Introduction
Steroid hormone production is the main function of
adrenocortical cells. A basic substrate for steroidogenesis is free
cholesterol which originates mainly from cholesteryl esters during
a hydrolysis reaction. The recruitment of cholesteryl esters may be
accomplished by the scavenger receptor class B, member 1 (Scarb1,
also known as SR-B1) -mediated uptake of HDL and the subsequent
conversion to free cholesterol by hormone-sensitive lipase (Lipe)
in the cytosol. LDL cholesterol, on the other hand, is transported
via receptor-mediated endocytosis and is then hydrolysed by acid
cholesteryl esterase in lysosomes (1–7).
Cholesteryl esters are also stored in cytosolic lipid droplets.
Free cholesterol is also produced by de novo cholesterol
biosynthesis from acetyl-coenzyme A in the endoplasmic reticulum by
sterol O-acyltransferase 1 (Soat1); however, it only constitutes
20% of the substrate for steroidogenesis (8). In rodents, the principal source of
cholesterol for steroidogenesis is circulating HDL. Both
cholesterol hydrolysis and steroid synthesis are controlled by
adrenocorticotropic hormone (ACTH) (9–11).
The enucleation of the adrenal gland consists of the
removal of the cortex and medulla, leaving only the capsule with
some zona glomerulosa and progenitor cells (12–14). It has been proven that cells left
under the capsule after surgery do not express cytochrome P450
aldosterone synthase (Cyp11b2) or cytochrome P450 11β-hydroxylase
(Cyp11b1) (15). The two enzymes
are markers of zona glomerulosa and zona fasciculata/reticularis
cells, respectively. Therefore, it has been suggested that cortical
cells of adrenal regeneration arise from de-differentiated zona
glomerulosa cells which are left attached to the capsule following
surgery, the phenotype of which is similar to that of cells of the
zona intermedia, adrenal progenitor cells (14–17).
It is well known that adrenal enucleation results in
an immediate decrease in plasma corticosterone levels, which leads
to a marked compensatory hypersecretion of ACTH. The regeneration
process is inhibited when one adrenal gland is left untouched and
plasma corticosterone levels are normal and ACTH secretion remains
under feedback control (15,18,19). Corticosteroid hormone
administration also prevents the regeneration of the cortex. It has
been previously reported that during the first week of
regeneration, the mRNA expression levels of aldosterone synthase
and 11β-hydroxylase remain low (hybridization data). Cyp11b2
expression levels remain lower than those of the control up to day
30 of adrenal regeneration, whereas Cyp11b1 and 3β-hydroxysteroid
dehydrogenase (Hsd3β) levels are similar to those of the controls
on the 20th and 30th day after enucleation (15).
In the present study, we aimed to determine the
expression profiles of genes involved in steroidogenesis during
enucleation-induced adrenocortical regeneration in rats. In order
to accomplish this goal, the Affymetrix microarray method was used,
as described in a recent study (20). This method enables the assay of
numerous (approximately 30,000) genes which are involved in any
aspect of adrenocortical function. Apart from well known genes with
steroidogenic functions, such as steroidogenic acute regulatory
protein (StAR, NM_031558.3), cholesterol side-chain cleavage enzyme
(cholesterol desmolase, Cyp11a1, NM_017286.2), Hsd3β
(NM_001042619.1), steroid 21-hydroxylase (Cyp21a1, NM_057101.2),
11β-hydroxylase (NM_012537.3) and aldosterone synthase
(NM_012538.2), genes encoding enzymes which are indirectly involved
in steroid hormone synthesis were also analyzed, such as fatty acid
binding protein 6 (Fabp6, NM_017098.1), Lipe (NM_012859.1), Soat1
(Acat-1, NM_031118.1), nuclear receptor subfamily 0, group B,
member 1 (Nr0b1, NM_053317.1), nuclear receptor subfamily 5, group
A, member 1 (Nr5a1, NM_001191099.1).
Materials and methods
Animals, reagents and experimental
design
Female Wistar rats (final body weight, 100–120 g)
were used, obtained from the Laboratory Animal Breeding Center,
Department of Toxicology, Poznan University of Medical Sciences.
The animals were maintained under standardized conditions of light
(14:10 h; light:dark cycle, illumination onset at 06.00 a.m.), at
23°C, with free access to standard pellets and tap water. The study
protocol was approved by the local ethics committee for animal
studies. Unless not otherwise stated, all reagents were obtained
from Sigma-Aldrich (St. Louis, MO, USA) or from Avantor Performance
Materials Poland S.A. (Gliwice, Poland).
Under standard ketamine and xylazine anaesthesia,
the rats were approached dorsally, in order to enucleate both
adrenal glands according to a classic method (20). The operated rats were administered
0.9% NaCl in their drinking water for 3 days. They were then
sacrificed 1, 2, 3, 5, 8 or 15 days after surgery, and their
regenerated adrenals were immediately removed, freed of adherent
fat and immersed in RNAlater. Other glands were frozen at −20°C for
gene expression studies [microarray and quantitative PCR (qPCR)] or
fixed in Bouin’s solution and embedded in paraffin for
immunohistochemistry. Adrenals from sham-operated rats (day 1
post-surgery) were applied as the control adrenal glands.
RNA isolation
The applied methods were described in previous
studies (21–25). Total RNA was extracted from the
regenerated tissue with the use of TRI Reagent (Sigma-Aldrich) and
then, purified on columns (Rneasy Mini Kit; Qiagen, Hilden,
Germany), as previously described (23,25–27). The amount of total mRNA was
determined by optical density at 260 nm and its purity was
estimated by a 260/280 nm absorption ratio (>1.8) on a NanodDrop
spectrophotometer; Thermo Scientific, Walthman, MA, USA).
Reverse transcription
Reverse transcription was performed using AMV
reverse transcriptase (Promega Corp., Madison, WI, USA) with
oligo(dT) (PE Biosystems, Warrington, UK) primers at 42°C for 60
min on a thermocycler (UNO II; Biometra, Goettingen, Germany). The
primers used were designed by Primer 3 software (Whitehead
Institute for Biomedical Research, Cambridge, MA, USA) (Table I). The primers were purchased from
the Laboratory of DNA Sequencing and Oligonucleotide Synthesis,
Institute of Biochemistry and Biophysics, Polish Academy of
Sciences, Warsaw, Poland.
 | Table IConventional RT-PCR and qPCR
analyses. |
Table I
Conventional RT-PCR and qPCR
analyses.
| cDNA | GenBank accession
no. | Primers | Primer sequence
(5′→3′) | Position | PCR product size
(bp) |
|---|
| Cyp11a1 | NM_017286 | S |
GATGACCTATTCCGCTTTGC | 592–611 | 357 |
| | A |
GTTGGCCTGGATGTTCTTG | 930–948 | |
| StAR | NM_031558 | S |
CCTGAGCAAAGCGGTGTCAT | 745–764 | 187 |
| | A |
GCAAGTGGCTGGCGAACTCTA | 911–931 | |
| Hsd3β | NM_001042619.1 | S |
GGCATCTCTGTTGTCATC | 375–392 | 189 |
| | A |
GGTCTTCTTGTAGGAGTTG | 545–563 | |
| Cyp11b1 | NM_012537 | S |
AGAGTATCCTCCCGCATCG | 311–329 | 102 |
| | A |
GCCAGTCTGCCCCATTTAG | 394–412 | |
| Cyp11b2 | NM_012538.2 | S |
TGGCAGCACTAATAACTCAGG | 875–895 | 276 |
| | A |
AAAAGCCACCAACAGGGTAG | 1131–1150 | |
| Fabp6 | NM_017098.1 | S |
GAAAGTGAGAAGAATTACGA | 76–95 | 153 |
| | A |
CATGATGTTGCCCCCAGAG | 210–228 | |
| Lipe | NM_012859.1 | S |
GCCCTCCAAACAGAAACCC | 967–985 | 135 |
| | A |
AAATCCATGCTGTGTGAGAA | 1082–1101 | |
| Soat1 | NM_031118.1 | S |
AAACAGTTGATAGCCAAGAAG | 209–229 | 137 |
| | A |
CCATTGTCCAGAGATGCAG | 327–345 | |
| Nr0b1 (Dax-1) | NM_053317.1 | S |
AGAGTACGCCTATCTGAAG | 1141–1159 | 199 |
| | A |
ATCGGTGTTGATGAATCTC | 1321–1339 | |
| Nr5a1 (Sf-1) | NM_001191099.1 | S |
ATGGCGGACCAGACCTTTATC | 949–969 | 165 |
| | A |
GCTGTCTTCCTTGCCGTACTG | 1093–1113 | |
| Hprt | NM_012583 | S |
CAGTCAACGGGGGACATAAAAG | 391–412 | 146 |
| | A |
ATTTTGGGGCTGTACTGCTTGA | 515–536 | |
Microarray RNA analysis
The Affymetrix® Rat Gene 1.1 ST Array
(Affymetrix, Santa Clara, CA, USA) was followed as described in one
of our recent studies (20).
