Introduction
Acute myocardial infarction is a serious threat to
human health. With the changes in dietary structure, the incidence
of and mortality rates due to myocardial infarction have shown an
increasing trend over the years (1). The utilization of genetic
engineering technology to facilitate angiogenesis in the infarcted
area, to rescue the dying myocardium and to improve cardiac
function has become a focus of research in recent years (2). A large number of experimental and
clinical studies have demonstrated that genetic engineering
technology can be used to improve local blood infusion and restore
impaired heart function in the ischemic myocardium (3–5).
However, previous studies have usually focused on the role of a
single factor, such as vascular endothelial growth factor (VEGF) or
basic fibroblast growth factor (bFGF) in angiogenesis. The
long-term and high expression of VEGF has been shown to result in
the continuous increase in the levels of VEGF in the blood, which
may cause side-effects, such as hemangioma, retinal disease and
various types of cancer. These side-effects (disadvantages) are the
obstacles of gene therapy (6).
Muinck et al suggested that the second generation of
vascular-derived factor and therapeutic gene transfer may enhance
the expression of multiple angiogenic factors. PR39 is an
angiogenic masterswitch protein (7), belonging to the second generation of
angiogenic growth factors. Li et al demonstrated that
exogenous PR39 can selectively inhibit the
ubiquitin/proteasome-dependent degradation of hypoxia-inducible
factor (HIF)-1α and can increase the intracellular expression of
VEGF, kinase insert domain receptor (KDR) and fibroblast growth
factor receptor 1 (FGFR1) (8).
In the present study, we successfully enforced the
expression of the PR39 fusion gene into CRL-1730 endothelial cells.
Subsequently, we investigated the effects of PR39 using cultured
CRL-1730 cells and an animal (pig) model of myocardial infarction.
Our results revealed that the adeno-associated virus (AAV)-mediated
expression of PR39 enhanced the expression of HIF-1α and inhibited
apoptosis under hypoxic conditions in the CRL-1730 cells. PR39
regulated the HIF-1α-induced expression of angiogenic growth
factors. Moreover, compared with the control group, the infarcted
areas of the pigs treated with AAV-PR39 were significantly
decreased and HIF-1α expression was markedly elevated, thus
indicating that treatment with AAV-PR39 exerts protective effects
against myocardial infarction.
Materials and methods
Strains, plasmids and cloning
vectors
E. coli TOP10 cells, T/TAT-His vector,
pBV220/NT4 vector, AAV plasmid (pSSHG-CMV), helper virus pAAV/Ad
and packaging plasmid PFG140 were purchased from Xi’an Huaguang
Bioengineering Co. (Xi’an, China). T4 DNA ligase, restriction
enzymes (NaeI, BamHI, EcoRI and
Eco721), Taq DNA polymerase, and the pGEM-T-Easy vector were
purchased from Amersham Life Sciences (Pittsburgh, PA, USA).
Cell culture and hypoxia
The CRL-1730 vascular endothelial cells were
purchased from the Cell Bank of Shanghai Institutes for Biological
Sciences, Chinese Academy of Sciences, Shanghai, China. The cells
were cultured in RPMI-1640 medium (Invitrogen, Carlsbad, CA, USA)
supplemented with 10% FBS. Experiments were performed on cells at 5
to 6 passages. After the cells had grown to confluence, they were
placed in a quiescent medium for 24 h. Hypoxia was induced with the
use of a modular incubator chamber provided by the Department of
Pathology at the Fourth Military Medical University, Xi’an, China.
The oxygen level in the chamber was monitored with an oxygen
analyzer and remained at 1–3% for up to 72 h.
Construction of expression box containing
NT4-TAT-His-PR39
The transmembrane peptide, TAT, was fused with the
C-terminus of the NT4 signal peptide and 6xHis-tag was directly
fused at the C-terminus of TAT. PR39 was connected downstream of
NT4-TAT-His, resulting in the expression box, NT4-TAT-His-PR39. The
R39 sequences were amplified using the following primers: forward
1, 3′-CCA CGT GAG GAG ACG TCC CCG ACC CCC ATA TTT GCC-5′ and
forward 2, 5′-CCA TAT TTG CCA AGG CCA AGG CCA CCT CCG TTC TTC CCA
CCA AGG CTC CCA CC-3′; reverse 1, 3′-CCG TGG TGG GAA CCT TGG TGG
GAA CCC TGG TGG AAT GCG TGG TGG GAG CC-5′ and reverse 2, 5′-CGG ATC
CTC AGG GGA ACC GTG GTG GGA ACC-3′. The middle region of PR39 was
amplified by primers F2 and R1, the 5-terminus of PR39 was
amplified by primer F1 and the 3-terminus of PR39 was amplified by
primer R2. Following two steps of PCR, complete sequences of PR39
were obtained. PCR included 30 cycles of 94°C for 60 sec, 40°C for
60 sec and 72°C for 90 sec followed by 10 min of extension. The PCR
product was ligated into the pGEM-T Easy vector, resulting in
pGEM-T/PR39, which was subsequently transfected into competent
E. coli TOP10 cells.
Construction of pBV220/NT4-TAT-His-PR39
vector
The pGEM-T/PR39 vector was double digested with
Eco721 and BamHI. The PR39 fragment was excised and
ligated into the T/TAT-His vector digested with identical
restriction enzymes. The ligation products were transformed into
the TOP10 strain and positive clones containing T/TAT-His-PR39 were
obtained. T/TAT-His-PR39 was double digested with NaeI and
BamHI and the excised product was ligated into pBV220/NT4,
thus creating the vector, pBV220/NT4-TAT-His-PR39. The successful
construction of the vector was confirmed by DNA sequencing.
