Introduction
Colorectal cancer (CRC) is the fourth most common
malignant tumor worldwide, leading to approximately 200,000 deaths
per year in Europe and the US (1). Although improved surgical techniques
combined with multi-disciplinary approaches have been applied in
therapy, the 5-year survival rate for patients with CRC remains
poor, mainly due to tumor cells acquiring multidrug resistance
properties. The overexpression of drug efflux proteins (2–4) is
considered a common mechanism of multidrug resistance, as shown in
preclinical studies. However, no significant improvement in the
chemotherapeutic effectiveness has been observed in clinical
practice by the inhibition of drug efflux proteins (5,6),
suggesting the existence of other chemoresistance mechanisms.
Irinotecan hydrochloride (CPT-11) is a water-soluble
derivative of camptothecin (7),
presenting a wide spectrum of antitumor activity by preventing DNA
religation, resulting in DNA double-strand breaks and eventually
leading to apoptosis (8). The
cytotoxic activity of CPT-11 has been reported in several malignant
tumors, including breast, lung, ovarian, and colon cancer (9–16).
CPT-11 serving as a DNA topoisomerase I inhibitor, combined with
5-fluorouracil (5-FU) and leucovorin has been accepted as a
first-line treatment for patients with advanced CRC. However, this
combination only offers a 2-month median survival advantage over
previous chemotherapeutic agents (8). Any treatment conferring a modest
survival benefit for CRC will have significant meaning.
Understanding the mechanisms of drug resistance will greatly
contribute to the development of more effective treatments for
improving survival in patients with CRC.
Basic fibroblast growth factor (bFGF) is a
pleiotropic factor involved in the processes of cell proliferation,
differentiation and anti-apoptosis in a wide variety of cells
derived from the mesoderm and neuroectoderm. It has been well
documented that bFGF plays an essential role in tumor growth and
progression by stimulating the proliferation of tumor cells and
promoting angiogenesis (17–19). During chemotherapy in CRC, CPT-11
enhances the levels of intratumoral bFGF, and upregulates bFGF
levels in recurrent tumors following chemotherapy, suggesting that
elevated levels of extracellular bFGF may mediate an epigenetic
mechanism of multidrug resistance. bFGF serving as a key factor
involved in an epigenetic mechanism by which cancer cells become
resistant to chemotherapeutic agents may provide a novel potential
target for designing strategies to sensitize tumor cells to
chemotherapeutic drugs.
In our previous studies, using phage display
technology, we obtained a novel bFGF antagonist peptide (named P7)
with strong inhibitory activity against bFGF-induced cell
proliferation and angiogenesis (20,21). Our results suggested that P7
peptides may bind to bFGF and block the biological activities of
bFGF. In this study, we aimed to investigate the effects and
mechanisms of action of our previously isolated P7 peptide on the
bFGF-induced resistance of CRC cells to CPT-11.
Materials and methods
Materials
The CRC cell line, HT-29, was kept in our
laboratory. Recombinant human bFGF was obtained from PeproTech,
Inc. (Rocky Hill, NJ, USA). P7 peptides (PLLQATLGGGS) with a purity
of >98% were synthesized at SBS Genetech Co., Ltd. (Beijing,
China). Dynabeads® M-280 Streptavidin, Dynamag-2 magnet,
RMPI-1640 medium and fetal bovine serum (FBS) were obtained from
Invitrogen (Carlsbad, CA, USA). Anti-phospho-Akt, anti-Akt,
anti-Bcl-2, anti-Bcl-xL, anti-Bax and anti-GAPDH antibodies were
purchased from Cell Signaling Technology (Danvers, MA, USA).
Polyvinylidene difluoride (PVDF) membranes were purchased from
Millipore (Billerica, MA, USA). The enhanced chemiluminescence
(ECL) detection kit was the product of Pierce (Rockford, IL, USA).
The Alexa Fluor® 488 Annexin V/PI kit was from
Invitrogen. The SYBR-Green qPCR Master Mix was the product of
Tiangen Biotech (Beijing, China). The materials for 2-dimensional
gel electrophoresis, including immobilized pH gradient (IPG) strips
(pH 3–10 non-linear), CHAPS, Bio-Lyte 3–10 Ampholyte 40% solution,
acrylamide, methylene-bisacrylamide, sodium dodecyl sulfate (SDS),
tetramethylethylenediamine (TEMED) and iodoracetamide were the
products of Bio-Rad (Hercules, CA, USA). Glycerol and ammonium
persulfate were purchased from Sigma (St. Louis, MO, USA).
Dithiothreitol (DTT) and urea were obtained from Promega (Madison,
WI, USA).
Cell viability assay
The cells were seeded into 96-well culture plates at
a density of 5×103 cells/well and cultured overnight.
After being starved in RMPI-1640 medium with 0.4% FBS for 24 h, the
cells were treated with serially diluted CPT-11 (15, 30, 60, 120,
180 μM) for 48 h. The IC50 of CPT-11 was determined by
MTT colorimetric assay.
After determining the IC50 of CPT-11 as
60 μM, the cells were further treated as follows: with 60 μM CPT-11
alone, 60 μM CPT-11 plus 20 ng/ml bFGF, or 60 μM CPT-11 plus 20
ng/ml bFGF combined with 4 μM P7. The viability of the cells was
determined by MTT colorimetric assay following incubation for 48
h.
Apoptosis assay
The cells were seeded in a 6-well plate at a density
of 4×105 cells/well, starved in RMPI-1640 medium with
0.4% FBS overnight, and then treated with 60 μM CPT-11 alone, 60 μM
CPT-11 plus 20 ng/ml bFGF, or 60 μM CPT-11 plus 20 ng/ml bFGF
combined with 4 μM P7 for 48 h. After being washed with cold PBS
twice, the cells were resuspended in 1X Annexin-binding buffer,
incubated with Alexa Fluor 488 Annexin V and PI for 15 min in the
dark at room temperature, and subjected to flow cytometric
analysis. FCS Express version 3 software was applied to analyze the
apoptotic ratio of the cells.
