Introduction
Pulmonary arterial hypertension (PAH) is a complex
pathological process, involving pulmonary vascular remodeling and
constriction, as well as lung inflammation. It is characterized by
a sustained and progressive elevation in pulmonary vascular
pressure and the remodeling of the pulmonary vasculature,
eventually leading to right ventricular failure and death (1). Vascular active substances, such as
serotonin [5-hydroxytryptamine (5-HT)], platelet-derived growth
factor (PDGF), interleukin (IL) and endothelin-1 (ET-1),
participate in the pathological processs of PAH (2).
The main causes of pulmonary vascular remodeling are
hypertrophy and hyperplasia of pulmonary arterial smooth muscle
cells (PASMCs), muscularization of normal non-muscular peripheral
arteries and deposition of the extracellular matrix (ECM) (3). The regulation of the ECM is involved
in cell growth, proliferation, differentiation and death (4). Matrix metalloproteinases (MMPs), a
family of zinc-dependent endopeptidases, play a substaintial role
in the degradation of the ECM. In previous studies, we demonstrated
that the levels of MMP-2 and MMP-9 in pulmonary arteries are
increased in monocrotaline (MCT)-induced PAH models (3,5).
MMP proteolytic activity is controlled by specific endogenous
inhibitors, which are the tissue inhibitors of metalloproteinases
(TIMPs) (6). The increased
deposition of matrix proteins in the pulmonary vessels has been
attributed to the change in the balance of MMP and TIMP activity
(7). Thus, the balance between
MMPs and TIMPs seems to be important for ECM regulation and
homeostasis (6).
Inflammatory mechanisms appear to play a significant
role in pulmonary hypertension (PH), including MCT-induced PH in
rats and PAH of various origins in humans (8). Perivascular inflammatory infiltrates
with macrophages and lymphocytes in the areas of occlusive lesions
can be observed in PAH (8,9).
The expression levels of cytokines, such as IL-1β, intercellular
adhesion molecule-1 (ICAM-1) and tumor necrosis factor-α (TNF-α),
have been shown to be increased in hypoxia- or MCT-induced PAH
(3,10–12).
The 5-HT transporter (5-HTT) and serotonin
transporter (SERT) play a prominent role in the progression of PAH
(13–16). It is known that SERT is involved
in pulmonary vascular remodeling and lung inflammation in PH
(17). The mitogenic and
comitogenic effects of 5-HT on PASMCs are associated with the
cellular internalization of serotonin, which is mediated by SERT
(5,17,18). The critical step in 5-HT
biosynthesis is catalyzed by the rate-limiting enzyme, tryptophan
hydroxylase (Tph) (19). Tph
activity, therefore, serves as a marker for 5-HT synthesis
(19). There are two isoforms of
the Tph enzyme regulating 5-HT synthesis. Tph-1 is mainly present
in enterochromaffin cells and spleen, whereas Tph-2 is
predominantly present in the brain stem and mesenteric plexus
neurons (20). Thus, 5-HT
synthesis is thought to be controlled mainly by Tph-2 in the
central nervous system and by Tph-1 in the peripheral organs
(19). The synthesis of 5-HT and
the expression of Tph-1 are increased in the pulmonary vascular
endothelium of patients with idiopathic PAH (IPAH) (16).
The inhibition of serotonin by
4-chloro-DL-phenylalanine (PCPA) has been shown to reduce
MCT-induced right ventricular hypertrophy in rats (21). However, it is not known whether
this Tph inhibitor (TphI) can protect against MCT-induced pulmonary
vascular remodeling and lung inflammation. Therefore, in the
present study, we aimed to investigate the effects of the TphI,
PCPA, on pulmonary vascular remodeling and lung inflammation in
rats with MCT-induced PAH.
Materials and methods
Monocrotaline-induced PAH in rats
Sixty-eight male Sprague-Dawley (SD) rats (weighing
180±10 g) were obtained from the Animal Resource Centre, China
Medical University (certificate no. Liaoning 034). The animal care
and experimental protocols were in accordance with the
Institutional Animal Care and Use Committee of China Medical
University. The rats were randomly divided into 4 groups: (i) the
control (untreated) group, (ii) the MCT-treated group, (iii) the
group treated with MCT + 50 mg/kg PCPA (MCT + P1), and (iv) the
group treated with MCT + 100 mg/kg PCPA (MCT + P2). Rats in the MCT
and 2 PCPA-treated groups (MCT + P1 and MCT + P2) were administered
intraperitoneal injections of MCT (60 mg/kg; Sigma-Aldrich, St.
Louis, MO, USA). PCPA (Sigma-Aldrich) was dissolved in
physiological (0.9%) saline. Rats in the PCPA-treated groups were
administered intraperitoneal injections of PCPA (50 or 100 mg/kg)
once a day for 21 days. Rats in the control and MCT groups (MCT
alone) received an equal volume of the vehicle (0.9% physiological
saline) by intraperitoneal injection over the same period of time.
All the rats were provided abundant food and water ad
libitum. They were kept in a 12-h light/dark cycle at 18–22°C
and 50–70% humidity. The parameters which we measured in the whole
experiment were performed in a blinded manner.
Assessment of PAH
On day 22, the rats in all the groups were
anaesthetized with 3% pentobarbital sodium (40 mg/kg). Systemic
arterial pressure (SAP) and pulmonary arterial pressure (PAP) were
recorded under the same conditions as previously described
(22–24). The right carotid artery was
isolated and cannulated with a polyethylene catheter to a
fluid-filled force transducer. A pressure transducer via the
carrier amplifier and multi-channel polygraph was connected. On
behalf of the recorded pressure system circulating blood, the SAP
was measured. Another polyvinyl PV-1 catheter was introduced into
the right jugular vein and pushed through the right ventricle into
the pulmonary artery for the measurement of PAP. When the pressure
increased and pulmonary arterial waveform baseline appeared, we
immediately fixed the PV-1 catheter and began measuring PAP. Prior
to catheterization, the catheters were filled with saline
containing 1% heparin. Hemodynamic variables were measured with a
pressure transducer and recorded on a polygraph system (RM6000;
Nihon Kohden Corp., Tokyo, Japan).
After the measurements were completed, the rats were
sacrificed with an overdose of pentobarbital sodium. The heart
tissue was separated into the right ventricle (RV) and left
ventricle plus septum (LV + S). The right ventricular index (RVI)
was calculated using the following formula: RVI = RV/(LV + S).
Histological examination
The animals were exsanguinated with sterile saline
and 4% paraform. Tissues from the right lower lobe of the lungs
were then cut and fixed with 4% paraform. Subsequently, the
paraffin-embedded lung tissues were subjected to sectioning to
yield 5-μm-thick sections and were then stained with haematoxylin
and eosin (H&E). A total of 12 pulmonary arteries were
investigated per rat using the MetaMorph (Universal Imaging Corp.,
West Chester, PA, USA)/DP10/BX51 (Olympus, Tokyo, Japan) imaging
system in 3 rats of each group. The pulmonary arteries (external
diameter, 60–80 μm) were randomly selected for high-power (x400)
evaluation. Pulmonary vascular remodeling was measured as the
percentage of medial thickness, which was calculated according to
previously described methods as follows: medial wall thickness (%)
= (external diameter - internal diameter)/external diameter) ×100%
(22); wall area (%) = (total
vessel area - lumen area)/total vessel area ×100% (25).
Immunohistochemisty
Paraffin-embedded lung tissue sections were stained
using ultrasensitive SP and diaminobenzidine (DAB) staining kits
(Maxin-Bio, Fuzhou, China). The primary rabbit polyclonal antibody
against Tph-1 (BS3727, Bioworld Technology, St. Louis Park, MN,
USA) was diluted 1:200, whereas the primary rabbit polyclonal
antibody against SERT protein (bs-1893R, Biosynthesis
Biotechnology, Beijing, China) was diluted 1:300. As a negative
control, samples were incubated with 0.01 M phosphate-buffered
saline (PBS) instead of the primary antibody. Digital images were
analyzed under a BX51 microscope (Olympus). At least 12 pulmonary
arteries (external diameter, 60–80 μm) per rat were examined in 3
rats from each group. The levels of Tph-1 and SERT protein were
calculated as the average optical density. Immunohistochemical
staining followed a basic indirect protocol using a citrate
antigen-retrieval method (5,22).