Construction of AAV vector containing
NT4-TAT-His-PR39
Both pSSCMV and pBV220/NT4-TAT-His-PR39 were double
digested with EcoRI and BamHI and the excised
NT4-TAT-His-PR39 fragment was ligated into linear pSSCMV that is
the AAV promoter, thus creating the vector,
pSSCMV/NT4-TAT-His-PR39. HEK-293 cells were co-transfected with
pSSCMV/NT4-TAT-His-PR39, packaging plasmid PFG10 and helper virus
plasmids pAAV/Ad for 72 h. The transfected cells were harvested,
and three turns of repeated freezing and thawing were performed.
The cells were subsequently lysed by sonication. Following
centrifugation at 5,000 rpm for 15 min, cell debris was removed and
the derived virus suspension was inactivated by heating at 56°C for
30 min. Purified recombinant AAV was then harvested.
Quantitative reverse transcription PCR
(qRT-PCR)
Total RNA was extracted using the RNA Plus kit
(Takara, Dalian, China). Total RNA (1 μg) was used for cDNA
synthesis with a QuantiTech Reverse Transcription kit (Takara)
according to the manufacturer’s instructions. The PCR mixture
contained cDNA, generated from 2 ng of total RNA, 0.1 nmol/l
forward and reverse primer mix and SYBR-Green I reagent. The
specific primers used were as follows: HIF-1α sense, 5′-AGC CAG ACG
ATC ATG CAG CTA CTA-3′ and antisense, 5′-GAG TAC TTG CGC TCA GGA
GGA -3′; angiopoietin-2 sense, 5′-GGG CAT AAT TGT GCT TGA CTG G-3′
and antisense, 5′-ATG GTC TTT AGA ATT GGG TCA CTG G-3′; endostatin
sense, 5′-CTC AAT GCA GAG CAC GAT GT-3′ and antisense, 5′-TGT TCT
CAG GCT CTG AGG GT-3′; thrombospondin-2 sense, 5′-TGG AAG GAC TAC
ACG GCC TAT AG-3′ and antisense, 5′-TAG GTT TGG TCA TAG ATA GGT CCT
GAG T-3′; and β-actin sense, 5′-ATT GCC GAC AGG ATG CAG A-3′ and
antisense, 5′-GAG TAC TTG CGC TCA GGA GGA-3′. Quantitative PRC
assays were performed using the Bio-Rad iQ5 Quantitative PCR system
(Bio-Rad, Hercules, MA, USA) and SYBR Premix Ex Taq kit (Takara).
Amplification was carried out for one cycle of 2 min at 50°C and
one cycle of 2 min at 94°C followed by 40 cycles of a 2-step loop:
15 sec at 95°C and 30 sec at 60°C. Relative quantification of the
PCR products was calculated following normalization to β-actin.
Western blot analysis
Total protein extracts were prepared using RIPA
lysis buffer (Beyotime, Nantong, China) according to the
manufacturer’s instructions. The protein concentration in the
lysates was evaluated using a BCA protein assay kit (Beyotime). For
western blot analysis, the proteins lysates (30 μg/lane) were
separated by SDS-PAGE and transferred onto polyvinylidene
difluoride membranes (Whatman Schleicher & Schuell, Middlesex,
UK). The membranes were blocked for 20 min at room temperature with
TBS blocking buffer. The blots were then incubated overnight at 4°C
with each primary antibody (anti-PR39, antiHIF-1α,
anti-angiopoietin-2, anti-endostatin and anti-thrombospondin-2
antibody) followed by incubation for 1 h with horseradish
peroxidase-conjugated secondary antibody. After washing, the sites
of antibody binding were visualized with chemiluminescence reagent
(Boehringer Mannheim, Mannheim, Germany) and the relative levels of
each protein to β-actin were calculated.
siRNA transfection
For the targeted knockdown of HIF-1α, siRNA-HIF-1α
was designed and synthesized by Genetimes Technology Inc.
(Shanghai, China). The nucleotide sequences were as follows:
siRNA-HIF-1α, 5′-TCG AGG AAG GAA CCT GAT GCT TTA TTC AAG AGA TAA
AGC ATC AGG TTC CTT CTT A-3′ (sense) and 5′-CTA GTA AGA AGG AAC CTG
ATG CTT TAT CTC TTG AAT AAA GCA TCA GGT TCC TTC C-3′ (antisense).
