Introduction
Esophagectomy is one of the most invasive treatments
in gastrointestinal surgery (1).
Post-operative pulmonary complications such as pneumonia, acute
lung injury (ALI) and acute respiratory distress syndrome (ARDS)
prolong the duration of mechanical ventilation and have been found
to be strongly associated with increased morbidity and mortality
after esophagectomy (2). Surgical
procedures for esophagectomy also induce systemic inflammatory
response syndrome (SIRS), characterized by the overproduction of
pro-inflammatory cytokines, which elicit excessive stress and may
trigger post-operative complications (2).
Previously, we showed that one-lung ventilation
(OLV) more potently induced pulmonary inflammation [interleukin
(IL)-6 production] in the ventilated-dependent lung (DL) compared
with the collapsed non-dependent lung (NDL) during lung resection
(3). Moreover, the inflammatory
response in the ventilated DL was significantly suppressed by
volatile anesthetic agents (such as sevoflurane and desflurane)
compared with an intravenous anesthetic agent (such as propofol) in
patients undergoing lung resection (4–7).
However, the effect of those anesthetic agents on the inflammatory
response in the ventilated DL and collapsed NDL induced by OLV has
yet to be evaluated, particularly during esophagectomy that causes
several complications in the lung. The aim of this study was to
compare the actions of anesthetic agents, sevoflurane and propofol
on inflammatory reaction in the ventilated DL and collapsed NDL
during esophagectomy. For this purpose, airway epithelial lining
fluid (ELF) was obtained by a bronchoscopic microsampling method
before and after esophagectomy anesthetized with sevoflurane or
propofol, and the in vivo production of inflammatory
cytokines and chemokine were measured using cytometric bead array
systems, and compared before and after the operation.
Materials and methods
Subjects
Twenty subjects [18 males and 2 females, age range
52–78; mean age ± standard deviation (SD), 68.6±6.4 years]
classified as American Society of Anesthesiologists Physical Status
category I–II, undergoing cervico-thoraco-abdominal three-field
lymph node dissection through a right thoracotomy were recruited in
this study. The study protocol was approved by the local ethics
committee (Juntendo University Hospital, Tokyo, Japan) and
conducted from December, 2011 to November, 2012 in accordance with
the principles of the amended Declaration of Helsinki and Ethical
Guidelines for Epidemiological Research (http://aje.oxfordjournals.org/content/170/11/1451.full).
Subjects provided written informed consent prior to participating
in the study. None of the subjects received premedication.
Exclusion criteria were neurologic or psychiatric
disease, cardiac disease classified as NYHA classes II–IV
(http://www.abouthf.org/questions_stages.htm),
preoperative severe impairment of respiratory function (such as a
vital capacity of <50% or a forced expiratory volume in 1 sec of
<50% of that predicted), and pre-existing coagulopathy or
thrombocytopenia. Subjects were also excluded if they exhibited
systemic or local active infections (either clinically defined or
evidenced by elevated C-reactive protein levels, leukocytosis or
body temperature of >38°C).
Subjects were randomly assigned to a sevoflurane
(n=10), and a propofol (n=10) group, using a list of random numbers
generated by computer software (Microsoft Excel; Microsoft Corp.,
Redmond, WA, USA).
Study protocols
All the patients underwent general anesthesia
combined with epidural anesthesia. Prior to surgery, a thoracic
epidural catheter was inserted into the intervertebral space
between T7 and T8, or T9 and T10 for pain management.
Protocol 1 (sevoflurane group) involved the
induction of anesthesia with intravenous injection of propofol (1–2
mg/kg; AstraZeneca, Osaka, Japan), rocuronium (0.9 mg/kg; MSD K.K.,
Tokyo, Japan) and remifentanil (0.15–0.3 μg/kg/min; Janssen
Pharmaceutical K.K., Tokyo, Japan). Each subject was then intubated
with an endotracheal tube (Rüsch® endotracheal tube;
Teleflex Medical Sdn Bhd, Kamunting, Malaysia), and the position of
the endotracheal tube was confirmed by a fiber-optic bronchoscopy
(Portable Intubation Fiberscope FI-10RBS; Pentax, Tokyo, Japan).
After end-tidal CO2 was confirmed, anesthesia was
maintained with the inhalation of sevoflurane (0.8–1.5 minimum
alveolar concentration; Maruishi Pharmaceutical Co., Ltd., Osaka,
Japan) and intravenous infusion of remifentanil (0.15–0.5
μg/kg/min).