Determination of MMP-2/−9 activity by
gelatin zymography
The gelatinolytic activity of both the latent and
mature forms of MMP-2 and MMP-9 was evaluated by gelatin
zymography. Soluble lung proteins (40 μg) were separated on 10%
sodium dodecyl sulphate polyacrylamide gel electrophoresis
(SDS-PAGE) containing 1.0 mg/ml gelatin under non-reducing
conditions. Following incubation for 1 h in washing buffer [50 mM
Tris-HCl (pH, 7.5), 2.5% Triton X-100, 5 mM CaCl2, 1 μM
ZnCl2] at room temperature, the gels were incubated for
40 h in incubation buffer [50 mM Tris-HCl (PH, 7.5), 200 mM NaCl, 5
mM CaCl2] at 37°C. The gels were then stained with 0.05%
Coomassie Blue R-250 for 3 h and destained with destaining solution
[methanol:acetic acid:ion-exchanged water (50:10:40)] until bands
were visible. Areas of MMP-2/−9 activity appear as clear bands
against a dark blue background where the protease has digested the
substrate. The strip was subjected to image analysis to determine
its gray value, representative of MMP-2/−9 activity. Each
experiment was repeated to detect >3 batches of different
samples.
Western blot analysis
The samples of the left lower lobe of the lungs from
the rats in each group were homogenized to extract the protein
using a polytron homogenizer (Kinematica, Lucerne, Switzerland).
The homogenate was centrifuged at 15,000 × g for 20 min at 4°C and
the supernatant was collected and stored at −70°C until analysis.
Equal amounts of protein were separated through a reducing SDS-PAGE
and electrotransferred onto a polyvinylidene difluoride (PVDF)
membrane. Following incubation in blocking buffer (5% non-fat dry
milk, TBS and 0.05% Tween-20) at room temperature for 2 h, the
membranes were incubated with primary antibodies overnight at 4°C.
Rabbit polyclonal anti-SERT antibody (bs-1893R, 1:300) was
purchased from Biosynthesis Biotechnology. Rabbit polyclonal
anti-Tph-1 antibody (BS3727, 1:800) and rabbit polyclonal
anti-IL-1β antibody (BS3506, 1:700) were purchased from Bioworld
Technology. Mouse monoclonal anti-MMP-2 antibody (zs-13595, 1:200),
goat polyclonal anti-MMP-9 antibody (zs-6840, 1:100), rabbit
polyclonal anti-TIMP-1 antibody (sc-5538, 1:300), rabbit polyclonal
anti-TIMP-2 antibody (zs-5539, 1:300), goat polyclonal anti-ICAM-1
antibody (zs-1511, 1:200), goat polyclonal anti-TNF-α antibody
(sc-1350, 1:200) and mouse polyclonal anti-β-actin antibody
(sc-47778, 1:2000) were purchased from Santa Cruz Biotechnology
(Santa Cruz, CA, USA). The immunoreactive bands were visualized
with the corresponding horseradish peroxidase-conjugated secondary
antibodies and Super ECL Plus (Applygen Technologies, Beijing,
China). Relative protein expression was quantified by densitometry
using Quantity One software (Bio-Rad Laboratories, Hercules, CA,
USA).
Statistical analysis
All statistical analyses was performed using SPSS
software (version 16.0; SPSS Inc., Chicago, IL, USA) and data are