As a control for siRNA-HIF-1α, a corresponding random siRNA
sequence was used (positive strand, 5′-TCG AGG GAG ACC GGA TTT GAT
CTA TTC AAG AGA TAG ATC AAA TCC GGT CTC CTT A-3′ and negative
strand, 5′-CTA GTA AGG AGA CCG GAT TTG ATC TAT CTC TTG AAT AGA TCA
AAT CCG GTC TCC C-3′). For transfection, the CRL-1730 cells were
seeded in each cell of a 24-well microplate, grown for 24 h till
they reached 50% confluence, and incubated with a mixture of 6 pmol
siRNA-HIF-1α and 1 μl Lipofectamine RNAi-MAX (Invitrogen, Carlsbad,
CA) in 100 μl serum-free medium 254 at 37°C with 5% CO2.
Twenty-four hours later, the transfection efficiency was examined
by measuring the levels of HIF-1α by qRT-PCR and western blot
analysis, as described above.
Immunohistochemistry
CRL-1730 cell suspension was transferred into the
culture dishes with coverslips and RPMI-1640 medium containing 10%
FBS was added. Following 24 h of culture, 100 μl of
AAV-NT4-TAT-His-PR39 (3.4×109 pfu) or control vector
were used to infect the CRL-1730 cells for 60 h. The coverslips
with the cells were rinsed with PBS twice and fixed with acetone
for 15 min. After three rinses with PBS, the coverslips were
cultured in an incubator containing 0.75%
H2O2 for 30 min, and then blocked with 20%
normal bovine serum in incubation buffer (0.5% Triton X-100 in 0.1
M PBS). The coverslips were then supplemented with primary
monoclonal anti-PR39 or anti-HIF-1α (1:500 dilution) antibody and
incubated at 4°C overnight. After extensive washes, secondary goat
anti-rabbit IgG was added. A diaminobenzidine (DAB) staining kit
was used to detect a positive reaction by producing a brown color.
The protein expression level was quantified by the optical density
(OD) of brown color calculated using Image Pro Plus (IPP)
software.
Detection of apoptosis by flow
cytometry
The CRL-1730 cells were washed twice with cold PBS
and resuspended at a concentration of 1×106/ml. The
cells (100 μl) were supplemented with 5 μl Annexin V-FITC and 5 μl
propidium iodide (PI). After gentle mixing, the cells were
incubated in the dark for 15 min. Staining buffer (400 μl) was then
added to the cells and apoptosis was analyzed immediately by flow
cytometry.
Development of animal (pig) model of
myocardial infarction
The animal experiments caried out in this study
conformed to the guidelines for Laboratory Animals issued by the
Fourth Military Medical University. The present study was performed
with the approval of the Animal Ethics Committee of the Fourth
Military Medical University (Certification no. 0145). All
surgerical procedures were performed under sodium pentobarbital
anesthesia, and all efforts were made to minimize the suffering of
the animals. A total of 18 Chinese mini pigs (weighing 20–25 kg,
provided by the Fourth Military Medical University Experimental
Animal Center) were randomly divided into an experimental group
(AAV-PR39-treated group, n=9) and a control group [normal saline
(PBS)-treated group, n=9]. After 12 h of pre-operative fasting and
4 h of water deprivation, 2 ml of sumianxin II (Animal Husbandry
Industry and Animal Health Products Co., Ltd., Jilin, China) and 18
ml of 5% pentobarbital sodium (1 ml/kg) (Fourth Military Medical
University) were injected intramuscularly. After successful
anesthesia, the pigs were fixed in a supine position on a homemade
wooden stand and placed on a DSA catheter bed (Axiom Artis dBA;
Siemens, Erlangen, Germany). Intravenous access was established and
atropine (1 mg) was injected intramuscularly. The the pig model of
myocardial infarction was then constructed as previously described
(6). One milliliter of
AAV/HRE-PR39 containing 3×109 pfu (experimental group)
or 1 ml of PBS (control group) was injected into the left anterior
descending artery of each mini pig via a 3F microcatheter. ECG
monitoring was performed during the surgery. MRI examination was
performed for the left ventricles after 0, 1, 2 and 3 weeks of the
bandaging of the right thigh or ligation of the right femoral
artery. All the pigs were sacrificed 3 weeks after surgery and the
normal as well as diseased cardiac tissues were obtained.
Cardiac MRI examination
A superconductive scanner (Magnetom Trio 3.0T MRI,
Siemens, AG, Germany) and body coil were used for the MRI
examination. The short and long axis of the heart (view of
two-chamber, four-chamber) was determined by conventional MRI
scanning. Subsequently, HASTE sequential scanning was performed for
axial and coronal views. The following scanning parameters were
used: repetition time (TR)/echo time (TE), 649/52 msec; thickness,
6 mm; no spacing; field of view (FOV), 380 mm; rectangular field of
view (RFOV), 68.8%; acquisition window, 673 msec; trigger pulse, 2;
trigger delay, 24 msec; flip, 160°. Left ventricular short axis
view and long axis view was examined by magnetic resonance
sensitive sequence (T2-weighted TrueFISP Imaging of Myocardium) to
understand the myocardial edema. The following scanning parameters
were used: TR/TE, 281.95/1.09 msec; thickness, 6 mm; no spacing;
Matrix, 256×256; FOV, 360 mm; RFOV, 75%; acquisition window, 715
msec; trigger pulse, 2; trigger delay, 433 msec; flip, 160°.