Protocol 2 (propofol group) involved the induction
of anesthesia with intravenous infusion of propofol (using a
target-controlled infusion technique with a target concentration of
3–4 μg/ml), rocuronium (0.9 mg/kg) and remifentanil (0.15–0.5
μg/kg/min). Each subject was then intubated with an endotracheal
tube, and end-tidal CO2 was confirmed. Anesthesia was
maintained with intravenous infusion of propofol (a target
concentration of 2–4 μg/ml) and remifentanil (0.15–0.5
μg/kg/min).
Following intubation, the patients were placed in a
left lateral position. The subjects were ventilated by
pressure-controlled ventilation with 4-cm H2O-positive
end-expiratory pressure, and peak inspiratory pressure was
maintained at >20 cm H2O with a tidal volume of 7–10
ml/kg, with the fraction of inspired oxygen (FIO2) being
maintained at 0.4. The oxygen saturation was adjusted to >97%,
and the respiratory rate was adjusted in order that normocapnia
could be maintained (normal arterial carbon dioxide pressure).
General anesthesia was maintained using sevoflurane or propofol,
with epidural anesthesia using ropivacaine (Maruishi Pharmaceutical
Co., Ltd.) and 3 mg morphine hydrate (Daiichi Sankyo Company, Ltd.,
Tokyo, Japan).
During esophagectomy, OLV was performed using a
Coopdech endobronchial blocker tube (Daiken Medical Co., Ltd,
Osaka, Japan), and the FIO2 of the left lung (DL) was
controlled to maintain SaO2 >90%. When the peak
inspiratory pressure was >30 cm H2O, the position of
an endobronchial blocker tube was confirmed using a fiber-optic
bronchoscope. Bronchial suction was subsequently performed, and the
tidal volume was reduced to 5–7 ml/kg, when required. Following the
thoracic esophagectomy and the posterior mediastinal lymph node
dissection, the endobronchial blocker tube was removed and the
right lung (collapsed NDL) was manually inflated until visible
atelectasis was ameliorated, and two-lung ventilation was resumed.
Thereafter, the patients were placed in the supine position, and
post-operative analgesia was provided with continuous epidural
infusion of 0.2% ropivacaine plus 5 mg morphine/day. A gastric or
colonic tube was constructed at laparotomy, and cervical
esophagogastrostomy was performed. After confirming that the forced
vital capacity was >10 ml/kg and the circulation was stable, the
endotracheal tube was removed, and then the patients were
transferred to the intensive care unit. ALI and ARDS were evaluated
throughout the post-surgical period. ALI and ARDS were defined
according to the American European Consensus Conference on ARDS
criteria (8). Additional criteria
included bilateral infiltrations on plain chest radiographs and no
clinical evidence of left atrial hypertension. The duration of SIRS
after surgery was evaluated according to the definition of the
American College of Chest Physicians/Society of Critical Care
Medicine. SIRS is characterized by two or more of the following
conditions: i) body temperature >38°C or <36°C, ii) heart
rate >90 beats/min, iii) respiratory rate >20 beats/min or
PaCO2 <32 mm Hg and iv) white blood cell count
>12,000 cells/mm3, <4,000 cells/mm3 or
10% immature (band) forms (9).
Bronchoscopic microsampling
ELF was obtained by a bronchoscopic microsampling
method before and after OLV. A bronchofiberscope was inserted into
the trachea through an endotracheal tube and placed at the
bifurcation. A bronchoscopic microsampling probe (BC-402C; Olympus,
Tokyo, Japan) was inserted into the right and left main bronchi
through the channel of the bronchofiberscope: the probe comprised a
2.6-mm outer-diameter polyethylene sheath and an inner 1.9-mm
diameter cotton probe (20 mm length) attached to a stainless steel
guidewire. The inner probe was gently advanced 7 cm from the
bifurcation into the right and left main bronchi until it made
contact with the mucosal surface. ELF was obtained from the mucosal
surface under direct observation. The inner probe was then
withdrawn, and the probe was sectioned at 30 mm from its tip and
stored at −80°C until analysis. Furthermore, peripheral blood was
collected before and after OLV, simultaneously with ELF sampling,
and one day after the surgery. Sera were prepared from blood
samples by centrifugation at 1,700 × g for 20 min and stored at
−80°C until analysis.