Total RNA was isolated from the adrenal glands with the use of TRI
Reagent (Sigma-Aldrich) and then purified on columns (RNeasy Mini
kit; Qiagen). RNA quantity and quality were analyzed by gel
electrophoresis and spectrophotometry (NanoDrop ND-1000
spectrophotometer; Thermo Scientific). Total RNA (100 ng) was then
subjected to 2 rounds of sense cDNA amplification
(Ambion® WT Expression kit). The obtained cDNA was used
for biotin labelling and fragmentation by Affymetrix
GeneChip® WT Terminal Labeling and Hybridization
(Affymetrix). By inserting the term ‘steroidogenesis’ as a query of
the description of the regeneration course in the Gene Ontology
(GO) database, 44 genes involved in steroidogenesis were identified
as either up- or downregulated. The investigated genes were
clustered according to an hierarchical clustering algorithm. The
following clusters were obtained: i) genes presenting the highest
expression levels from regeneration day 5 to regeneration day 15
(15 genes); ii) genes presenting the highest expression levels from
day 3 to day 8 of the experiment (5 genes); iii) genes presenting
the highest expression levels in the control glands and iv) genes
presenting the highest expression levels at the beginning of the
regeneration process. Gene expression values were presented as a
heat map according to their hierarchical clustering. Since not all
steroidogenesis-associated genes presented a 2 fold alteration in
their expression levels under the applied experimental conditions,
genes not identified by the GO database were also analyzed.
qPCR
qPCR was performed using the LightCycler®
2.0 instrument (Roche Diagnostics, Basel, Switzerland) with version
4.05 software. Using the primers presented in Table I, the YBR-Green detection system
was applied. In each 20 μl of reaction mixture, 4 μl of template
cDNA (standard or control), 0.5 μM of each gene-specific primer and
3.5 μM MgCl2 (a concentration determined by
pre-optimization experiments).LightCycler FastStart DNA Master
SYBR-Green I mix (Roche) was used. The qPCR program included a
10-min denaturation step in order to activate the Taq DNA
polymerase, followed by a 3-step amplification program:
denaturation at 95°C for 10 sec, annealing at 56°C for 5 sec and
extension at 72°C for 10 sec. The specificity of the amplification
products was determined by melting curve analysis (0.1°C/sec
transition rate).
Immunohistochemistry
A standard immunohistochemistry method (using
peroxidase) with ABC reaction (avidin biotin complex) was used
(20,25,28). The adrenals were fixed in Bouin’s
solution for 24 h and embedded in paraffin. 6 μm-thick sections of
adrenals were used. The sections were then boiled in citrate buffer
(pH 6; 2.5 min, 2.5 min, 3.5 min and 3×20 min cooling). The
sections were incubated with the primary antibody for 1 h at room
temperature (anti-Cyp11a1, anti-Cyp11b1, 1:200) or overnight at 4°C
(anti-StAR, 1:200). All antibodies were purchased from Abcam
(Cambridge, MA, USA) and Bioss (Scotland, UK). Subsequently, the
sections were washed and incubated with the secondary
(peroxidase-conjugated) antibody for 60 min at 37°C. Peroxidase
activity was detected using the DAB technique (liquid DAB
substrate-chromogen system; Dako, Glostrup, Denmark). The nuclei
were counterstained with haematoxylin. The control sections
included similarly treated adjacent sections with the omission of
the primary antibody.
Statistical analysis
Data are expressed as the means ± SE and
statistically significant differences between the control and
experimental groups was assessed by the Student’s t-test.
Results
Microarray analysis
Using the Affymetrix microarray method we examined
the expression levels of approximately 30,000 genes in each sample
of regenerated adrenals (days 1, 2, 3, 5, 8 and 15 after
enucleation) and in the control glands (3 adrenals per day). The
microarray data were compared by fold change calculations, relative
to the control glands. From all up- or downregulated genes that
were examined, only those involved in steroidogenesis were
selected. The selection was performed using the Gene Ontology (GO)
database, where the word ‘steroidogenesis’ was used as a query of
the description of biological processes. It was noted that 32 genes
involved in steroid hormone production were found to have altered
expression levels on day 1 of regeneration (up-/downregulation by
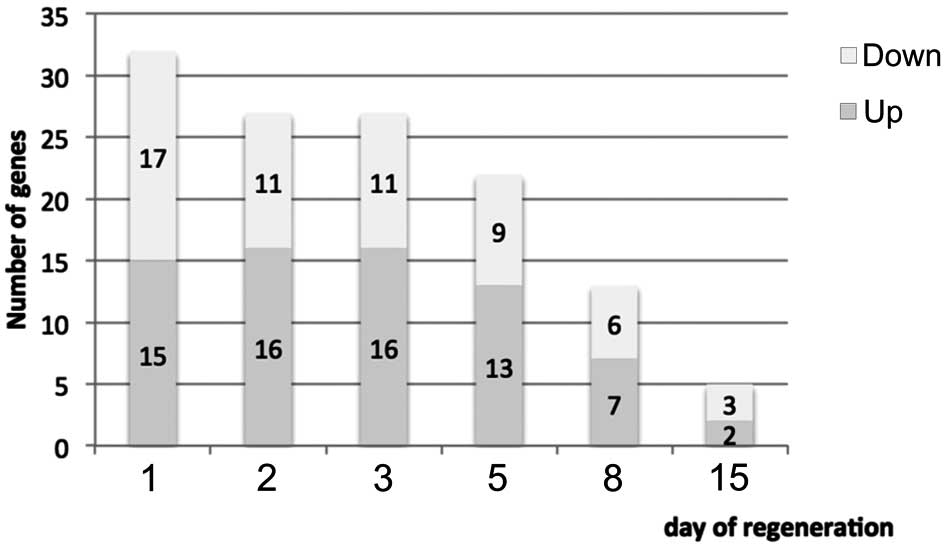
>2-fold, p<0.05) (Fig. 1).
Twenty-four hours after enucleation, only 15 genes were found
upregulated, including the gene for insulin-like growth factor 1
(Igf1), insulin-induced gene 1 (Insig1), genes for cyclin E1
(Ccne1) and UDP glucuronosyltransferase 1 family, polypeptide A5
(Ugt5a1). At the same time point, the expression levels of 17 genes
were decreased (Hsd3β, Nr0b1, Cyp11b2 and Soat1). By contrast,
increased expression levels were observed in only 2 genes, whereas
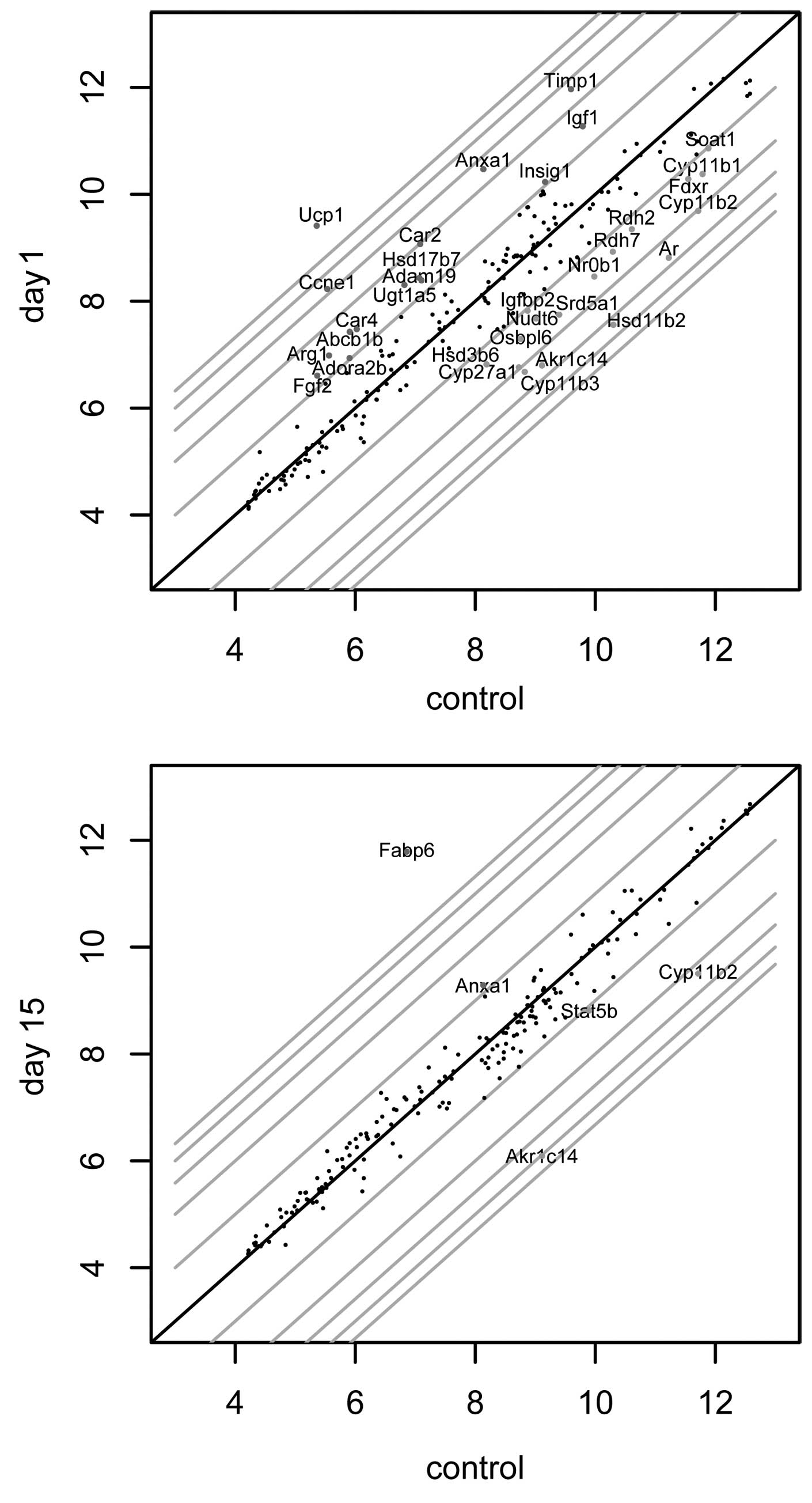
3 genes showed a decrease in expression on day 15 following adrenal
enucleation (Fig. 2,
scaterplot).