Myocardial delayed perfusion imaging was performed by 2D
TrueFISP-PSIR sequences. Contrast agents were injected at a speed
of 1 ml/sec and 10 min after injection, scanning commenced. The
following scanning parameters were used: TR/TE, 382.24/1.09 msec;
thickness, 6 mm; no spacing; matrix, 256×256; FOV, 360 mm; RFOV,
75%; acquisition window, 750 msec; trigger pulse, 2; trigger delay,
300 msec; flip, 60°.
Statistical analysis
Data were analyzed using SPSS 13.0 software. The
data of each group were tested for normal distributions first.
Subsequently, the Student’s t-test was carried out for paired
samples. The results are presented as the means ± SD. All reported
P-values were two tailed, and P<0.05 was considered to indicate
a statistically significant difference.
Results
Expression of exogenous PR39 in
endothelial cells
The NT4-TAT-His-PR39 fragment was ligated into the
linear pSSCMV that is the AAV promoter, which triggered the
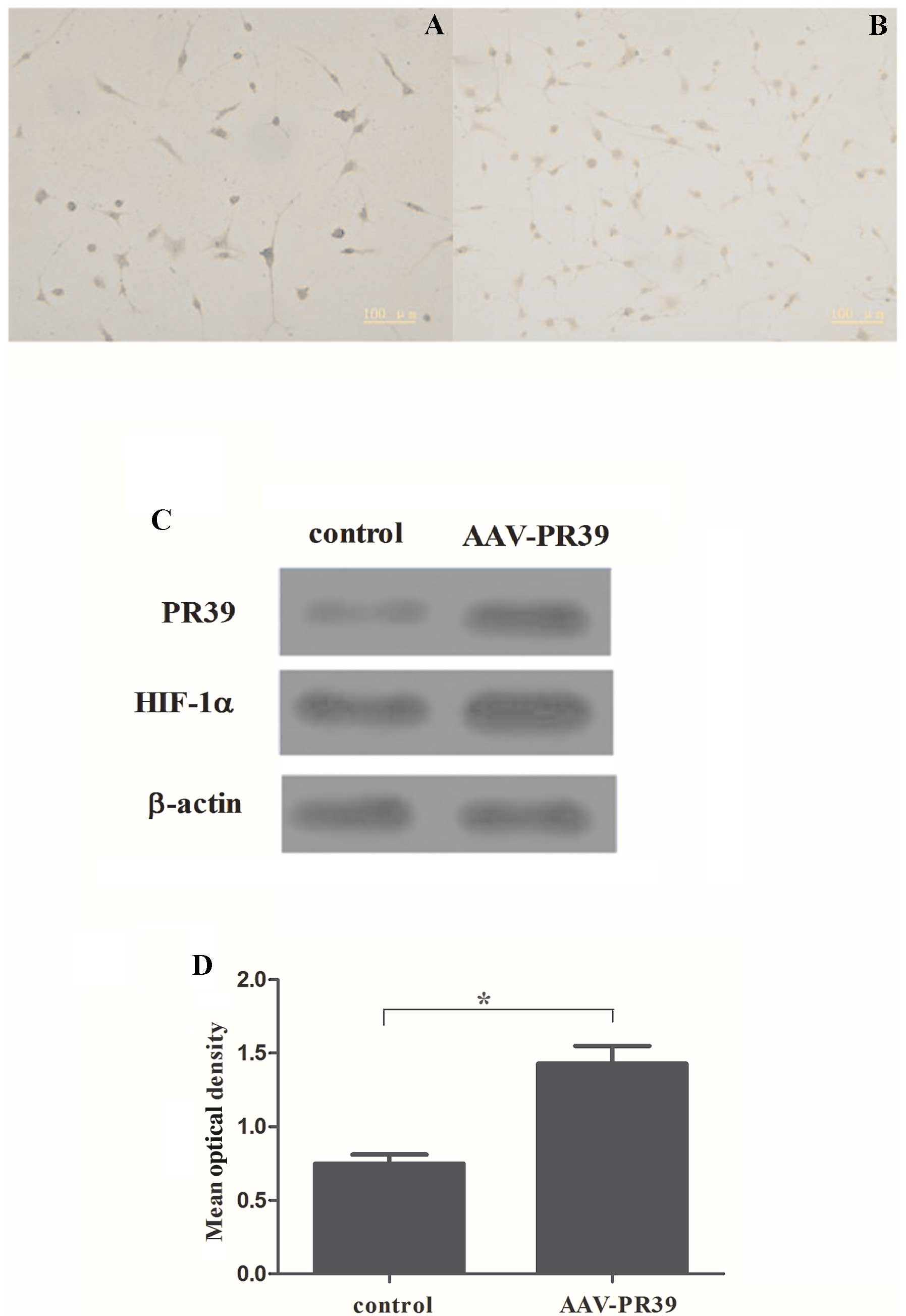
expression of PR39 in the CRL-1730 cells. As shown by
immunohistochemistry, a large amount of brown particles were
observed in the AAV-PR39-treated group, while only a few brown
particles were observed in the control group (Fig. 1A and B). Western blot analysis
revealed that the protein level of PR39 was markedly increased in
the AAV-PR39-treated group (Fig.
2C). Moreover, quantitative analysis indicated that the OD
value of PR39 was markedly elevated in the AAV-PR39-treated group,
as compared with the control group (P<0.001) (Fig. 1C). These results indicated that
the AAV vector containing NT4-TAT-His-PR39 was successfully
transfected into the CRL-1730 cells.
