Introduction
N,N-Dimethyl-D-erythro-sphingosine (DMS) (Fig. 1) is biologically derived from
sphingosine and has been detected in several tissues (1). It has been reported that DMS can
modulate phosphorylation events by inhibiting protein kinase
(2) along with sphingosine kinase
(3). DMS can inhibit the activity
of sphingosine kinase 1 (SPHK1), resulting in an increase in
ceramide levels and a decrease in sphingosine-1-phosphate (S1P)
levels within the cell events involved in cell differentiation and
apoptosis (4–6).
The nuclear factor-κB (NF-κB) family is involved in
cellular responses to stimuli such as stress, cytokines, free
radicals, ultraviolet irradiation, oxidized LDL and bacterial or
viral antigens. Upon induction, it can transfer to the nucleus and
stimulate the expression of various target genes, which play
several crucial functions, including resistance to apoptosis and
promotion of cell survival. Increased SPHK activity can alter the
sphingolipid signal and NF-κB p65 expression and can eventually
lead to the drug resistance of breast cancer cells (7). Inhibition of basal SPHK1 activity
has been shown to induce apoptosis in A549 cells by interfering
with constitutive NF-κB activity (8).
Calcium is an ubiquitous second messenger that
controls a broad range of cellular functions. Previous studies have
reported that DMS increases Ca2+ concentration within
cells, including T lymphocytes, monocytes, astrocytes, neuronal
cells (9–12) and HCT116 human colon cancer cells
(13).
Lung cancer is a common aggressive malignancy
worldwide with limited treatment options available. Experimental
research has shown that SPHK1 inhibitor can significantly improve
the curative effects of chemotherapy drugs on lung cancer cells, as
well as other types of cancer cells (14–16). However, its mechanistic action has
yet to be extensively investigated. In the present study, the
effects of DMS on human lung cancer cells were examined. Further,
the mechanistic link between DMS and SPHK1 expression, the NF-κB
pathway and intracellular Ca2+ concentration in A549
cells were investigated.
Materials and methods
Reagents
DMS was purchased from Enzo Life Sciences (Enzo, New
York, NY, USA) and was dissolved in dimethyl sulfoxide (DMSO). The
final concentrations of DMSO were ≤0.1% in the drugs. Antibodies to
SPHK1, NF-κB p65, poly-ADP-ribose polymerase (PARP) and GAPDH were
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). All other reagents were purchased from Sigma-Aldrich (St.
Louis, MO, USA).
Cell culture
The human lung cancer cell line, A549, was
maintained in RPMI-1640 culture medium (Invitrogen, Carlsbad, CA,
USA) supplemented with streptomycin (100 μg/ml) and penicillin (100
U/ml), glutamine (2 mM) and 10% (v/v) fetal bovine serum. The cells
were grown at 37ºC in a humidified atmosphere containing 5%
CO2.
Morphological observations
Cells (5×105/well) were plated in 6-well
plates. After 12 h, the cells were exposed to serial dilutions of
DMS (1, 2, 4 and 8 μmol/l) for the times indicated. The changes in
cell morphology were observed under an inverted microscope at 48
h.
Cell proliferation assay
Cells (6×103/well) were transferred in 5
replicates to 96-well plates in 100 μl medium. All cells were
incubated at 37ºC in 5% CO2 for 12 h to allow the cells
to attach to the bottom of the wells. Serial dilutions of DMS (0.5,
1, 2, 4, 8 μmol/l) were added and the control group was
supplemented with equal volumes of phosphate-buffered saline (PBS).
At the culture times of 24, 48 and 72 h, the viability of the cells
was analyzed after the addition of MTT and DMSO, respectively.
Absorbance was determined using an enzyme mark instrument at a
wavelength of 490 nm.
Colony formation assay
The cells were washed, trypsinized and resuspended
in culture medium, then counted and plated in 60-mm dishes (200
cells/dish) in triplicate and cultured in medium with serial
dilutions of DMS, grown for 3 weeks, fixed with 10% methanol and
stained with 2% crystal violet for 20 min. The dishes were washed
and dried, and the colonies were counted to obtain a cloning
efficiency for each DMS concentration.
Analysis of cell apoptosis
The number of apoptotic cells was measured by
staining the cell nuclei with Hoechst 33342 dye and the apoptotic
cells were identified as those with condensed, fragmented nuclei.
The cells were stained with 5 μl Hoechst 33342 (1 g/l) and
incubated at 37ºC in the dark for 30 min following treatment with
DMS at concentrations of 1 and 2 μmol/l for 24 and 48 h. Cell
morphology was observed under a fluorescence microscope.
Flow cytometric analysis of apoptosis was performed
by staining the cells with Annexin V-FITC and propidium iodide
(PI). Cells (5×105/well) were plated in 6-well plates
and incubated overnight to attach to the bottom of the wells.
Serial dilutions of DMS were then added and the control group was
supplemented with equal volumes of PBS. After 48 h, the control or
treated cells were resuspended in Annexin V-binding buffer, stained
with fluorescein-conjugated Annexin V and PI (Annexin V-FITC kit;
Becton Dickinson, Franklin Lakes, NJ, USA) and incubated at room
temperature for 15 min. The cells stained only with Annexin V-FITC
were used as the positive controls to set the apoptotic window, and
the cells stained only with PI were used as the positive controls
to set the necrotic window.
DNA fragmentation analysis
Cells were plated in a 6-well plate and treated with
serial dilutions of DMS. Twenty-four and 48 h later, the cells were
collected by scraping and centrifuged at 600 × g for 10 min. The
cells were then washed twice with PBS and DNA fragmentation was
extracted using the Genome extraction kit (Generay Biotechnology,
Shanghai, China) according to the manufacturer’s instructions. The
DNA samples were subjected to electrophoresis on a 2% agarose gel
and were then visualized under UV light after staining with
ethidium bromide.
