) and play important roles in regulating apoptosis
(39). The increased expression
of Bax can induce apoptosis through the release of cytochrome
c from the mitochondria, while Bcl-2 protects cells from
apoptosis through teh stabilization of the mitochondrial membrane
potential (40). As described
above, our results suggest that saponin B is a potent compound,
exerting apoptotic effects on tumor cells. Several studies have
revealed that apoptosis induced by saponin is modulated through a
mitochondrial-dependent pathway, which was ascertained in our study
by the upregulated Bax protein and downregulated Bcl-2 protein
expression.
Furthermore, we employed western blot analysis to
elucidate the involvement of the Fas-l protein in saponin
B-mediated apoptosis in U87MG cells. It is widely known that the
Fas-l/Fas-signaling-mediated death receptor apoptotic pathway is a
potential target of antitumor therapy (41); thus, targeting the death receptor
pathway may provide more significant benefits for U87MG GBM cells.
Our results testified that the Fas-l/Fas-signaling pathway played a
crucial role in saponin B-induced apoptosis. As is also widely
known, caspase-3 activation plays a key role in the execution phase
of apoptosis and is often preceded by Bcl-2, Bax and caspase-9, via
the mitochondrial pathway (42),
and has been identified as being a key mediator of apoptotic events
in either cancerous or non-cancerous mammalian cells, such as
neuronal cells and GBM cells (43). Therefore, caspase-3 is ascertained
to be a common executive proteolytic enzyme in both the extrinsic
and intrinsic apoptotic pathways. In this sudy, we examined the
increase in active caspase-3 fragments and caspase-3 activity
following treatment with saponin B. Saponin B was most effective in
increasing caspase-3 activation and activity after 8 and 24 h of
treatment.
Collectively, our data suggest that saponin B exerts
a strong time- and dose-dependent apoptotic effect on highly
malignant human glioma cell lines, rapidly inducing apoptosis
through the activation of both the death receptor-mediated and
mitochondrial-mediated proteolytic pathways in U87MG cells. A
better understanding of the mechanisms behind the chemotherapeutic
activity of saponin B would benefit future animal experiments and
clinical studies, thereby increasing the prospects for the clinical
treatment of this highly malignant tumor.
Acknowledgements
The authors would like to thank Xiaoyan Chen for her
excellent technical assistance. This study was supported by grants
from the National Natural Science Foundation of China (nos.
30873402 and 81274029) and the Natural Science Foundation of
Shaanxi Province (no. 2012JM4010).
References
|
1
|
DeAngelis LM: Brain tumors. N Engl J Med.
344:114–123. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A,
Lacombe D, Cairncross JG, Eisenhauer E and Mirimanoff RO:
Radiotherapy plus concomitant and adjuvant temozolomide for
glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ballman KV, Buckner JC, Brown PD, Giannini
C, Flynn PJ, LaPlant BR and Jaeckle KA: The relationship between
six-month progression-free survival and 12-month overall survival
end points for phase II trials in patients with glioblastoma
multiforme. Neuro Oncol. 9:29–38. 2007. View Article : Google Scholar
|
|
4
|
Lamborn KR, Yung WK, Chang SM, Wen PY,
Cloughesy TF, DeAngelis LM, Robins HI, Lieberman FS, Fine HA, Fink
KL, Junck L, Abrey L, Gilbert MR, Mehta M, Kuhn JG, Aldape KD,
Hibberts J, Peterson PM and Prados MD: Progression-free survival:
an important end point in evaluating therapy for recurrent
high-grade gliomas. Neuro Oncol. 10:162–170. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Curtin JF, King GD, Candolfi M, Greeno RB,
Kroeger KM, Lowenstein PR and Castro MG: Combining cytotoxic and
immune-mediated gene therapy to treat brain tumors. Curr Top Med
Chem. 5:1151–1170. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fulda S, Jeremias I, Steiner HH, Pietsch T
and Debatin KM: Betulinic acid: a new cytotoxic agent against
malignant brain-tumor cells. Int J Cancer. 82:435–441. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee YS, Jin DQ, Kwon EJ, Park SH, Lee ES,
Jeong TC, Nam DH, Huh K and Kim JA: Asiatic acid, a triterpene,
induces apoptosis through intracellular Ca2+ release and
enhanced expression of p53 in HepG2 human hepatoma cells. Cancer
Lett. 186:83–91. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cragg GM and Newman DJ: Plants as a source
of anti-cancer agents. J Ethnopharmacol. 100:72–79. 2005.PubMed/NCBI
|
|
9
|
Gordaliza M: Natural products as leads to
anticancer drugs. Clin Transl Oncol. 9:767–776. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang XY, Chen XL, Tang HF, Gao H, Tian XR
and Zhang PH: Cytotoxic triterpenoid saponins from the rhizomes of
Anemone taipaiensis. Planta Med. 77:1550–1554. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Geske FJ and Gerschenson LE: The biology
of apoptosis. Hum Pathol. 32:1029–1038. 2001. View Article : Google Scholar
|
|
12
|
Kumar R, Herbert PE and Warrens AN: An
introduction to death receptors in apoptosis. Int J Surg.