Effect of PR39 on the expression of
HIF-1α under hypoxicconditions
To determine whether PR39 induces HIF-1α expression,
we determined the HIF-1α expression levels in CRL-1730 cells. Under
hypoxic conditions, the HIF-1α protein was observed and was mainly
expressed in the nucleus. Brown staining of the CRL-1730 cells in
the AAV-PR39-treated group was stronger than that in the control
group (Fig. 2A and B). Western
blot analysis indicated that the level of HIF-1α expression was
markedly elevated in the AAV-PR39-treated group (Fig. 2C). Compared with the control
(PBS-treated) group, the OD value for HIF-1α protein expression in
the AAV-PR39-treated group was markedly elevated (P<0.05)
(Fig. 2D). These results
demonstrated that PR39 enhanced the expression of HIF-1α in the
CRL-1730 cells.
PR39 regulates the HIF-1α-induced
expression of angiogenic growth factors
We found that PR39 enhanced the expression of HIF-1α
in CRL-1730 cells. Hypoxia regulates the expression of various
genes participating in the various steps of angiogenesis (9). Therefore, in this study, the levels
of angiopoietin-2, endostatin and thrombospondin-2 were analyzed to
determine the effects of HIF-1α on the expression of angiogenic
growth factors. qRT-PCR analysis revealed that the HIF-1α and
angiopoietin-2 mRNA levels were markedly decreased by siRNA-HIF-1α,
whereas the endostatin and thrombospondin-2 mRNA levels were
markedly increased by siRNA-HIF-1α (P<0.05) (Fig. 3A). Consistent with the results
from qRT-PCR, the downregulation of HIF-1α and angiopoietin-2 was
also observed following treatment of the CRL-1730 cells with
siRNA-HIF-1α, whereas endostatin and thrombospondin-2 levels were
markedly increased by siRNA-HIF-1α (Fig. 3B). In addition, siRNA-HIF-1α
combined with PR39 upregulated the expression of HIF-1α and
angiopoietin-2 and downregulated the expression of endostatin and
thrombospondin-2 (*P<0.05), as compared with the
siRNA-HIF-1α-treated group (Fig. 3A
and B). Taken together, these results demonstrated that PR39
regulated the HIF-1α-induced expression of angiogenic growth
factors.
PR39 inhibits hypoxia-induced
apoptosis
A previous study reported that PR39 inhibits
hypoxia-induced apoptosis in bovine aortic endothelial cells
(19). Thus, in this study, we
examined the effects of AAV-PR39 on hypoxia-induced apoptosis in
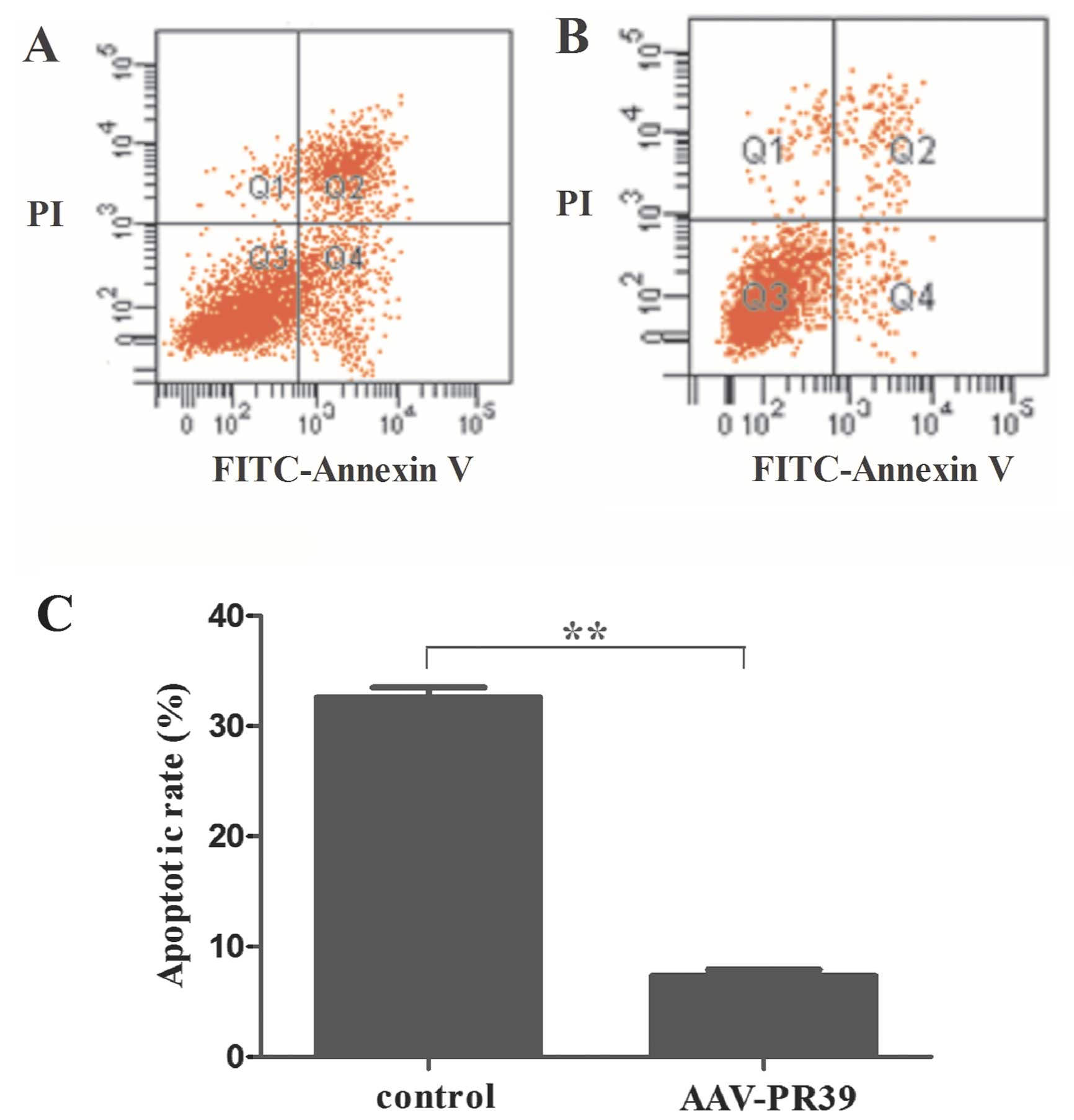
CRL-1730 cells. Flow cytometry revealed that the number of
apoptotic cells markedly decreased in the AAV-PR39-treated CRL-1730
cells, as compared with the control group (Fig. 4A and B). Quantitative analysis
demonstrated that the percentage of apoptotic cells after 72 h of
hypoxia was 32.58±0.39% in the untreated cells and 7.35±0.43% in
the cells pre-treated with AAV-PR39 (P<0.01) (Fig. 4C). These results indicated that
PR39 inhibited apoptosis under hypoxic conditions in the CRL-1730
cells.
Effect of PR39 on the expression of
HIF-1α in the areas surrounding areas the infarcted area
