Introduction
A high incidence of lung, breast, prostate, and
colorectal cancers has been previously reported (1). Prostate cancer continues to be a
major problem in developing countries, the United States and Europe
(2,3). In western countries it is a common
malignancy and the third most frequently diagnosed form of cancer
in males (1,3). In the United States, prostate cancer
is the second leading cause of cancer mortality in males (4). In 2012 an estimated 241,740 males in
the United States were diagnosed with prostate cancer, and ~28,660
of those cases resulted in death (5). Prostate cancer also accounts for
~24% of new case diagnoses and 13% of cancer deaths in the United
Kingdom (6). Although the
pathogenesis of prostate cancer has not yet been determined, some
known contributing risk factors include age, ethnicity and diet
(7).
Developing prostate cancers require immediate
therapies due to their androgen dependency (8,9).
However, 18–36 months after initial treatments most patients with
prostate cancer become castration-resistant to androgen deprivation
therapy, leading to metastasis and the development of
hormone-refractory prostate cancer (2,10).
When prostatic cancer progresses from being androgen-dependent to
-independent, the patient usually succumbs to the disease (11). Androgen-refractory prostate cancer
is chemoresistant and grows prior to androgen-dependent cancer
(12,13). For this reason, the development of
innovative and effective therapeutic options that inhibit
androgen-dependent and -independent prostate cancers is needed
(3,14).
Isoprenoids are important for cell proliferation,
apoptosis, cell differentiation, lipid biosynthesis, and cell cycle
arrest (15–24). Sterol and non-sterol isoprenoids
are essential for a number of cell functions, including cell
signaling, protein synthesis, maintaining membrane integrity, cell
proliferation and apoptosis (22). Both types of isoprenoid are
natural products produced from a commonplace precursor, mevalonate.
Farnesol is a non-sterol isoprenoid produced by the
dephosphorylation of farnesol pyrophosphate, a catabolite of the
cholesterol biosynthetic pathway (22,23).
Farnesol is a 15-carbon isoprenoid found in a
variety of plant products, such as orange peel, lemon-grass oil,
strawberries and chamomile (25–27). Farnesol has been shown to have
anti-bacterial (28,29), anticancer (16,21,30,31) and olfactory nerve effects
(32). The anticancer action of
farnesol has been reported to be mediated by farnesoid X receptor
activity (33), wherein it
induces the expression of thyroid hormone receptor-β1 mRNA,
regulates gene transcription (34), and facilitates the activities of
peroxisome proliferator-activated receptor-α and -γ (35).
The phosphatidylinositol-3-kinase
(PI3K)/serine/threonine kinase (Akt) signaling pathway is essential
for the mediation of several cell activities, including cell
proliferation, growth, the inflammatory response, chemotaxis,
survival and apoptosis (36,37). Increased activation of the
PI3K/Akt signaling pathway is involved in the development of
malignant tumors and resistance to chemotherapy (38). In addition, activation of Akt
frequently occurs in endometrial and pancreatic cancers and has
been linked to a poor prognosis (39–42). In vitro and in vivo
studies have reported that inhibition of the PI3K/Akt signaling
pathway is important for the success of chemotherapy-induced
apoptosis of pancreatic cancer cells (43–45).
Mitogen-activated protein kinases (MAPKs) are
serine/threonine-specific. They interrupt intracellular signaling
related to a variety of activities, including cell proliferation,
cell survival, cell death and transformation (46,47). MAPKs respond to extracellular
stimuli such as growth factors, cytokines, and stress (48). The activities of the major members
of the MAPK family, i.e., mitogen-activated protein kinase (p38),
extracellular signal-regulated protein kinase 1/2 (ERK1/2), and
c-Jun N-terminal kinase (JNK), are regulated by different MAPK
cascades (49).
The primary aim of this study was to identify the
mechanism of farnesol-induced apoptosis in DU145 prostate cancer
cells. Analysis of cell viability by 4′,6-diamidino-2-phenylindole
(DAPI) staining and Annexin V/propidium iodide (PI) staining
determined that farnesol-induced death of DU145 prostate cancer
cells was due to apoptosis. The protein levels of phosphorylated
JNK, phosphorylated ERK, phosphorylated p38, p53, Bcl-2, Bax,
cleaved caspase-3, and phosphorylated Akt were also determined.
In vivo, tumor size was measured twice per week for up to 5
weeks. Farnesol-induced cell death was also examined by the
terminal deoxynucleotidyltransferase-mediated dUTP nick-end
labeling (TUNEL) assay.
Materials and methods
Cells, materials, antibodies and
reagents
Human prostate cancer DU145 cells were obtained from
the Korean Cell Line Bank. Farnesol (Fig. 1) and
3-(4,5-dimethyl-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) were
purchased from Sigma-Aldrich (St. Louis, MO, USA). RPMI-1640
medium, trypsin-EDTA, penicillin-streptomycin, and fetal bovine
serum (FBS) were obtained from HyClone Laboratories (Logan, UT,
USA). Primary antibodies were purchased from Cell Signaling
Technology (Danvers, MA, USA). Dimethyl sulfoxide (DMSO) was
purchased from Merck (Darmstadt, Germany). Tween-20 was purchased
from Sigma-Aldrich. Cell lysis buffer and DAPI were obtained from
Invitrogen (Carlsbad, CA, USA). A fluorescein isothiocyanate
(FITC)-conjugated Annexin V apoptosis detection kit was purchased
from BD Biosciences (San Diego, CA, USA).
Preparation of farnesol and cell
culture
Farnesol was dissolved in a 1:14.6 mixture of
Tween-20 and DMSO prior to administration. Final concentrations
ranged from 0 to 100 μM. Test agents were added to the culture
medium at concentrations of 0, 10, 20, 30, 40, 50, 60, 70, 80, 90
and 100 μM with a final DMSO concentration of <0.1%.