expressed as the means ± SD. Statistical analyses were performed
using one-way ANOVA with Fisher’s least significant difference
(LSD) or Dunnett’s T3 test. A value of P<0.05 was considered to
indicate a statistically significant difference.
Results
Effects of PCPA on MCT-induced changes in
hemodynamics and RVI
The rats were fed for 3 weeks; during this period, 8
out of 17 rats died in the MCT group (mortality rate, 47.1%;
P<0.01 vs. control). One out of 17 rats died in the MCT + P1
group (mortality rate, 5.9%; P<0.01 vs. MCT).
Compared with the control group, PAP and RV/LV + S
in the MCT group were markedly elevated, and body weight was
decreased. Compared with the MCT group, PCPA significantly
decreased PAP and RV/LV + S. The heart rate and SAP showed no
significant differences among the 4 groups (Table I).
 | Table IComparison of haemodynamic
measurements and right ventricular index between the different
groups. |
Table I
Comparison of haemodynamic
measurements and right ventricular index between the different
groups.
| Parameter | Control (n=17) | MCT (n=9) | MCT + P1
(n=16) | MCT + P2
(n=17) |
|---|
| Body weight
(g) | 292±23 | 205±46b | 201±23b | 184±26b |
| SAP (mmHg) | 130±22 | 120±24 | 123±18 | 119±18 |
| PAP (mmHg) | 14.7±1.9 | 25.6±7.4b | 18.4±3.5a,c | 16.9±4.1c |
| Heart rate
(bpm) | 374±30 | 393±19 | 372±40 | 355±42 |
| RV/LV + S (%) | 30.7±4.2 | 44.0±11.3b |
34.3±3.7d | 32.1±3.6c |
Effects of PCPA on pulmonary vascular
histology
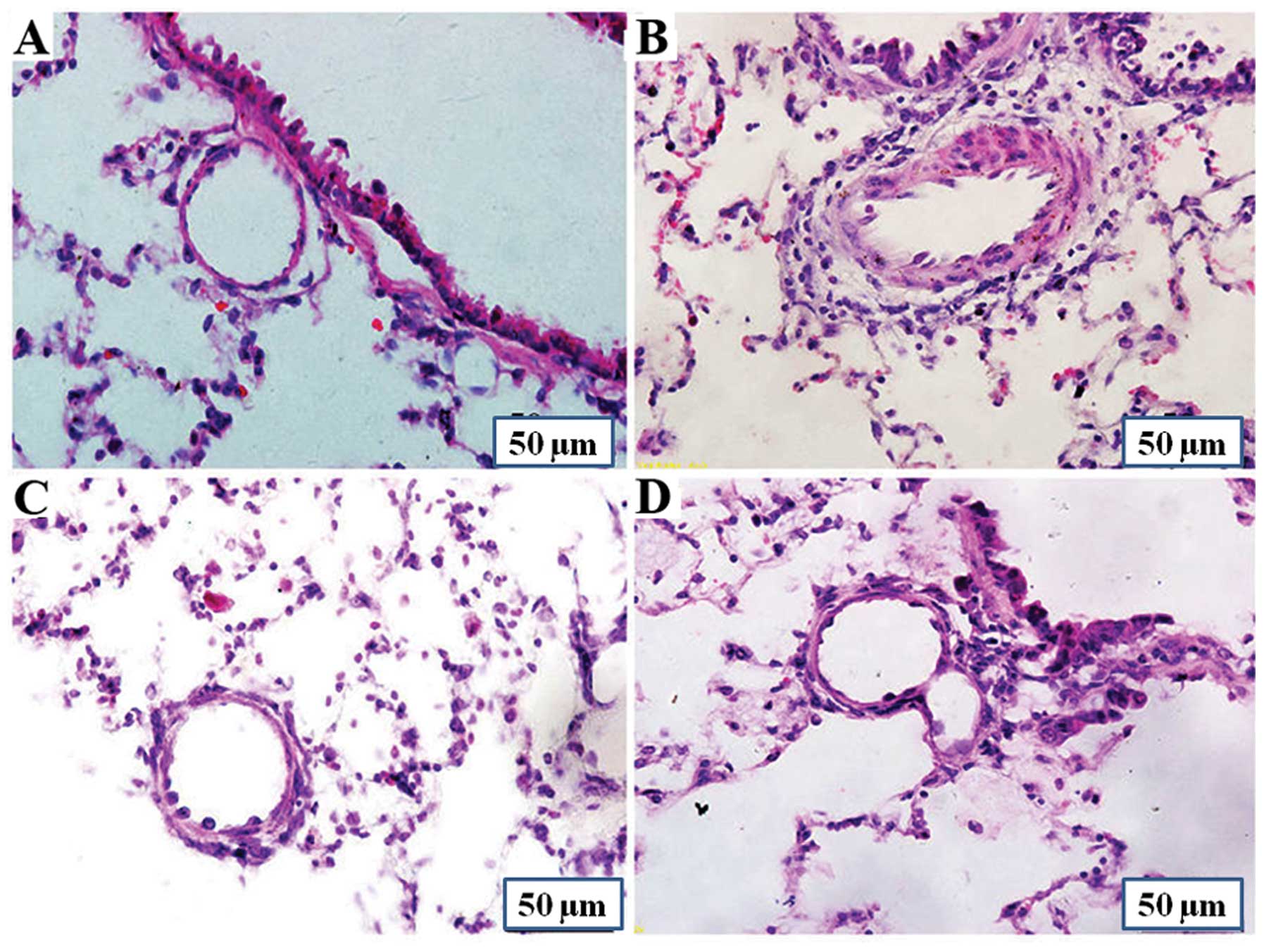
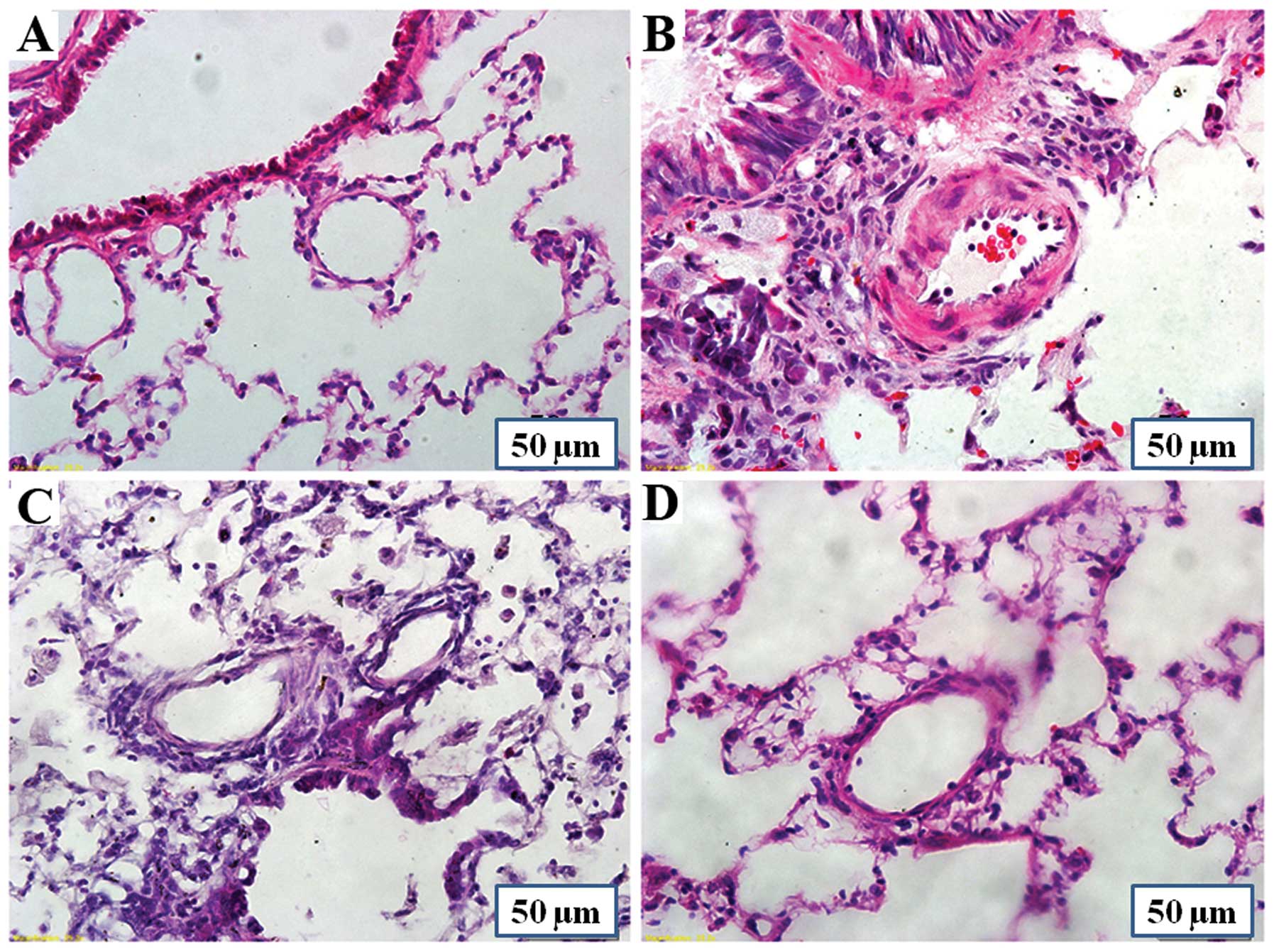
Rats in the MCT group showed a marked increase in
the average medial wall thickness of the pulmonary arteries. The
percentage of medial wall thickness increased from 19.1±7.7%
(control group) to 49.7±9.2% (MCT group) (P<0.01), and the
percentage in the MCT + P1 and MCT + P2 groups decreased to
34.3±8.2% (P<0.01 vs. MCT) and 28.1±10.7% (P<0.01 vs. MCT),
respectively (Fig. 1). The
percentage of the wall area of pulmonary arteries in the MCT group
markedly increased from 34.4±9.2% to 60.5±8.0% (P<0.01 vs.
control). Compared with the MCT group, the percentage of medial
wall thickness of pulmonary arteries in the MCT + P1 and MCT + P2
groups decreased to 51.4±4.5% (P<0.05) and 43.8±5.8%
(P<0.01), respectively. These results demonstrated that MCT
induced pulmonary vascular remodeling, whereas PCPA attenuated the
effects of MCT (Fig. 1).
Effect of PCPA on Tph-1 expression in rat
lungs
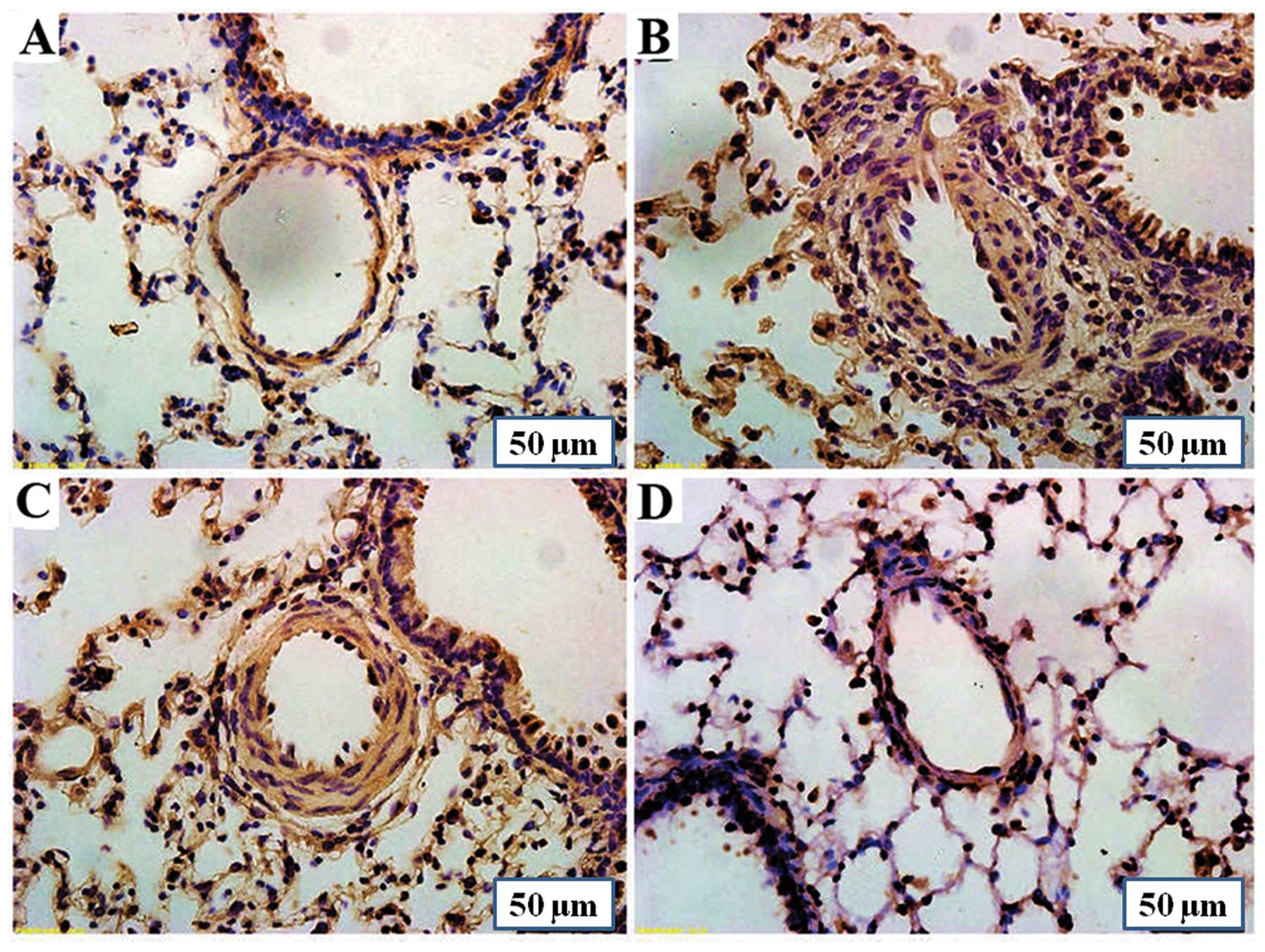
Immunohistochemistry using anti-Tph-1 antibodies
revealed a strong Tph-1 expression in the lungs of rats with
MCT-induced PAH compared with the control group. Treatment with
PCPA (50 mg/kg/day) slightly reversed the MCT-induced increase in
Tph-1 protein expression in the lungs. However, treatment with PCPA
(100 mg/kg/day) reduced the MCT-induced increase in Tph-1
expression significantly (Fig.