The expression levels of the above-mentioned genes
were also presented as a heat map (Fig. 3). The investigated genes were
clustered based on an hierarchical clustering algorithm. The
following 4 clusters were obtained: i) 15 genes with the highest
expression levels between days 5–15 of regeneration; ii) 5 genes
with the highest expression levels between days 3–8 of the
experiment; iii) 6 genes with the highest expression in the control
glands; iv) genes with the highest expression at the beginning of
the regeneration process. Signal intesities were expressed in a
color scale, where green represents the upregulated expression, and
red the downregulated expression levels relative to the control
group. All these genes are listed in detail in Fig. 3. In addition, graphs depicting the
expression profiles of several genes, represented the distinguished
gene groups and were validated by qPCR (Fig. 3).
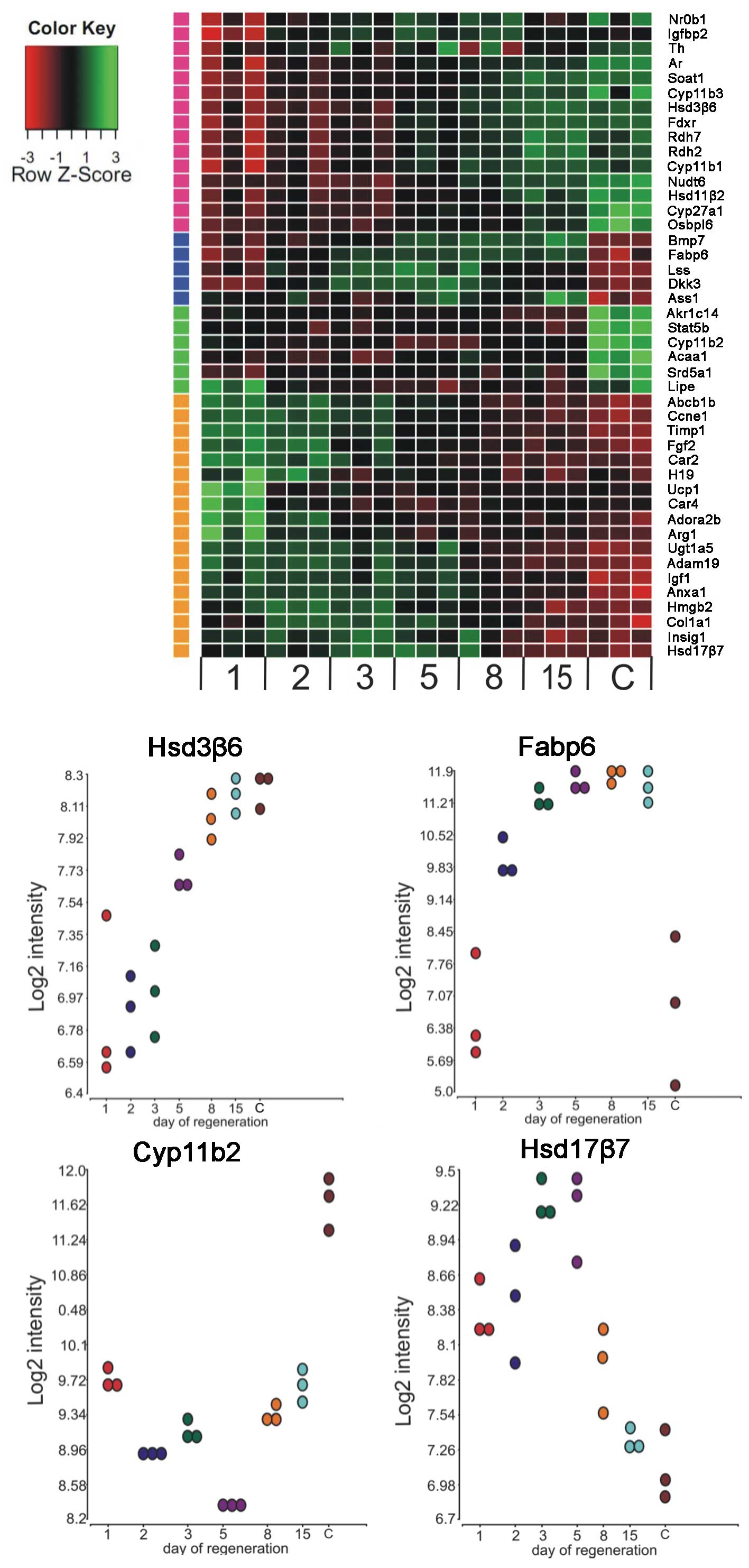 | Figure 3The heatmap presents microarray
expression data of 52 genes involved in steroidogenesis. Raw
expression data of each gene in regenerating adrenals along
different days of the experiment (1, 2, 3, 5, 8 and 15) and in
control glands (C) are shown. Signal intesities are expressed in a
color scale where green represents the upregulated expression and
red the downregulated expression levels relative to the control
group (color bar). The investigated genes were clustered based on
an hierarchical clustering algorithm. The following clusters were
obtained: i) genes with the highest expression levels observed
between days 5–15 of regeneration (15 genes: Nr0b1, Igfbp2, Th, Ar,
Soat1, Cyp11b3, Hsd3β6, Fdxr, Rdh7, Rdh2, Cyp11b1, Nudt6, Hsd11β2,
Cyp27a1, Osbpl6); ii) genes with the highest expression levels
observed between days 3–8 of the experiment (only 5 genes: Bmp7,
Fabp6, Lss, Dkk3, Ass1); iii) genes with the highest expression
levels observed in the control glands (only 6 genes: Akr1c14,
Stat5b, Cyp11b2, Acaa1, Srd5a1, Lipe); iv) genes with the highest
expression levels observed at the beginning of the regenerating
process (Abcb1b, Ccne1, Timp1, Fgf2, Car2, H19, Ucp1, Car4,
Adora2b, Arg1, Ugt1a5, Adam19, Igf1, Anxa1, Hmgb2, Col1a1, Insig1,
Hsd17β7). Dot graphs presents the expression profiles of of 4
selected genes (Hsd3β6, Fabp6, Cyp11b2, Hsd17β7) as a
representative of each heat map cluster (validated by qPCR). |
qPCR