Caspase-3 activity assay
Cells were plated in 6-well plates and treated with
serial dilutions of DMS for 24 and 48 h. Subsequently, the cells
were washed twice with cold PBS and lysed in lysis buffer (Beyotime
Institute of Biotechnology, Shanghai, China) and placed on ice for
15 min. A sample of cytosolic protein was formed by centrifugation
at 5,000 × g for 10 min and protein concentration was determined by
the Bradford method. Cell extracts (30 μg protein) were incubated
in reaction buffer containing Ac-DEVD-pNA (2 mM) at 37ºC for 2 h.
Cleavage of the pNA fluorescence was detected using an enzyme mark
instrument at an excitation wavelength of 405 nm. Caspase-3
activity was presented as units of fluorescence/(mg of protein ×
h).
Quantitative RT-PCR (qRT-PCR)
Cells were washed with cold PBS and then harvested
using TRIzol reagent (Invitrogen, Carlsbad, CA, USA); total RNA was
extracted according to the manufacturer’s instructions. The
extracted RNA was reverse transcribed into cDNA. The reverse
transcription reaction system was 10 μl: 5X reaction buffer 2 μl,
M-MuLV reverse transcriptase 0.5 μl, primer mix 0.5 μl, RNA 1 μg
and DEPC added to a final volume of 10 μl. The samples were kept at
37ºC for 15 min and were then incubated at 98ºC for 5 min. The PCR
components were set up as follows: 1 μl cDNA product, 12.5 μl
master mix, 10 pmol/μl forward primer, 10 pmol/μl reverse primer
and DEPC added to a final volume of 25 μl. PCR was performed with
30 cycles of denaturation: 5 min at 95ºC, 30 sec at 94ºC, 40 sec at
57ºC, and extension 30 sec at 72ºC. The primers were designed using
primer 5 software. SPHK1 forward, 5′-gtt cca aga cac ctg cct cc-3′
and reverse, 5′-cac gca acc gct gac cat-3′; GAPDH forward, 5′-ggt
gtg aac cat gag aag tat gac-3′ and reverse, 5′-tgg cag tga tgg cat
gga ctg tg-3′. The amplified DNA fragment was separated on gel
electrophoresis and analyzed by Applied Biosystems 7300 real-time
PCR software.
Western blot analysis
Cells were washed with cold PBS gently, supplemented
with 100 μl/well cell lysis buffer (Beyotime Institute of
Biotechnology) and placed on ice for 15 min. A sample of cytosolic
protein was formed by centrifugation at 14,000 × g for 10 min and
protein concentration were determined by the BCA method. The
proteins (40 μg) were separated by 12% SDS-PAGE and then