2).
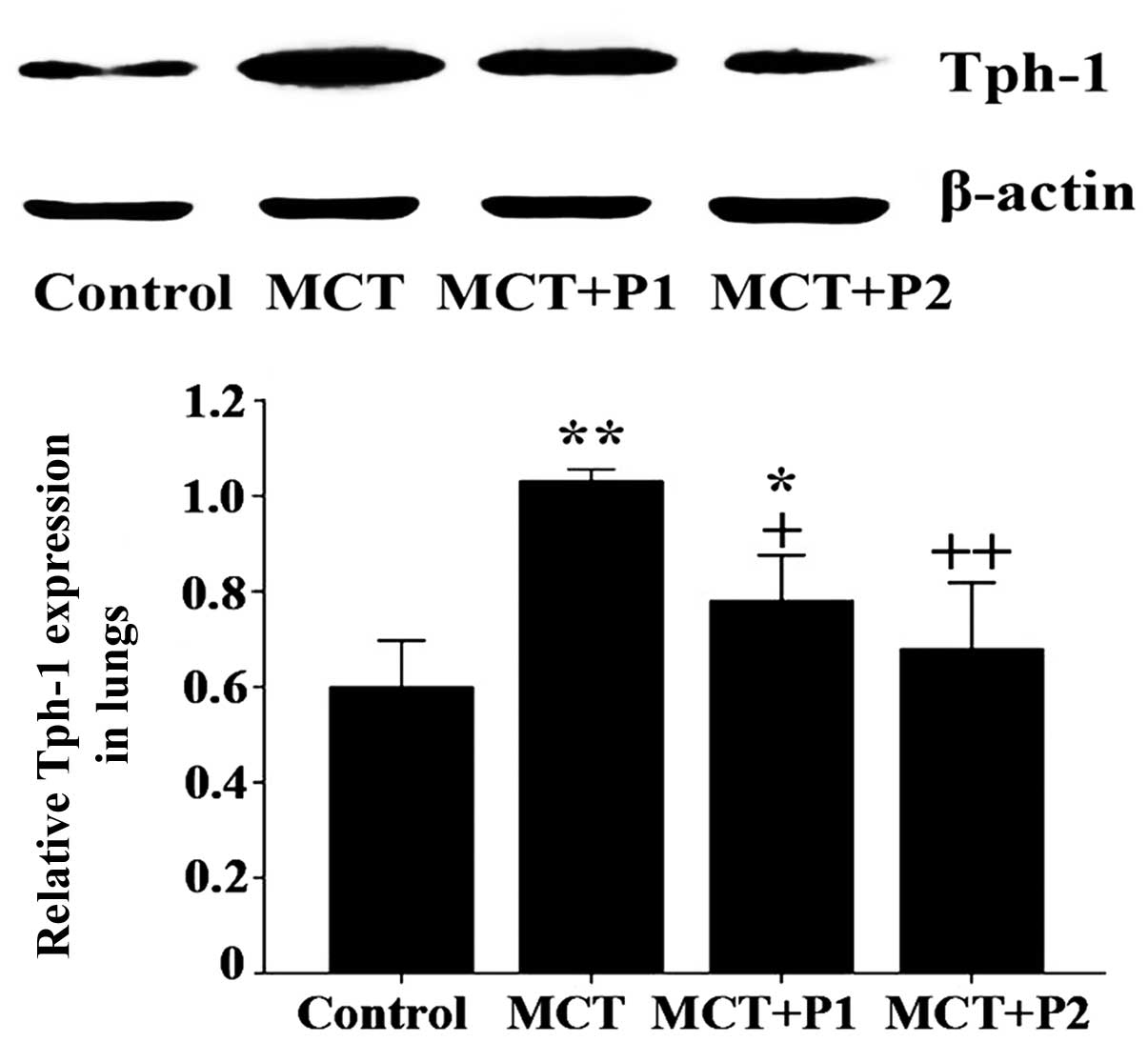
Western blot analysis revealed that the level of
Tph-1 protein expression significantly increased in the MCT group
compared with the control group (1.03±0.01 vs. 0.59±0.04,
respectively, P<0.01). At 50 mg/kg, PCPA attenuated the
MCT-induced increase in the expression of Tph-1 protein in the rat
lungs (0.78±0.04, P<0.05 vs. MCT). At 100 mg/kg, PCPA
significantly suppressed the MCT-induced expression of Tph-1
protein (0.67±0.05, P<0.01 vs. MCT, Fig. 3).
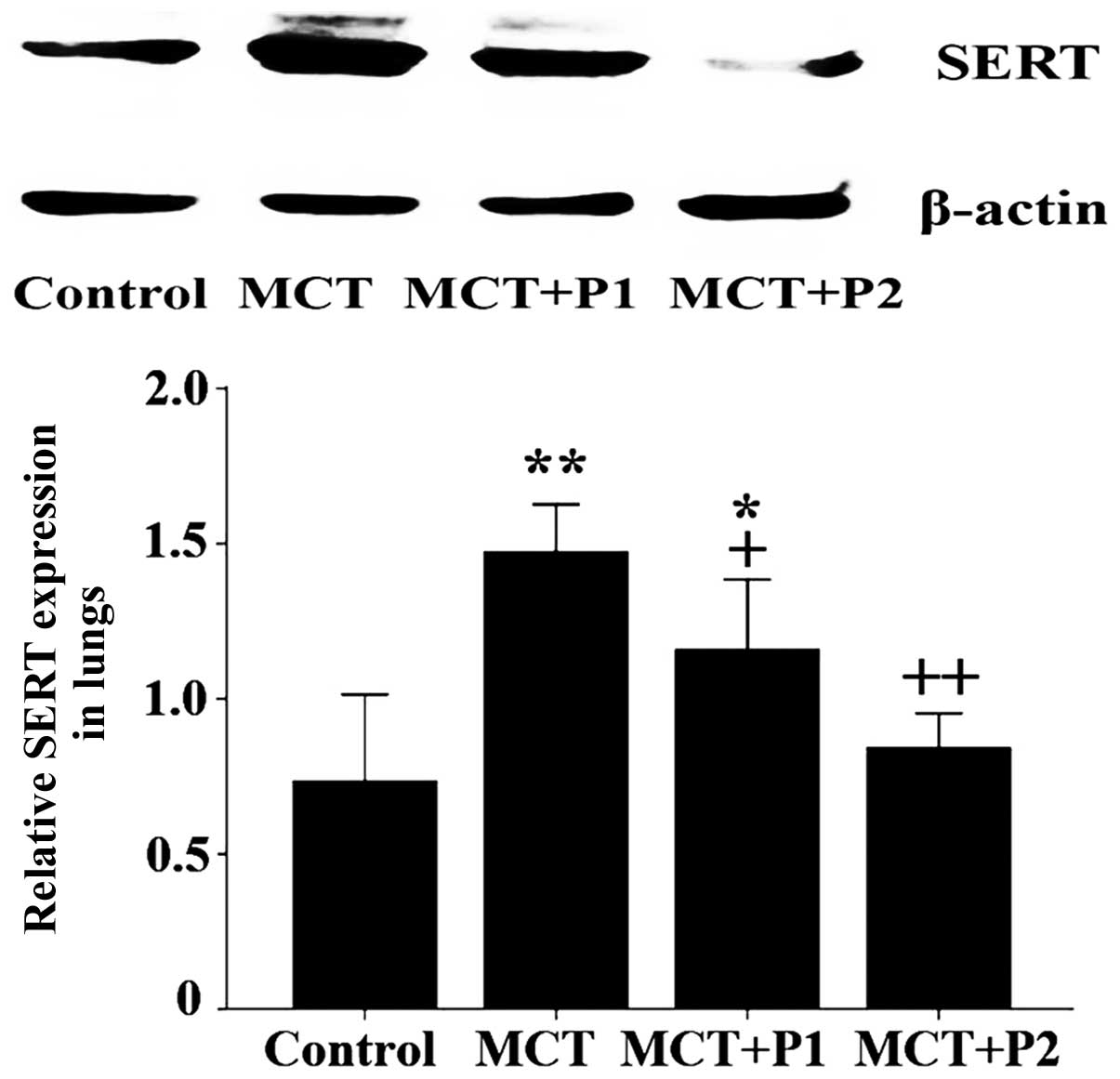
Effect of PCPA on SERT expression in rat
lungs
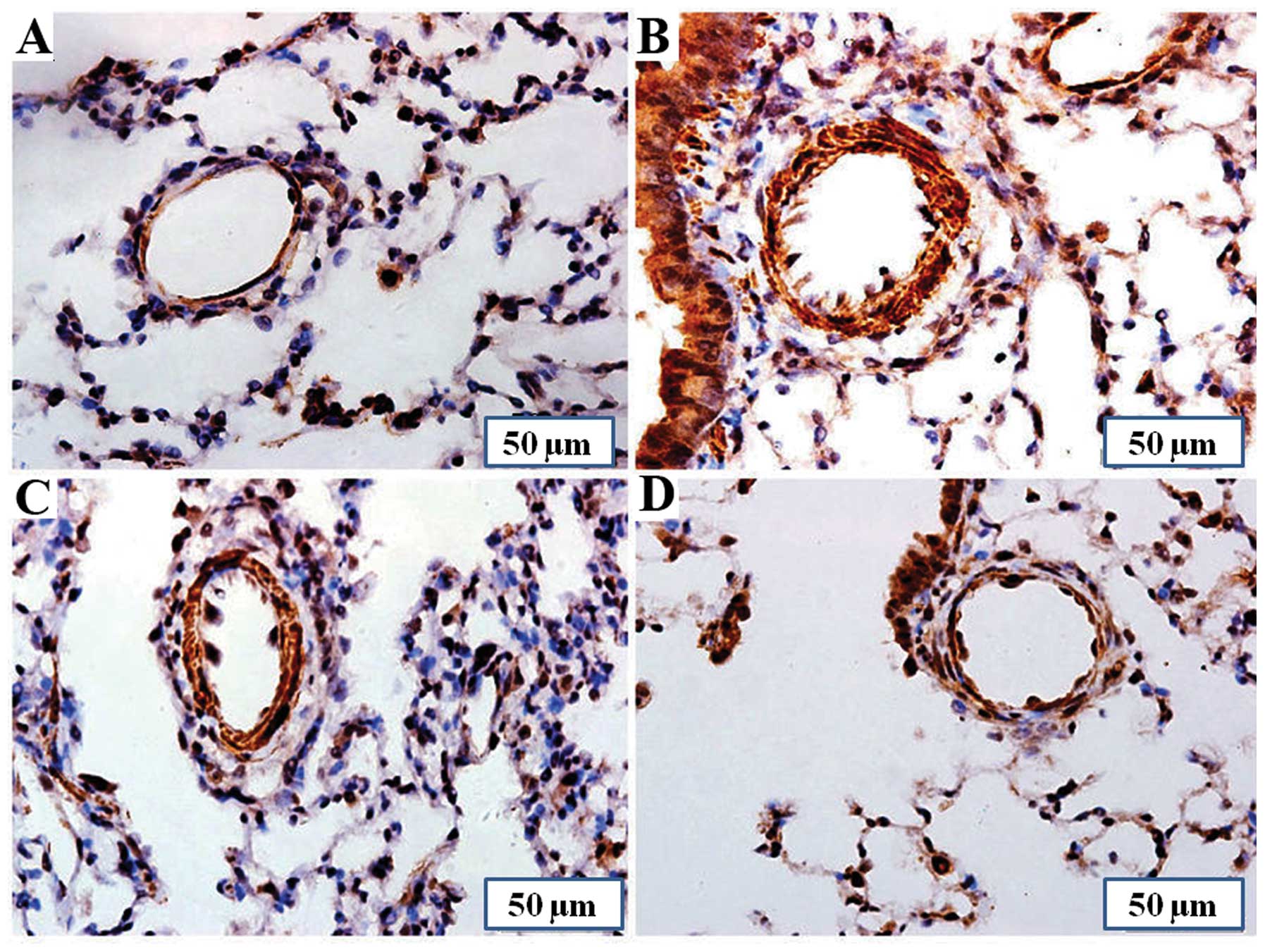
The results of immunohistochemical analysis revealed
that the expression of SERT was markedly upregulated in the lung
tissues of the MCT group compared with the control group. Treatment
with PCPA attenuated the MCT-induced increase in SERT expression
(Fig. 4).
Consistent with the results of immunohistochemical
analysis, western blot analysis revealed that SERT protein
expression was significantly increased in the MCT group compared
with the control group (1.47±0.07 vs. 0.73±0.01, respectively,
P<0.01). At 50 mg/kg, PCPA attenuated the MCT-induced expression
of SERT protein in the rat lungs (1.16±0.09, P<0.05 vs. MCT). At
100 mg/kg, PCPA significantly suppressed the MCT-induced expression
of SERT protein (0.84±0.05, P<0.01 vs. MCT, Fig. 5).
Effects of PCPA on MMP activity and
protein expression
Gelatin zymography was used to evaluate the effects
of PCPA on the activity of MMP-2 and MMP-9 in MCT-induced PAH. The
activity of MMP-2 and MMP-9 significantly increased with MCT
treatment (745±37 and 701±90, P<0.01 and P<0.05,
respectively), compared with the control group (n=5). Following