DU145 cells were cultured in RPMI-1640 supplemented
with 10% FBS, 100 μg/ml penicillin and 100 μg/ml streptomycin in 5%
CO2 at 37°C. Cells were then subcultured in a
175-cm2 flask (Nunc, Fisher Scientific, Loughborough,
UK) at a density of 80–90%.
Cell viability assay
The effect of farnesol on DU145 cell survival was
determined by MTT assay. Cells were seeded in 96-well microplates
at two densities: wells containing 2×104 cells/ml were
incubated for 24 h and those containing 1×104 cells/ml
were incubated for 48 h. The two groups underwent treatment with
0–100 μM farnesol. Following incubation, the medium was removed and
the cells were incubated for 2 h with 40 μl of a solution
containing 5 mg/ml MTT in phosphate-buffered saline (PBS). Then,
100 μl of DMSO was added to each well to dissolve the formazan
crystal. Absorbance at 570 nm was measured using an ELISA reader
(Bio-Rad, Hercules, CA, USA). Cell viability was expressed as the
ratio of optical density values of the treatment and control
groups.
Nuclear morphology
To assess the extent of apoptosis, DU145 cell nuclei
were stained with DAPI and observed using fluorescence microscopy.
DU145 cells were seeded in 12-well microplates at a density of
4×104 cells/well and incubated for 24 h with 0, 30 or 60
μM farnesol. Following treatment the cells were fixed and incubated
in PBS containing 4% paraformaldehyde for 30 min. After fixation,
the cells were washed twice with PBS and nuclei were stained with
DAPI. Fluorescence signals were visualized using a fluorescence
microscope at a ×200 magnification.
Western blotting
DU145 cells were incubated with 0, 30 or 60 μM
farnesol for 24 h. Cells were harvested by washing the flasks once
with ice-cold PBS and then treating cells for 3 min with
trypsin-EDTA. Floating cells were collected, transferred to 50-ml
conical centrifuge tubes, and centrifuged for 7 min at 260 × g. The
resulting cell pellets were washed twice with PBS and resuspended
in 200 μl of lysis buffer. Lysates were prepared and centrifuged at
15,920 × g for 5 min at 4°C.
Protein concentrations were determined by a Bradford
protein assay (Bio-Rad). Protein (30 μg) was separated by 12%
SDS-PAGE and transferred electrophoretically to polyvinylidene
difluoride membranes (Amersham Biosciences). The transferred
membranes were blocked with Tris-buffered saline containing 5%
non-fat dry milk and 0.1% Tween-20 for 2 h at 4°C. After blocking,
but prior to incubation, the blocked membranes were treated
individually with the following antibodies: anti-β-actin,
anti-cleaved caspase-3, anti-cleaved caspase-9, anti-Bcl-2,
anti-Akt, anti-phospho-Akt, anti-Bax, anti-phospho-PI3K,
anti-phospho-JNK, anti-phospho-ERK, anti-phospho-p38 and anti-p53.
Membranes were then incubated overnight with gentle agitation at
4°C.
Following incubation the membranes were washed with
Tris-buffered saline containing 0.1% Tween-20, treated with
secondary antibodies (horseradish peroxidase-conjugated goat
anti-mouse or -rabbit IgG; Cell Signaling Technology), and
incubated with gentle agitation at room temperature for 2 h. The
membranes were washed once more with Tris-buffered saline
containing 0.1% Tween-20, and bands were subsequently visualized
using the ECL detection reagents (Pierce, Rockford, IL, USA).
Apoptosis analysis
Following treatment with farnesol, DU145 cell
apoptosis was measured using an Annexin V staining kit
(Becton-Dickinson, Franklin Lakes, NJ, USA). Cells were incubated
with 0, 30 or 60 μM farnesol for 24 h and harvested by exposure to
trypsin-EDTA for 3 min. The harvested cells were washed once with
PBS, resuspended in 100 μl of Annexin V binding buffer, and mixed
with 5 μl of fluorescein isothiocyanate-conjugated Annexin V and
phycoerythrin-conjugated PI. The resuspended cells were then
incubated at room temperature in the dark for 15 min. The frequency
of apoptotic cells was analyzed using a FACSCalibur™ flow cytometer
(Becton-Dickinson).
Animals
Five-week-old male nude mice weighing 20–30 g were
purchased from Orient Bio (Gyeonggi-do, Korea). They were
maintained at 23±5°C and a relative humidity of 40±10% with
artificial lighting from from 800 to 2,000 lx in an animal
laboratory approved by the Companion and Laboratory Animal Science
Department of Kong-Ju National University. The mice were housed in
cages and allowed free access to autoclaved water, food and
commercial rodent chow (Biopia, Seoul, Korea). Animal experiments
were performed with the approval of the Institutional Animal Care
and Use Committee and according to the guidelines of Kong-Ju
National University.
Xenografts
DU145 cells were grown in media composed of
RPMI-1640 supplemented with 10% FBS, 100 μg/ml penicillin and 100
μg/ml streptomycin. The cells were then harvested by washing with
ice-cold PBS and exposure to trypsin-EDTA. Collected cells were
washed twice with PBS and resuspended in RPMI-1640 medium.
DU145 prostate cancer cells at a density of
5×106 cells/0.2-ml medium were injected subcutaneously
into the flanks of donor nude mice. When the resulting tumors
reached 2,000 mm3 in size, the mice were anesthetized
with diethyl ether and the tumor masses were removed surgically.
After slicing the mass into 2×2-mm sections, tumor fragments were
implanted subcutaneously into the right flank of each mouse. After
tumor implantation, mice were randomly divided into two groups (n=3
each).
Preparation and administration of
farnesol
Three mice were divided randomly into different
treatment groups. Oral treatment of each group began on the day of
tumor fragment implantation and continued for 5 weeks. Control mice
received 0.2-ml corn oil daily for 5 weeks. Mice in the treatment
group received 50 mg/kg farnesol daily for 5 weeks.
Measurement of tumor size
Mice were observed for 5 weeks following tumor
implantation. Tumor sizes were measured twice per week using
vernier calipers (Mitutoyo, Kawasaki, Japan). Tumor sizes were
calculated as: width2 × length/2.