Akt activation assay
The cells were seeded in a 12-well plate at a
density of 5×105 cells/well and cultured overnight.
After being starved for 24 h, the cells were pre-treated with 20
ng/ml bFGF, or 20 ng/ml bFGF combined with 4 μM P7 for 4 h prior to
treatment with 60 μM CPT-11. After being washed twice with cold
PBS, the cells were harvested and centrifuged at 12,000 × g for 10
min at 4°C to remove the insoluble components. The resultant
protein samples were separated by 10% SDS-PAGE gel and were then
transferred onto a PVDF membrane. In order to block non-specific
binding, the membrane was incubated with 5% non-fat milk in TBST
(25 mM Tris, pH 7.4, 150 mM NaCl and 0.1% Tween-20) buffer for 1 h.
After being washed with TBST 3 times, the membrane was incubated
with primary antibody [an anti-phospho-Akt rabbit monoclonal
antibody (mAb) or an anti-Akt rabbit mAb] overnight followed by
probing with goat anti-rabbit IgG, HRP-linked antibody for 1 h at
room temperature. The ECL detection kit was used to detect the
proteins on the blots. The relative ratio was determined by
Quantity One software.
bFGF internalization assay
The biotinylation of bFGF was performed on cysteine
residues according to the manufacturer’s instructions (Vector
Laboratories, Inc., Burlingame, CA, USA). The modification of bFGF
at cysteine residues has no effect on the biological activity of
the growth factor (22). After
being starved for 24 h, the HT-29 cells were pre-treated with 4 μM
peptides for 5 min prior to stimulation with 20 ng/ml biotinylated
bFGF (biot-bFGF) for 4 h. The cells were harvested by
centrifugation at 250 × g for 5 min at 4°C, and washed twice in
cold PBS. The Nuclear and Cytoplasmic Protein Extraction kit was
used to extract the nuclear and the cytoplasmic proteins according
to the manufacturer’s instructions (KeyGen Biotech. Co. Ltd.,
Nanjing, China). The protein concentrations were determined using
the Bradford method, as previously described (23). An equal amount of protein sample
was incubated in the presence of streptavidin beads for 30 min at
room temperature with gentle rotation. After extensive washing with
PBS containing 0.1% BSA 5 times, the bound proteins were directly
resuspended in 0.1% SDS by boiling the beads for 5 min and they
were subsequently analyzed by western blot analysis using anti-bFGF
antibody.
Analysis of the expression of
apoptosis-related proteins
The cells were seeded in a 12-well plate at a
density of 5×105 cells/well and cultured overnight.
After being starved for 24 h, the cells were pre-treated with 20
ng/ml bFGF, or 20 ng/ml bFGF combined with 4 μM P7 for 4 h prior to
treatment with 60 μM CPT-11 for 48 h. The cells were harvested and
lysed in 1X SDS-PAGE loading buffer. The resultant protein samples
were separated by 10% SDS-PAGE gel and were then transferred onto a
PVDF membrane, which was incubated in 5% non-fat milk in TBST
buffer for 1 h, and subsequently with polyclonal antibodies against
Bcl-2, Bax, Bcl-xL and GAPDH overnight, and then with
HRP-conjugated secondary antibody for 1 h. The ECL detection kit
was used to visualize the blots. The results were analyzed using
Quantity One software to determine the relative ratio.
2-Dimensional gel electrophoresis (2-DE)
and mass spectrometric analysis
The HT-29 cells (1×106 cells) were grown
in cell culture flasks (75 cm2) overnight. After being
starved for 24 h, the cells were treated with 60 μM CPT-11 alone,
60 μM CPT-11 plus 20 ng/ml bFGF, or 60 μM CPT-11 plus 20 ng/ml bFGF
combined with 4 μM P7 for 48 h. The samples for 2-DE were prepared
as follows: 1 mg of protein sample was resuspended in rehydration
buffer (7 M urea, 2 M thiourea, 4% CHAPS, 65 mM DTT, 0.2% pH 3–10
ampholyte, and 0.001% bromophenol blue) to a total volume of 350
μl. The resuspended proteins were loaded on a 17-cm immobilized pH
gradient strip and separated first according to the isoelectric
point of the proteins. After reduction for 15 min in equilibration
buffer (6 M urea, 30% glycerol, 2% SDS, 50 mM Tris-HCL, pH 8.8 and
2% DTT) for 15 min and alkylation for an additional 15 min in the
same solution (except that 2% DTT was substituted with 2.5%
iodoacetamide), the proteins were then separated in 12% SDS-PAGE
gels, followed by staining with Coomassie brilliant blue G-250.
After being analyzed using PDQuest 8.0 software, the
protein spots were excised and subjected to in-gel trypsin
digestion as previously described (21). The digested samples were dried and
analyzed by tandem time-of-flight (TOF) mass spectrometry (ABI 4800
TOF/TOF). Mascot protein identification software was used for
peptide detection in the IPI human database.
Real-time PCR (qPCR)
Total RNA was isolated from the HT-29 cells using
TRIzol reagent according to the manufacturer’s instructions
(Invitrogen). First-strand cDNA was synthesized from the isolated
total RNA using random primers. Relative qPCR was performed with
SYBR-Green PCR Master Mix using a MiniOpticon real-time PCR
detector. The sequences of the primers used for PCR were as
follows: cytokeratin 8 (CK8) forward, 5′-ATCAGCTCCTCGAGCTTCTC-3′
and reverse, 5′-TCCAGGAACCGTACCTTGTC-3′; and GAPDH forward,
5′-GAAGGTGAAGGTCGGAGTC-3′ and reverse,
5′-AAGATGGTGATGGGATTTC-3′.