We investigated whether PR39 affects the infarct
area in vivo using a pig model of myocardial infarction.
Three weeks after the induction of myocardial infarction, we
measured the infarct areas and the results revealed that the size
of the infarct areas (167.4±5.42 mm2) was significantly
smaller in the AAV-PR39-treated group, as compared with the control
(PBS-treated) group (325.17±6.42 mm2) (P<0.01).
Furthermore, the size of the infarct area was not significantly
altered at the different time points (0 h,
342.6±6.81mm2; 1 week, 337.4±6.22 mm2; 3
weeks, 325.17±6.42 mm2, P>0.05) in the control
(PBS-treated) group. By contrast, in the AAV-PR39-treated group,
the size of the infarct area was markedly reduced as time
progressed after the induction of myocardial infarction (0 h,
326.25±5.92 mm2; 1 week, 273.6±4.86 mm2; 3
weeks, 167.4±5.42 mm2, P<0.01) (Fig. 5). To investigate the mechanisms
involved, we detected the expression of HIF-1α. At 21 days after
the induction of myocardial infarction, HIF-1α expression was
detected by immunohistochemistry, as illustrated in Fig. 6A and B. Brown color indicates
HIF-1α protein staining and blue color indicates myocardial nuclear
staining in the diseased cardiac tissues. We then examined the
expression of HIF-1α by western blot analysis; the upregulation of
HIF-1α in cardiac tissues was also demonstrated by western blot
analysis (Fig. 6C). Quantitative
analysis using IPP software indicated that the OD value for HIF-1α
expression in the AAV-PR390-treated group was significantly higher
than that in the control group (P<0.01) (Fig. 6D). These results suggested that
PR39 decreased the size of the infarct area by upregulating the
expression of HIF-1α in the areas surrounding the infarct area.
Discussion
PR39, a proline (P)- and arginine (R)-rich peptide
implicated in wound healing and in the protection against
myocardial ischemia, was originally isolated from the pig intestine
and identified in neutrophil azurophilic granules and macropages
(10,11). During the early stages of
myocardial infarction, PR39 can reduce the infarct size (12), promote angiogenesis in ischemic
tissue, establish an effective collateral circulation and prevent
microcirculation dysfunction (13). Currently, studies on the
expression of biological short peptides are limited. Thus, in this
study, we fused the NT4 signal peptide upstream of PR39. The NT4
signal peptide can recognize the intracellular activation center
(14), thus achieving the
eukaryotic expression of PR39. To date, the AAV vector is
frequently used to investigate the biological effects of the PR39
peptide. The advantage of AAV lies in its non-toxicity,
non-immunogenicity, non-pathogenicity and infectivity of
non-dividing cells. In addition, the long-term and stable
expression of the genes carried by AAV can be achieved in host
cells (15,16). Therefore, in this study, we
utilized AAV as a vector to carry and deliver the PR39 gene into
the host cells.