For selected genes of known steroidogenic function,
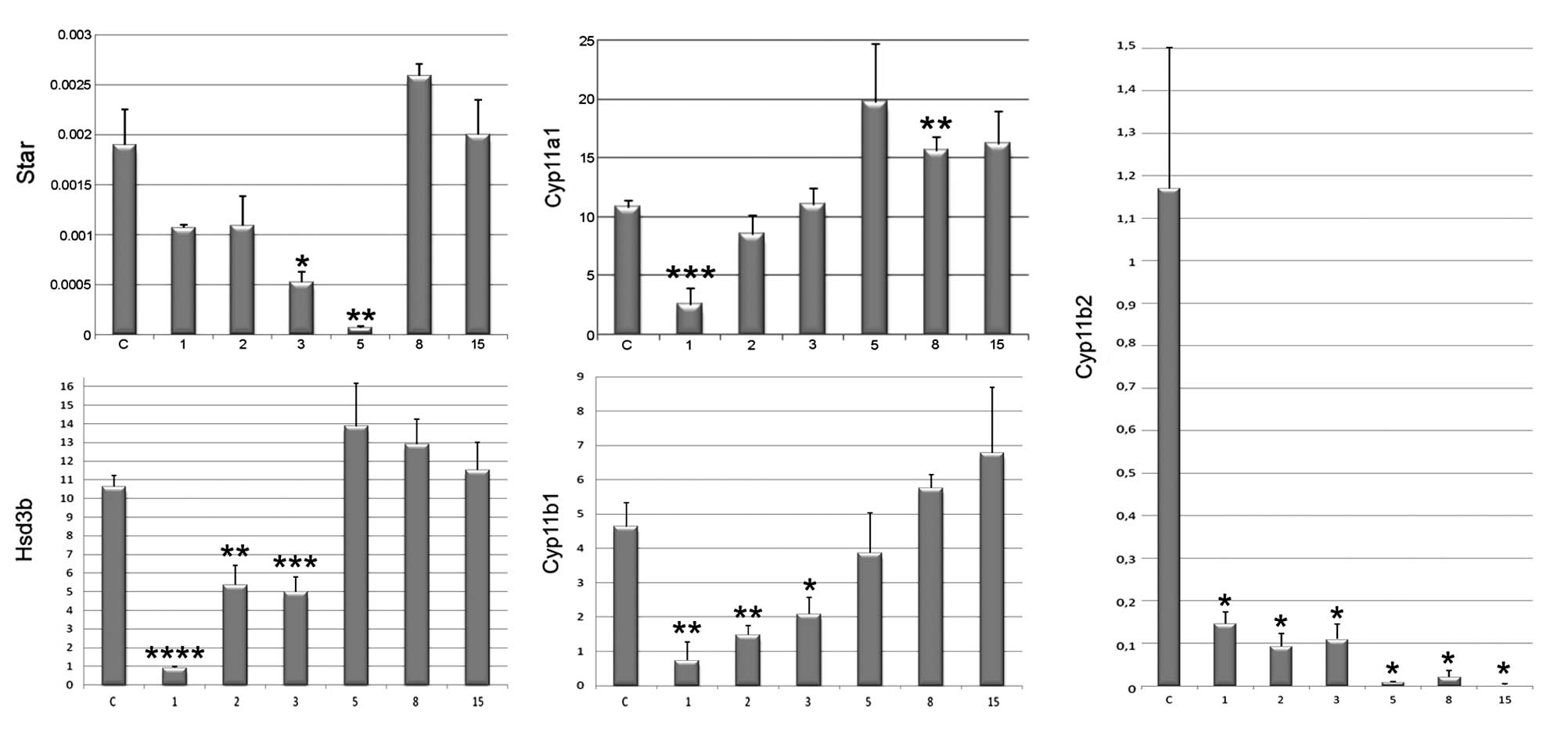
the microarray data were validated by qPCR (Fig. 4). As demonstrated, the mRNA
expression levels of StAR were much lower during the regeneration
process compared to the control. The Cyp11a1 and Hsd3β genes
presented a similar expression profile during the regeneration
process. On the 1st day after enucleation, the expression levels of
both genes significantly decreased compared to those of the control
(p<0.001). Moreover, on days 2 and 3 of regeneration, the
expression levels remained low; however, between days 5–15 they
increased and remained higher than those of the control. As
depicted in Fig. 4, the Cyp11b1
mRNA levels were very low during the first 3 days of regeneration,
but on days 8 and 15 of the experimental procedure, the expression
levels reached similar levels to those of the control. The mRNA
expression levels of Cyp11b2, on the other hand, were very low
throughout the period following adrenal regeneration.
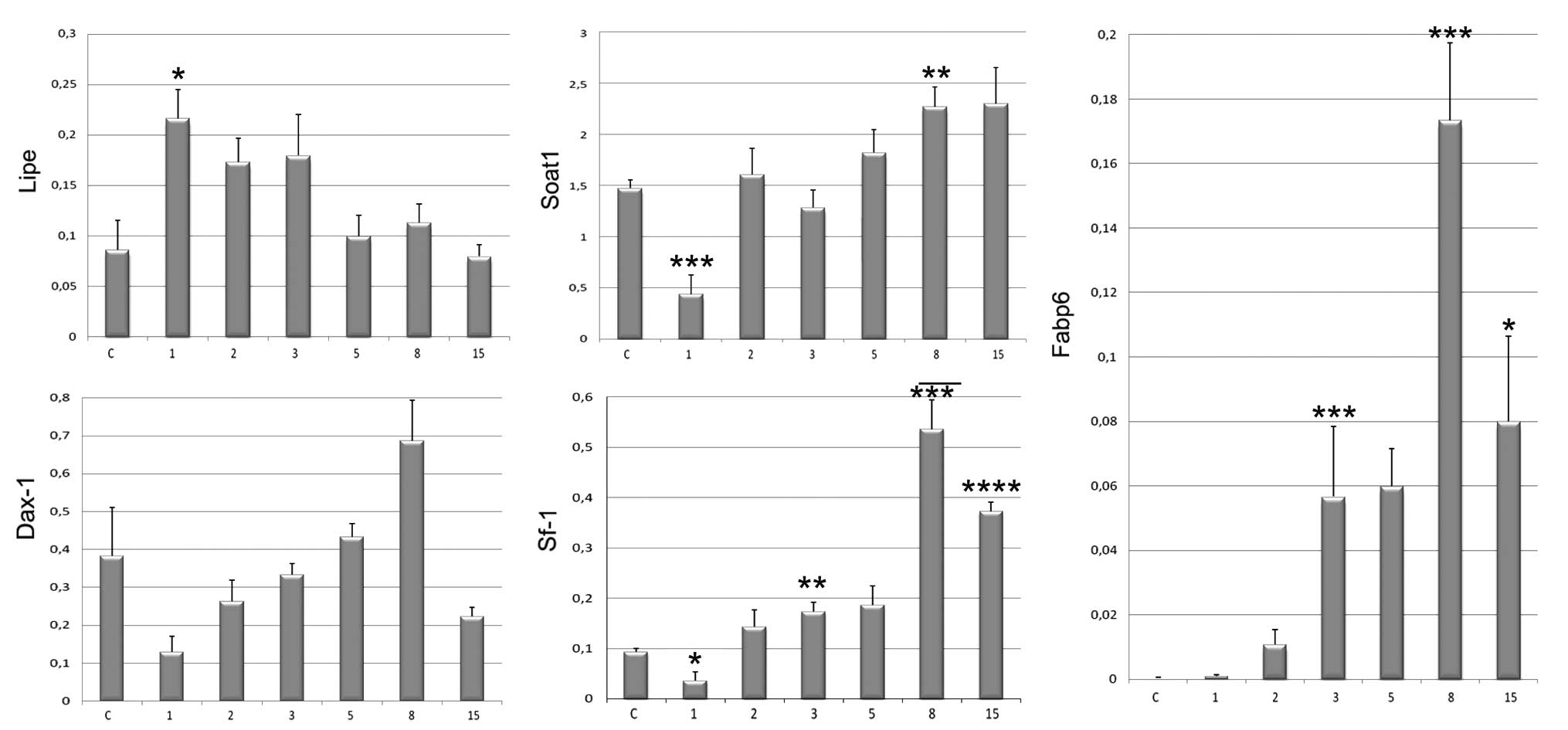
Among the genes that were indirectly involved in
steroidogenesis, Fabp6 was markedly upregulated in the regenerating
adrenals (Fig. 5). The highest
expression levels of Fabp6 were observed on day 8 of regeneration
(p<0.001). Its expression levels on day 1 of regeneration were
similar to those of the control, but from day 2 until day 8 they
significantly increased. On day 15, decreased Fabp6 mRNA levels
were observed; however, in spite of this they were still much
higher compared to those of the control. Contrary to Fabp6, Lipe
gene expression levels, on day 1 following enucleation, were the
highest compared to those of the control. Thereafter, they
gradually decreased, failing to reach the control values. Soat1
expression levels were the lowest on day 1 of regeneration and
subsequently increased, reaching values higher than those in the
control adrenals. Dosage-sensitive sex reversal, adrenal hypoplasia
critical region, on chromosome X, gene 1 (Dax-1) mRNA levels were
the lowest on day 1 of the experiment; however, during the entire
experimental period, the differences were statistically
insignificant. After an initial drop in the expression levels on
day 1 of the experiment, Sf-1 mRNA levels underwent a notable
upregulation, particularly on days 8 and 15 after enucleation,
where values were 4- to 5-fold higher than those in the control
adrenals.