Statistical analysis
Data are presented as the means ± standard
deviations (SD) from at least 3 independent experiments and
statistical analysis was performed using GradPad Prism software
version 5.0. A value of P<0.05 was considered to indicate a
statistically significant difference.
Results
P7 peptides reverse bFGF-induced
resistance to CPT-11
The effects of P7 peptides on bFGF-induced CPT-11
resistance were evaluated by MTT assay. The results are presented
in Fig. 1. CPT-11 inhibited cell
growth in a dose-dependent manner, with an IC50 of 60
μM. At the fixed CPT-11 concentration (IC50), bFGF
induced resistance to CPT-11, as indicated by the increase in the
the survival rate of the HT-29 cells treated with CPT-11 plus bFGF.
In the presence of bFGF, the addition of P7 peptides reversed the
bFGF-induced resistance to CPT-11.
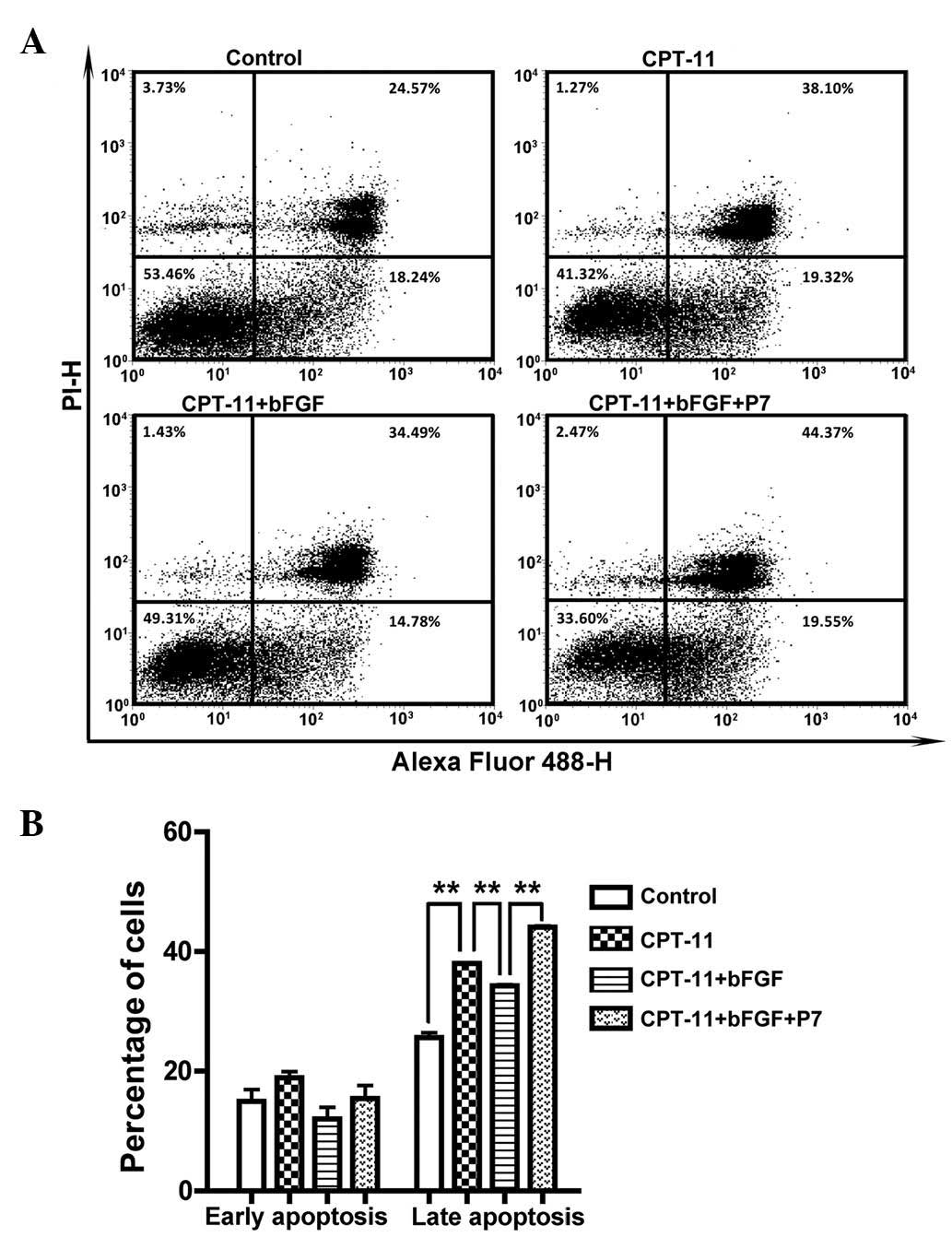
P7 attenuates the anti-apoptotic effects
of bFGF on CPT-11-treated cells
It has been known that the failure to activate the
apoptotic programme is an essential mode of drug resistance in
tumor cells. bFGF has anti-apoptotic potential, and is involved in
the induction of the resistance of tumor cells to chemotherapeutic
drugs. Therefore, in order to investigate whether the P7 peptide
reversed bFGF-induced resistance to CPT-11 by attenuating the
anti-apoptotic effects of bFGF, dual staining with Alexa Fluor 488
Annexin V and PI followed by flow cytometric analysis was carried
out to determine the effects of peptide P7 on apoptosis. As
illustrated in Fig. 2, CPT-11
markedly enhanced the apoptotic rate, with the late-stage apoptotic
rate increasing from 25.64±1.33% to 38.01±0.12%. Treatment with
bFGF decreased the late-stage apoptotic rate to 34.30±0.27%; this
was then increased to 44.09±0.29% by the addition of P7 peptides,
suggesting that P7 peptides may reverse bFGF-induced CPT-11
resistance by counteracting the anti-apoptotic effects of bFGF on
CPT-11-treated cells.