transferred onto PVDF membranes (Millipore, Bedford, MA, USA). The
blots were blocked with 5% non-fat milk and then probed with
primary antibodies (1:1,000 dilution) against the SPHK1 and GAPDH
protein at 4ºC overnight. After washing, the membranes were
incubated with secondary antibody (1:10,000 dilution) at room
temperature. Antibodies were diluted in TBS containing 0.05% (v/v)
Tween-20 and 5% BSA. Proteins were analyzed using the near infrared
laser imaging system.
Measurement of [Ca2+]i
concentration
The intracellular [Ca2+]i concentration
was measured using the fluorescent dye, Fluo-4/AM (Dojindo
Laboratories, Kumamoto, Japan). The cells were treated with serial
dilutions of DMS for 24 and 48 h, then resuspended in PBS
containing 1% bovine serum and incubated for 30 min with 5 μM
Fluo-4/AM in the dark. After washing with PBS, the
Fluo-4/AM-labeled cells were observed under an inverted
fluorescence microscope.
Results
Morphological alteration of A549 cells
following treatment with DMS
To explore the effects of DMS on the A549 cells, the
cells were exposed to serial dilutions of DMS for 24 h. Cell
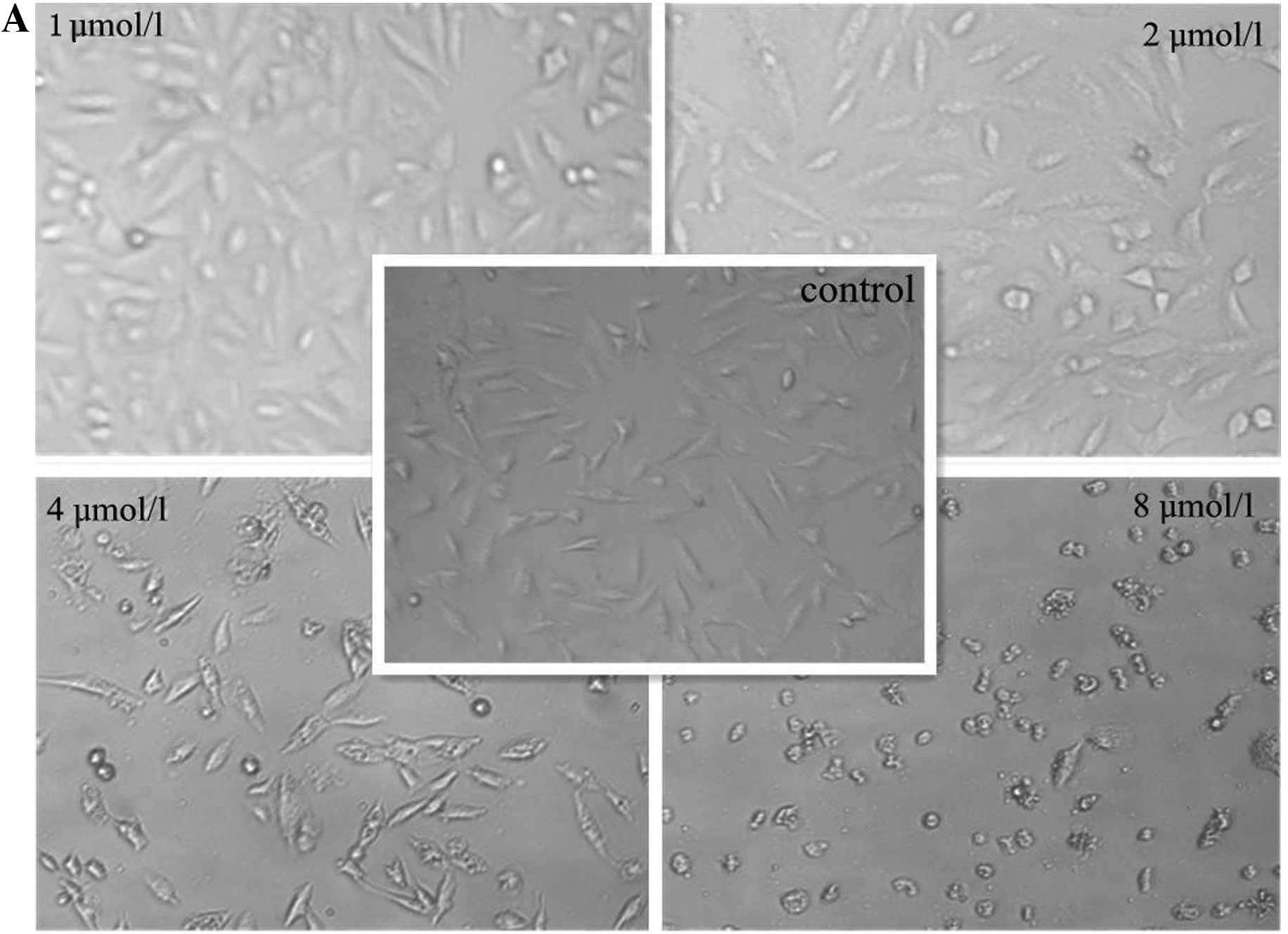
morphology was observed under an inverted microscope. DMS
dose-dependently altered the morphology of the A549 cells. At the
concentration of 4 μmol/l, cell shrinkage and rounding was
observed. Vacuoles were also observed in the cytoplasm (Fig. 2). The induction of apoptosis was
further confirmed by Hoechst 33342 staining. Under a fluorescence
microscope, with increasing concentrations of DMS, cell shrinkage,
nuclear fragmentation, nuclear dissolution and apoptotic bodies
were observed (Fig. 3).
Cytotoxicity of DMS in A549 cells
The A549 cells were treated with serial dilutions of
DMS for 24, 48 and 72 h. MTT assays were used to measure the
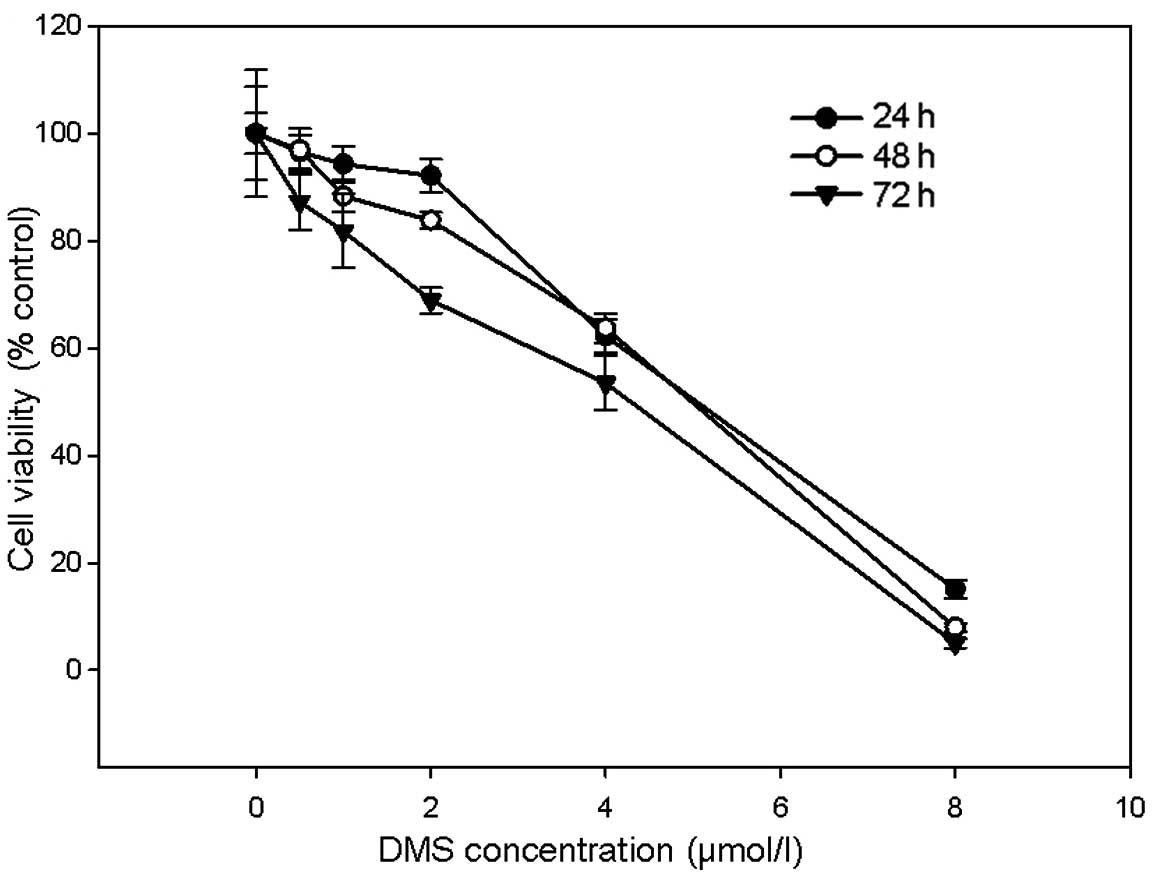
cytotoxicity of DMS in the A549 cells. Treatment with DMS decreased
the viability of the A549 cells in a dose- and time-dependent
manner (Fig. 4). When DMS
concentration reached 4 μmol/l, the cell survival rates were
significantly decreased by 37.74±3.1, 36.25±2.82 and 46.5±5.11%
(mean ± SD, n=6, P<0.01) at 24, 48 and 72 h, respectively
(Fig. 4). The IC50
values for 24, 48 and 72 h were 4.864, 4.788 and 4.456 μmol/l
respectively, calculated using SPSS 16.0 software.