TUNEL assay
Apoptotic cells were detected using the Dead End™
Colorimetric TUNEL System (Promega, Madison, WI, USA). Tumor
paraffin blocks were separated into 5-μm sections using a microtome
(Shandon Inc., Pittsburgh, PA, USA) and attached to microscope
slides.
After washing in running water overnight, slides
were deparaffinized by immersion in fresh xylene. The slides were
then washed with 100% ethanol and the tumor tissues were rehydrated
by soaking in a graded ethanol series. The samples were washed with
PBS for 5 min and treated with 20 μg/ml proteinase for 20 min at
room temperature. Endogenous peroxidase was inactivated by 0.3%
H2O2 in PBS for 5 min. The samples were then
washed with PBS for 5 min and the slides were treated with
streptavidin HRP in PBS for 30 min.
The TUNEL sections were visualized using
3,3′-diaminobenzidine tetrahydrochloride (DAB) solution. A total of
50 μl of 20× DAB substrate buffer was added to 950 μl of deionized
water, 50 μl of 20× DAB chromogen and 50 μl of 20× hydrogen
peroxide. This DAB solution (100 μl) was added to each slide and
the sections were stained with methyl green. Randomly selected
fields were visualized under a light microscope at a ×400
magnification. The number of cells positively stained by each
antibody was counted.
Statistical analysis
Data were expressed as the means ± standard
deviation (SD). One-way analysis of variance was used to analyze
differences in multiple comparisons. P<0.05 was considered to
indicate statistical significance.
Results
Farnesol-induced inhibition of cell
proliferation
Treatment with farnesol results in inhibition of the
proliferation of DU145 cells. The chemical structure of farnesol is
shown in Fig. 1.
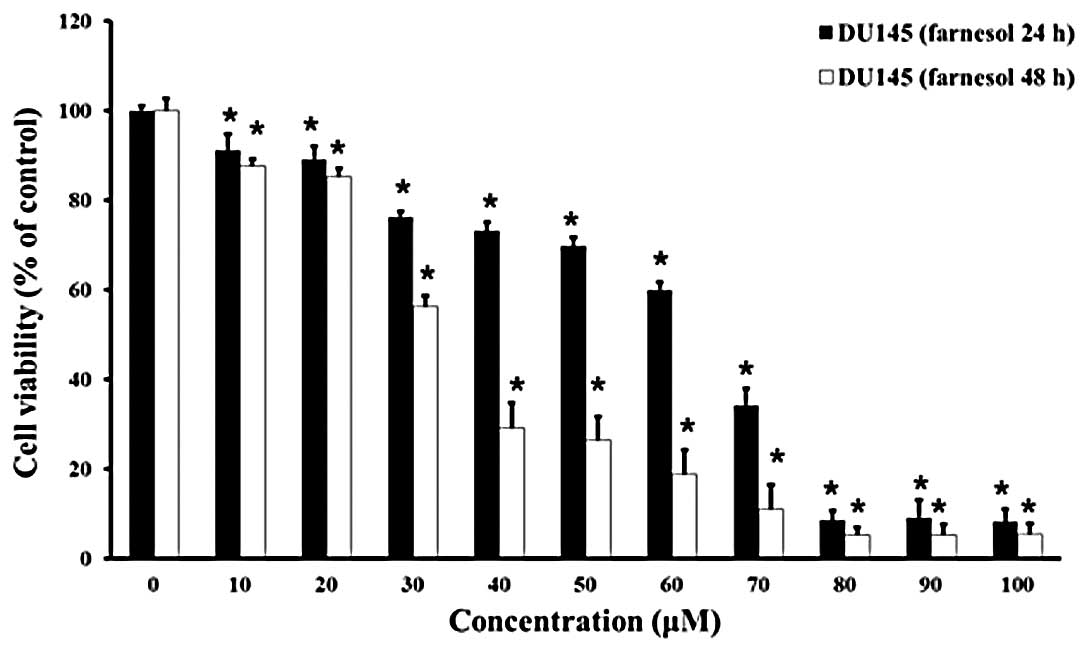
To determine the effect of farnesol on their growth,
DU145 cells were treated with 0–100 μM farnesol for 24 or 48 h, and
viability was determined by MTT assay (Fig. 2).
Farnesol decreased the viability of DU145 cells in a
dose- and time-dependent manner. In comparison to the control
group, cell viability began to decrease significantly at 10 μM in
the 24- and 48-h groups (P<0.05). The resulting cell viabilities
after treatment with 30, 60 or 100 μM farnesol were 76.3±1.1,
60.0±1.7 and 8.1±2.8%, respectively, at 24 h; and 56.3±2.3,
18.8±5.4 and 5.4±2.3%, respectively, at 48 h. Farnesol
concentrations >80 μM for 24 or 48 h resulted in an ~90%
reduction in cell viability.
These results suggested that farnesol induces cell
death in DU145 cells in a dose- and time-dependent manner.
According to the MTT assay, 30 and 60 μM farnesol were used in
subsequent experiments.
Farnesol increases chromatin condensation
in DU145 prostate cancer cells
The cell proliferation results suggested that the
number of dead cells increased in a dose- and time-dependent manner
after farnesol treatment (Fig.
2). Chromatin condensation increased following treatment with
30 or 60 μM farnesol.
DAPI staining distinguishes viable and apoptotic
cells based on nuclear morphology. To investigate the chromatin
condensation effects, DU145 cells were treated with 30 or 60 μM
farnesol for 24 h and chromatin condensation was visualized by DAPI
staining. Changes in the morphology of farnesol-treated DU145 cells
were detected using a fluorescence microscope at a ×200
magnification (Fig. 3A).
Chromatin condensation was detected in DU145 cells treated with 30
or 60 μM farnesol in a dose-dependent manner. Characteristic
apoptotic cell features were confirmed in DU145 cells treated with
farnesol, including cytoplasmic condensation, loss of cell volume,
nuclear fragmentation, convoluted nuclei, and apoptotic bodies. The
untreated group did not exhibit features of apoptosis (Fig. 3A). These DAPI staining results
suggested that farnesol induces apoptosis of DU145 cells in a
dose-dependent manner.