Carmeliet et al demonstrated that HIF-1α is
highly expressed under hypoxic conditions. In addition, hypoxic
conditions can induce an upregulation in the expression of a number
of angiogenesis-related genes, including VEGF, VEGF receptor
[Fms-related tyrosine kinase 1 (FLT1) and KDR] and FGFR1 (17,18). In this study, we demonstrated that
HIF-1α was maintained at a relatively high level in endothelial
cells under hypoxic conditions. In addition, HIF-1α expression in
the AAV-PR39-treated group was higher than that in the control
(PBS-treated) group, indicating that PR39 enhanced the expression
of HIF-1α in the cells under hypoxic conditions. One of the
possible mechanisms involved may be that PR39 selectively inhibits
the ubiquitin/proteasome-dependent degradation of HIF-1α and
enhances the expression of VEGF, KDR, FLT-1 and FGFR1 (8), thereby initiating the expression of
a series of growth factors.
Hypoxia is the cellular stress which occurs when
oxygen demand exceeds supply. As a homeostatic response to this
challenge, several classes of genes are upregulated, which encode
proteins involved in angiogenesis, such as VEGF and FGF (9). In this study, we found that PR39
regulated the HIF-1α-induced expression of angiogenic growth
factors. The observed profile of gene expression is an accordance
with the observed pro-angiogenic effect, such as increased regional
blood flow. It has been reported that PR39 augments the expression
of HIF-1α-dependent genes, such as VEGF and its receptor, VEGF-R1,
as well as that of HIF-1α-independent genes, such as VEGF-R2,
FGFR-1 and syndecan-4 (8). These
results suggested that PR39 regulated the HIF-1α-induced expression
of angiogenic growth factors.
We further demonstrated that the apoptotic rate was
significantly lower in the AAV-PR39-treated group, suggesting that
PR39 inhibited apoptosis. A previous study reported that exogenous
PR39 inhibits apoptosis in endothelial cells subjected to hypoxic
injury, and that the anti-apoptotic effects of PR39 are mediated by
the increased inhibitor of apoptosis protein (IAP)-2 expression via
transcriptional and post-transcriptional regulation (12). The establishment of an effective
collateral circulation (through the induction of anti-apoptotic
effects) during the early stages of myocardial infarction is
conducive for the reduction of the infarct size and the protection
of heart functions.
Previous studies have demonstrated that both
apoptosis and necrosis exist in myocardial infarction. During the
early stages of myocardial infarction, apoptosis is the main type
of cell death observed (17,19). Thus, during the early stages of
acute myocardial infarction, the disruption of the apoptotic signal
transduction pathways appears to be an effective approach to
reducing cell death in the ischemic area and to protect heart
functions (13,19). Damage to the myocardial cells
surrounding the myocardial infarct area is reversible and part of
the myocardial tissues maintains the systolic and diastolic
function (20). At this point,
timely blood supply can recover the function of the myocardial
tissues. However, if the ischemic injury is continuously increased,
myocardial damage becomes irreversible (21). Therefore, prompt treatment can
limit the myocardial infarct size and reduce mortality in patients
with myocardial infarction. Reversing the damage to myocardial
cells is dependent on the supply of oxygen and the removal of
metabolites. Rapid establishment of collateral circulation in the
ischemic region can markedly improve the blood supply and reduce
the myocardial infarct size.
During the early stages of myocardial infarction,
PR39 can rescue the cells in the ischemic region through its
anti-hypoxic and anti-apoptotic functions (22). It also enhances the expression of
myocardial HIF-1α, which further promotes local angiogenesis and
accelerates the establishment of collateral circulation in the
ischemic areas. Therefore, PR39 has a wide range of clinical
applications in the early treatment of the ischemic myocardium and
can be used to prevent myocardial infarction. In this study, we
demonstrated that the left ventricular myocardial infarct area in
the pigs in the AAV-PR39-treated group was significantly reduced,
further demonstrating the safety and efficacy of PR39 in the
treatment of ischemic heart disease.
In conclusion, the expression of recombinant PR39
elevates the levels of HIF-1α under hypoxic conditions and
decreases the infarct size by upregulating the expression of HIF-1α
in the surrounding areas of the infarct. As a masterswitch of
angiogenesis, PR39 can effectively initiate angiogenic mechanisms,
rapidly establish collateral circulation, protect the viability of
myocardial cells and reduce the infarct size. The results obtained
in this study provide new insight into the genetic therapy of
coronary heart disease.
Acknowledgements
The authors thank Mr. Guangxiao Yang and Ms.
Quanying Wang (Xi’an Huaguang Bioengineering Corp., Xi’an, Shaanxi,
China) for assisting with the experiments.
References
|
1
|
Thygesen K, Alpert JS, Jaffe AS, Simoons
ML, Chaitman BR and White HD: Third universal definition of
myocardial infarction. J Am Coll Cardiol. 60:1581–1598. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kanashiro-Takeuchi RM, Schulman IH and
Hare JM: Pharmacologic and genetic strategies to enhance cell
therapy for cardiac regeneration. J Mol Cell Cardiol. 51:619–625.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Carmeliet P and Jain RK: Molecular
mechanisms and clinical applications of angiogenesis. Nature.