Inhibition of cell colony formation by
DMS
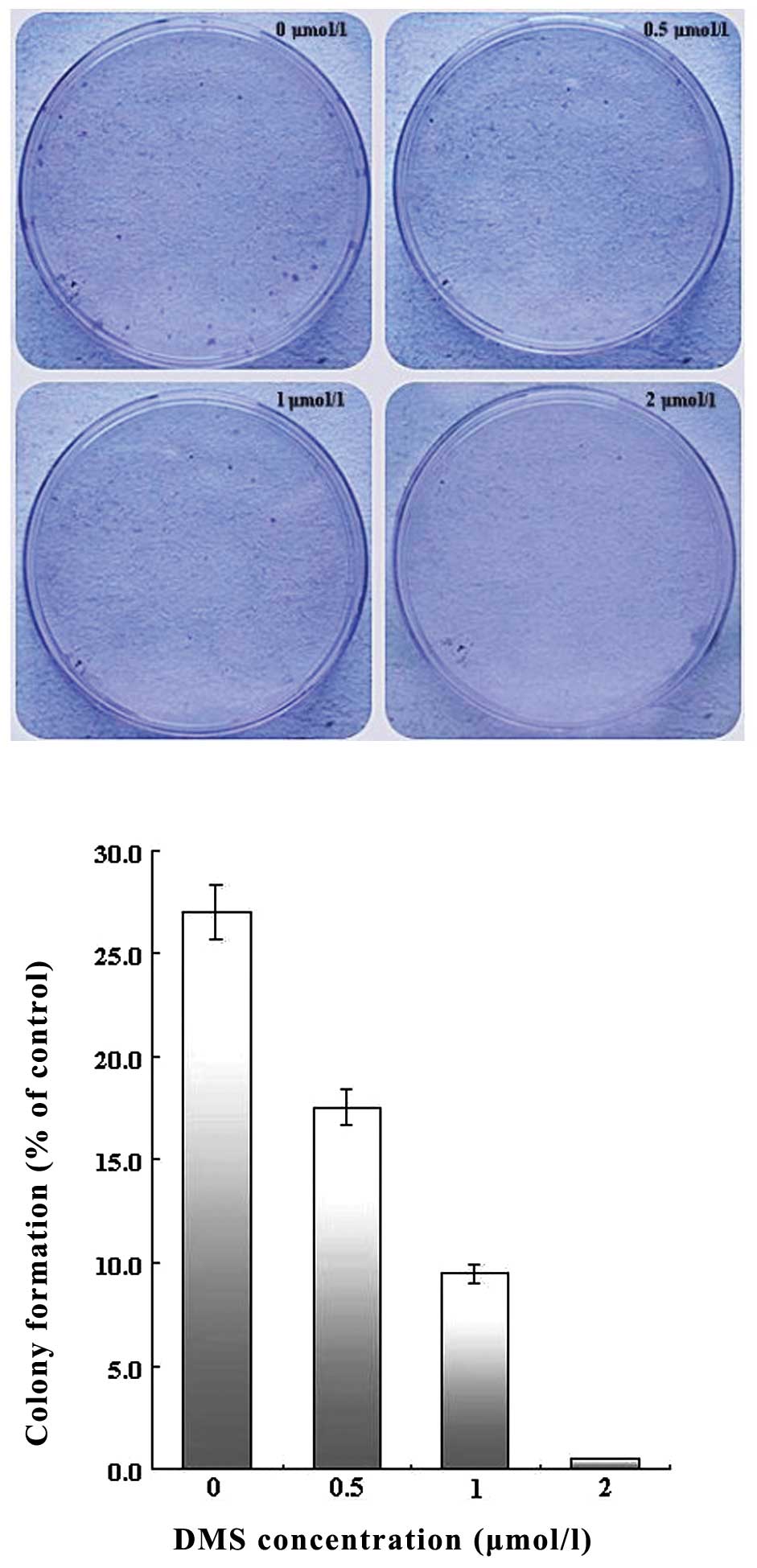
To determine the effects of DMS on the ability of
single cell proliferation, colony formation assay was performed.
Colony formation efficiency was calculated with the number of
visible colonies divided by the number of plated cells. Treatment
with DMS suppressed colony formation in a dose-dependent manner.
Once the concentration was >2 μmol/l, the growth of the A549
cells was almost completely inhibited, with a colony formation rate
of <1% (Fig. 5).
DMS induces cell apoptosis by activating
the apoptotic signaling pathway
The A549 cells were treated with various
concentrations of DMS for 24 and 48 h, stained with Annexin V-FITC
and PI, and analyzed by flow cytometry (Fig. 6A). FITC single-positive cells
represent early apoptotic cells, FITC/PI double-positive cells
represent apoptotic cells and PI single-positive cells represent
dead cells. The percentages of apoptotic cells increased with the
increasing DMS concentrations and with the prolonged exposure time.
However, following treatment with 4 μmol/l DMS for 48 h, the
percentage of dead cells reached 40.5% and the number of apoptotic
cells in turn decreased. These results were further confirmed by
DNA fragmentation assay. Treatment of the cells with DMS (4 μmol/l
for 24 h, 2 and 4 μmol/l for 48 h) resulted in a classical
laddering pattern, whereas no DNA laddering was observed in the
controls (Fig. 6B).