Farnesol induces apoptosis in DU145
prostate cancer cells
The cause of farnesol-induced cell death was
identified as apoptosis or necrosis. Apoptosis of DU145 cells
treated with 30 or 60 μM farnesol was confirmed by Annexin V/PI
double staining. As shown in Fig.
3B, farnesol induced apoptosis in DU145 cells in a
dose-dependent manner. At 24 h treatment with 30 and 60 μM farnesol
induced apoptosis in 11.79 and 14.55% of cells, respectively. Flow
cytometry confirmed that farnesol induces apoptosis of DU145
prostate cancer cells.
Farnesol induces apoptosis through the
PI3K/Akt pathway
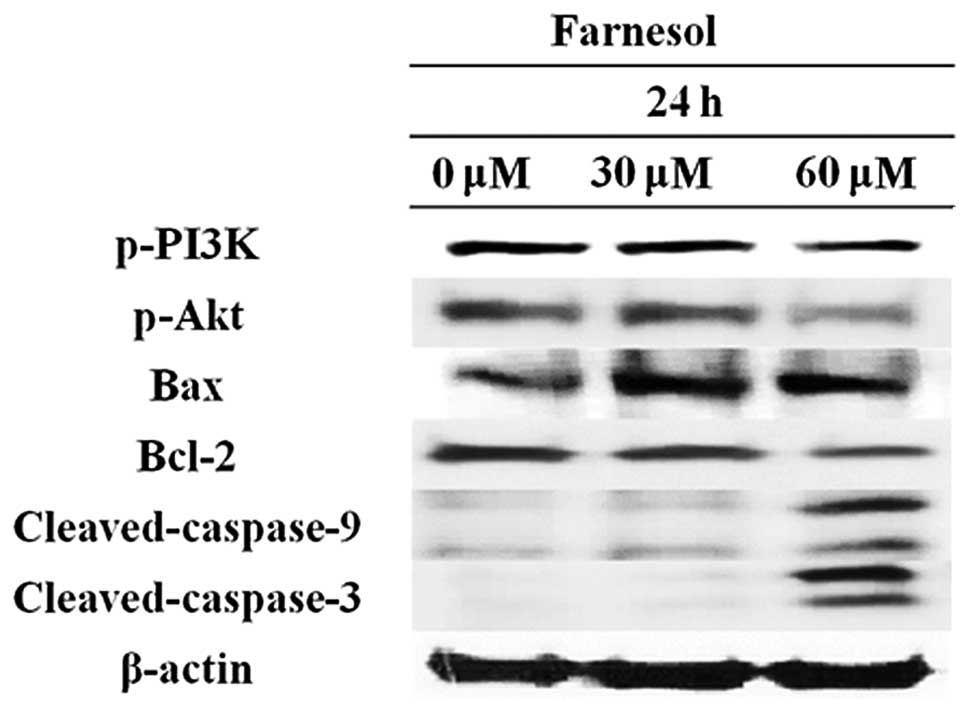
The effect of farnesol on the PI3K/Akt pathway was
subsequently examined. DU145 cells were treated with 30 or 60 μM
farnesol for 24 h and apoptosis-related proteins assayed by western
blotting. As shown in Fig. 4,
PI3K phosphorylation was lower in the farnesol-treated group
compared to the control group. The farnesol treatment group
exhibited decreased Akt and Bcl-2 phosphorylation (Fig. 4).
By contrast, Bax, cleaved caspase-9 and cleaved
caspase-3 levels increased in the farnesol-treated group (Fig. 4). These results suggested that
farnesol induced apoptosis via inhibition of the PI3K/Akt pathway
and activation of the caspase chain reaction.
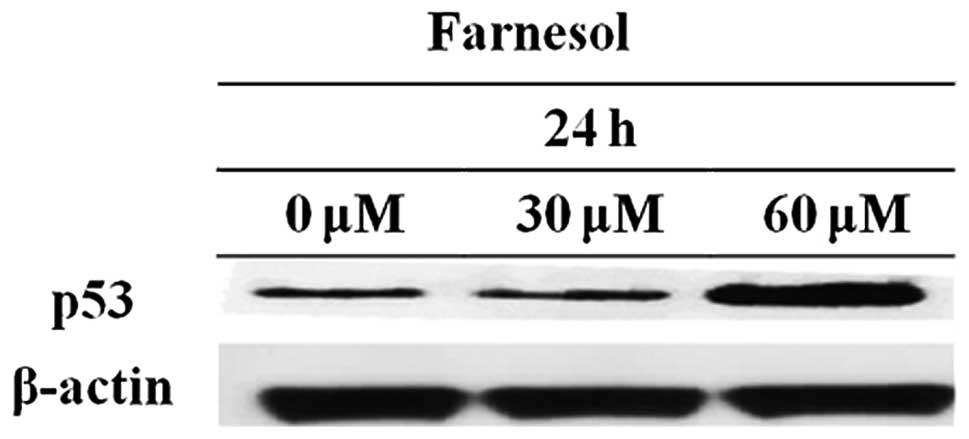
Farnesol increases tumor suppressor gene
p53 expression
p53 arrests the cell cycle (50,51) and induces apoptosis (52,53). p53 induces apoptosis by increasing
the expression of apoptosis-related proteins (54–56). DU145 cells were treated with 30 or
60 μM farnesol for 24 h and the p53 level was assayed by western
blotting. p53 expression was increased in the farnesol-treated
group compared to the control group (Fig. 5).