treatment with PCPA (50 mg/kg/day), the increased MMP-2 activity
showed a decreasing trend (636±92, n=5); however, the effect was
not obvious. Following treatment with a higher dose of PCPA (100
mg/kg/day), the increased MMP-2 activity markedly decreased to a
level similar to that of the control (472±82, P<0.05 vs. MCT).
There was also a decreasing trend in the activity of MMP-9
following PCPA treatment; in the MCT + P1 group, MMP-9 activity
decreased to 592±68 and in the MCT + P2 group, it decreased to
504±73 (P<0.05 vs. MCT, Fig.
6).
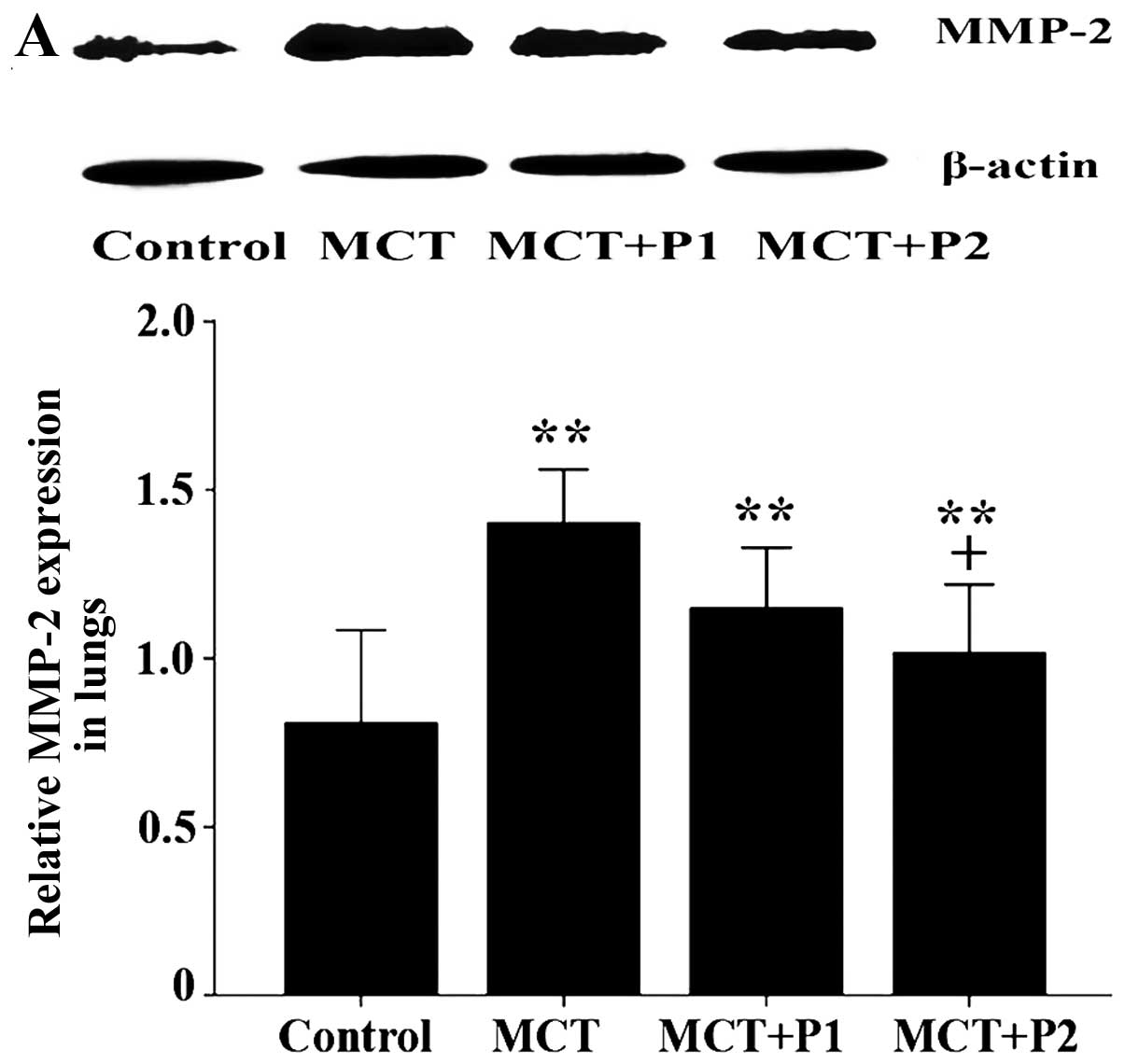
The protein expression of MMP-2, MMP-9, TIMP-1 and
TIMP-2 was determined by western blot analysis. Compared with the
control group, the protein expression of MMP-2, MMP-9, TIMP-1 and
TIMP-2 in the MCT group was increased from 0.81±0.13 to 1.40±0.07
(P<0.01 vs. control, Fig. 7A),
0.72±0.07 to 1.24±0.02 (P<0.01 vs. control, Fig. 7B), 0.48±0.10 to 1.18±0.08
(P<0.01 vs. control, Fig. 7C),
and from 0.67±0.12 to 1.37±0.20 (P<0.01 vs. control, Fig. 7D), respectively (n=5). Of note,
PCPA inhibited the MCT-induced increase in MMP-2/−9, and TIMP-1/−2
expression in a dose-dependent manner. In the MCT + P1 group, the
MMP-9 and TIMP-1 levels decreased to 0.95±0.06 and 0.86±0.08,
respectively (both P<0.05 vs. MCT). However, the MMP-2 and
TIMP-2 levels decreased to 1.14±0.08 and 1.01±0.1, respectively,
although the differences did not reach statistical significance
compared with the MCT group. In the MCT + P2 group, the MMP-2,
MMP-9, TIMP-1 and TIMP-2 levels significantly decreased to 1.02±0.1
(P<0.05 vs. MCT), 0.88±0.02 (P<0.01 vs. MCT), 0.76±0.08
(P<0.01 vs. MCT), and 0.84±0.05 (P<0.05 vs. MCT),
respectively (Fig. 7).