Immunohistochemistry
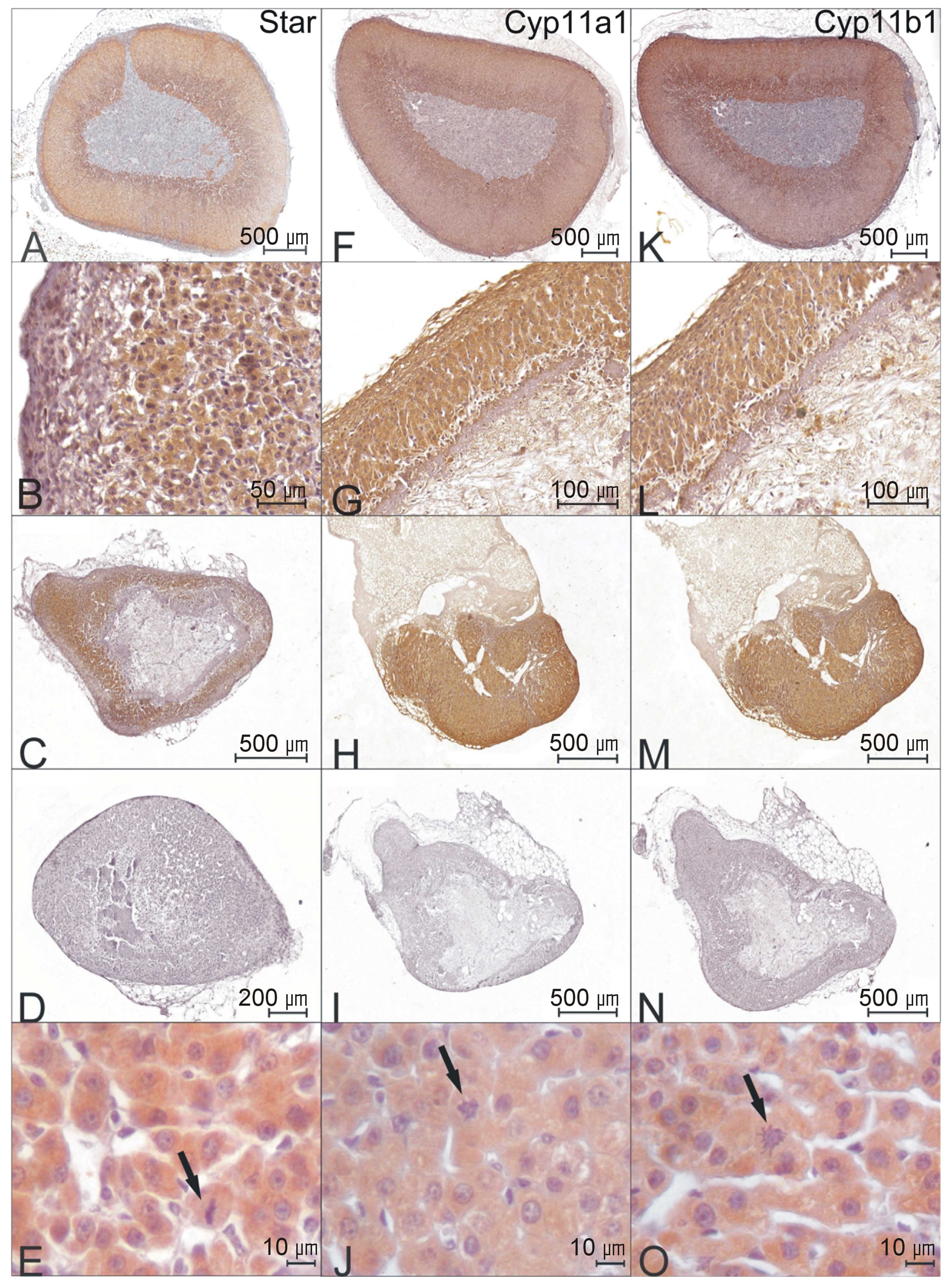
Immunohistochemistry revealed Cyp11a1-, Cyp11b1- and
StAR-like immunoreactivity in the cytoplasm of all adrenocortical
cells of control rats (Fig. 6).
At the beginning of enucleation-induced adrenal regeneration (days
2–5) StAR-like immunoreactivity was not observed in cells adjacent
to the capsule. It should be mentioned that these cells express
visinin-like immunoreactivity [visinin-like 1 (Vsnl1),
NM_012686.2], a marker of zona glomerulosa cells (data not shown).
Cyp11a1-like and Cyp11b1-like immunoreactivity was observed in all
adrenocortical cells of the regenerating cortex.
Discussion
Enucleation-induced adrenal regeneration is a fine
model of rapid adrenal cell growth, in which stages of
proliferation, differentiation and specialization occur in a
relatively short time. Therefore, in this study, using this
experimental model, we aimed to characterize the expression
profiles of genes directly and indirectly involved in
steroidogenesis. The global expression profiles of genes and of
selected genes involved in steroidogenesis were evaluated by
Affymetrix microarray analysis, as well as by qPCR and
immunohistochemistry, as described in one of our recent studies
(20). We used the GO database
with the following criteria: use of the word ‘steroidogenesis’ in
the search bar of the description of biological processes and with
a qualification cut-off of p<0.05 and a >2-fold change in
expression, led to the selection of 44 genes involved in steroid
hormone production that were either up-/downregulated. The number
of selected genes decreased during adrenal regeneration. On the 1st
day following enucleation, 15 genes were found upregulated, while
17 were downregulated. The genes with elevated expression levels
included Insig1, which may play a role in regulating intracellular
cholesterol concentrations; Ccne1, involved in cellular growth
stimulation; and Ugt5a1, coding UDP-glucuronosyltransferase, an
enzyme of the glucuronidation pathway which transforms small
lipophilic molecules into water-soluble metabolites. By contrast,
on day 15 of adrenocortical regeneration, the expression levels of
only 2 genes were increased (e.g., Fabp6), while 3 genes were found
downregulated [Cyp11b2; aldo-keto reductase family 1, member C14
(Akr1c14), involved in steroid metabolism; and signal transducer
and activator of transcription 5B (Stat5b), a transcription factor
which mediates the function of growth hormones and several
interleukins]. Genes indirectly involved in steroidogenesis
prevailed among all the genes selected by the GO database and they
are responsible for providing substrates for steroidogenesis. This
group combines, for example, genes involved in the uptake,
transport and hydrolysis of lipoproteins (Fabp6, Lipe, Ugt1a5 and
Insig1), or those involved in cholesterol synthesis [lanosterol
synthase (2,3-oxidosqualene-lanosterol cyclase) (Lss)] or in
mitochondrial ion transport [uncoupling protein 1 (Ucp1) and
ferredoxin reductase (Fdxr)]. Of note, the GO database with search
descriptor ‘steroidogenesis’ only revealed some genes directly
involved in steroid hormone production, for example the Hsd3β gene,
Cyp11b1 and Cyp11b2, whereas other genes, such as StAR and Cyp11a1
were not selected. Thus, the data obtained from the GO database
requires careful analysis to identify specific genes, not
identified by GO analysis.
As demonstrated in the current study, adrenal
enucleation in rats resulted in a rapid and marked decrease in the
expression levels of the prevailing number of steroidogenic genes
(until day 5), while less or more notable increases in their mRNA
levels were observed in the following days. These data are in
agreement with those of earlier reports. For example, labelled
areas of cells which expressed Cyp11b1 and Cyp11b2 mRNA remained
very small during the first days post-surgery (hybridization and
immunohistochemistry data) (15,29,30). The labelled areas of the cells
which expressed Cyp11b1 and Hsd3β mRNA increased between days 5 and
10 of regeneration, compared to day 2, and were comparable to those
of the control on day 20 of the experiment. On the other hand,
labelled areas of the cells which expressed Cyp11b2 mRNA were
smaller than those of the control even on day 30 of
regeneration.
In the present study, microarray data were validated
by qPCR and such an approach provided a more precise
characterization of gene expression involved in steroidogenesis.