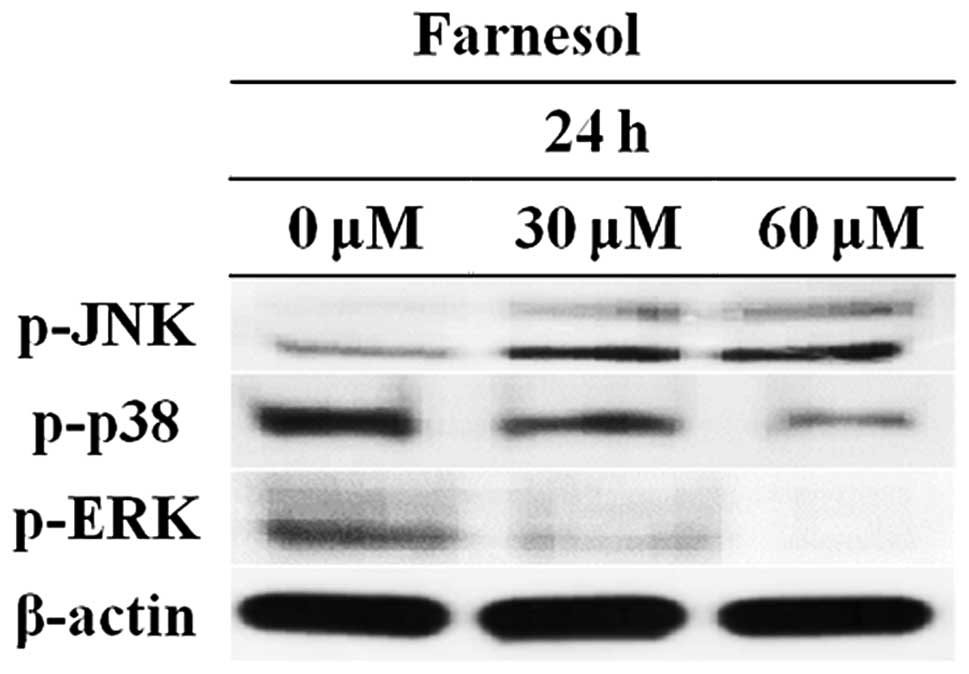
Farnesol regulates the expression of MAPK
pathway genes in DU145 prostate cancer cells
The three main MAPK members are stress-activated
protein kinase JNK, stress-activated protein kinase 2 (p38), and
ERK. MAPKs are associated with a variety of cellular
activities.
The p38 MAPK signaling pathway is activated in
cancer cells. Therefore, carcinostatic substances should inhibit
activation of the p38 MAPK pathway. DU145 cells were treated with
30 or 60 μM farnesol for 24 h and p-JNK, p-p38 and p-ERK levels
were determined by western blotting. As shown in Fig. 6, p-JNK expression was increased in
the farnesol-treated group compared to the control group. However,
the treatment group exhibited decreased levels of p38 and p-ERK
phosphorylation. These results indicated that farnesol induced
apoptosis via regulation of the MAPK pathway.
Farnesol inhibits the growth of DU145
prostate tumors
The apoptosis-inducing effects of farnesol in DU145
cells were confirmed prior to in vivo investigations using
an animal model. To assess the antitumor effect of farnesol, a
mouse xenograft model with DU145 prostate cancer cells was used.
After subcutaneous inoculation of DU145 cells into athymic nude
mice, farnesol was administered for 5 weeks and tumor sizes were
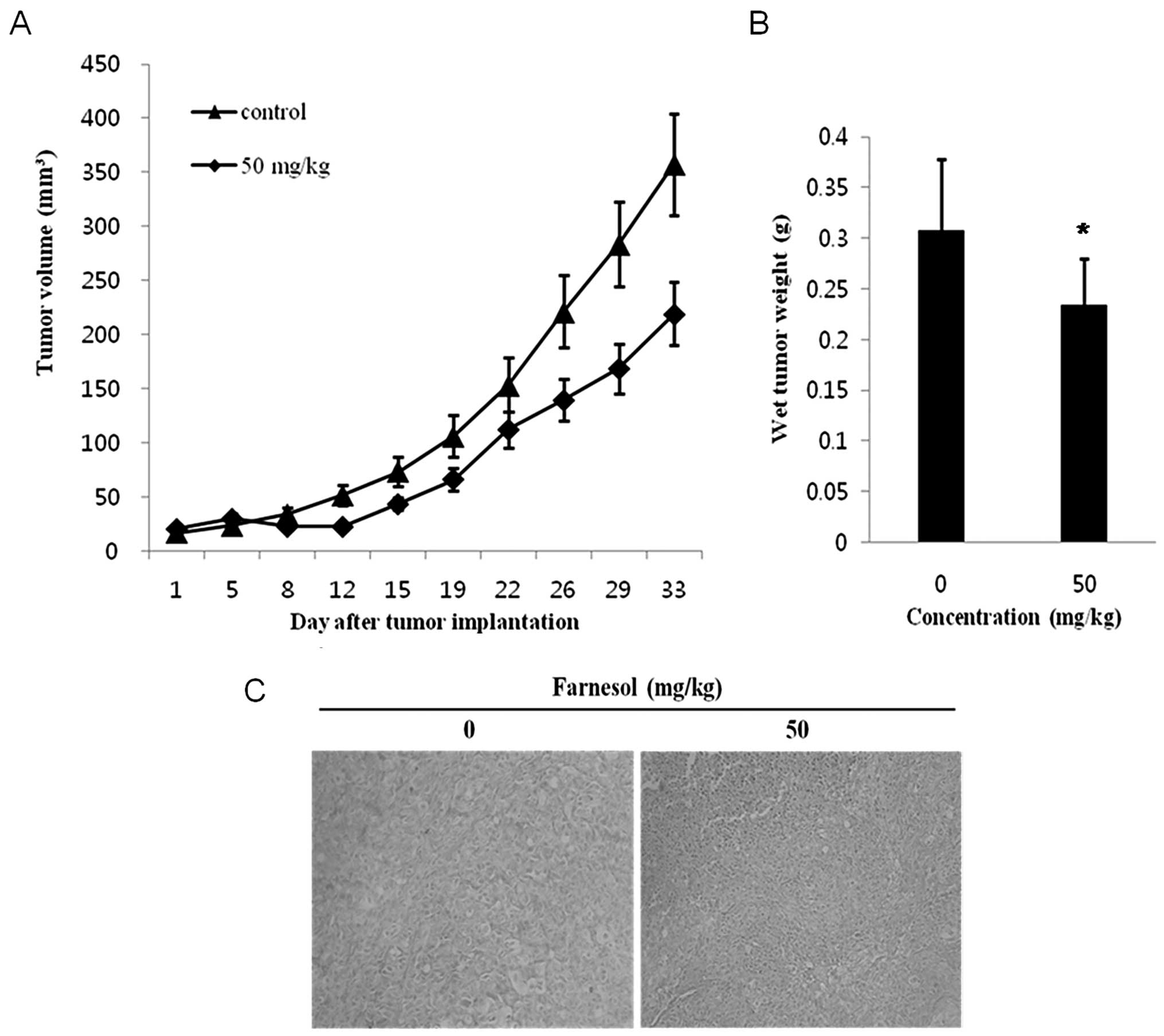
measured at 2-day intervals. As shown in Fig. 7A, the tumor volume in animals
treated with 50 mg/kg farnesol was reduced by 50% compared with the
control group (P<0.05). The average tumor volumes at 5 weeks
were 1,000 mm3 in the control group and 800
mm3 in the farnesol group (Fig. 7A). Administration of farnesol
reduced tumor weights compared to the control group (Fig. 7B).