Effects of PCPA on lung inflammation
As shown in Fig.
8, a marked perivascular inflammatory cell infiltration and a
significant increase in the number of inflammatory cells was
observed in the MCT group. PCPA at a dose of 50 and 100 kg/day
markedly decreased MCT-induced lung inflammation.
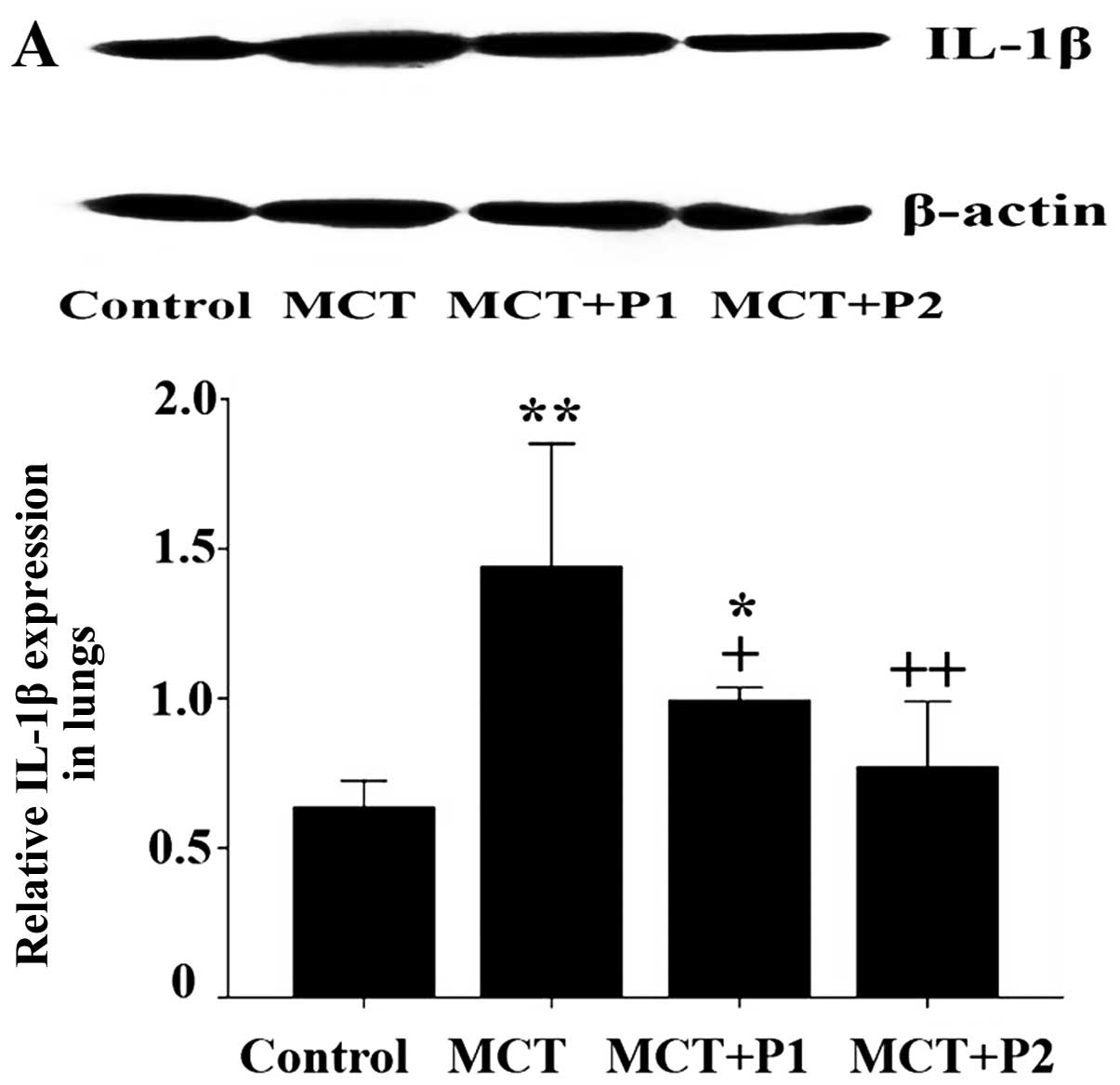
Western blot analysis revealed that the levels of
IL-1β, TNF-α and ICAM-1 in the MCT group were notably increased
compared with control group. IL-1β expression increased in the MCT
group from 0.63±0.04 to 1.44±0.11 (P<0.01 vs. control, n=5). In
the MCT + P1 and MCT + P2 groups, IL-1β expression decreased to
0.99±0.02 (P<0.05 vs. MCT) and 0.77±0.11 (P<0.01 vs. MCT,
Fig. 9A), respectively. TNF-α
expression increased in the MCT group from 0.64±0.08 to 1.14±0.09
(P<0.01 vs. control, n=5). In the MCT + P1 and MCT + P2 groups,
TNF-α expression decreased to 0.84±0.06 (P<0.05 vs. MCT) and
0.77±0.07 (P<0.05 vs. MCT, Fig.
9B), respectively. ICAM-1 expression increased in the MCT group
from 0.45±0.02 to 1.61±0.26 (P<0.01 vs. control, n=5). In the
MCT + P1 and MCT + P2 groups, ICAM-1 expression decreased to
1.24±0.12 and 0.98±0.15 (P<0.05 vs. MCT, Fig. 9C), respectively. PCPA inhibited
the MCT-induced increase in the expression of these inflammatory
cytokines (Fig. 9).
Discussion
The results of the present study demonstrated that
MCT markedly elevated PAP, RV/LV + S, pulmonary vascular
remodeling, as well as lung inflammation and mortality, accompanied
with an increased expression of Tph-1, SERT, MMPs/TIMPs and
inflammatory cytokines (IL-1β, TNF-α and ICAM-1). Our results also
revealed that PCPA dose dependently inhibited the MCT-induced
increase in PAP, RV/LV + S, pulmonary vascular remodeling and lung
inflammation, and decreased the mortality rate, accompanied by the
suppression of Tph-1, SERT, MMPs/TIMPs and inflammatory cytokine
(IL-1β, TNF-α and ICAM-1) expression.