Measurement of cytokines
The probe was weighed and mixed with 500 μl
distilled water by vortexing for 1 min, and the solution was
recovered. The probe was then dried and reweighed to estimate the
recovered ELF by subtracting the weight of dried probes from that
of wet probes (the difference of the weight of wet probe and dried
probe was ~10 mg), after which the dilution factor was calculated
(10).
Inflammatory cytokine and chemokine levels in ELF
were measured using the cytometric bead array systems (Human
Inflammatory Cytokine CBA kit; Becton-Dickinson, Franklin Lakes,
NJ, USA). The human Inflammatory Cytokine kit included six
fluorescently distinguishable capture microbeads coated with
antibodies against the analytes, tumor necrosis factor (TNF)-α,
IL-1β, IL-6, IL-8, IL-10 and IL-12p70. This method can detect
cytokines bound onto microbeads by using an enzyme-linked
immunosorbent assay. The minimum quantifiable levels of cytokines
detected with the Human Inflammatory Cytokine kit were 3.7 for
TNF-α, 7.2 for IL-1β, 2.5 for IL-6, 3.6 for IL-8, 3.3 for IL-10 and
1.9 pg/ml for IL-12p70. The cytokine levels in sera before and
after OLV were also measured using the cytometric bead array
systems without dilution.
Statistical analysis
Data are shown as the means ± SD. Statistical
significance was determined by one-way ANOVA, the Student’s t-test
or the Fisher’s exact test (GraphPad Prism 5; GraphPad, San Diego,
CA, USA). P<0.05 was considered to be significant.
Results
Patient characteristics
Patient characteristics are shown in Table I. Gender, age, body mass indices,
vital capacity, forced expiratory volume in 1 sec (FEV1.0), partial
pressure of oxygen in arterial blood (PaO2), the
durations of surgery, anesthesia and OLV, estimated blood loss and