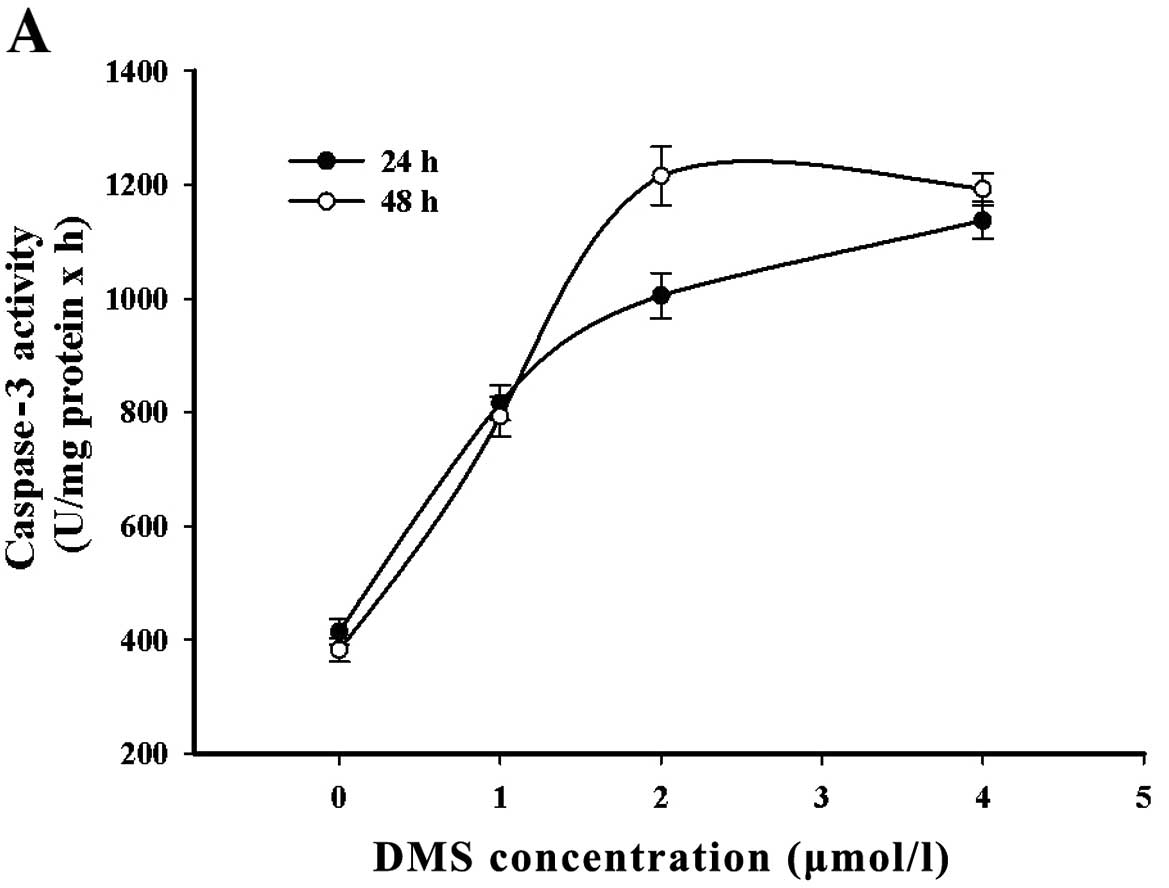
In order to investigate the signaling involved in
DMS stimulation, caspase-3 activation in response to DMS was
measured and the expression of PARP was analyzed by western blot
analysis. The results revealed an increase in caspase-3 activity
following treatment with DMS (1 μmol/l). It reached a plateau from
2 to 4 μmol/l. In addition, as a substrate of caspase-3, PARP was
cleaved in a time- and concentration-dependent manner (Fig. 7).
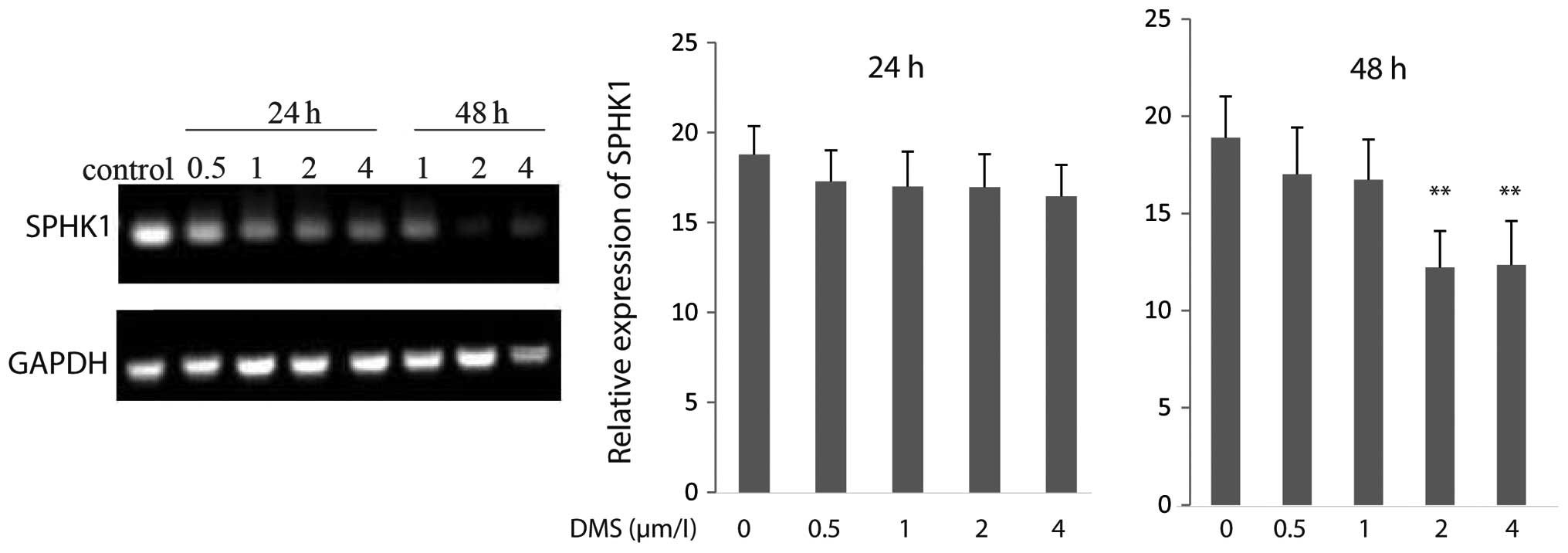
DMS suppresses gene expression of
SPHK1
To investigate the effects of DMS on SPHK1
gene expression, we analyzed the mRNA levels of SPHK1 in
A549 cells by RT-PCR. The results revealed that the mRNA levels of
SPHK1 were markedly downregulated following treatment with
DMS at 2 and 4 μmol/l for 48 h. In addition, qRT-PCR revealed that
the mRNA levels of SPHK1 decreased by 35.28 and 34.64% when
the cells were treated with 2 and 4 μmol/l DMS, respectively for 48
h (Fig. 8), indicating that DMS
inhibits the expression of SPHK1 at the transcriptional
level.