Farnesol induces apoptosis in DU145
prostate tumors
The effect of farnesol was studied in two groups of
mice. When tumors in control mice reached 2,000 mm3, the
animals in the two groups were sacrificed and 5-μM sections were
prepared. As shown in Fig. 7C, a
significant increase in the number of TUNEL-positive cells was
observed in mice treated with 50 mg/kg farnesol compared to the
control group (P<0.05).
Discussion
Farnesol has been reported to induce cell death in
numerous cancer cell lines (16,21,30,31). Several studies have demonstrated
that farnesol also has anti-bacterial effects (28,29). However, the effect of farnesol in
DU145 prostate cancer cells is not well understood. This study
investigated the anticancer effects of farnesol in DU145 prostate
cancer cells mediated by PI3K/Akt and MAPK signaling. Farnesol
induced dose- and time-dependent apoptosis in DU145 prostate cancer
cells.
To assess the effect of farnesol on the viability of
DU145 cells, the MTT assay was performed. Farnesol significantly
inhibited the growth of DU145 prostate cancer cells in a dose- and
time-dependent manner (Fig.
2).
Apoptosis occurs as the result of a highly complex
cascade of cellular events characterized by cell shrinkage,
chromatin condensation and cell fragmentation (57,58). To investigate the effect of
farnesol on chromatin condensation, DAPI staining was performed. As
shown in Fig. 3A, the frequency
of apoptotic bodies increased in the farnesol treatment group
compared to the untreated group. Cells treated with 60 μM farnesol
fluoresced more intensely, indicating chromatin condensation. Thus,
farnesol the potentially able to induce apoptosis.
The results of Annexin V/PI staining were similar.
As shown in Fig. 3B, 24-h
treatment with 30 and 60 μM farnesol induced apoptosis in 11.79 and
14.55% of the cells, respectively. These results indicate that
farnesol increased apoptosis in a dose-dependent manner.
Western blot assays revealed that treatment of DU145
cells with farnesol altered the expression of PI3K/Akt pathway
proteins. As shown in Fig. 4,
farnesol treatment inhibited Akt and PI3K phosphorylation and
decreased the level of Bcl-2. By contrast, Bax, cleaved caspase-3
and cleaved caspase-9 expression increased compared to the control
group (Fig. 4), suggesting that
farnesol induces apoptosis by altering the expression of PI3K/Akt
pathway proteins.
p53 is a well-known tumor suppressor gene.
Upregulation of p53 is associated with significant inhibition of
Akt phosphorylation (59). As
shown in Fig. 5, p53 activity
increased following farnesol treatment. Therefore, farnesol induced
apoptosis by the inhibition of p-Akt.
MAPKs are associated with a variety of cell
activities, including cell proliferation, survival, death, and
transformation (46,47). Western blotting was performed to
determine the involvement of the MAPK pathway in farnesol-induced
apoptosis. As shown in Fig. 6,
expression of p-JNK was increased in the farnesol-treated group
compared to the control group. By contrast, p-ERK and p-p38 levels
decreased as a result of farnesol treatment. These results indicate
that farnesol induces apoptosis by regulating proteins related to
the MAPK pathway.
To determine its effects on DU145 prostate tumor
volume, farnesol was administered to groups of 3 mice for 5 weeks.
After 5 weeks tumor volumes were 35% lower in the farnesol-treated
group compared to the control group (P<0.05) (Fig. 7A). Farnesol also significantly
reduced tumor weight (Fig. 7B).
TUNEL-positive cell numbers were increased in tumors from mice
treated with farnesol compared to the control mice (Fig. 7C). These results suggest that
farnesol significantly reduced tumor size, weight, and increased
TUNEL-positive cell numbers (Fig.
7).
Collectively, the results of this study have shown
farnesol to be a potential chemopreventive or therapeutic agent
against DU145 prostate cancer.
Acknowledgements
This study was supported by the research grant of
the Kongju National University in 2012.
References
|
1
|
Cohen LA: Nutrition and prostate cancer: a
review. Ann NY Acad Sci. 963:148–155. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim EJ, Lim SS, Park SY, et al: Apoptosis
of DU145 human prostate cancer cells induced by dehydrocostus
lactone isolated from the root of Saussurea lappa. Food Chem
Toxicol. 46:3651–3658. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jain G, Voogdt C, Tobias A, et al: IκB
kinases modulate the activity of the androgen receptor in prostate
carcinoma cell lines. Neoplasia. 14:178–189. 2012.
|
|
4
|
Hellerstedt BA and Pienta KJ: The current
state of hormonal therapy for prostate cancer. CA Cancer J Clin.