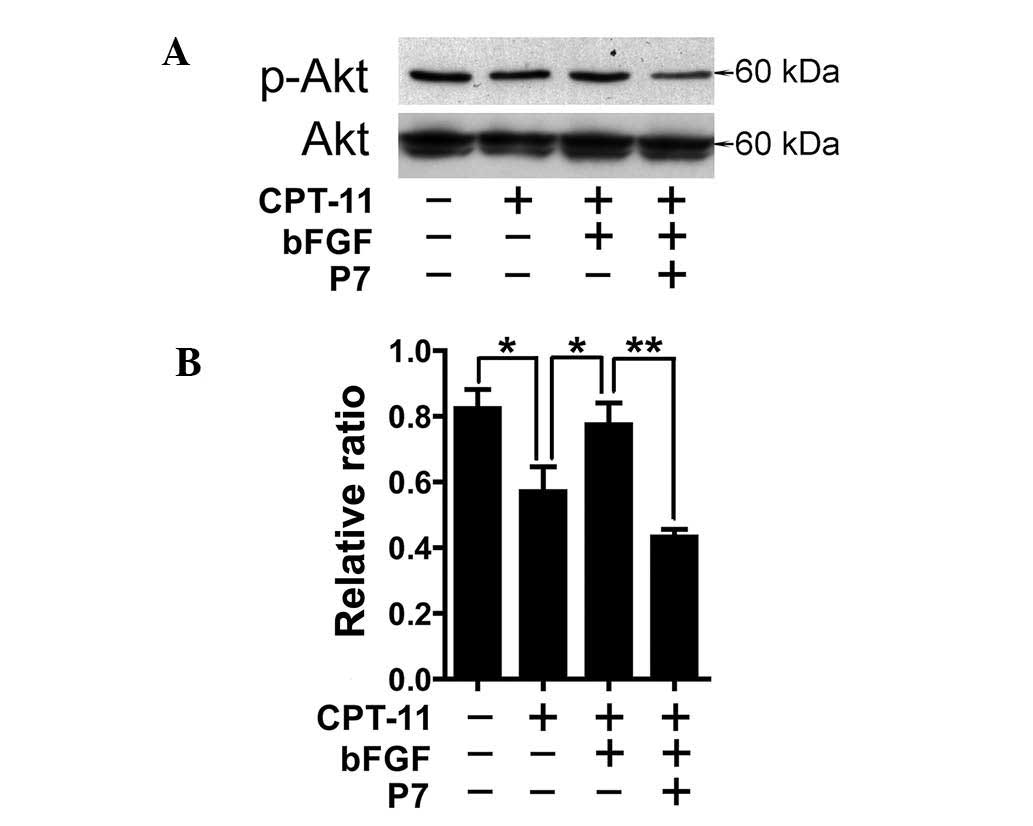
P7 blocks bFGF-induced Akt
activation
Since the PI3K/Akt signaling pathway decisively
contributes to drug resistance by mediating anti-apoptotic signals,
we further examined the effects of P7 peptides on the activation of
the PI3K/Akt signaling pathway by western blot analysis. As shown
in Fig. 3, CPT-11 decreased the
activation of Akt; however, the addition of bFGF enhanced the
phosphorylation of Akt which was downregulated by CPT-11, whereas
P7 peptides attenuated the activation of Akt induced by bFGF,
revealing that the PI3K/Akt signaling pathway is involved in
mediating the reversal effects of P7 peptides on bFGF-induced
apoptosis and resistance to CPT-11.
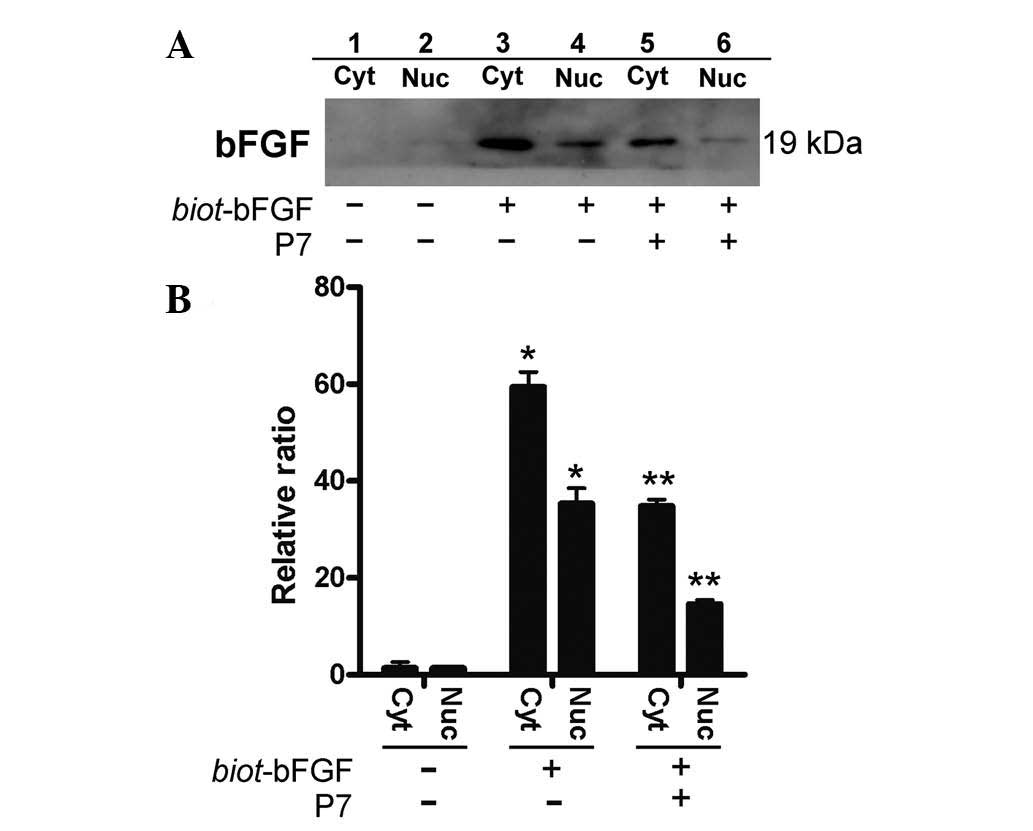
P7 inhibits the internalization of
bFGF
The binding of bFGF with its receptors unleashes its
full biological activity, including its anti-apoptotic properties,
by activating signal transduction pathways and enabling its
internalization into cells (24).