the volume of fluid administration (crystalloid solution and
Hespander fluid solution containing hydroxyethyl starch; Otsuka
Pharmaceutical Factory, Inc., Tokushima, Japan) and blood
transfusion during surgery did not significantly differ between the
sevoflurane and propofol groups.
 | Table IClinical characteristics and surgical
data of the sevoflurane and propofol groups. |
Table I
Clinical characteristics and surgical
data of the sevoflurane and propofol groups.
|
Characteristics | Sevoflurane
(n=10) | Propofol
(n=10) |
|---|
| Gender
(male/female) | 9/1 | 9/1 |
| Age (years) | 70.7±5.1 | 66.4±7.1 |
| Body mass index
(kg/m2) | 22.0±2.5 | 21.7±2.1 |
| Vital capacity
(%) | 104±13 | 103±13 |
| FEV1.0 (%) | 72±8 | 71±8 |
| PaO2 (mm
Hg) | 88±7 | 93±13 |
| Duration of
anesthesia (min) | 485.2±60.7 | 529.6±78.4 |
| Duration of surgery
(min) | 389.5±59.6 | 422.7±35.4 |
| Duration of OLV
(min) | 190.4±39.6 | 191.2±39.1 |
| Blood loss
(ml) | 777±582 | 1052±889 |
| Fluid
administration (ml) | 4432±660 | 5607±1690 |
| Blood transfusion
(ml) | 106±257 | 144±455 |
Comparison of cytokine levels in ELF
between the sevoflurane and propofol groups before and after
OLV
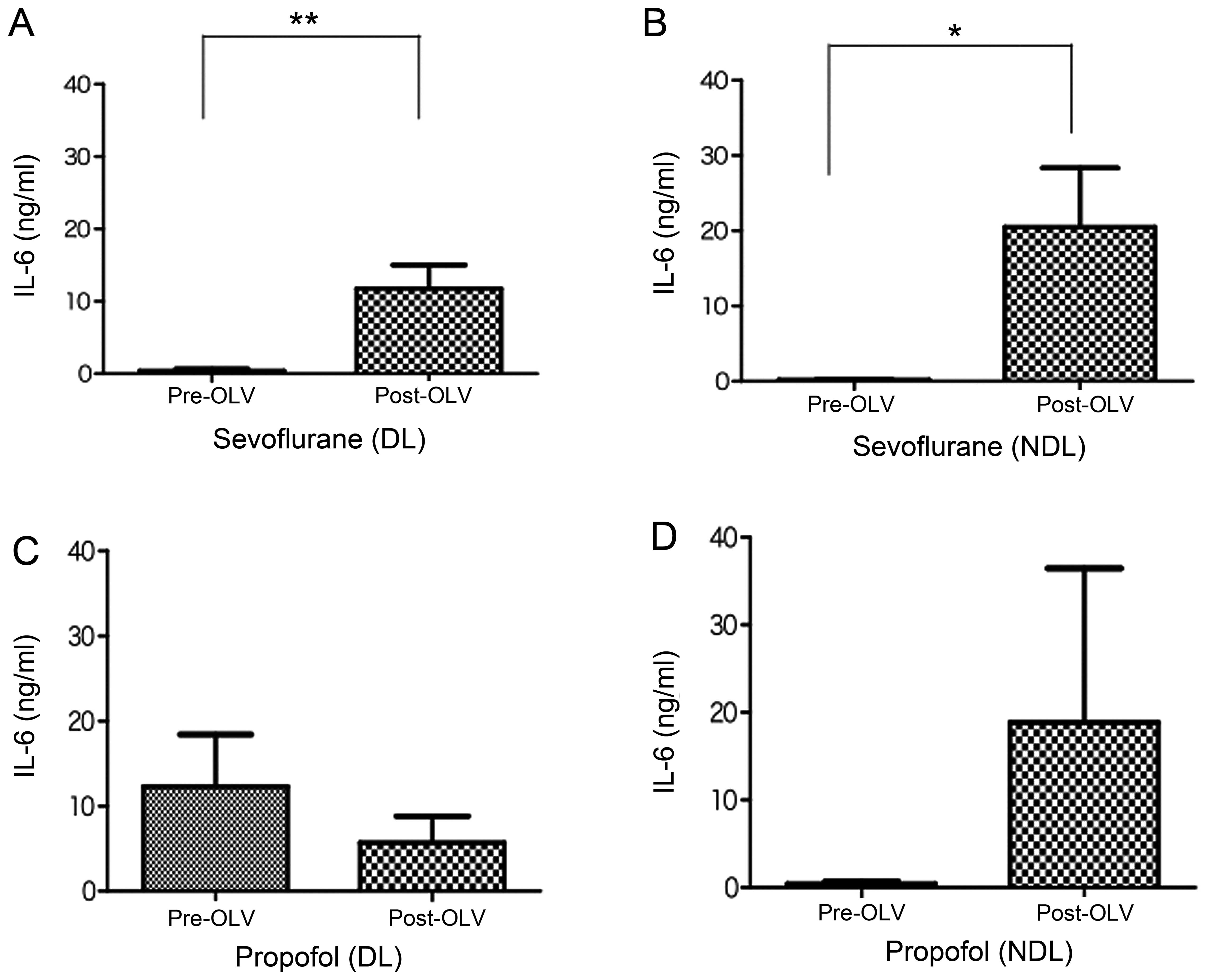
Fig. 1 shows the
changes in the levels of IL-6 in ELF recovered before and after OLV
from the ventilated DL and collapsed NDL of the sevoflurane and
propofol groups. The levels of IL-6 in ELF were significantly
increased in the ventilated DL and collapsed NDL after OLV compared
with levels prior to OLV in the sevoflurane group (P<0.05)
(Fig. 1A and B). In contrast,
there was no significant change in the IL-6 levels in the propofol
group in both the ventilated DL and collapsed NDL before and after
OLV (Fig. 1C and D), although the
IL-6 level was slightly decreased in the ventilated DL after OLV
(Fig. 1C).