3:268–277. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Abd-Elrahman I, Hershko K, Neuman T,
Nachmias B, Perlman R and Ben-Yehuda D: The inhibitor of apoptosis
protein Livin (ML-IAP) plays a dual role in tumorigenicity. Cancer
Res. 69:5475–5480. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mickisch G, Fajta S, Keilhauer G, Schlick
E, Tschada R and Alken P: Chemosensitivity testing of primary human
renal cell carcinoma by a tetrazolium based microculture assay
(MTT). Urol Res. 18:131–136. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vindelov L and Christensen IJ: An
integrated set of methods for routine flow cytometric DNA analysis.
Methods Cell Biol. 33:127–137. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Scaife RM: G2 cell cycle arrest,
down-regulation of cyclin B, and induction of mitotic catastrophe
by the flavoprotein inhibitor diphenyleneiodonium. Mol Cancer Ther.
3:1229–1237. 2004.PubMed/NCBI
|
|
17
|
Handrick R, Rudner J, Muller I, Eibl H,
Belka C and Jendrossek V: Bcl-2 mediated inhibition of
erucylphosphocholine-induced apoptosis depends on its subcellular
localisation. Biochem Pharmacol. 70:837–850. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Narayan P, Mentzer RJ and Lasley RD:
Annexin V staining during reperfusion detects cardiomyocytes with
unique properties. Am J Physiol Heart Circ Physiol.
281:H1931–H1937. 2001.PubMed/NCBI
|
|
19
|
Huigsloot M, Tijdens IB, Mulder GJ and van
de Water B: Differential regulation of doxorubicin-induced
mitochondrial dysfunction and apoptosis by Bcl-2 in mammary
adenocarcinoma (MTLn3) cells. J Biol Chem. 277:35869–35879. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Malerba I, Gribaldo L, Diodovich C,
Carloni M, Meschini R, Bowe G and Collotta A: Induction of
apoptosis and inhibition of telomerase activity in human bone
marrow and HL-60 p53 null cells treated with anti-cancer drugs.
Toxicol In Vitro. 19:523–532. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cheng G, Zhang X, Tang HF, Zhang Y, Zhang
XH, Cao WD, Gao DK, Wang XL and Jin BQ: Asterosaponin 1, a
cytostatic compound from the starfish Culcita novaeguineae,
functions by inducing apoptosis in human glioblastoma U87MG cells.
J Neurooncol. 79:235–241. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xiao XY, Hao M, Yang XY, Ba Q, Li M, Ni
SJ, Wang LS and Du X: Licochalcone A inhibits growth of gastric
cancer cells by arresting cell cycle progression and inducing
apoptosis. Cancer Lett. 302:69–75. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ha ES, Lee EO, Yoon TJ, Kim JH, Park JO,
Lim NC, Jung SK, Yoon BS and Kim SH: Methylene chloride fraction of
Spatholobi Caulis induces apoptosis via caspase dependent
pathway in U937 cells. Biol Pharm Bull. 27:1348–1352.
2004.PubMed/NCBI
|
|
24
|
Edinger AL and Thompson CB: Death by
design: apoptosis, necrosis and autophagy. Curr Opin Cell Biol.