The StAR gene encodes a protein involved in the acute regulation of
steroid hormone synthesis. This protein is responsible for the
transport of cholesterol from the outer to the inner mitochondrial
membrane. An earlier study revealed notable downregulation of both
StAR gene and protein expression levels in regenerating rat
adrenals on experimental day 5 (31). The current study confirmed these
data and revealed a significant upregulation in gene expression
between experimental days 8–15. During the initial stages of
regeneration, StAR-like immunoreactivity was absent in
capsule-adjacent cells (Fig. 5C),
while cells of the deeper layers of the regenerating cortex
exhibited an intense cytoplasmic reaction.
Cyp11a1 (cholesterol desmolase) gene expression
levels were notably downregulated only on day 1 post-adrenal
enucleation, which was followed by prompt and marked increases in
the following days (even exceeding values found in the control
glands). During the course of regeneration, immunohistochemistry
revealed Cyp11a1-like immunoreactivity in all adrenocortical cell
cytoplasms. These data suggest that the restoration of Cyp11a1 gene
expression levels occurs very rapidly during rat adrenal gland
regeneration and may be necessary in order to maintain a suitable
level of steroidogenesis, which depends on the pregnenolone
formation rate.
In rats, adrenal enucleation has been shown to
result in a marked decrease in Hsd3β gene expression on day 2
following surgery and Hsd3β mRNA levels gradually increased between
days 5–10 of the experiments (15). Our observations confirmed this
earlier report.
Aldosterone synthase gene expression was described
in rats during enucleation-induced adrenal regeneration, by
Engeland et al (15,29,30). The expression of this gene serves
as a marker of the adrenal zona glomerulosa. By means of in
situ hybridization, following enucleation, they observed a
marked decrease in Cyp11b2 gene expression levels between days
2–20. The present study confirmed this observation and suggested
that the morphofunctional maturation of zona glomerulosa in
regenerating adrenals involves a very long process, compared with
the zona fasciculata cells. Thus, the restoration of the
mineralocorticoid synthesis pathway is notably delayed, when
compared with that of the glucocorticoid pathway. These
observations were supported by the quantification of aldosterone
and corticosterone blood levels in the rats. In animals with
regenerating adrenal glands, the restoration of blood
corticosterone levels to those of the control values was notably
shorter when compared with aldosterone concentrations (32,33).
Of the enzymes involved in the glucocorticoid
synthesis pathway, Cyp11b1 (11β-hydroxylase) gene expression levels
were examined, as its protein product is responsible for the
transformation of 11-deoxycorticosterone (11-DOC) into
corticosterone. As previously reported, 11β-hydroxylase-like
immunoreactivity was expressed during the first week following
enucleation, reflecting the presence of fasciculata cells (30). The current study revealed that
Cyp11b1 gene expression levels during adrenal regeneration were low
until day 5 following surgery and subsequently increased reaching
values comparable to those of the control adrenals.
According to the above discussion, in
enucleation-induced adrenal regeneration, after a rapid decrease,
the expression levels of genes which are directly involved in
glucocorticoid synthesis reach control levels or even higher around
experimental day 5. Contrary to glucocorticoid synthesis, even
after day 15 of regeneration, Cyp11b2 gene expression levels remain
lower than those of the control. These time differences regarding
the recovery of expression levels of genes responsible for
corticosterone and aldosterone synthesis may depend on high
proliferation, as well as on the varying differentiation rates of
zona glomerulosa cells and zona fasciculata cells. It is possible
that the proliferation and differentiation of these cells prevent
the expression of genes regulating their highly specific function,
in other words aldosterone synthesis.
It is well known that enucleation of adrenals
results in profound changes in the expression profiles of genes
directly involved in steroidogenesis. However, to the best of our
knowledge, no data are available on gene expression profiling
indirectly involved in this process. This group includes, among
others, genes encoding proteins which bind, transport and
metabolize fatty acids required for steroidogenesis. Fabp6 is a
member of the FABP superfamily, which was detected in enterocytes
of the ileum, luteal cells, as well as steroid-producing cells of
the adrenal glands. Experimental data have suggested that it may
mediate steroid transport and metabolism in steroid-producing cells
(34,35). Using both microarray analysis and
qPCR, in this study, we found that during rat adrenal regeneration,
Fabp6 mRNA expression levels notably increased, reaching levels
significantly higher than those of the controls. Thus, the
expression levels of the Fabp6 gene in adrenal regeneration are
similar to those of genes directly involved in steroid hormone
synthesis.
The adrenal steroid hormones are synthesized from
cholesteryl esters, derived mostly from plasma HDL or LDL
lipoproteins. Cholesterol uptake is mediated by specific receptors,
Scarb1 and low density lipoprotein receptor (Ldlr) (.5,36–39). Our
microarray analysis of Scarb1 and Ldlr genes failed to demonstrate
significant changes in their expression levels during rat adrenal
regeneration. On the other hand, during regeneration days 2–8, Lss
gene expression levels were found to be high. Lss codes lanosterol
synthase which catalyzes the cyclization of squalene to lanosterol
during the biosynthesis of cholesterol from acyl-CoA. Lss
expression levels were found significantly higher (by >2 to
3-fold higher) than those of the control (microarray data not
shown), suggesting that the source of cholesterol in adrenal cell
regeneration may be de novo cholesterol synthesis rather
than its uptake from lipoproteins, at least during the first days
of regeneration. This is considered a very interesting finding,
since it is well known that in a normal rat adrenal gland, only 20%
of cholesterol originates from de novo synthesis in the
endoplasmic reticulum (8,40,41).
Lipe is a major cholesterol hydrolase of the adrenal
glands. It cleaves cholesterol esters, stores triglycerides,
diacylglycerides, monoacylglycerides, fatty acids and phospholipids
that are also found in other tissues, such as the liver, white
adipose tissue, the brain, kidneys and heart (42–47). In the current study, with the use
of microarray analysis, we revealed that the expression profile of
the Lipe gene was not altered significantly in adrenal cell
regeneration during the examined period. However, the results from
qPCR revealed a significant upregulation of Lipe mRNA levels on day
1 of regeneration compared to the control, followed by a gradual
decrease in the expression levels observed in the control rats.
This finding suggests that Lipe is involved in supplying
regenerating adrenocortical cells with cholesterol for steroid
production. Previous studies (4,48)
have indicated that Lipe is a major enzyme of the adrenal glands.
Lipe-deficient adrenal glands of mice are characterized by the
accumulation of lipid droplets in zona glomerulosa and fasciculata
cells and by the agglomeration of necrotic lipoid cells near the
medulla, as well as between medulla cells. Although these
morphological changes do not significantly influence basal
corticosterone expression levels, in Lipe-deficient mice, the
response to ACTH stimulation is impaired. In this case, free
cholesterol for steroid production may be provided by a
compensatory decrease in Soat1 activity or an increase in LDL
uptake or de novo synthesis of cholesterol (4). On the other hand, Osuga et al
reported that Lipe deficiency had no effect on the adrenal
corticosterone production under ACTH-stimulated conditions
(49). Shen et al provided
evidence of the interaction of Lipe with StAR in the transfer
process of cholesterol in adrenocortical cells (50).
Recent data presented by Ohta et al (51) suggested that neutral cholesterol
ester hydrolase 1 (Nceh1, NM_001127524.2) is an additional esterase
of lipid metabolism in mouse adrenal glands. Lipe deficiency led to
great limitations in cholesterol hydrolase activity in these cells;
however, the additional inactivation of the Nceh1 gene completely
inhibited esterase activity. Nceh1/Lipe double-deficient mice
present with enlarged adrenal glands. Although the current study
did not include qPCR data on Nceh1 expression levels in rat adrenal
gland regeneration, microarray analysis demonstrated no significant
changes in the expression profile of the Nceh1 gene during adrenal
regeneration (data not shown).
An important result of the present study involves
Soat1, the gene expression profile of which is opposite to that of
Lipe. The expression levels of this gene were very low on day 1
after adrenal enucleation and were subsequently increased to levels
higher than those observed in the control adrenals. Soat1 belongs
to the acyltransferase family and is localized on the membranes of
the endoplasmic reticulum, where it catalyzes the conversion of
free cholesterol and fatty acyl-CoA to cholesteryl esters.
Soat1-deficient mice are characterized by markedly reduced
cholesteryl ester levels in the adrenocortical cells; however,
their response to ACTH challenge remains unaffected (52). The results of the current study
suggest that Soat1 may play a role in the storage of cholesteryl
esters in lipid droplets during the adrenocortical cell
regeneration process; such lipid vacuoles were observed form day 5
of the experiment.