Therefore, the effects of P7 peptides on bFGF internalization were
further analyzed by anti-bFGF antibody probing exogenous biot-bFGF
in the cytoplasmic and nuclear fractions. The intense signals of
biot-bFGF were detected in both the cytoplasmic and nuclear
proteins extracted from the biot-bFGF treated cells (Fig. 4, lanes 3 and 4), whereas
pre-treatment of the cells with P7 peptides (4 μM) for 5 min prior
to stimulation with biot-bFGF weakened the signals detected in the
cytoplasms and nuclei of the cells (Fig. 4, lanes 5 and 6), indicating that
P7 peptides significantly inhibited bFGF internalization, which may
partly contribute to the counteracting effects of P7 peptides,
preventing the blockade of apoptosis by bFGF in the cells treated
with CPT-11, and further reducing bFGF-induced resistance to
CPT-11.
P7 counteracts the regulatory effects of
bFGF on the expression of apoptosis-related proteins in
CPT-11-treated cells
Given that P7 peptides attenuated the anti-apoptotic
effects of bFGF in CPT-11-treated cells, and Bcl-2 family proteins
play essential roles in modulating the process of apoptosis,
western blot analysis was carried out to elucidate the involvement
of Bcl-2 family proteins in the effects of P7 peptides on the
apoptosis of HT-29 cells. As shown in Fig. 5, when the HT-29 cells were exposed
to 60 μM CPT-11, the ratio of Bax to Bcl-2 increased. Pre-treatment
with bFGF for 4 h prior to the administration of CPT-11
downregulated the ratio of Bax to Bcl-2, which was rescued by the
addition of P7 peptides. Compared with the control, no significant
change in Bcl-xL expression was observed in all the groups. These
results suggest that the downregulation of the anti-apoptotic
protein, Bcl-2, and the upregulation of the pro-apoptotic protein,
Bax, may correlate with the attenuation of the anti-apoptotic
effects of bFGF by P7 peptides in CPT-11-treated cells.
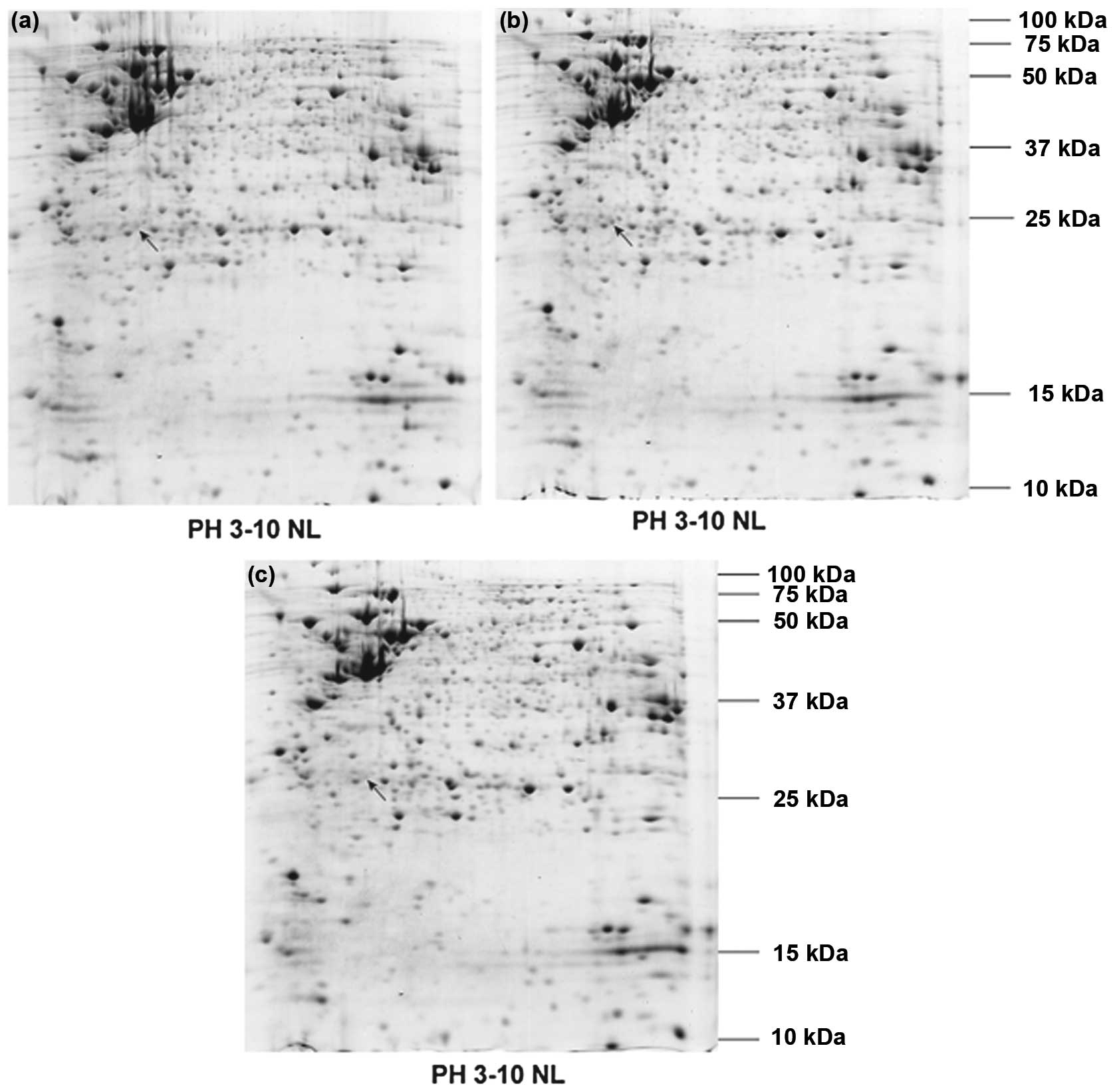
Identification of proteins associated
with the effects of P7 peptides on bFGF-induced resistance to
CPT-11
A proteomic approach based on 2-DE coupled with mass
spectrometry was applied to identify proteins participating in the
effects of P7 peptides on bFGF-induced resistance to CPT-11 in
HT-29 cells. The protein profiles presented in Fig. 6 were first compared between
CPT-11-treated and bFGF-stimulated cells to select the specific
proteins involved in the bFGF regulation of resistance to CPT-11,
and then compared the intensities of the selected protein spots
between bFGF-stimulated and P7 peptide-treated groups to determine
the proteins associated with resistance to CPT-11 which were
affected by P7 peptides targeting bFGF (Fig. 7). The selected differentially
expressed protein spots were excised from the gels and then
subjected to trypsin digestion; they were then analyzed by
matrix-assisted laser desorption/ionization (MALDI)-TOF-TOF mass
spectrometry. As shown in Table
I, the selected proteins were confidently identified by
searching the data collected from MALDI-TOF-TOF against the IPI
human database with the Mascot online search tool. The expression
of the identified protein, CK8, which was increased by bFGF
stimulation, was downregulated by the addition of the P7
peptides.
