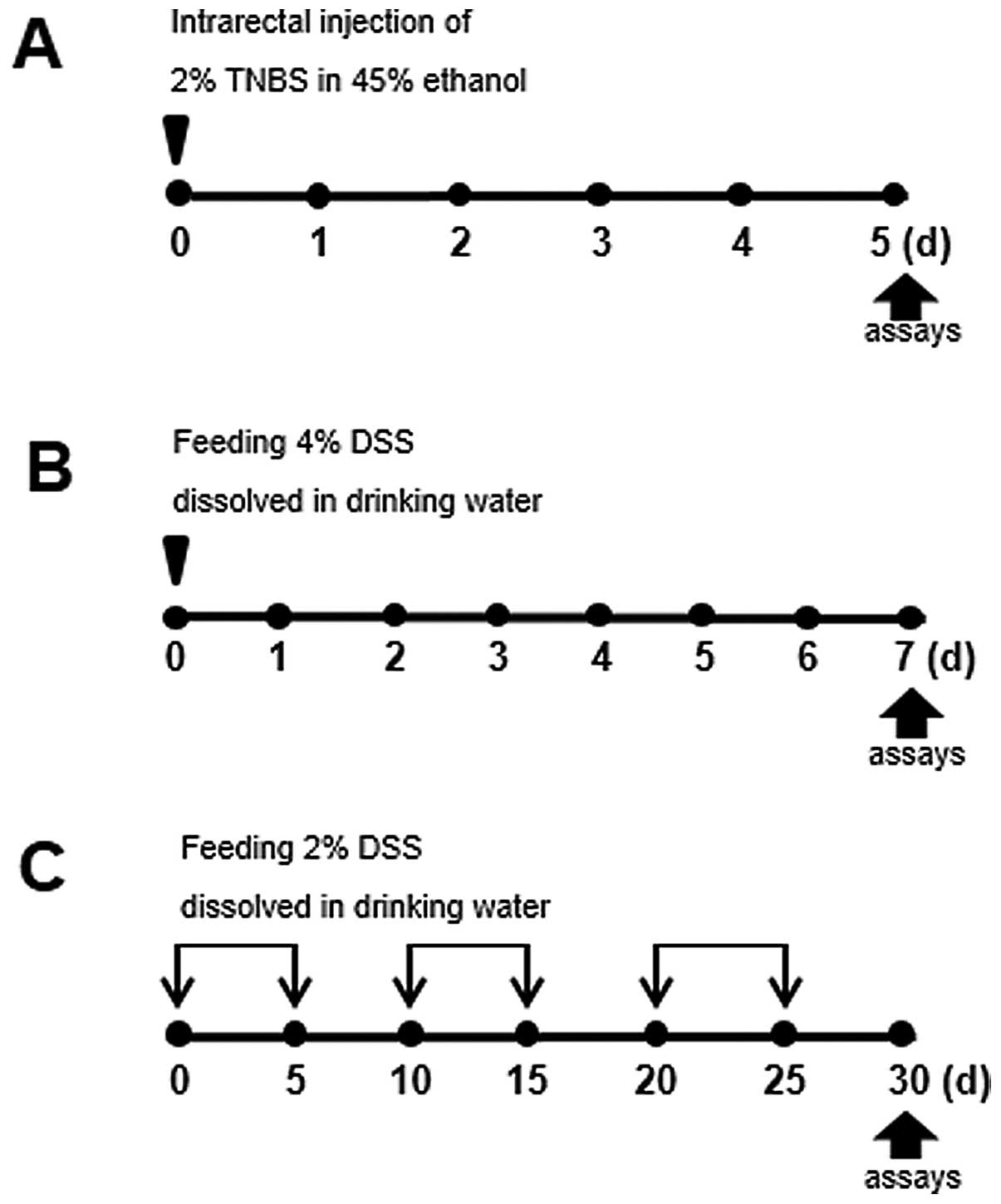
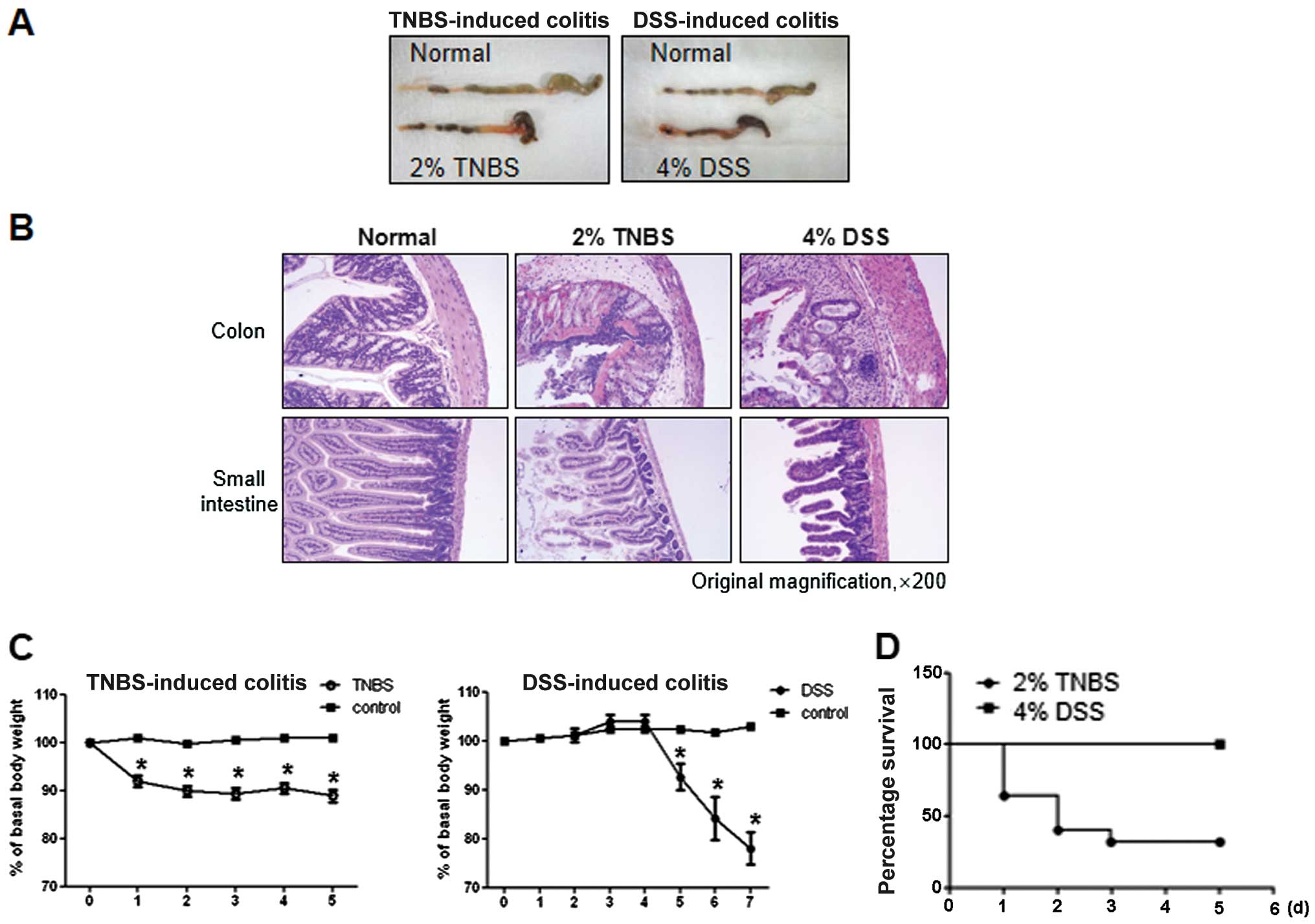
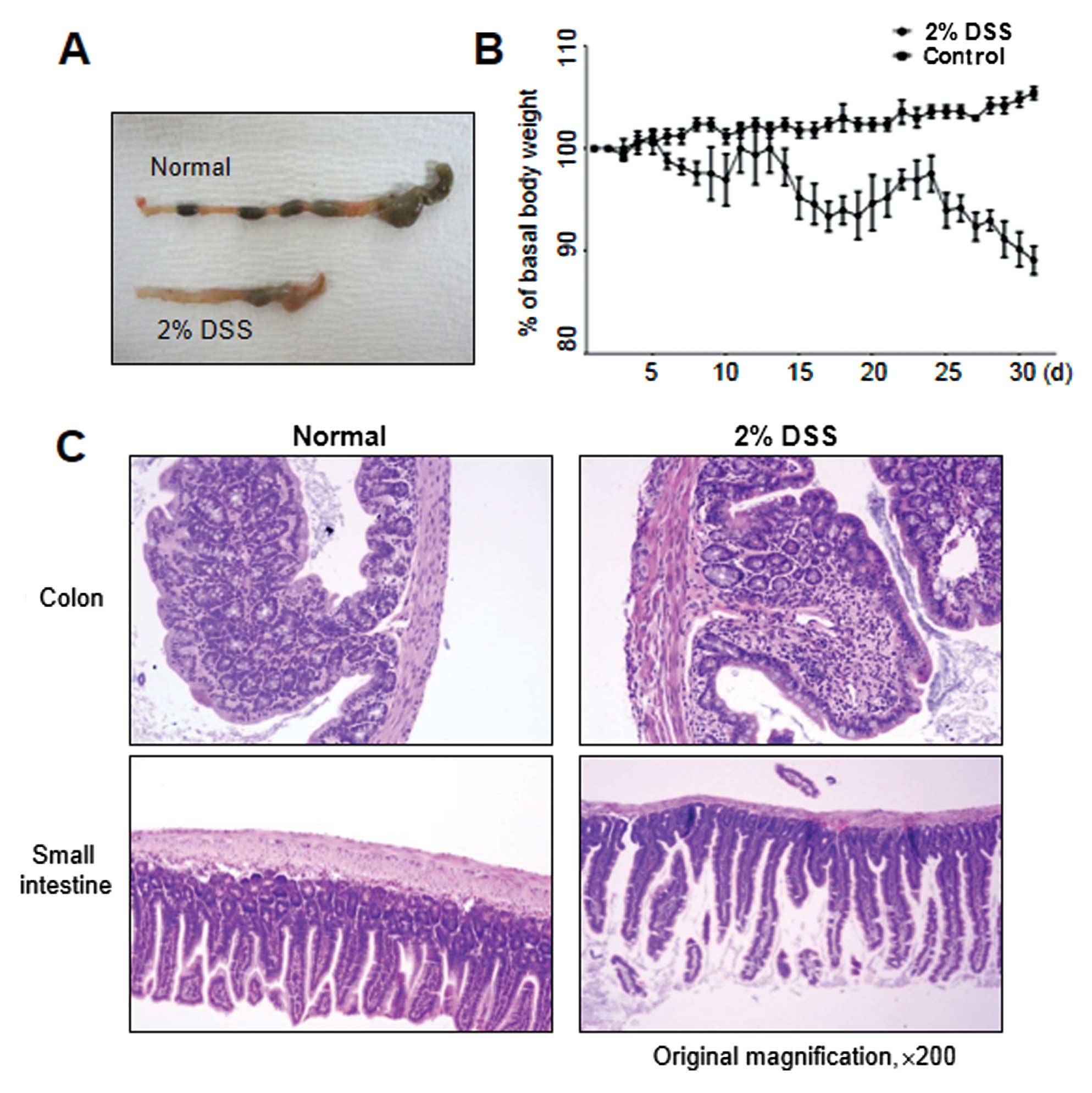
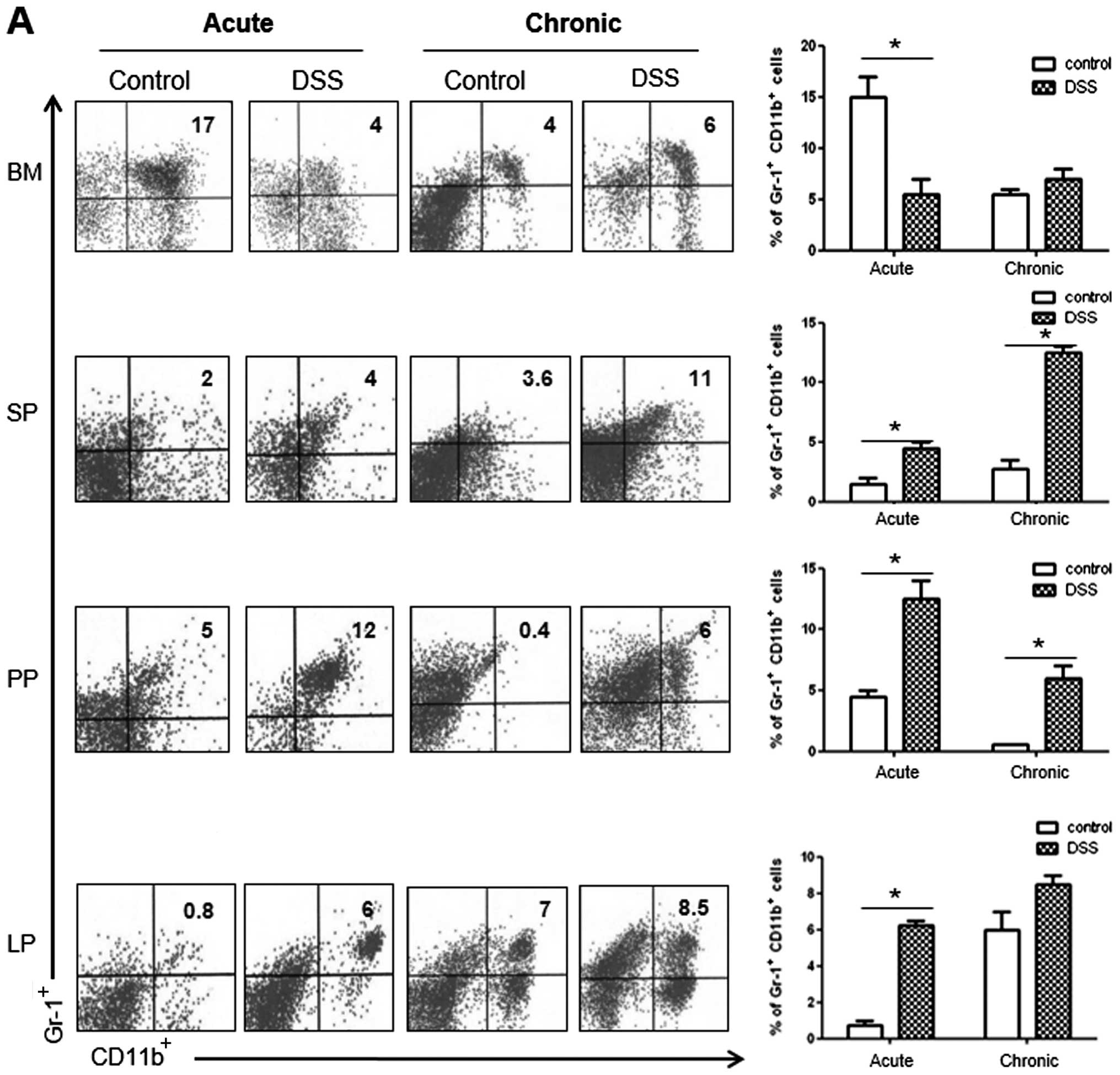