Serotonin is recognized as one of the most important
growth factors in PAH, and the levels of plasma 5-HT have been
shown to be markedly elevated in patients with PAH (26). 5-HT is involved in the development
of PAH with pulmonary vascular remodeling, including the migration
and proliferation of smooth muscle cells (27). It is known that the critical step
in 5-HT biosynthesis is catalyzed by the rate-limiting enzyme, Tph
(19), and that Tph-1 is the
chief enzyme responsible for the synthesis of peripheral 5-HT
(31). Tph expression and 5-HT
synthesis have been shown to be increased in pulmonary endothelial
cells from patients with IPAH and hypoxia-induced PAH in rats
(16,19). The adenovirus-mediated knockdown
of pulmonary endothelial Tph-1 has been shown to attenuate
hypoxia-induced PH (28); in
addition, Tph−/− mice display markedly reduced plasma
levels of 5-HT (29). These data
indicate that Tph-1 plays an important role in the development of
PAH. On the other hand, the overexpression of SERT is a common
pathogenic mechanism in various forms of human PH and animal
pathogenic models (5,15,30). These data from other studies are
accordance with those of our present study, namely that the
expression of Tph-1 and SERT was markedly increased in the lungs of
rats in the MCT group.
As previously demonstrated, PCPA markedly inhibited
5-HT synthesis through the inhibition of Tph-1. Valzelli et
al (32) reported that PCPA
acted as a competitive and irreversible inhibitor of Tph, the
rate-limiting enzyme in 5-HT synthesis, used to deplete brain
serotonin. Treatment of TPH1+/+ mice with PCPA
significantly decreased the level of 5-HT in the colon (33). When a theta-burst stimulation was
applied in layer 2/3 of 5-HT depleted cortical slices (following
in vivo treatment with the Tph inhibitor, PCPA), it was
observed that there was a persistent shift in the ratio between
excitation and inhibition, in the favour of inhibition (34). An intraperitoneal injection of
PCPA has been shown to selectively impair 5HT-containing nerve
terminals and fibers (35). In
our previous studies, we demonstrated that the inhibition of SERT
by the selective serotonin reuptake inhibitor (SSRI), fluoxetine,
significantly inhibited MCT-induced PAH and the 5-HT-induced
proliferation of PASMCs (25,36,37). In the present study, we observed
that PCPA markedly suppressed the expression of Tph-1 and SERT. The
modulation of SERT levels can influence the availability of 5-HT in
the synaptic cleft (38). In a
previous study, after a single intraperitoneal injection of PCPA,
there was a rapid downregulation in SERT mRNA levels in the cell
bodies (38). These findings
suggest that PCPA inhibits 5-HT by suppressing Tph-1, regulating
SERT and the serotonin downstream signaling pathway.
The MMP axis plays a crucial role in the development
of PH, as well as in the disease states specifically with regard to
ECM remodeling and vascular homeostasis (39). Previous studies had shown that
increased MMP activity and expression enhances vascular remodeling
in human and animal models of PAH (40,41). The activity of MMPs is regulated
by TIMPs and the balance between MMPs and TIMPs plays an important
role in vascular remodeling, angiogenesis and vasodilatation
(41). A higher expression of
MMP-2, MMP-9, TIMP-1 and TIMP-2 in the lungs has been found in rats
with MCT-induced PAH (3). This
may be associated with the damage to pulmonary arterial endothelial
cells induced by MCT and pulmonary inflammation (42). Consistent with previous results,
the present results demonstrated that MCT induced an increased in
the expression of MMP-2, MMP-9, TIMP-1 and TIMP-2 in the lung
tissue. We also found that PCPA markedly attenuated pulmonary
vascular remodeling, suppressed MMP-2, MMP-9 enzymatic activity,
and decreased MMP-2, MMP-9/TIMP-1, TIMP-2 expression in a
dose-dependent manner.
Certain studies have reported that serotonin induces
MMP production via SERT, protein kinase C, phospholipase C, the
extracellular signal-regulated kinase (ERK) 1/2 pathway in smooth
muscle cells and the RhoA-ROCK and Akt signaling pathways (25,43). The serotonin intracellular
signaling pathway may be involved in the inhibition of ECM
remodeling by fluoxetine (3).
Based on the inhibitory effects of PCPA on Tph-1 and 5-HT, we
hypothesized that the PCPA-induced regulation of MMP-2,
MMP-9/TIMP-1, TIMP-2 is ascribed to the suppression of pulmonary
vascular remodeling, in which the serotonin intracellular signaling
pathway may be involved.
Inflammatory mechanisms play a prominent role in the
pathogenesis of PAH by contributing to the development and
progressino of PAH; they have also been implicated as a triggering
factor in PAH (44,45). It had been demonstrated that
serotonin is closely related to inflammation, including the
activation of alveolar macrophages, the development and maintenance
of vascular remodeling, and the induction of mast cell adhesion and
migration through the release of cytokines (46–48). In our previous study, we
demonstrated that SSRI fluoxetine protected against MCT-induced
lung inflammation by inhibiting SERT (3), which indicated that SERT may
participate in the process of inflammation. The levels of
inflammatory cytokines, such as monocyte chemoattractant protein-1
(MCP-1), IL-6, IL-1β, ICAM-1 and TNF-α are markedly increased in
patients with IPAH and in animal experimental models of PAH
(3,49,50). Further evidence indicates that
there are significantly higher amounts of IL-1β and IL-6 in the
culture supernatants of lipopolysaccharide-stimulate macrophages
following incubation with 5-HT (33). The inhibition of 5-HT synthesis by
PCPA not only attenuates the severity of inflammation associated
with dextran sodium sulfate (DSS)-induced colitis, but also reduces
the production of pro-inflammatory mediators in the gut (33). These findings are consistent with
the observation of the reduction in colonic IL-1β, IL-6 and TNF-α
levels in Tph1−/− mice treated with PCPA following DSS
administration (33). The present
study on MCT-induced lung inflammation also demonstrated an
increase in ICAM-1, IL-1β and TNF-α levels in the lungs, which is
in accordance with the above-mentioned evidence. This study also
demonstrated that PCPA markedly inhibited lung inflammatory
responses in MCT-induced PAH in rats and showed that this
inhibition was accompanied by the attenuated expression of
inflammatory cytokines, including IL-1β, TNF-α and ICAM-1.
In conclusion, the Tph-1 inhibitor, PCPA, protects
against MCT-induced pulmonary vascular remodeling and lung
inflammation, which is associated with the downregulation in the
expression of Tph-1, SERT, MMPs/TIMPs and inflammatory cytokines in
rats.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (No. 81273511 and No. 30572194)
and No. 30973533.
References
|
1
|
MacLean MR: Pulmonary hypertension and the
serotonin hypothesis: where are we now? Int J Clin Pract Suppl.
27–31. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dewachter L, Adnot S, Fadel E, et al:
Angiopoietin/Tie2 pathway influences smooth muscle hyperplasia in
idiopathic pulmonary hypertension. Am J Respir Crit Care Med.
174:1025–1033. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li XQ, Wang HM, Yang CG, Zhang XH, Han DD
and Wang HL: Fluoxetine inhibited extracellular matrix of pulmonary
artery and inflammation of lungs in monocrotaline-treated rats.
Acta Pharmacol Sin. 32:217–222. 2010.PubMed/NCBI
|
|
4
|
Crapo PM, Medberry CJ, Reing JE, Tottey S,
Van der Merwe Y, Jones KE and Badylak SF: Biologic scaffolds
composed of central nervous system extracellular matrix.
Biomaterials. 33:3539–3547. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang Y, Han DD, Wang HM, Liu M, Zhang XH
and Wang HL: Downregulation of osteopontin is associated with
fluoxetine amelioration of monocrotaline-induced pulmonary
inflammation and vascular remodelling. Clin Exp Pharmacol Physiol.
38:365–372. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Giantin M, Aresu L, Benali S, et al:
Expression of matrix metalloproteinases, tissue inhibitors of
metalloproteinases and vascular endothelial growth factor in canine
mast cell tumours. J Comp Pathol. 147:419–429. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Umesh A, Paudel O, Cao YN, Myers AC and
Sham JS: Alteration of pulmonary artery integrin levels in chronic
hypoxia and monocrotaline-induced pulmonary hypertension. J Vasc
Res. 48:525–537. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Price LC, Wort SJ, Perros F, et al:
Inflammation in pulmonary arterial hypertension. Chest.
141:210–221. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tuder RM, Groves B, Badesch DB and Voelkel
NF: Exuberant endothelial cell growth and element of inflammation
are present in plexiform lesions of pulmonary hypertension. Am J
Pathol. 144:275–285. 1994.PubMed/NCBI
|
|
10
|
Bauer EM, Zheng H, Comhair S, Erzurum S,
Billiar TR and Bauer PM: Complement C3 deficiency attenuates
chronic hypoxia-induced pulmonary hypertension in mice. PLoS One.
6:e285782011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rashid M, Fahim M and Kotwani A: Efficacy
of tadalafil in chronic hypobaric hypoxia-induced pulmonary
hypertension: possible mechanisms. Fundam Clin Pharmacol.