 | Table IDifferentially expressed proteins
identified by MALDI- TOF-TOF mass spectrometry. |
Table I
Differentially expressed proteins
identified by MALDI- TOF-TOF mass spectrometry.
| Protein name | Accession no. | Theor. Mw/pI | Score | CI |
|---|
| CK8 | IPI00792642 | 24809.4/4.7 | 366 | 100 |
qPCR was performed to validate the expression levels
of CK8 identified by proteomic analysis. CK8 expression was
upregulated by bFGF stimulation and decreased by the addition of
the P7 peptides (Fig. 8). The
result coincided with that obtained from proteomic analysis.
Discussion
CRC is a type of lethal gastrointestinal malignancy.
The main reason for its high mortality rate is the frequent relapse
due to resistance to chemotherapeutic drugs. The development of
novel targeted therapies will open up new opportunities to combat
drug resistance. It was recently reported that bFGF plays an
essential role in mediating chemoresistance, and thus may be served
as a potential target for the development of new therapeutic
strategies to combat the relapse of cancer (25,26).
We previously obtained a novel bFGF antagonist
peptide, P7, using phage display technology (20,21). Our results indicated that P7
exerts strong inhibitory effects on bFGF-induced cell proliferation
and angiogenesis by specifically inhibiting the binding of bFGF to
its receptors. We speculated that the previously isolated P7
peptides targeting bFGF may also have potent inhibitory effects on
bFGF-induced chemoresistance. Therefore, in this study, MTT assay
was first carried out to investigate the effects of P7 peptides on
bFGF-induced resistance to CPT-11. The results revealed that bFGF
increased the survival rate of CPT-11-treated HT-29 cells; however,
the survival rate decreased by the addition of P7 peptides,
suggesting that P7 peptides reversed bFGF-induced resistance to
CPT-11. Further analysis of the effects of P7 peptides on
apoptosis, which is closely related to drug resistance in tumor
cells, indicated that P7 peptides counteracted the anti-apoptotic
effects of bFGF in CPT-11-treated cells.
The mechanisms responsible for the P7 peptides
counteracting the anti-apoptotic effects of bFGF in CPT-11-treated
cells were further explored by analyzing the effects of P7 peptides
on the anti-apoptotic PI3K/Akt signaling pathway, bFGF
internalization, and the expression of Bcl-2 family members
involved in regulating the intrinsic pathway for the activation of
apoptotic effectors. The role of Bcl-2 family members in regulating
the response to chemotherapy has been extensively investigated. It
has been demonstrated that the overexpression of the anti-apoptotic
Bcl-2 family protein, Bcl-2, or the loss of the pro-apoptotic Bcl-2
family member, Bax, expression decreases sensitivity to
chemotherapeutic agents (27–30), whereas the downregulation of Bcl-2
or the overexpression of Bax sensitizes cancer cells to
chemotherapeutic agents (29).
The ratio of Bax to Bcl-2 is usually applied to determine the
susceptibility of cancer cells to chemotherapy-mediated apoptosis
(31). Our results demonstrated
that decrease in Akt activation, the suppression of bFGF
internalization and the increase in the Bax to Bcl-2 ratio induced
by P7 peptides contributed to the counteracting of the
anti-apoptotic effects of bFGF, and the reversal of bFGF-induced
resistance to CPT-11.
Proteomic analysis has been proven to be an
efficient strategy for the identification of proteins involved in
various pathologies. Therefore, we applied this approach to
identify proteins associated with the effects of P7 peptides on
bFGF-induced resistance of HT-29 cells to CPT-11. The
differentially expressed protein spots identified by 2-DE were
subjected to analysis by MALDI-TOF-TOF mass spectrometry, and were
confidently identified by searching the data collected from
MALDI-TOF-TOF against the IPI human database with the Mascot online
search tool (Table I). The
identified protein, CK8, whose expression level was further
confirmed by qPCR, belongs to the keratin family of intermediate
filament proteins, located on the cell surface of a variety of
tumor cells, including colon cancer, breast cancer and liver cancer
cells. Wang et al reported that the suppression of CK8
enchanced the sensitivity of the cells to cisplatin, while the
overexpression of CK8 provided resistance to cisplatin-mediated
apoptosis in human nasopharyngeal carcinoma cells (32). Jaquemar et al demonstrated
that CK8-deficient embryos were exquisitely sensitive to apoptosis
(33). Previous studies have
implicated CK8 in the resistance to tumor necrosis factor family
receptor- and Fas-induced apoptosis (34,35). Moreover, the activation of Akt
increases CK8 expression at the post-transcriptional level, and the
knockdown of Akt decreases CK8 protein levels (36). Our proteomic analysis revealed
that P7 peptides reversed the upregulation of CK8 expression which
was induced by bFGF. Combined with the results that P7 peptides
decreased Akt activation induced by bFGF, it is reasonable to
speculate that the downregulation of Akt activation by P7 peptides
may result in the decreased expression of CK8, which would partly
counteract the anti-apoptotic effects of bFGF, and would thus
attenuate the resistance of HT-29 cells to CPT-11 induced by
bFGF.