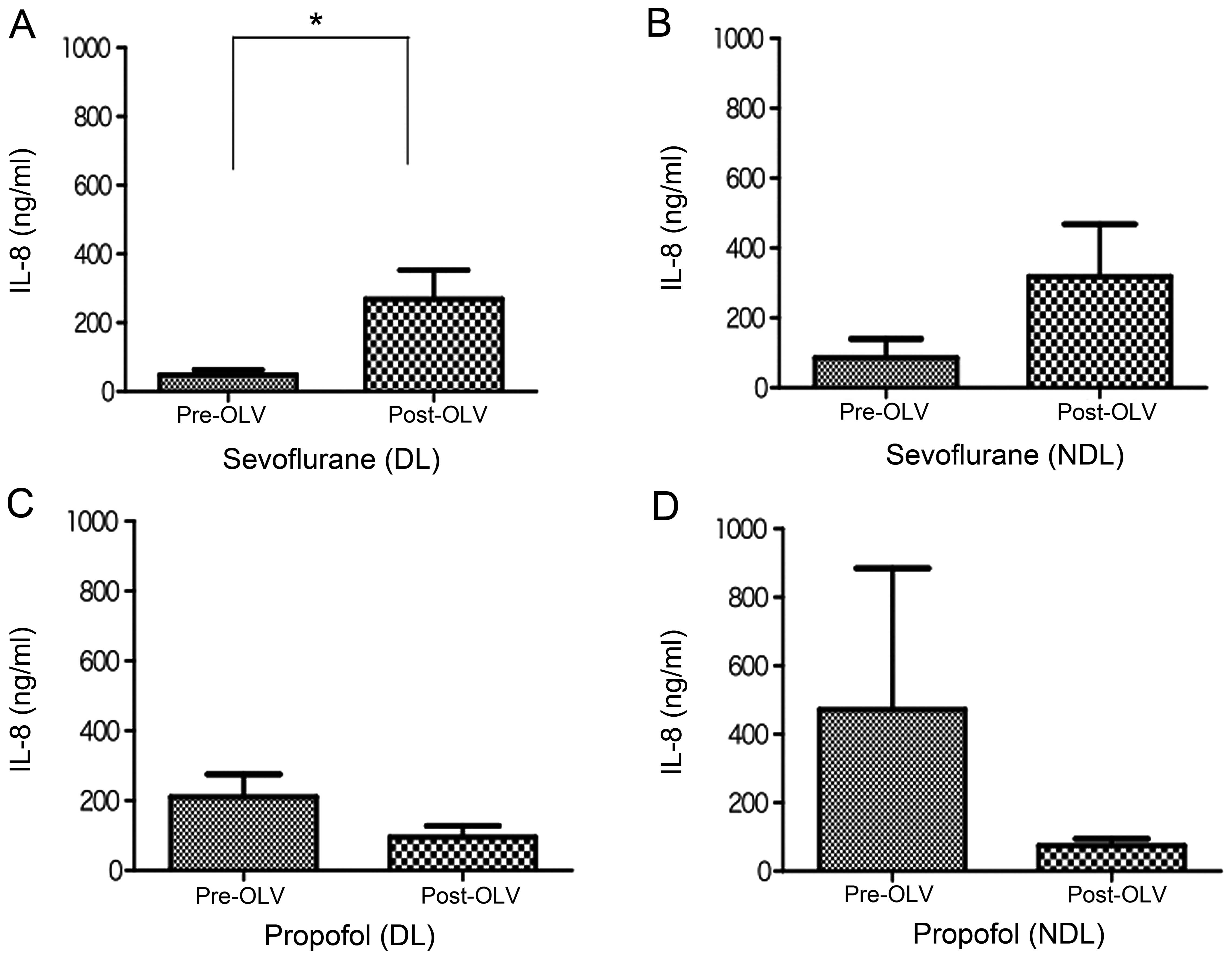
Fig. 2 shows the
changes in the levels of IL-8 in ELF recovered before and after OLV
from the ventilated DL and collapsed NDL of the sevoflurane and
propofol groups. Similar to the changes in IL-6 levels, the IL-8
levels were markedly increased in the ventilated DL and collapsed
NDL after OLV compared with those prior to OLV in the sevoflurane
group (Fig. 2A and B), and the
increase in the IL-8 level after OLV was significant in the
ventilated DL (P<0.05) (Fig.
2A). By contrast, there was essentially no significant change
in the IL-8 levels in the propofol group in the ventilated DL and
collapsed NDL before and after OLV (Fig. 2C and D), although the IL-8 levels
were slightly decreased in the ventilated DL and collapsed NDL
after the OLV.
Fig. 3 shows the
changes in the levels of IL-10 in ELF recovered before and after
OLV from the ventilated DL and collapsed NDL of the sevoflurane and
propofol groups. In contrast to the changes in IL-6 and IL-8
levels, no clear change was observed in the IL-10 levels in the
ventilated DL and collapsed NDL before and after OLV in the
sevoflurane group (Fig. 3A and
B). IL-10 levels in the propofol group, however, were increased
in the ventilated DL and collapsed NDL after OLV compared with
those prior to OLV (Fig. 3C and
D), although the changes were not significant. The levels of
TNF-α, IL-1β and IL-12p70 in ELF were below the detection
limits.