The Nr5a1 gene, which encodes steroidogenic factor 1
(Sf-1) and the Nr0b1 gene, which encodes transcription factor Dax-1
have been documented as 2 genes of critical importance in the
regulation of adrenal development and function. In the present
study, their expression profiles were characterized during rat
enucleation-induced adrenal regeneration. According to the obtained
microarray data, Sf-1 expression was decreased compared to that of
the control during rat adrenal regeneration. However, the results
from qPCR demonstrated an upregulation of Sf-1 gene expression
commencing from day 3 of regeneration. On days 8 and 15, its
expression levels were significantly higher than those in the
control adrenals. On the other hand, the expression levels of the
Dax-1 gene presented no change when compared to the control on a
daily basis during the period of adrenal regeneration. As is known,
both genes interact with each other and regulate the development
and function of adrenocortical cells. Mutations in either gene
result in the defective function of both human and mouse adrenal
glands (53–57). Our data suggest that the Sf-1 gene
plays an important role in rat enucleation-induced adrenal
regeneration.
Overall, our results indicate that the expression of
several genes directly and indirectly involved in steroidogenesis
is altered during rat adrenal regeneration. Our data strongly
suggest that Fabp6, Lipe and Soat1 play a role in supplying
regenerating adrenocortical cell with substrates for steroid
synthesis. All genes examined in the current study were either
found to be up- or downregulated, as indicated by their mRNA
expression level changes. Our results indicated that during the
first days of adrenal regeneration, an intense synthesis of
cholesterol may occur, which is then supplemented by the conversion
of cholesterol into cholesteryl esters and stored in lipid
droplets, which were already visible on day 5 following enucleation
(20).
Acknowledgements
This study is in partial fulfillment of the Ph.D.
thesis requirement of the Poznan University of Medical Sciences for
M. Tyczewska. This study was supported by a grant (no. N401 334639)
from the National Science Centre, Poland.
References
|
1
|
Balasubramaniam S, Goldstein JL and Brown
MS: Regulation of cholesterol synthesis in rat adrenal gland
through coordinate control of 3-hydroxy-3-methylglutaryl coenzyme A
synthase and reductase activities. Proc Natl Acad Sci USA.
74:1421–1425. 1977. View Article : Google Scholar
|
|
2
|
Nishikawa T, Mikami K, Saito Y, Tamura Y
and Kumagai A: Studies on cholesterol esterase in the rat adrenal.
Endocrinol. 108:932–936. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gwynne JT and Mahaffee DD: Rat adrenal
uptake and metabolism of high density lipoprotein cholesteryl
ester. J Biol Chem. 264:8141–8150. 1989.PubMed/NCBI
|
|
4
|
Li H, Brochu M, Wang SP, Rochdi L, Côté M,
Mitchell G and Gallo-Payet N: Hormone-sensitive lipase deficiency
in mice causes lipid storage in the adrenal cortex and impaired
corticosterone response to corticotropin stimulation. Endocrinol.
143:3333–3340. 2002. View Article : Google Scholar
|
|
5
|
Azhar S and Reaven E: Scavenger receptor
class BI and selective cholesteryl ester uptake: partners in the
regulation of steroidogenesis. Mol Cell Endocrinol. 195:1–26. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Connelly MA and Williams DL: SR-BI and HDL
cholesteryl ester metabolism. Endocr Res. 30:697–703. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Miller WL and Bose HS: Early steps in
steroidogenesis: intracellular cholesterol trafficking. J Lipid
Res. 52:2111–2135. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kraemer FB: Adrenal cholesterol
utilization. Mol Cell Endocrinol. 265–266:42–45. 2007.
|
|
9
|
Trzeciak WH and Boyd GS: Activation of
cholesteryl esterase in bovine adrenal cortex. Eur J Biochem.
46:201–207. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Malendowicz LK: A correlated steorological
and functional studies on the long-term effects of ACTH on rat
adrenal cortex. Folia Histochem Cytobiol. 24:203–211.
1986.PubMed/NCBI
|
|
11
|
Schimmer BP, Cordova M, Cheng H, Tsao A,
Goryachev AB, Schimmer AD and Morris Q: Global profiles of gene
expression induced by adrenocorticotropin in Y1 mouse adrenal
cells. Endocrinology. 147:2357–2367. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Greep RO and Deane HW: Histological,
cytochemical and physiological observations on the regeneration of
the rat’s adrenal gland following enucleation. Endocrinology.
45:42–56. 1949.PubMed/NCBI
|
|
13
|
Ingle DJ and Higgins GM: Regeneration of
the adrenal gland following enucleation. Am J Med Sci. 196:232–239.
1938. View Article : Google Scholar
|
|
14
|
Mitani F, Mukai K, Miyamoto H, Suematsu M
and Ishimura Y: The undifferentiated cell zone is a stem cell zone
in adult rat adrenal cortex. Biochim Biophys Acta. 1619:317–324.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Engeland WC and Levay-Young BK: Changes in
the glomerulosa cell phenotype during adrenal regeneration in rats.
Am J Physiology. 276:R1374–R1382. 1999.PubMed/NCBI
|
|
16
|
Mitani F, Suzuki H, Hata J, Ogishima T,
Shimada H and Ishimura Y: A novel cell layer without
corticosteroid-synthesizing enzymes in rat adrenal cortex:
histochemical detection and possible physiological role.
Endocrinology. 135:431–438. 1994.
|
|
17
|
Mitani F, Ogishima T, Miyamoto H and
Ishimura Y: Localization of P450aldo and P45011 beta in normal and
regenerating rat adrenal cortex. Endocr Res. 21:413–423. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Holzwarth MA, Shinsako J and Dallman MF:
Adrenal regeneration. Time course, effect of hypothalamic
hemi-islands and response to unilateral adrenalectomy.
Neuroendocrinology. 31:168–176. 1980.PubMed/NCBI
|
|
19
|
Buckingham JC and Hodges JR:
Interrelationships of pituitary and plasma corticotrophin and
plasma corticosterone in adrenalectomized and stressed,
adrenalectomized rats. J Endocrinol. 63:213–222. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tyczewska M, Rucinski M, Trejter M,
Ziolkowska A, Szyszka M and Malendowicz LK: Angiogenesis in the
course of enucleation-induced adrenal regeneration-expression of
selected genes and proteins involved in development of capillaries.
Peptides. 38:404–413. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Albertin G, Carraro G, Parnigoto PP,
Conconi MT, Ziolkowska A, Malendowicz LK and Nussdorfer GG: Human
skin keratinocytes and fibroblasts express adrenomedullin and its
receptors, and adrenomedullin enhances their growth in vitro by
stimulating proliferation and inhibiting apoptosis. Int J Mol Med.
11:635–639. 2003.
|
|
22
|
Tortorella C, Macchi C, Spinazzi R,
Malendowicz LK, Trejter M and Nussdorfer GG: Ghrelin, an endogenous
ligand for the growth hormone-secretagogue receptor, is expressed
in the human adrenal cortex. Int J Mol Med. 12:213–217.
2003.PubMed/NCBI
|
|
23
|
Rucinski M, Albertin G, Spinazzi R,
Ziolkowska A, Nussdorfer GG and Malendowicz LK: Cerebellin in the
rat adrenal gland: gene expression and effects of CER and
[des-Ser1]CER on the secretion and growth of cultured
adrenocortical cells. Int J Mol Med. 15:411–415. 2005.
|
|
24
|
Rucinski M, Andreis PG, Ziolkowska A,
Nussdorfer GG and Malendowicz LK: Differential expression and
function of beacon in the rat adrenal cortex and medulla. Int J Mol
Med. 16:35–40. 2005.PubMed/NCBI
|
|
25
|
Ziolkowska A, Rucinski M, Tortorella C,
Tyczewska M, Nussdorfer GG and Malendowicz LK: Cultured rat
calvarial osteoblast-like cells are provided with orexin type 1
receptors. Int J Mol Med. 20:779–782. 2007.PubMed/NCBI
|
|
26
|
Rucinski M, Ziolkowska A, Tyczewska M and
Malendowicz LK: Expression of prepro-ghrelin and related receptor
genes in the rat adrenal gland and evidences that ghrelin exerts a
potent stimulating effect on corticosterone secretion by cultured
rat adrenocortical cells. Peptides. 30:1448–1455. 2009. View Article : Google Scholar
|
|
27
|
Albertin G, Rucinski M, Carraro G,
Forneris M, Andreis P, Malendowicz LK and Nussdorfer GG:
Adrenomedullin and vascular endothelium growth factor genes are
overexpressed in the regenerating rat adrenal cortex, and AM and
VEGF reciprocally enhance their mRNA expression in cultured rat
adrenocortical cells. Int J Mol Med. 16:431–435. 2005.
|
|
28
|
Hochol A, Belloni AS, Rucinski M,
Ziolkowska A, Di Liddo R, Nussdorfer GG and Malendowicz LK:
Expression of neuropeptides B and W and their receptors in
endocrine glands of the rat. Int J Mol Med. 18:1101–1106.