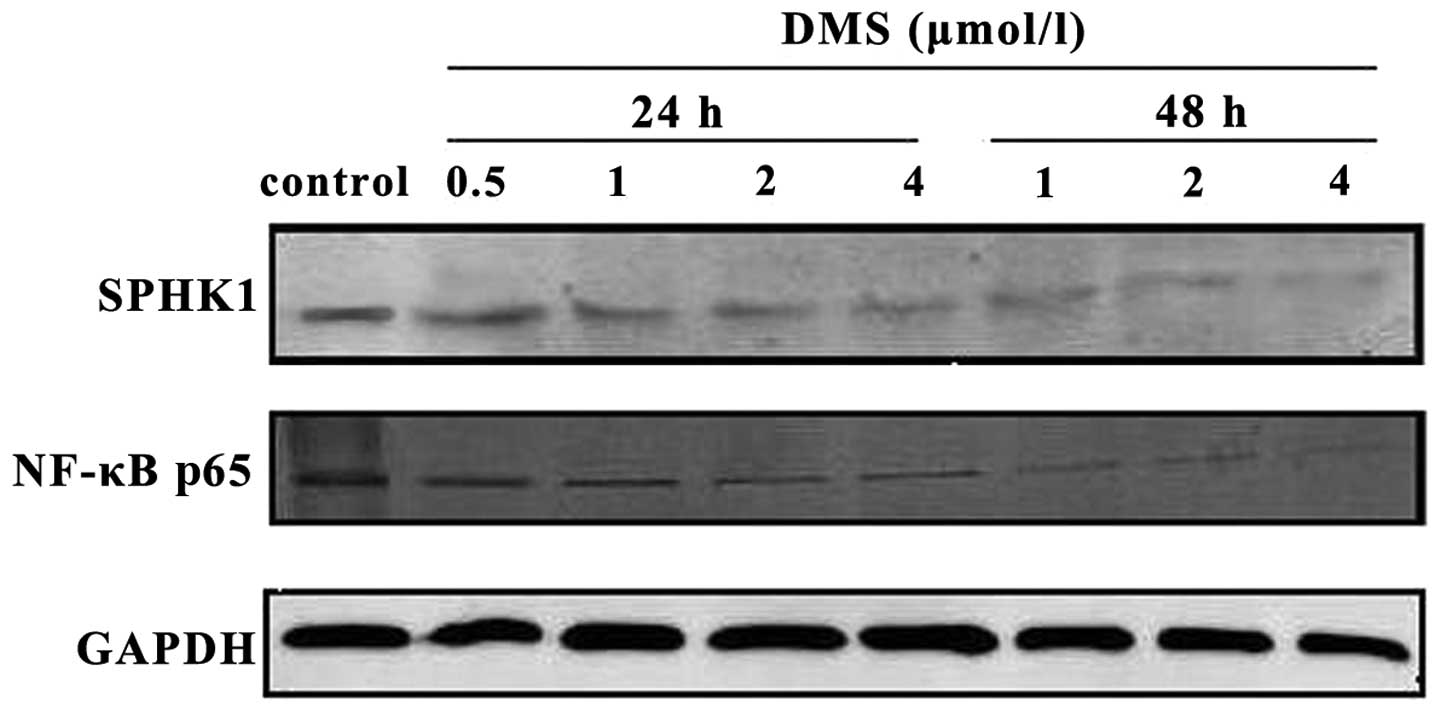
DMS inhibits SPHK1 and NF-κB
activation
Western blot analysis indicated that the expression
of SPHK1 and the NF-κB p65 subunit decreased, with the increasing
DMS concentrations and the prolonged treatment time. Therefore, the
inhibition of NF-κB activity and SPHK1 expression may be
responsible for the induction of cell apoptosis by DMS (Fig. 9).
DMS increases intracellular
Ca2+ concentration
The A549 cells were treated with various
concentrations of DMS for 24 and 48 h and then incubated with
Fluo-4-AM for 30 min. Fluo-4-AM can conjugate with
[Ca2+]i and thus generate strong fluorescence in 405 nm
after excitation light; therefore, intracellular [Ca2+]i
levels can be indirectly visualized under an inverted fluorescence
microscope. We observed that DMS increased intracellular
[Ca2+]i concentrations in the A549 cells (Fig. 10).
Discussion
Tumor progression depends mainly on the degree of
cell proliferation and cell loss, and apoptosis is the main source
of cell loss. SPHK1 is highly expressed in several types of tumor
cells (approximately 2–3-fold higher) and its ability to prevent
apoptosis has been extensively demonstrated (Table I) (17). There is evidence that the
overexpression of SPHK1 contributes to cellular resistance to
chemotherapy drugs (7). As an
inhibitor of SPHK1, the anticancer properties of DMS have been
widely investigated in preclinical models. The inhibition of tumor
cell growth and migration by DMS has been reported (18–20) with a Ki value of 5 μmol/l
(21,22). Moreover, the dose of DMS and tumor
growth inhibition positively correlated in animal model of
tumor-burdened nude mice.
 | Table ISummary of changes in SPHK1
expression in cancer tissues. |
Table I
Summary of changes in SPHK1
expression in cancer tissues.
| Tumor type | SPHK1 expression
(Refs.) | Prognostic
association (Refs.) | Associated with
drug resistance (Refs.) |
|---|
| Breast | Increase (23,24) | Yes (23) | Yes (25) |
| Prostate | Increase (26) | | Yes (27) |
| Ovary | Increase (28) | | |
| Glioblastoma | | Yes (29) | |
| Liver | Increase (30) | | |
|
Gastrointestinal | Increase (31) | | |
| AML | Increase (32) | | Yes (16) |
| Lung | Increase (33) | | Yes (34) |
| Melanoma | | Yes (35) | Yes (36) |
The NF-κB signaling pathway in tumor biology has
attracted considerable attention. It has been reported that cells
expressing high levels of NF-κB are resistant to chemotherapy and
radiotherapy (37). The
inhibition of NF-κB activation sensitizes tumor cells to
chemotherapy (38,39) and eventually lead to apoptosis.