473:298–307. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Potente M, Gerhardt H and Carmeliet P:
Basic and therapeutic aspects of angiogenesis. Cell. 146:873–887.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lykouras D, Flordellis C and Dougenis D:
Gene therapy targets and the role of pharmacogenomics in heart
failure. Targets in Gene Therapy. You Y: InTech; View Article : Google Scholar : 2011, Available at:
http://www.intechopen.com/books/targets-in-gene-therapy/gene-therapy-targets-and-the-role-of-pharmacogenomics-in-heart-failure.
|
|
6
|
Lee RJ, Springer ML, Blanco-Bose WE, Shaw
R, Ursell PC and Blau HM: VEGF gene delivery to myocardium:
deleterious effects of unregulated expression. Circulation.
102:898–901. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Muinck E, Nagy N, Tirziu D, Murakami M,
Gurusamy N, Goswami SK, Ghatpande S, Engelman RM, Simons M and Das
DK: Protection against myocardial ischemia-reperfusion injury by
the angiogenic Masterswitch protein PR 39 gene therapy: the roles
of HIF1alpha stabilization and FGFR1 signaling. Antioxid Redox
Signal. 9:437–445. 2007. View Article : Google Scholar
|
|
8
|
Li J, Post M, Volk R, Gao Y, Li M, Metais
C, Sato K, Tsai J, Aird W and Rosenberg RD: PR39, a peptide
regulator of angiogenesis. Nat Med. 6:49–55. 2000. View Article : Google Scholar
|
|
9
|
Cavadas MA, Nguyen LK and Cheong A:
Hypoxia-inducible factor (HIF) network: insights from mathematical
models. Cell Commun Signal. 11:42–56. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee PH, Ohtake T, Zaiou M, Murakami M,
Rudisill JA, Lin KH and Gallo RL: Expression of an additional
cathelicidin antimicrobial peptide protects against bacterial skin
infection. Proc Natl Acad Sci USA. 102:3750–3755. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shen F, Fan Y, Su H, Zhu Y, Chen Y, Liu W,
Young WL and Yang GY: Adeno-associated viral vector-mediated
hypoxia-regulated VEGF gene transfer promotes angiogenesis
following focal cerebral ischemia in mice. Gene Ther. 15:30–39.
2007. View Article : Google Scholar
|
|
12
|
Bao J, Sato K, Li M, Gao Y, Abid R, Aird
W, Simons M and Post MJ: PR-39 and PR-11 peptides inhibit
ischemia-reperfusion injury by blocking proteasome-mediated IκBα
degradation. Am J Physiol Heart Circ Physiol. 281:H2612–H2618.
2001.PubMed/NCBI
|
|
13
|
Korthuis RJ, Gute DC, Blecha F and Ross
CR: PR-39, a proline/arginine-rich antimicrobial peptide, prevents
postischemic microvascular dysfunction. Am J Physiol.
277:H1007–H1013. 1999.PubMed/NCBI
|
|
14
|
Li Y, Qiu S, Song L, Yan Q and Yang G:
Secretory expression of p53 (N15)-Ant following lentivirus-mediated
gene transfer induces cell death in human cancer cells. Cancer
Invest. 26:28–34. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Garrett DJ, Cohen JC and Larson JE: Long
term physiologic modification using rAAV in utero gene-therapy.
Genet Vaccines Ther. 2:42004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zaiss AK, Liu Q, Bowen GP, Wong NC,
Bartlett JS and Muruve DA: Differential activation of innate immune
responses by adenovirus and adeno-associated virus vectors. J
Virol. 76:4580–4590. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Carmeliet P, Dor Y, Herbert JM, Fukumura
D, Brusselmans K, Dewerchin M, Neeman M, Bono F, Abramovitch R,
Maxwell P, Koch CJ, Ratcliffe P, Moons L, Jain RK, Collen D and
Keshert E: Role of HIF-1alpha in hypoxia-mediated apoptosis, cell
proliferation and tumour angiogenesis. Nature. 394:485–490. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gerber HP, Condorelli F, Park J and
Ferrara N: Differential transcriptional regulation of the two
vascular endothelial growth factor receptor genes Flt-1, but not
Flk-1/KDR, is up-regulated by hypoxia. J Biol Chem.
272:23659–23667. 1997. View Article : Google Scholar
|
|
19
|
Wu J, Parungo C, Wu G, Kang PM, Laham RJ,
Sellke FW, Simons M and Li J: PR39 inhibits apoptosis in hypoxic
endothelial cells. Circulation. 109:1660–1667. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brevoord D, Kranke P, Kuijpers M, Weber N,
Hollmann M and Preckel B: Remote ischemic conditioning to protect
against ischemia-reperfusion injury: a systematic review and
meta-analysis. PLoS One. 7:e421792012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Masci PG, Francone M, Desmet W, Ganame J,
Todiere G, Donato R, Siciliano V, Carbone I, Mangia M and Strata E:
Right ventricular ischemic injury in patients with acute ST-segment
elevation myocardial infarction clinical perspective
characterization with cardiovascular magnetic resonance.
Circulation. 122:1405–1412. 2010. View Article : Google Scholar
|
|
22
|
Post MJ, Sato K, Murakami M, Bao J, Tirziu
D, Pearlman JD and Simons M: Adenoviral PR39 improves blood flow
and myocardial function in a pig model of chronic myocardial
ischemia by enhancing collateral formation. Am J Physiol Regul
Integr Comp Physiol. 290:R494–R500. 2006. View Article : Google Scholar : PubMed/NCBI
|