Comparison of cytokine levels in sera
between the sevoflurane and propofol groups before and after
OLV
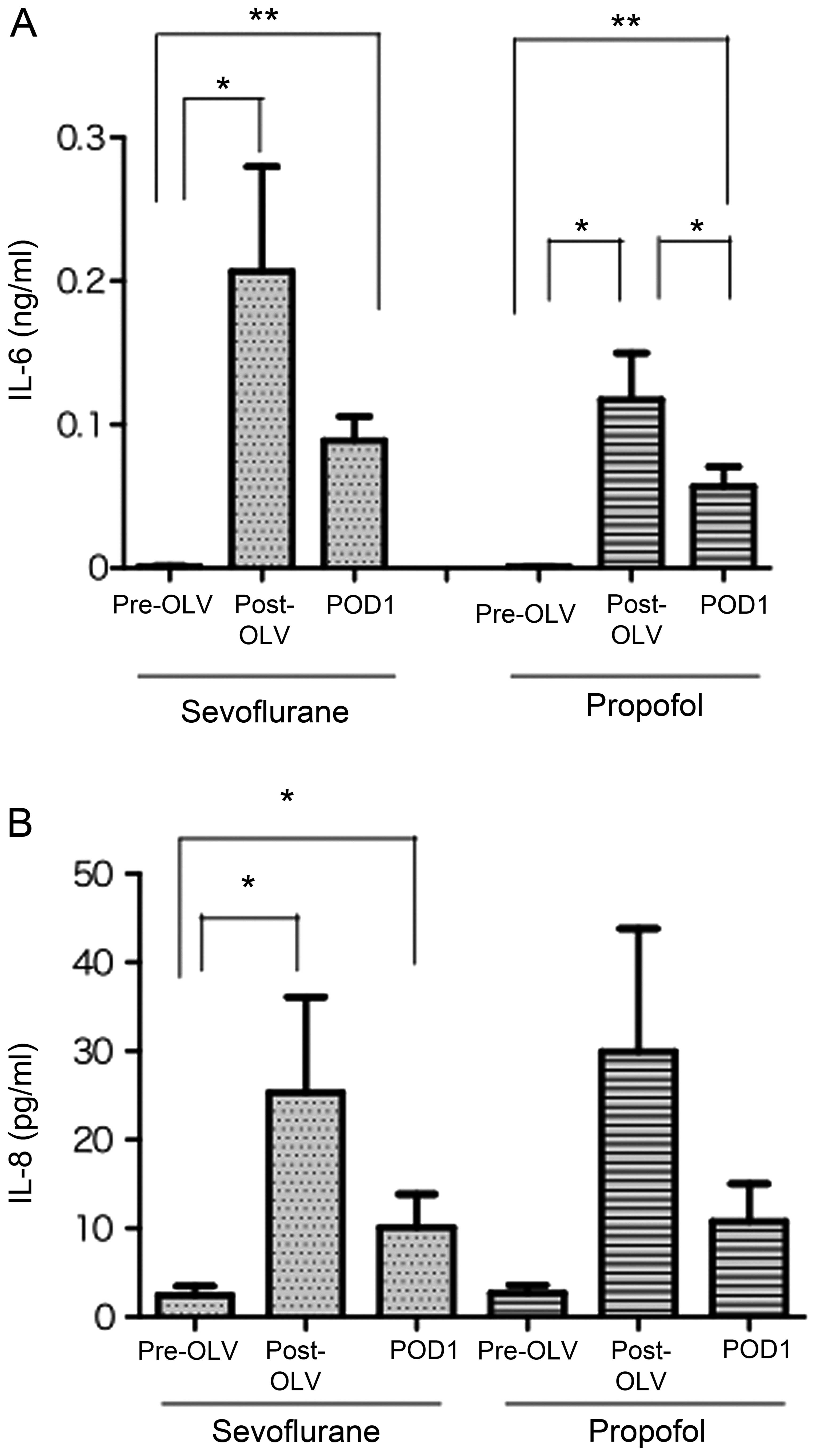
Fig. 4 shows the
changes in the serum levels of IL-6 and IL-8 before and after OLV,
and one day after the surgery in the sevoflurane and propofol
groups. In the sevoflurane and propofol groups, the serum levels of
IL-6 and IL-8 were markedly increased after OLV compared with those
prior to OLV, and slightly decreased one day after the surgery
(Fig. 4A and B). Notably, the
serum levels of IL-6 and IL-8 before and after OLV and one day
after the surgery were not significantly different between the
sevoflurane and propofol groups. The levels of TNF-α, IL-1β, IL-10
and IL-12p70 in sera were below the detection limits.