Consistently, in our study, we observed that triggering apoptosis
in the A549 cells was associated with the inhibition of NF-κB
activation. In fact, NF-κB is a calcium-dependent transcription
factor (40). The disturbance of
intracellular calcium triggers the elevation of reactive oxygen
species in the mitochondria and leads to the translocation of NF-κB
into the nucleus (41). A
previous study reported that DMS increases the [Ca2+]i
concentration in U937 and HCT116 cells (13). In the present study, we confirmed
that DMS increased intracellular [Ca2+]i levels in A549
cells.
Billich et al(8) reported that the suppression of SPHK1
activation by DMS diminished NF-κB activity due to the reduced
nuclear translocation of RelA (p65), resulting in spontaneous
apoptosis in A549 cells. This is consistent with our experimental
results. However, in our study, NF-κB activity was not increased,
despite the increase in intracellular [Ca2+]i levels in
A549 cells after treatment of DMS. These results suggest that other
mechanisms may exist between the SPHK1 pathway and intracellular
calcium signaling in terms of regulating NF-κB activity. When the
SPHK1 pathway plays a major role, NF-κB activity may be diminished.
By contrast, when intracellular calcium signaling plays a dominant
role, NF-κB activity may be increased. However, the exact role of
the SPHK1 pathway, calcium channels and the NF-κB signaling network
in regulating the growth of cancer cells remains to be further
elucidated.
Acknowledgements
This study was supported by the Science and
Technology Department of Zhejiang Province Commonwealth Technology
Applied Research Projects (grant no. 2012F82G2060018), the National
Natural Science Foundation of China (grant no. 51272236) and the
Zhejiang Provincial Natural Science Foundation of China (grant no.
LZ13H160004).
References
|
1
|
Igarashi Y and Hakomori S: Enzymatic
synthesis of N, N-dimethyl-sphingosine: demonstration of the
sphingosine: N-methyltransferase in mouse brain. Biochem Biophys
Res Commun. 164:1411–1416. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Megidish T, Cooper J, Zhang L, Fu H and
Hakomori S: A novel sphingosine-dependent protein kinase (SDK1)
specifically phosphorylates certain isoforms of 14-3-3 protein. J
Biol Chem. 273:21834–21845. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
zu Heringdorf DM, Lass H, Alemany R, et
al: Sphingosine kinase-mediated Ca2+signalling by
G-protein-coupled receptors. EMBO J. 17:2830–2837. 1998.
|
|
4
|
Edsall LC, Van Brocklyn JR, Cuvillier O,
Kleuser B and Spiegel S: N,N-dimethylsphingosine is a potent
competitive inhibitor of sphingosine kinase but not of protein
kinase C: modulation of cellular levels of sphingosine 1-phosphate
and ceramide. Biochemistry. 37:12892–12898. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sakakura C, Sweeney EA, Shirahama T, et
al: Selectivity of sphingosine-induced apoptosis. Lack of activity
of DL-erythyro-dihydrosphingosine. Biochem Biophys Res Commun.
246:827–830. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cuvillier O, Pirianov G, Kleuser B, et al:
Suppression of ceramide-mediated programmed cell death by
sphingosine-1-phosphate. Nature. 381:800–803. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Antoon JW, White MD, Slaughter EM, et al:
Targeting NFκB mediated breast cancer chemoresistance through
selective inhibition of sphingosine kinase-2. Cancer Biol Ther.
11:678–689. 2011.
|
|
8
|
Billich A, Bornancin F, Mechtcheriakova D,
Natt F, Huesken D and Baumruker T: Basal and induced sphingosine
kinase 1 activity in A549 carcinoma cells: function in cell
survival and IL-1beta and TNF-alpha induced production of
inflammatory mediators. Cell Signal. 17:1203–1217. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Alfonso A, De la Rosa L, Vieytes M and
Botana L: Dimethylsphingosine increases cytosolic calcium and
intracellular pH in human T lymphocytes. Biochem Pharmacol.
65:465–478. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee EH, Lee YK, Im YJ, Kim JH, Okajima F
and Im DS: Dimethylsphingosine regulates intracellular pH and
Ca(2+) in human monocytes. J Pharmacol Sci. 100:289–296. 2006.
|
|
11
|
Shin Y, Daly J and Choi O: Diverse effects
of sphingosine on calcium mobilization and influx in differentiated
HL-60 cells. Cell Calcium. 27:269–280. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chang YJ, Lee YK, Lee EH, Park JJ, Chung
SK and Im DS: Structure-activity relationships of
dimethylsphingosine (DMS) derivatives and their effects on
intracellular pH and Ca2+in the U937 monocyte cell line.
Arch Pharm Res. 29:657–665. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim HL and Im DS:
N,N-dimethyl-D-erythro-sphingosine increases intracellular
Ca2+concentration via
Na+-Ca2+-exchanger in HCT116 human colon
cancer cells. Arch Pharm Res. 31:54–59. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pchejetski D, Golzio M, Bonhoure E, et al:
Sphingosine kinase-1 as a chemotherapy sensor in prostate
adenocarcinoma cell and mouse models. Cancer Res. 65:11667–11675.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Morales A, Paris R, Villanueva A, Llacuna
L, Garcia-Ruiz C and Fernandez-Checa J: Pharmacological inhibition
or small interfering RNA targeting acid ceramidase sensitizes
hepatoma cells to chemotherapy and reduces tumor growth in vivo.
Oncogene. 26:905–916. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bonhoure E, Pchejetski D, Aouali N, et al:
Overcoming MDR-associated chemoresistance in HL-60 acute myeloid
leukemia cells by targeting shingosine kinase-1. Leukemia.