52:154–179. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar
|
|
6
|
Rowan S, Rachet B, Alexe DM, et al:
Survival from prostate cancer in England and Wales up to 2001. Br J
Cancer. 99(Suppl 1): S55–S57. 2008. View Article : Google Scholar
|
|
7
|
Spitz MR, Strom SS, Yamamura Y, et al:
Epidemiologic determinants of clinically relevant prostate cancer.
Int J Cancer. 89:259–264. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bostwick DG and Qian J: Effect of androgen
deprivation therapy on prostatic intraepithelial neoplasia.
Urology. 58(Suppl 1): S91–S93. 2001. View Article : Google Scholar
|
|
9
|
Ezekwudo D, Shashidharamurthy R, Devineni
D, et al: Inhibition of expression of anti-apoptotic protein Bcl-2
and induction of cell death in radioresistant human prostate
adenocarcinoma cell line (PC-3) by methyl jasmonate. Cancer Lett.
270:277–285. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Feldman BJ and Feldman D: The development
of androgen-independent prostate cancer. Nat Rev Cancer. 1:34–45.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jemal A, Siegel R, Ward E, et al: Cancer
statistics, 2008. CA Cancer J Clin. 58:71–96. 2008. View Article : Google Scholar
|
|
12
|
Akao Y, Kusakabe S, Banno Y, et al:
Ceramide accumulation is independent of camptothecin-induced
apoptosis in prostate cancer LNCaP cells. Biochem Biophys Res
Commun. 294:363–370. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Akao Y, Banno Y, Nakagawa Y, et al: High
expression of sphingosine kinase 1 and S1P receptors in
chemotherapy-resistant prostate cancer PC3 cells and their
camptothecin-induced up-regulation. Biochem Biophys Res Commun.
342:1284–1290. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rabi T and Bishayee A: d-Limonene
sensitizes docetaxel-induced cytotoxicity in human prostate cancer
cells: Generation of reactive oxygen species and induction of
apoptosis. J Carcinog. 8:92009. View Article : Google Scholar
|
|
15
|
Hanley K, Kömüves LG, Ng DC, et al:
Farnesol stimulates differentiation in epidermal keratinocytes via
PPARalpha. J Biol Chem. 275:11484–11491. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rioja A, Pizzey AR, Marson CM and Thomas
NS: Preferential induction of apoptosis of leukaemic cells by
farnesol. FEBS Lett. 467:291–295. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bifulco M: Role of the isoprenoid pathway
in ras transforming activity, cytoskeleton organization, cell
proliferation and apoptosis. Life Sci. 77:1740–1749. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Miquel K, Pradines A and Favre G: Farnesol
and geranylgeraniol induce actin cytoskeleton disorganization and
apoptosis in A549 lung adenocarcinoma cells. Biochem Biophys Res
Commun. 225:869–876. 1996. View Article : Google Scholar
|
|
19
|
Wright MM, Henneberry AL, Lagace TA, et
al: Uncoupling farnesol-induced apoptosis from its inhibition of
phosphatidylcholine synthesis. J Biol Chem. 276:25254–25261. 2001.
View Article : Google Scholar
|
|
20
|
McTaggart SJ: Isoprenylated proteins. Cell
Mol Life Sci. 63:255–267. 2006. View Article : Google Scholar
|
|
21
|
Ong TP, Heidor R, de Conti A, et al:
Farnesol and geraniol chemopreventive activities during the initial
phases of hepatocarcinogenesis involve similar actions on cell
proliferation and DNA damage, but distinct actions on apoptosis,
plasma cholesterol and HMGCoA reductase. Carcinogenesis.
27:1194–1203. 2006. View Article : Google Scholar
|
|
22
|
Goldstein JL and Brown MS: Regulation of
the mevalonate pathway. Nature. 343:425–430. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Edwards PA and Ericsson J: Sterols and
isoprenoids: signaling molecules derived from the cholesterol
biosynthetic pathway. Annu Rev Biochem. 68:157–185. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Miquel K, Pradines A, Tercé F, Selmi S and
Favre G: Competitive inhibition of choline phosphotransferase by
geranylgeraniol and farnesol inhibits phosphatidylcholine synthesis
and induces apoptosis in human lung adenocarcinoma A549 cells. J
Biol Chem. 273:26179–26186. 1998. View Article : Google Scholar
|
|
25
|
Burke YD, Stark MJ, Roach SL, et al:
Inhibition of pancreatic cancer growth by the dietary isoprenoids
farnesol and geraniol. Lipids. 32:151–156. 1997. View Article : Google Scholar
|
|
26
|
Crowell PL: Prevention and therapy of
cancer by dietary monoterpenes. J Nutr. 129:S775–S778.
1999.PubMed/NCBI
|
|
27
|
Chagas CE, Vieira A, Ong TP and Moreno FS:
Farnesol inhibits cell proliferation and induces apoptosis after
partial hepatectomy in rats. Acta Cir Bras. 24:377–382. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bharate SB, Bhutani KK, Khan SI, et al:
Biomimetic synthesis, antimicrobial, antileishmanial and
antimalarial activities of euglobals and their analogues. Bioorg
Med Chem. 14:1750–1760. 2006. View Article : Google Scholar
|
|
29
|
Katsuyama M, Ichikawa H, Ogawa S and
Ikezawa Z: A novel method to control the balance of skin
microflora. Part 1 Attack on biofilm of Staphylococcus
aureus without antibiotics. J Dermatol Sci. 38:197–205. 2005.
View Article : Google Scholar
|
|
30
|
McAnally JA, Jung M and Mo H:
Farnesyl-O-acetylhydroquinone and geranyl-O-acetylhydroquinone
suppress the proliferation of murine B16 melanoma cells, human
prostate and colon adenocarcinoma cells, human lung carcinoma
cells, and human leukemia cells. Cancer Lett. 202:181–192. 2003.
View Article : Google Scholar
|
|
31
|
Burke YD, Ayoubi AS, Werner SR, et al:
Effects of the isoprenoids perillyl alcohol and farnesol on
apoptosis biomarkers in pancreatic cancer chemoprevention.