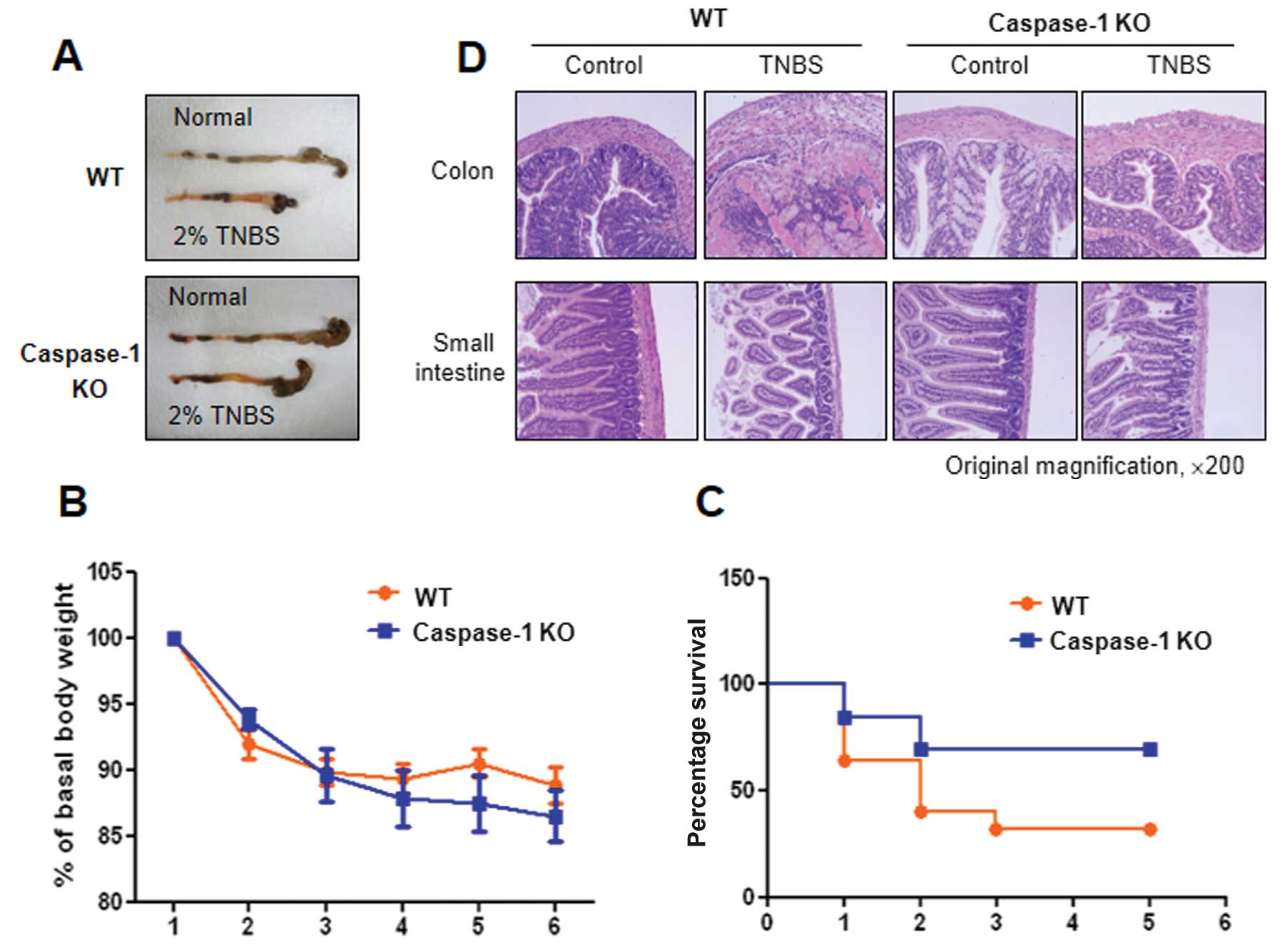
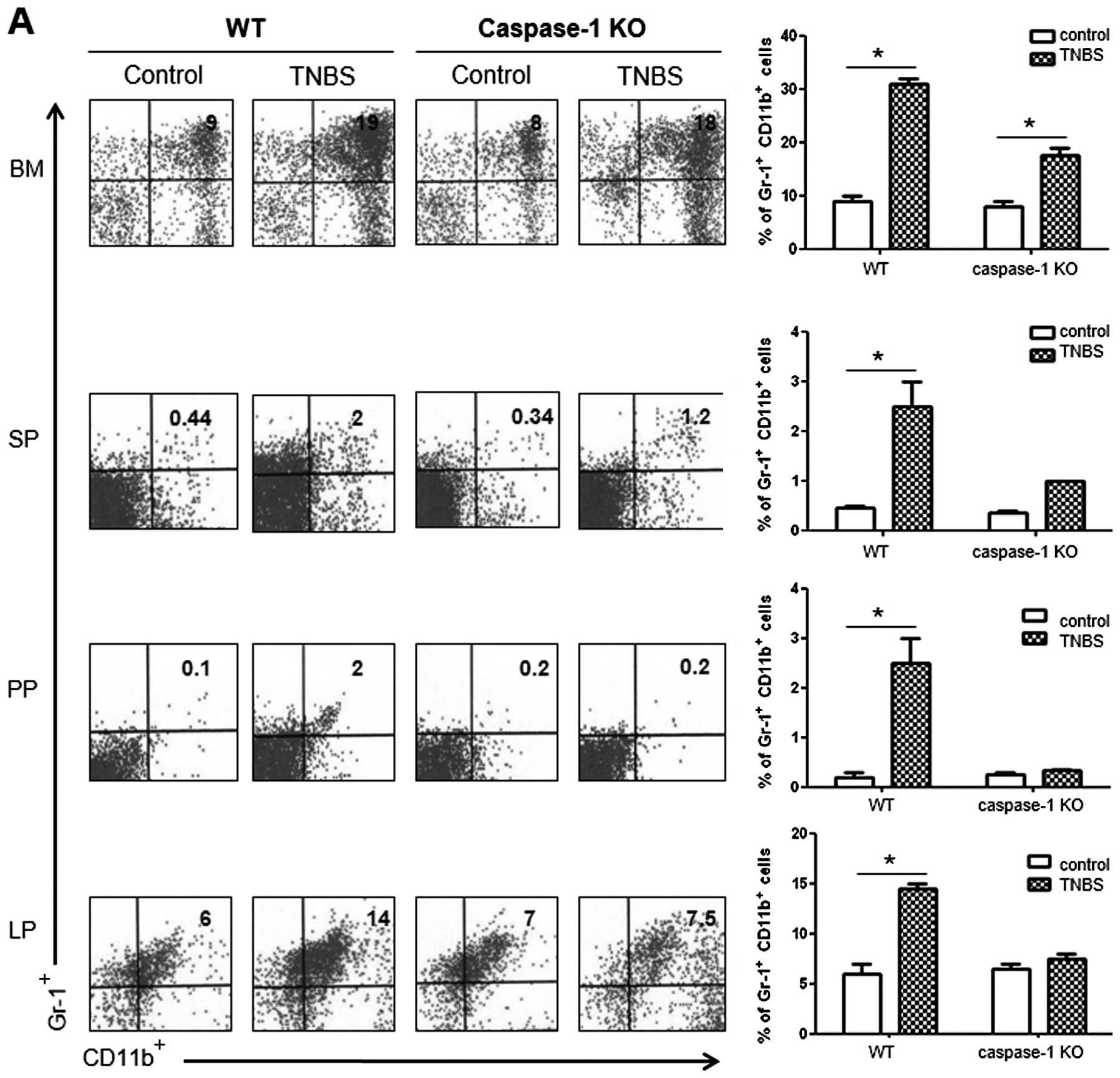
|
1
|
Morris GP, Beck PL, Herridge MS, Depew WT,
Szewczuk MR and Wallace JL: Hapten-induced model of chronic
inflammation and ulceration in the rat colon. Gastroenterology.
96:795–803. 1989.PubMed/NCBI
|
|
2
|
Wirtz S, Neufert C, Weigmann B and Neurath
MF: Chemically induced mouse models of intestinal inflammation. Nat
Protoc. 2:541–546. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Neurath MF, Fuss I, Kelsall BL, Stuber E
and Strober W: Antibodies to interleukin 12 abrogate established
experimental colitis in mice. J Exp Med. 182:1281–1290. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Elson CO, Beagley KW, Sharmanov AT, et al:
Hapten-induced model of murine inflammatory bowel disease: mucosa
immune responses and protection by tolerance. J Immunol.