27:271–278. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li M, Riddle SR, Frid MG, et al: Emergence
of fibroblasts with a proinflammatory epigenetically altered
phenotype in severe hypoxic pulmonary hypertension. J Immunol.
187:2711–2722. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guignabert C, Raffestin B, Benferhat R, et
al: Serotonin transporter inhibition prevents and reverses
monocrotaline-induced pulmonary hypertension in rats. Circulation.
111:2812–2819. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fanburg BL and Lee SL: A new role for an
old molecule: serotonin as a mitogen. Am J Physiol. 272:L795–L806.
1997.PubMed/NCBI
|
|
15
|
Marcos E, Fadel E, Sanchez O, et al:
Serotonin-induced smooth muscle hyperplasia in various forms of
human pulmonary hypertension. Circ Res. 94:1263–1270. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Eddahibi S, Guignabert C, Barlier-Mur AM,
et al: Cross talk between endothelial and smooth muscle cells in
pulmonary hypertension: critical role for serotonin-induced smooth
muscle hyperplasia. Circulation. 113:1857–1864. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Morrell NW, Adnot S, Archer SL, et al:
Cellular and molecular basis of pulmonary arterial hypertension. J
Am Coll Cardiol. 54(Suppl 1): S20–S31. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rothman RB, Cadet JL, Dersch CM, et al:
Altered gene expression in pulmonary tissue of tryptophan
hydroxylase-1 knockout mice: implications for pulmonary arterial
hypertension. PLoS One. 6:e177352011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Izikki M, Hanoun N, Marcos E, et al:
Tryptophan hydroxylase 1 knockout and tryptophan hydroxylase 2
polymorphism: effects on hypoxic pulmonary hypertension in mice. Am
J Physiol Lung Cell Mol Physiol. 293:L1045–L1052. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Margolis KG and Pothoulakis C: Serotonin
has a critical role in the pathogenesis of experimental colitis.
Gastroenterology. 137:1562–1566. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tucker A, Bryant SE, Frost HH and Miqally
N: Chemical sympathectomy and serotonin inhibition reduce
monocrotaline-induced right ventricular hypertrophy in rats. Can J
Physiol Pharmacol. 61:356–362. 1983. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu M, Wang Y, Wang HM, Bai Y, Zhang XH,
Sun YX and Wang HL: Fluoxetine attenuates chronic
methamphetamine-induced pulmonary arterial remodeling: possible
involvement of serotonin transporter and serotonin 1B receptor.
Basic Clin Pharmacol Toxicol. 112:77–82. 2013. View Article : Google Scholar
|
|
23
|
Agard C, Rolli-Derkinderen M,
Dumas-de-La-Roque E, et al: Protective role of the antidiabetic
drug metformin against chronic experimental pulmonary hypertension.
Br J of Pharmacol. 158:1285–1294. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang XH, Chen L, Wang HL and Gao J:
Methodological study of determination of rat pulmonary artery
pressure. J Chin Med Univ. 33:388–389. 2004.(in Chinese).
|
|
25
|
Wang HM, Wang Y, Liu M, Bai Y, Zhang XH
and Wang HL: Fluoxetine inhibits monocrotaline-induced pulmonary
arterial remodeling involved in inhibition of RhoA-Rho kinase and
Akt signalling pathways in rats. Can J Physiol Pharmacol.
90:1506–1515. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Herve P, Launay JM, Scrobohaci ML, et al:
Increased plasma serotonin in primary pulmonary hypertension. Am J
Med. 99:249–254. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hironaka E, Hongo M, Sakai A, et al:
Serotonin receptor antagonist inhibits monocrotaline-induced
pulmonary hypertension and prolongs survival in rats. Cardiovasc
Res. 60:692–699. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Morecroft I, White K, Caruso P, et al:
Gene therapy by targeted adenovirus-mediated knockdown of pulmonary
endothelial Tph1 attenuates hypoxia-induced pulmonary hypertension.
Mol Ther. 20:1516–1528. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fligny C, Fromes Y, Bonnin P, et al:
Maternal serotonin influences cardiac function in adult offspring.
FASEB J. 22:2340–2349. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
MacLean MR and Dempsie Y: Serotonin and
pulmonary hypertension-from bench to bedside? Curr Opin Pharmacol.
9:281–286. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Walther DJ, Peter JU, Bashammakh S,
Hortnaql H, Voits M, Fink H and Bader M: Synthesis of serotonin by
a second tryptophan hydroxylase isoform. Science. 299:762003.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Valzelli L, Bernasconi S and Dalessandro
M: Time-courses of p-CPA-induced depletion of brain serotonin and
muricidal aggression in the rat. Pharmacol Res Commun. 15:387–395.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ghia JE, Li N, Wang H, et al: Serotonin
has a key role in pathogenesis of experimental colitis.
Gastroenterology. 137:1649–1660. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Moreau AW, Amar M, Callebert J and Fossier
P: Serotonergic modulation of LTP at excitatory and inhibitory
synapses in the developing rat visual cortex. Neuroscience.
238:148–158. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nakadate K, Imamura K and Watanabe Y:
C-fos activity mapping reveals differential effects of
noradrenaline and serotonin depletion on the regulation of ocular
dominance plasticity in rats. Neuroscience. 235:1–9. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Song D, Wang HL, Wang S and Zhang XH:
5-Hydroxytryptamine-induced proliferation of pulmonary artery
smooth muscle cells are extracellular signal-regulated kinase
pathway dependent. Acta Pharmacol Sin. 26:563–567. 2005. View Article : Google Scholar
|
|
37
|
Zhai FG, Zhang XH and Wang HL: Fluoxetine
protects against monocrotaline-induced pulmonary arterial
hypertension: potential roles of induction of apoptosis and
upregulation of kv1.5 channels in rats. Clin Exp Pharmacol Physiol.
36:850–856. 2009. View Article : Google Scholar
|
|
38
|
Rattray M, Baldessari S, Gobbi M, Mennini
T, Samanin R and Bendotti C: p-Chlorophenylalanine changes
serotonin transporter mRNA levels and expression of the gene
product. J Neurochem. 67:463–472. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chelladurai P, Seeger W and Pullamsetti
SS: Matrix metalloproteinases and their inhibitors in pulmonary
hypertension. Eur Respir J. 40:766–782. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Okada M, Kikuzuki R, Harada T, Hori Y,
Yamawaki H and Hara Y: Captopril attenuates matrix
metalloproteinase-2 and −9 in monocrotaline-induced right
ventricular hypertrophy in rats. J Pharmacol Sci. 108:487–494.
2008.
|
|
41
|
Karthikeyan VJ, Lane DA, Beevers DG, Lip
GY and Blann AD: Matrix metalloproteinases and their tissue
inhibitors in hypertension-related pregnancy complications. J Hum
Hypertens. 27:72–78. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang HL: The serotonin receptor and
transporter are potential therapeutic targets for pulmonary
hypertension. Curr Opin Investig Drugs. 5:963–966. 2004.PubMed/NCBI
|
|
43
|
Shum JK, Melendez JA and Jeffrey JJ:
Serotonin-induced MMP-13 production is mediated via phospholipase
C, protein kinase C, and ERK1/2 in rat uterine smooth muscle cells.
J Biol Chem. 277:42830–42840. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Witzenrath M, Ahrens B, Kube SM, et al:
Allergic lung inflammation induces pulmonary vascular
hyperresponsiveness. Eur Respir J. 28:370–377. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hassoun PM, Mouthon L, Barbera JA, et al:
Inflammation, growth factors, and pulmonary vascular remodeling. J
Am Coll Cardiol. 54(Suppl 1): S10–S19. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mikulski Z, Zaslona Z, Cakarova L, et al:
Serotonin activates murine alveolar macrophages through 5-HT2C
receptors. Am J Physiol Lung Cell Mol Physiol. 299:L272–L280. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lima C, Souza VM, Soares AL, Macedo MS,
Tavares-de-Lima W and Vargaftig BB: Interference of methysergide, a
specific 5-hydroxytryptamine receptor antagonist, with airway
chronic allergic inflammation and remodeling in a murine model of
asthma. Clin Exp Allergy. 37:723–734. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kushnir-Sukhov NM, Gilfillan AM, Coleman
JW, Brown JM, Bruening S, Toth M and Metcalfe DD:
5-hydroxytryptamine induces mast cell adhesion and migration. J
Immunol. 177:6422–6432. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lawrie A, Waterman E, Southwood M, et al:
Evidence of a role for osteoprotegerin in the pathogenesis of
pulmonary arterial hypertension. Am J Pathol. 172:256–264. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wilkins MR: Pulmonary hypertension: the
science behind the disease spectrum. Eur Respir Rev. 21:19–26.
2012. View Article : Google Scholar : PubMed/NCBI
|