It has been previously demonstrated that P7 peptides
inhibit tumor growth and progression through the suppression of
cell proliferation and angiogenesis stimulated by bFGF (20,21,37,38). In this study, we demonstrated that
P7 peptides have the potential to reverse bFGF-induced
chemoresistance. It seems reasonable to speculate that P7 peptides
combined with chemotherapeutic agents, such as CPT-11, may not only
exert inhibitory effects on cell proliferation and angiogenesis,
but may also enhance the antitumor efficacy of chemotherapeutic
agents, resulting in greater therapeutic benefits in cancers
characterized by bFGF-induced chemoresistance.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (81071800, 30973671), the
Guangdong Provincial Science and Technology Program
(2010B060900040), the Natural Science Foundation of Guangdong
Province of China (9151064001000031), the Natural Science
Foundation of Zhejiang Province of China (Y2110492), the Science
and Technology Planning Project of Wenzhou (Y20090244), the
Fundamental Research Funds for the Central Universities (X. Wu),
Guangdong Provincial ‘Thousand-Hundred-Ten Talent Project’ (X. Wu)
and the Key Laboratory of Functional Protein Research of Guangdong
Higher Education Institutes, Jinan University.
References
|
1
|
Kronborg O, Ugstad M, Fuglerud P, Johne B,
Hardcastle J, Scholefield JH, Vellacott K, Moshakis V and Reynolds
JR: Faecal calprotectin levels in a high risk population for
colorectal neoplasia. Gut. 46:795–800. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lum BL, Fisher GA, Brophy NA, Yahanda AM,
Adler KM, Kaubisch S, Halsey J and Sikic BI: Clinical trials of
modulation of multidrug resistance. Pharmacokinetic and
pharmacodynamic considerations. Cancer. 72(Suppl 11): 3502–3514.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Barrand MA, Bagrij T and Neo SY: Multidrug
resistance-associated protein, a protein distinct from
P-glycoprotein involved in cytotoxic drug expulsion. Gen Pharmacol.
28:639–645. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fidler IJ: Critical determinants of cancer
metastasis: rationale for therapy. Cancer Chemother Pharmacol.
43:S3–S10. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Broxterman HJ, Lankelma J and Pinedo HM:
How to probe clinical tumour samples for P-glycoprotein and
multidrug resistance-associated protein. Eur J Cancer.
32A:1024–1033. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ferry DR, Traunecker H and Kerr DJ:
Clinical trials of P-glycoprotein reversal in solid tumours. Eur J
Cancer. 32A:1070–1081. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hertzberg RP, Caranfa MJ and Hecht SM: On
the mechanism of topoisomerase I inhibition by camptothecin:
evidence for binding to an enzyme-DNA complex. Biochemistry.
28:4629–4638. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Saltz LB, Cox JV, Blanke C, Rosen LS,
Fehrenbacher L, Moore MJ, Maroun JA, Ackland SP, Locker PK, Pirotta
N, Elfring GL and Miller LL: Irinotecan plus fluorouracil and
leucovorin for metastatic colorectal cancer. Irinotecan Study
Group. N Engl J Med. 343:905–914. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ichiki M, Gohara R, Rikimaru T, Kitajima
T, Fujiki R, Shimada A and Aizawa H: Combination chemotherapy with
irinotecan and ifosfamide as second-line treatment of refractory or
sensitive relapsed small cell lung cancer: a phase II study.
Chemotherapy. 49:200–205. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takeuchi S, Takamizawa H, Takeda Y, Ohkawa
T, Tamaya T, Noda K, Sugawa T, Sekiba K, Yakushiji M and Taguchi T:
An early phase II study of CPT-11 in gynecologic cancers. Research
Group of CPT-11 in Gynecologic Cancers. Gan To Kagaku Ryoho.
18:579–584. 1991.(In Japanese).
|
|
11
|
Wall ME: Camptothecin and taxol: discovery
to clinic. Med Res Rev. 18:299–314. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fukuoka M, Niitani H, Suzuki A, Motomiya
M, Hasegawa K, Nishiwaki Y, Kuriyama T, Ariyoshi Y, Negoro S and
Masuda N: A phase II study of CPT-11, a new derivative of
camptothecin, for previously untreated non-small-cell lung cancer.
J Clin Oncol. 10:16–20. 1992.PubMed/NCBI
|
|
13
|
Ohno R, Okada K, Masaoka T, Kuramoto A,
Arima T, Yoshida Y, Ariyoshi H, Ichimaru M, Sakai Y and Oguro M: An
early phase II study of CPT-11: a new derivative of camptothecin,
for the treatment of leukemia and lymphoma. J Clin Oncol.