157:2174–2185. 1996.PubMed/NCBI
|
|
5
|
Okayasu I, Hatakeyama S, Yamada M, Ohkusa
T, Inagaki Y and Nakaya R: A novel method in the induction of
reliable experimental acute and chronic ulcerative colitis in mice.
Gastroenterology. 98:694–702. 1990.PubMed/NCBI
|
|
6
|
Dieleman LA, Ridwan BU, Tennyson GS,
Beagley KW, Bucy RP and Elson CO: Dextran sulfate sodium-induced
colitis occurs in severe combined immunodeficient mice.
Gastroenterology. 107:1643–1652. 1994.PubMed/NCBI
|
|
7
|
Yamada M, Ohkusa T and Okayasu I:
Occurrence of dysplasia and adenocarcinoma after experimental
chronic ulcerative colitis in hamsters induced by dextran sulphate
sodium. Gut. 33:1521–1527. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ishiguro Y: Mucosal proinflammatory
cytokine production correlates with endoscopic activity of
ulcerative colitis. J Gastroenterol. 34:66–74. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Monteleone G, Trapasso F, Parrello T, et
al: Bioactive IL-18 expression is up-regulated in Crohn’s disease.
J Immunol. 163:143–147. 1999.PubMed/NCBI
|
|
10
|
Kwon KH, Murakami A, Hayashi R and
Ohigashi H: Interleukin-1beta targets interleukin-6 in progressing
dextran sulfate sodium-induced experimental colitis. Biochem
Biophys Res Commun. 337:647–654. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bauer C, Duewell P, Mayer C, et al:
Colitis induced in mice with dextran sulfate sodium (DSS) is
mediated by the NLRP3 inflammasome. Gut. 59:1192–1199. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Elinav E, Strowig T, Kau AL, et al: NLRP6
inflammasome regulates colonic microbial ecology and risk for
colitis. Cell. 145:745–757. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nam JH: Regulation of obesity and
non-alcoholic fatty liver disease by modulation of the gut
micobiota through inflammasome; its mechanism and potential for
clinical use. J Bacteriol Virol. 42:359–362. 2012. View Article : Google Scholar
|
|
14
|
Siegmund B, Lehr HA, Fantuzzi G and
Dinarello CA: IL-1beta-converting enzyme (caspase-1) in intestinal
inflammation. Proc Natl Acad Sci USA. 98:13249–13254. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gabrilovich DI and Nagaraj S:
Myeloid-derived suppressor cells as regulators of the immune
system. Nat Rev Immunol. 9:162–174. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang R, Ito S, Nishio N, Cheng Z, Suzuki
H and Isobe KI: Dextran sulphate sodium increases splenic
Gr1(+)CD11b(+) cells which accelerate recovery from colitis
following intravenous transplantation. Clin Exp Immunol.
164:417–427. 2011.PubMed/NCBI
|
|
17
|
Haile LA, von Wasielewski R,
Gamrekelashvili J, et al: Myeloid-derived suppressor cells in
inflammatory bowel disease: a new immunoregulatory pathway.
Gastroenterology. 135:871–881. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rivollier A, He J, Kole A, Valatas V and
Kelsall BL: Inflammation switches the differentiation program of
Ly6Chi monocytes from antiinflammatory macrophages to inflammatory
dendritic cells in the colon. J Exp Med. 209:139–155. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ostanin DV, Kurmaeva E, Furr K, et al:
Acquisition of antigen-presenting functions by neutrophils isolated
from mice with chronic colitis. J Immunol. 188:1491–1502. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tu S, Bhagat G, Cui G, et al:
Overexpression of interleukin-1beta induces gastric inflammation
and cancer and mobilizes myeloid-derived suppressor cells in mice.
Cancer Cell. 14:408–419. 2008. View Article : Google Scholar : PubMed/NCBI
|