20:95–102. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vadas M, Xia P, McCaughan G and Gamble J:
The role of sphingosine kinase 1 in cancer: Oncogene or
non-oncogene addiction? Biochimica et Biophysica Acta (BBA).
Biochim Biophys Acta. 1781:442–447. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nava VE, Cuvillier O, Edsall LC, et al:
Sphingosine enhances apoptosis of radiation-resistant prostate
cancer cells. Cancer Res. 60:4468–4474. 2000.PubMed/NCBI
|
|
19
|
Nava VE, Hobson JP, Murthy S, Milstien S
and Spiegel S: Sphingosine kinase type 1 promotes
estrogen-dependent tumorigenesis of breast cancer MCF-7 cells. Exp
Cell Res. 281:115–127. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sweeney EA, Sakakura C, Shirahama T, et
al: Sphingosine and its methylated derivative
N,N-dimethylsphingosine (DMS) induce apoptosis in a variety of
human cancer cell lines. Int J Cancer. 66:358–366. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu H, Sugiura M, Nava VE, et al:
Molecular cloning and functional characterization of a novel
mammalian sphingosine kinase type 2 isoform. J Biol Chem.
275:19513–19520. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Olivera A, Kohama T, Tu Z, Milstien S and
Spiegel S: Purification and characterization of rat kidney
sphingosine kinase. J Biol Chem. 273:12576–12583. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ruckhäberle E, Rody A, Engels K, et al:
Microarray analysis of altered sphingolipid metabolism reveals
prognostic significance of sphingosine kinase 1 in breast cancer.
Breast Cancer Res Treat. 112:41–52. 2008.PubMed/NCBI
|
|
24
|
French KJ, Schrecengost RS, Lee BD, et al:
Discovery and evaluation of inhibitors of human sphingosine kinase.
Cancer Res. 63:5962–5969. 2003.PubMed/NCBI
|
|
25
|
French KJ, Upson JJ, Keller SN, Zhuang Y,
Yun JK and Smith CD: Antitumor activity of sphingosine kinase
inhibitors. J Pharmacol Exp Ther. 318:596–603. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Akao Y, Banno Y, Nakagawa Y, et al: High
expression of sphingosine kinase 1 and S1P receptors in
chemotherapy-resistant prostate cancer PC3 cells and their
camptothecin-induced up-regulation. Biochem Biophys Res Commun.
342:1284–1290. 2006. View Article : Google Scholar
|
|
27
|
Leroux M, Auzenne E, Evans R, et al:
Sphingolipids and the sphingosine kinase inhibitor, SKI II, induce
BCL-2-independent apoptosis in human prostatic adenocarcinoma
cells. Prostate. 67:1699–1717. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sutphen R, Xu Y, Wilbanks GD, et al:
Lysophospholipids are potential biomarkers of ovarian cancer.
Cancer Epidemiol Biomarkers Prev. 13:1185–1191. 2004.PubMed/NCBI
|
|
29
|
Van Brocklyn JR, Jackson CA, Pearl DK,
Kotur MS, Snyder PJ and Prior TW: Sphingosine kinase-1 expression
correlates with poor survival of patients with glioblastoma
multiforme: roles of sphingosine kinase isoforms in growth of
glioblastoma cell lines. J Neuropathol Exp Neurol. 64:695–705.
2005.PubMed/NCBI
|
|
30
|
Suzuki H, Riley RT and Sharma RP:
Inducible nitric oxide has protective effect on fumonisin B1
hepatotoxicity in mice via modulation of sphingosine kinase.
Toxicology. 229:42–53. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kawamori T, Osta W, Johnson KR, et al:
Sphingosine kinase 1 is up-regulated in colon carcinogenesis. FASEB
J. 20:386–388. 2006.PubMed/NCBI
|
|
32
|
Sobue S, Nemoto S, Murakami M, et al:
Implications of sphingosine kinase 1 expression level for the
cellular sphingolipid rheostat: relevance as a marker for
daunorubicin sensitivity of leukemia cells. Int J Hematol.
87:266–275. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Johnson KR, Johnson KY, Crellin HG, et al:
Immunohistochemical distribution of sphingosine kinase 1 in normal
and tumor lung tissue. J Histochem Cytochem. 53:1159–1166. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Min J, Van Veldhoven PP, Zhang L, Hanigan
MH, Alexander H and Alexander S: Sphingosine-1-phosphate lyase
regulates sensitivity of human cells to select chemotherapy drugs
in a p38-dependent manner. Mol Cancer Res. 3:287–296. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sauer B, Ruwisch L and Kleuser B:
Antiapoptotic action of 1alpha,25-dihydroxyvitamin D3 in primary
human melanocytes. Melanoma Res. 13:339–347. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bektas M, Jolly PS, Müller C, Eberle J,
Spiegel S and Geilen CC: Sphingosine kinase activity counteracts
ceramide-mediated cell death in human melanoma cells: role of Bcl-2
expression. Oncogene. 24:178–187. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Han S and Roman J: Suppression of
prostaglandin E2 receptor subtype EP2 by PPARgamma ligands inhibits
human lung carcinoma cell growth. Biochem Biophys Res Commun.
314:1093–1099. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mukogawa T, Koyama F, Tachibana M, et al:
Adenovirus-mediated gene transduction of truncated I kappa B alpha
enhances radiosensitivity in human colon cancer cells. Cancer Sci.
94:745–750. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Weaver KD, Yeyeodu S, Cusack JC Jr,
Baldwin AS Jr and Ewend MG: Potentiation of chemotherapeutic agents
following antagonism of nuclear factor kappa B in human gliomas. J
Neurooncol. 61:187–196. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lin JC, Yang SC, Hong TM, et al:
Phenanthrene-based tylophorine-1 (PBT-1) inhibits lung cancer cell
growth through the Akt and NF-kappaB pathways. J Med Chem.
52:1903–1911. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gong G, Waris G, Tanveer R and Siddiqui A:
Human hepatitis C virus NS5A protein alters intracellular calcium
levels, induces oxidative stress, and activates STAT-3 and NF-kappa
B. Proc Natl Acad Sci USA. 98:9599–9604. 2001. View Article : Google Scholar
|