Anticancer Res. 22:3127–3134. 2002.
|
|
32
|
Tanida M, Niijima A, Shen J, et al:
Olfactory stimulation with scent of lavender oil affects autonomic
neurotransmission and blood pressure in rats. Neurosci Lett.
398:155–160. 2006. View Article : Google Scholar
|
|
33
|
Kikuzaki H and Nakatani N: Antioxidant
effects of some ginger constituents. J Food Sci. 58:1407–1410.
1993. View Article : Google Scholar
|
|
34
|
Duncan RE and Archer MC: Farnesol induces
thyroid hormone receptor (THR) beta1 but inhibits THR-mediated
signaling in MCF-7 human breast cancer cells. Biochem Biophys Res
Commun. 343:239–243. 2006. View Article : Google Scholar
|
|
35
|
Takahashi N, Kawada T, Goto T, et al: Dual
action of isoprenols from herbal medicines on both PPARgamma and
PPARalpha in 3T3-L1 adipocytes and HepG2 hepatocytes. FEBS Lett.
514:315–322. 2002. View Article : Google Scholar
|
|
36
|
Cantley LC: The phosphoinositide 3-kinase
pathway. Science. 296:1655–1657. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Luo J, Manning BD and Cantley LC:
Targeting the PI3K-Akt pathway in human cancer: rationale and
promise. Cancer Cell. 4:257–262. 2003. View Article : Google Scholar
|
|
38
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
Schlieman MG, Fahy BN, Ramsamooj R, et al:
Incidence, mechanism and prognostic value of activated Akt in
pancreas cancer. Br J Cancer. 89:2110–2115. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yamamoto S, Tomita Y, Hoshida Y, et al:
Prognostic significance of activated Akt expression in pancreatic
ductal adenocarcinoma. Clin Cancer Res. 10:2846–2850. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chadha KS, Khoury T, Yu J, et al:
Activated Akt and Erk expression and survival after surgery in
pancreatic carcinoma. Ann Surg Oncol. 13:933–939. 2006. View Article : Google Scholar
|
|
42
|
Terakawa N, Kanamori Y and Yoshida S: Loss
of PTEN expression followed by Akt phosphorylation is a poor
prognostic factor for patients with endometrial cancer. Endocr
Relat Cancer. 10:203–208. 2003. View Article : Google Scholar
|
|
43
|
Ng SS, Tsao MS, Nicklee T and Hedley DW:
Wortmannin inhibits pkb/akt phosphorylation and promotes
gemcitabine antitumor activity in orthotopic human pancreatic
cancer xenografts in immunodeficient mice. Clin Cancer Res.
7:3269–3275. 2001.
|
|
44
|
Bondar VM, Sweeney-Gotsch B, Andreeff M,
et al: Inhibition of the phosphatidylinositol 3′-kinase-AKT pathway
induces apoptosis in pancreatic carcinoma cells in vitro and in
vivo. Mol Cancer Ther. 1:989–997. 2002.
|
|
45
|
Fahy BN, Schlieman M, Virudachalam S and
Bold RJ: Akt inhibition is associated with chemosensitisation in
the pancreatic cancer cell line MIA-PaCa-2. Br J Cancer.
89:391–397. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
McCubrey JA, Lahair MM and Franklin RA:
Reactive oxygen species-induced activation of the MAP kinase
signaling pathways. Antioxid Redox Signal. 8:1775–1789. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kholodenko BN and Birtwistle MR:
Four-dimensional dynamics of MAPK information processing systems.
Wiley Interdiscip Rev Syst Biol Med. 1:28–44. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Liu X, Li Q, Dowdell K, et al:
Varicella-Zoster virus ORF12 protein triggers phosphorylation of
ERK1/2 and inhibits apoptosis. J Virol. 86:3143–3151. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Junttila MR, Li SP and Westermarck J:
Phosphatase-mediated crosstalk between MAPK signaling pathways in
the regulation of cell survival. FASEB J. 22:954–965. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
El-Deiry WS, Harper JW, O’Connor PM, et
al: WAF1/CIP1 is induced in p53-mediated G1 arrest and apoptosis.
Cancer Res. 54:1169–1174. 1994.PubMed/NCBI
|
|
51
|
Dulić V, Kaufmann WK, Wilson SJ, et al:
p53-dependent inhibition of cyclin-dependent kinase activities in
human fibroblasts during radiation-induced G1 arrest. Cell.
76:1013–1023. 1994.
|
|
52
|
Yonish-Rouach E, Resnitzky D, Lotem J, et
al: Wild-type p53 induces apoptosis of myeloid leukaemic cells that
is inhibited by interleukin-6. Nature. 352:345–347. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lowe SW, Schmitt EM, Smith SW, et al: p53
is required for radiation-induced apoptosis in mouse thymocytes.
Nature. 362:847–849. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Oda E, Ohki R, Murasawa H, et al: Noxa, a
BH3-only member of the Bcl-2 family and candidate mediator of
p53-induced apoptosis. Science. 288:1053–1058. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Nakano K and Vousden KH: PUMA, a novel
proapoptotic gene, is induced by p53. Mol Cell. 7:683–694. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Sheu MJ, Chou PY, Huang CS, et al:
Pipoxolan inhibits proliferation of HL-60 human leukaemia cancer
cells by arresting the cell cycle at the G0/G1 phase. Clin Exp
Pharmacol Physiol. 37:605–612. 2010. View Article : Google Scholar
|
|
57
|
Benson RS, Heer S, Dive C and Watson AJ:
Characterization of cell volume loss in CEM-C7A cells during
dexamethasone-induced apoptosis. Am J Physiol. 270:C1190–C1203.
1996.
|
|
58
|
Allen RT, Hunter WJ and Agrawal DK:
Morphological and biochemical characterization and analysis of
apoptosis. J Pharmacol Toxicol Meth. 37:215–228. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Prives C and Hall PA: The p53 pathway. J
Pathol. 187:112–126. 1999. View Article : Google Scholar
|