2006.PubMed/NCBI
|
|
29
|
Engeland WC, Levay-Young BK, Paul JA and
Fitzgerald DA: Expression of cytochrome P450 aldosterone synthase
and 11 beta-hydroxylase mRNA during adrenal regeneration. Endocr
Res. 21:449–454. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Engeland WC, Gomez-Sanchez CE, Fitzgerald
DA, Rogers LM and Holzwarth MA: Phenotypic changes and
proliferation of adrenocortical cells during adrenal regeneration
in rats. Endocr Res. 22:395–400. 1996.PubMed/NCBI
|
|
31
|
Rucinski M, Tortorella C, Ziolkowska A,
Nowak M, Nussdorfer GG and Malendowicz LK: Steroidogenic acute
regulatory protein gene expression, steroid-hormone secretion and
proliferative activity of adrenocortical cells in the presence of
proteasome inhibitors: In vivo studies on the regenerating rat
adrenal cortex. Int J Mol Med. 21:593–597. 2008.
|
|
32
|
Hochol A, Markowska A, Meneghelli V,
Jedrzejczak N, Majchrzak M, Nowak M, Nussdorfer GG and Malendowicz
LK: Effects of neurotensin and bombesin on the secretory and
proliferative activity of regenerating rat adrenal cortex. Histol
Histopathol. 14:1073–1078. 1999.PubMed/NCBI
|
|
33
|
Markowska A, Nussdorfer GG and Malendowicz
LK: Effects of bombesin and neuromedin-B on the proliferative
activity of the rat adrenal cortex. Histol Histopathol. 8:359–362.
1993.PubMed/NCBI
|
|
34
|
Amano O, Kanda T, Ono T and Iseki S:
Immunocytochemical localization of rat intestinal 15 kDa protein, a
member of cytoplasmic fatty acid-binding proteins. Anat Rec.
234:215–222. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Iseki S, Amano O, Kanda T, Fujii H and Ono
T: Expression and localization of intestinal 15 kDa protein in the
rat. Mol Cell Biochem. 123:113–120. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Landschulz KT, Pathak RK, Rigotti A,
Krieger M and Hobbs HH: Regulation of scavenger receptor, class B,
type I, a high density lipoprotein receptor, in liver and
steroidogenic tissues of the rat. J Clin Invest. 98:984–995. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Temel RE, Trigatti B, DeMattos RB, Azhar
S, Krieger M and Williams DL: Scavenger receptor class B, type I
(SR-BI) is the major route for the delivery of high density
lipoprotein cholesterol to the steroidogenic pathway in cultured
mouse adrenocortical cells. Proc Natl Acad Sci USA. 94:13600–13605.
1997. View Article : Google Scholar
|
|
38
|
Rodrigueza WV, Thuahnai ST, Temel RE,
Lund-Katz S, Phillips MC and Williams DL: Mechanism of scavenger
receptor class B type I-mediated selective uptake of cholesteryl
esters from high density lipoprotein to adrenal cells. J Biol Chem.
274:20344–20350. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Out R, Hoekstra M, Spijkers JA, Kruijt JK,
van Eck M, Bos IS, Twisk J and Van Berkel TJ: Scavenger receptor
class B type I is solely responsible for the selective uptake of
cholesteryl esters from HDL by the liver and the adrenals in mice.
J Lipid Res. 45:2088–2095. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Azhar S, Leers-Sucheta S and Reaven E:
Cholesterol uptake in adrenal and gonadal tissues: the SR-BI and
‘selective’ pathway connection. Front Biosc. 8:998–1029. 2003.
|
|
41
|
Hołlysz M, Derebecka-Hołysz N and Trzeciak
WH: Transcription of LIPE gene encoding hormone-sensitive
lipase/cholesteryl esterase is regulated by SF-1 in human
adrenocortical cells: involvement of protein kinase A signal
transduction pathway. J Mol Endocrinol. 46:29–36. 2011.
|
|
42
|
Cook KG, Lee FT and Yeaman SJ:
Hormone-sensitive cholesterol ester hydrolase of bovine adrenal
cortex: identification of the enzyme protein. FEBS Lett. 132:10–14.
1981. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Beckett GJ and Boyd GS: Purification and
control of bovine adrenal cortical cholesterol ester hydrolase and
evidence for the activation of the enzyme by a phosphorylation. Eur
J Biochem. 72:223–233. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cook KG, Yeaman SJ, Strålfors P,
Fredrikson G and Belfrage P: Direct evidence that cholesterol ester
hydrolase from adrenal cortex is the same enzyme as
hormone-sensitive lipase from adipose tissue. Eur J Biochem.
125:245–249. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yeaman SJ: Hormone-sensitive lipase-new
roles for an old enzyme. Biochem J. 379:11–22. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kraemer FB and Shen WJ: Hormone-sensitive
lipase: control of intracellular tri-(di-)acylglycerol and
cholesteryl ester hydrolysis. J Lipid Res. 43:1585–1594. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Okazaki H, Igarashi M, Nishi M, Sekiya M,
Tajima M, Takase S, Takanashi M, Ohta K, Tamura Y, Okazaki S,
Yahagi N, Ohashi K, Amemiya-Kudo M, Nakagawa Y, Nagai R, Kadowaki
T, Osuga J and Ishibashi S: Identification of neutral cholesterol
ester hydrolase, a key enzyme removing cholesterol from
macrophages. J Biol Chem. 283:33357–33364. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kraemer FB, Shen WJ, Harada K, Patel S,
Osuga J, Ishibashi S and Azhar S: Hormone-sensitive lipase is
required for high-density lipoprotein cholesteryl ester-supported
adrenal steroidogenesis. Mol Endocrinol. 18:549–557. 2004.
View Article : Google Scholar
|
|
49
|
Osuga J, Ishibashi S, Oka T, Yagyu H,
Tozawa R, Fujimoto A, Shionoiri F, Yahagi N, Kraemer FB, Tsutsumi O
and Yamada N: Targeted disruption of hormone-sensitive lipase
results in male sterility and adipocyte hypertrophy, but not in
obesity. Proc Natl Acad Sci USA. 97:787–792. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Shen WJ, Patel S, Natu V, Hong R, Wang J,
Azhar S and Kraemer FB: Interaction of hormone-sensitive lipase
with steroidogenic acute regulatory protein: facilitation of
cholesterol transfer in adrenal. J Biol Chem. 278:43870–43876.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ohta K, Sekiya M, Uozaki H, Igarashi M,
Takase S, Kumagai M, Takanashi M, Takeuchi Y, Izumida Y, Kubota M,
Nishi M, Okazaki H, Iizuka Y, Yahagi N, Yagyu H, Fukayama M,
Kadowaki T, Ohashi K, Ishibashi S and Osuga J: Abrogation of
neutral cholesterol ester hydrolytic activity causes adrenal
enlargement. Biochem Biophys Res Commun. 404:254–260. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Meiner VL, Cases S, Myers HM, Sande ER,
Bellosta S, Schambelan M, Pitas RE, McGuire J, Herz J and Farese RV
Jr: Disruption of the acyl-CoA: cholesterol acyltransferase gene in
mice: evidence suggesting multiple cholesterol esterification
enzymes in mammals. Proc Natl Acad Sci USA. 93:14041–14046. 1996.
View Article : Google Scholar
|
|
53
|
Hammer GD and Ingraham HA: Steroidogenic
factor-1: its role in endocrine organ development and
differentiation. Front Neuroendocrinol. 20:199–223. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Parker KL and Schimmer BP: Steroidogenic
factor 1: a key determinant of endocrine development and function.
Endocr Rev. 18:361–377. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Muscatelli F, Strom TM, Walker AP, Zanaria
E, Récanx D, Meindl A, Bardoni B, Guioli S, Zehetner G, Rabl W,
Schwarz HP, Kaplan JC, Camerino G, Meitinger T and Monaco AP:
Mutations in the DAX-1 gene give rise to both X-linked adrenal
hypoplasia congenita and hypogonadotropic hypogonadism. Nature.
372:672–676. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lala DS, Rice DA and Parker KL:
Steroidogenic factor I, a key regulator of steroidogenic enzyme
expression, is the mouse homolog of fushi tarazu-factor I. Mol
Endocrinol. 6:1249–1258. 1992.PubMed/NCBI
|
|
57
|
Beuschlein F, Keegan CE, Bavers DL, Mutch
C, Hutz JE, Shah S, Ulrich-Lai YM, Engeland WC, Jeffs B, Jameson JL
and Hammer GD: SF-1, DAX-1, and acd: molecular determinants
ofadrenocortical growth and steroidogenesis. Endocr Res.
28:597–607. 2002. View Article : Google Scholar : PubMed/NCBI
|