Changes in the PaO2/FiO2 ratio during and
after the surgery
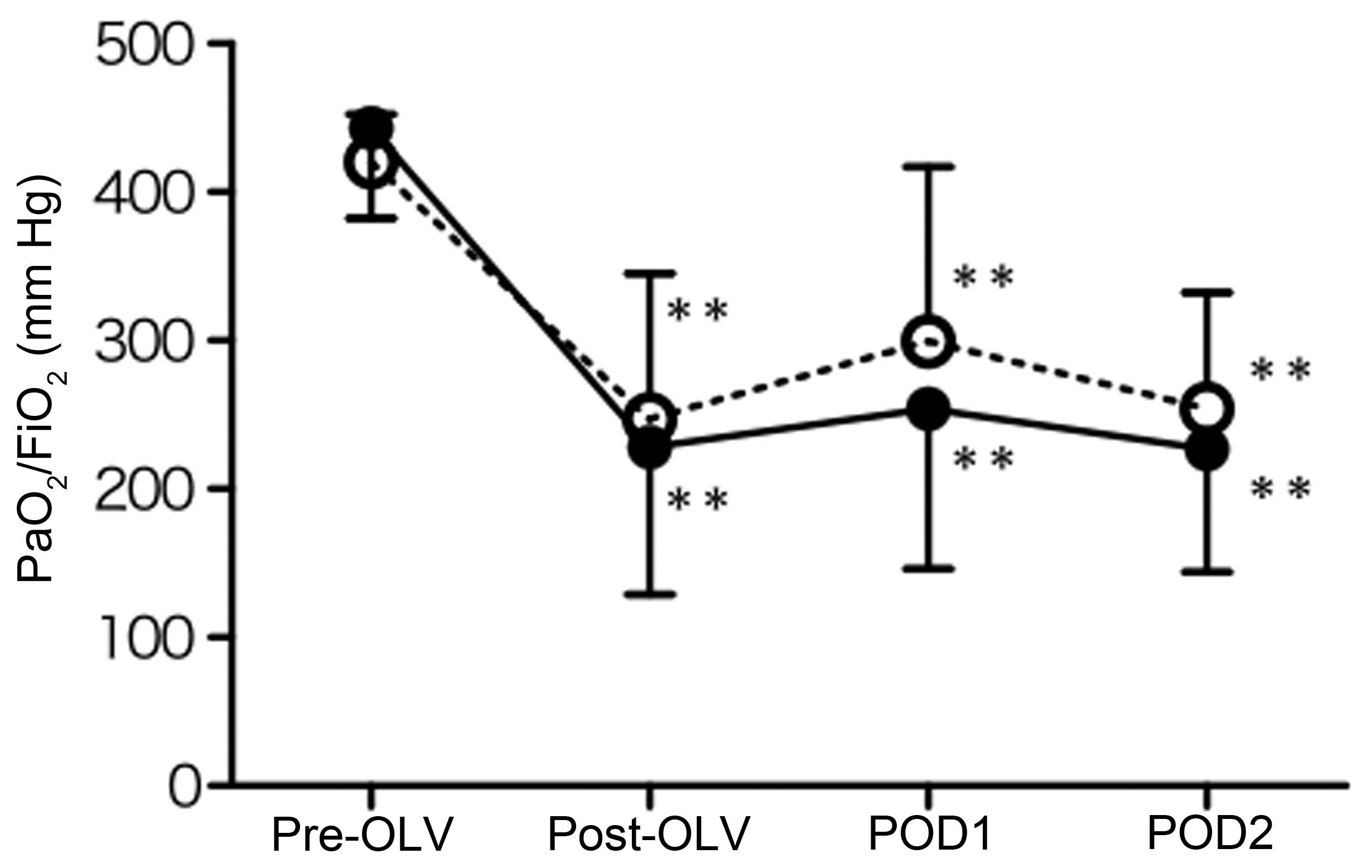
Fig. 5A shows the
changes in the ratio of PaO2/FiO2, one of the
indicators for ALI and ARDS, before and after OLV, one and two days
after the surgery in the sevoflurane and propofol groups. The
PaO2/FiO2 ratio was significantly decreased
after OLV compared with those before OLV (P<0.01), and
maintained at almost the same level one and two days after the
surgery in the sevoflurane and propofol groups. The
PaO2/FiO2 ratio was not significantly
different between the sevoflurane and propofol groups at each point
(Fig. 5).