8:1907–1912. 1990.PubMed/NCBI
|
|
14
|
Shimada Y, Yoshino M, Wakui A, Nakao I,
Futatsuki K, Sakata Y, Kambe M, Taguchi T and Ogawa N: Phase II
study of CPT-11, a new camptothecin derivative, in metastatic
colorectal cancer. CPT-11 Gastrointestinal Cancer Study Group. J
Clin Oncol. 11:909–913. 1993.PubMed/NCBI
|
|
15
|
Shirao K, Shimada Y, Kondo H, Saito D,
Yamao T, Ono H, Yokoyama T, Fukuda H, Oka M, Watanabe Y, Ohtsu A,
Boku N, Fujii T, Oda Y, Muro K and Yoshida S: Phase I-II study of
irinotecan hydrochloride combined with cisplatin in patients with
advanced gastric cancer. J Clin Oncol. 15:921–927. 1997.PubMed/NCBI
|
|
16
|
Takeuchi S, Dobashi K, Fujimoto S, Tanaka
K, Suzuki M, Terashima Y, Hasumi K, Akiya K, Negishi Y and Tamaya
T: A late phase II study of CPT-11 on uterine cervical cancer and
ovarian cancer. Research Groups of CPT-11 in Gynecologic Cancers.
Gan To Kagaku Ryoho. 18:1681–1689. 1991.(In Japanese).
|
|
17
|
Rusnati M and Presta M: Fibroblast growth
factors/fibroblast growth factor receptors as targets for the
development of anti-angiogenesis strategies. Curr Pharm Des.
13:2025–2044. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cronauer MV, Schulz WA, Seifert HH,
Ackermann R and Burchardt M: Fibroblast growth factors and their
receptors in urological cancers: basic research and clinical
implications. Eur Urol. 43:309–319. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gross JL, Herblin WF, Dusak BA, Czerniak
P, Diamond MD, Sun T, Eidsvoog K, Dexter DL and Yayon A: Effects of
modulation of basic fibroblast growth factor on tumor growth in
vivo. J Natl Cancer Inst. 85:121–131. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu X, Yan Q, Huang Y, Huang H, Su Z, Xiao
J, Zeng Y, Wang Y, Nie C, Yang Y and Li X: Isolation of a novel
basic FGF-binding peptide with potent antiangiogenetic activity. J
Cell Mol Med. 14:351–356. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang C, Lin S, Nie Y, Jia X, Wang J, Xiao
J, Wu J, Li X and Wu X: Mechanism of antitumor effect of a novel
bFGF binding peptide on human colon cancer cells. Cancer Sci.
101:1212–1218. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bonnet H, Filhol O, Truchet I, Brethenou
P, Cochet C, Amalric F and Bouche G: Fibroblast growth factor-2
binds to the regulatory beta subunit of CK2 and directly stimulates
CK2 activity toward nucleolin. J Biol Chem. 271:24781–24787. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sorensen V, Nilsen T and Wiedlocha A:
Functional diversity of FGF-2 isoforms by intracellular sorting.
Bioessays. 28:504–514. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gan Y, Wientjes MG and Au JL: Expression
of basic fibroblast growth factor correlates with resistance to
paclitaxel in human patient tumors. Pharm Res. 23:1324–1331. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Walsh CT, Wei Y, Wientjes MG and Au JL:
Quantitative image analysis of intra-tumoral bFGF level as a
molecular marker of paclitaxel resistance. J Transl Med. 6:42008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang Q, Maloof P, Wang H, Fenig E, Stein
D, Nichols G, Denny TN, Yahalom J and Wieder R: Basic fibroblast
growth factor downregulates Bcl-2 and promotes apoptosis in MCF-7
human breast cancer cells. Exp Cell Res. 238:177–187. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Burchill SA and Westwood G: Mechanism of
basic fibroblast growth factor-induced cell death. Apoptosis.
7:5–12. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Coleman AB: Positive and negative
regulation of cellular sensitivity to anti-cancer drugs by FGF-2.
Drug Resist Updat. 6:85–94. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Maloof P, Wang Q, Wang H, Stein D, Denny
TN, Yahalom J, Fenig E and Wieder R: Overexpression of basic
fibroblast growth factor (FGF-2) downregulates Bcl-2 and promotes
apoptosis in MCF-7 human breast cancer cells. Breast Cancer Res
Treat. 56:153–167. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Raisova M, Hossini AM, Eberle J, Riebeling
C, Wieder T, Sturm I, Daniel PT, Orfanos CE and Geilen CC: The
Bax/Bcl-2 ratio determines the susceptibility of human melanoma
cells to CD95/Fas-mediated apoptosis. J Invest Dermatol.
117:333–340. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang Y, He QY, Tsao SW, Cheung YH, Wong A
and Chiu JF: Cytokeratin 8 silencing in human nasopharyngeal
carcinoma cells leads to cisplatin sensitization. Cancer Lett.
265:188–196. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jaquemar D, Kupriyanov S, Wankell M, Avis
J, Benirschke K, Baribault H and Oshima RG: Keratin 8 protection of
placental barrier function. J Cell Biol. 161:749–756. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Caulin C, Ware CF, Magin TM and Oshima RG:
Keratin-dependent, epithelial resistance to tumor necrosis
factor-induced apoptosis. J Cell Biol. 149:17–22. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gilbert S, Loranger A, Daigle N and
Marceau N: Simple epithelium keratins 8 and 18 provide resistance
to Fas-mediated apoptosis. The protection occurs through a
receptor-targeting modulation. J Cell Biol. 154:763–773. 2001.
View Article : Google Scholar
|
|
36
|
Fortier AM, Van Themsche C, Asselin E and
Cadrin M: Akt isoforms regulate intermediate filament protein
levels in epithelial carcinoma cells. FEBS Lett. 584:984–988. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang C, Yu Y, Li Q, et al: P7 peptides
suppress the proliferation of K562 cells induced by basic
fibroblast growth factor. Tumour Biol. 33:1085–1093. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yu Y, Gao S, Li Q, et al: The FGF2-binding
peptide P7 inhibits melanoma growth in vitro and in vivo. J Cancer
Res Clin Oncol. 138:1321–1328. 2012. View Article : Google Scholar : PubMed/NCBI
|