16:663–669. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sperandio S, de Belle I and Bredesen DE:
An alternative, nonapoptotic form of programmed cell death. Proc
Natl Acad Sci USA. 97:14376–14381. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhai D, Jin C, Huang Z, Satterthwait AC
and Reed JC: Differential regulation of Bax and Bak by
anti-apoptotic Bcl-2 family proteins Bcl-B and Mcl-1. J Biol Chem.
283:9580–9586. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rega MF, Leone M, Jung D, Cotton NJ,
Stebbins JL and Pellecchia M: Structure-based discovery of a new
class of Bcl-xL antagonists. Bioorg Chem. 35:344–353. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tse C, Shoemaker AR, Adickes J, Anderson
MG, Chen J, Jin S, Johnson EF, Marsh KC, Mitten MJ, Nimmer P,
Roberts L, Tahir SK, Xiao Y, Yang X, Zhang H, Fesik S, Rosenberg SH
and Elmore SW: ABT-263: a potent and orally bioavailable Bcl-2
family inhibitor. Cancer Res. 68:3421–3428. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Leist M and Jaattela M: Four deaths and a
funeral: from caspases to alternative mechanisms. Nat Rev Mol Cell
Biol. 2:589–598. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lovborg H, Nygren P and Larsson R:
Multiparametric evaluation of apoptosis: effects of standard
cytotoxic agents and the cyanoguanidine CHS 828. Mol Cancer Ther.
3:521–526. 2004.PubMed/NCBI
|
|
31
|
Narayan P, Mentzer RJ and Lasley RD:
Annexin V staining during reperfusion detects cardiomyocytes with
unique properties. Am J Physiol Heart Circ Physiol.
281:H1931–H1937. 2001.PubMed/NCBI
|
|
32
|
Kaur B, Tan C, Brat DJ, Post DE and Van
Meir EG: Genetic and hypoxic regulation of angiogenesis in gliomas.
J Neurooncol. 70:229–243. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Newman DJ and Cragg GM: Natural products
as sources of new drugs over the last 25 years. J Nat Prod.
70:461–477. 2007.PubMed/NCBI
|
|
34
|
Shackelford RE, Kaufmann WK and Paules RS:
Cell cycle control, checkpoint mechanisms, and genotoxic stress.
Environ Health Perspect. 107(Suppl 1): 5–24. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mork CN, Faller DV and Spanjaard RA: A
mechanistic approach to anticancer therapy: targeting the cell
cycle with histone deacetylase inhibitors. Curr Pharm Des.
11:1091–1104. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhou J, Cheng G, Cheng G, Tang HF and
Zhang X: Novaeguinoside II inhibits cell proliferation and induces
apoptosis of human brain glioblastoma U87MG cells through the
mitochondrial pathway. Brain Res. 1372:22–28. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee SH, Meng XW, Flatten KS, Loegering DA
and Kaufmann SH: Phosphatidylserine exposure during apoptosis
reflects bidirectional trafficking between plasma membrane and
cytoplasm. Cell Death Differ. 20:64–76. 2013. View Article : Google Scholar
|
|
38
|
Korsmeyer SJ, Shutter JR, Veis DJ, Merry
DE and Oltvai ZN: Bcl-2/Bax: a rheostat that regulates an
anti-oxidant pathway and cell death. Semin Cancer Biol. 4:327–332.
1993.PubMed/NCBI
|
|
39
|
Juin P, Geneste O, Raimbaud E and Hickman
JA: Shooting at survivors: Bcl-2 family members as drug targets for
cancer. Biochim Biophys Acta. 1644:251–260. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cory S and Adams JM: The Bcl2 family:
regulators of the cellular life-or-death switch. Nat Rev Cancer.
2:647–656. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ziegler DS and Kung AL: Therapeutic
targeting of apoptosis pathways in cancer. Curr Opin Oncol.
20:97–103. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kleibl Z, Raisová M, Novotný J, Pohlreich
P and Matous B: Apoptosis and its importance in the development and
therapy of tumors (review). Sb Lek. 103:1–13. 2002.(In Czech).
|
|
43
|
Sulejczak D, Grieb P, Walski M and
Frontczak-Baniewicz M: Apoptotic death of cortical neurons
following surgical brain injury. Folia Neuropathol. 46:213–219.
2008.PubMed/NCBI
|