Durations of ALI, ARDS and SIRS after the
surgery
The durations of ALI, ARDS and SIRS were not
statistically different between the sevoflurane and propofol groups
after the surgery (Fig. 6). In
addition, there were a few incidences of SIRS in the two groups
(Table II), although there was
no significant difference between the two groups.
 | Table IIDevelopment of SIRS during the
post-operative course in sevoflurane and propofol groups. |
Table II
Development of SIRS during the
post-operative course in sevoflurane and propofol groups.
| Groups | Post-OLV | POD1 | POD2 |
|---|
| Sevoflurane | 2 | 2 | 3 |
| Propofol | 1 | 2 | 4 |
Discussion
Findings of previous studies have shown that airway
epithelial cells express and secrete various inflammatory and
immune molecules including cytokines (TNF-α, IL-1β, IL-6 and IL-10)
and chemokines (IL-8 and MCP-1) (5,11,12). TNF-α, IL-1β and IL-6 function as
proinflammatory molecules, whereas IL-8 and MCP-1 act as
chemoattractants that are responsible for the recruitment of
effector cells such as neutrophils and monocytes (13,14). Moreover, IL-10 suppresses the
pro-inflammatory cytokine production and the antigen-presenting
capacity of monocytes/macrophages and dendritic cells (15). The expression and production of
these molecules signifies that the airway epithelium plays an
important role in the initiation and exacerbation of inflammatory
responses within the airways (16).
Anesthesia and surgical trauma induce immunological
and inflammatory responses by stimulating airway epithelial cells
to produce pro- and anti-inflammatory cytokines in the lung
(17). In this context,
anesthetic agents such as sevoflurane and propofol modulate the
inflammatory reactions in the local milieu of the airway (4). Moreover, esophagectomy is one of the
most invasive treatments in gastrointestinal surgery (1), triggering the inflammatory reaction,
with the increased levels of pro-inflammatory cytokines IL-6 and
IL-8, and an anti-inflammatory cytokine IL-10 in bronchoalveolar
lavage (18,19). In addition, OLV, an anesthetic
procedure, induces the inflammatory reaction more potently in the
ventilated DL compared with the collapsed NDL (3). Thus, in the present study, we
compared the actions of anesthetic agents, sevoflurane and propofol
on the inflammatory reaction in the ventilated DL and collapsed NDL
during esophagectomy before and after OLV.
The present results show that the levels of IL-6 in
ELF were significantly increased in the ventilated DL and collapsed
NDL after OLV compared with those prior to OLV in the sevoflurane
group. By contrast, no significant change was observed in the IL-6
levels in the propofol group in the ventilated DL and collapsed NDL
before and after OLV. Similar to the changes in IL-6 levels, the
IL-8 levels were markedly increased in the ventilated DL and
collapsed NDL after OLV compared with those prior to OLV in the
sevoflurane group, whereas there was no significant change in the
IL-8 levels in the propofol group in the ventilated DL and
collapsed NDL before and after OLV. In contrast to the changes in
IL-6 and IL-8 levels, no clear change was evident in the IL-10
levels in the ventilated DL and collapsed NDL before and after OLV
in the sevoflurane group. IL-10 levels in the propofol group,
however, were increased in the ventilated DL and collapsed NDL
after OLV compared with those before OLV. These observations
suggest that propofol anesthesia more potently suppresses surgical
stress-induced inflammatory perturbation compared with sevoflurane
anesthesia during esophagectomy. In this context, it is of note
that propofol decreases the secretion of IL-8 from
lipopolysaccharide (LPS)-stimulated human neutrophils (20,21). It is reported that propofol, at
clinically relevant concentrations, can reduce inflammatory
responses in LPS-induced alveolar type II cell injury through the
downregulation of CD14 and Toll-like receptor 4 expression
(22). Furthermore, propofol is
indicated to protect endothelial cells against toxic-free radicals
in vitro (23,24). By contrast, it has been shown that
anesthesia with sevoflurane leads to the depressed bronchociliary
clearance compared with propofol in vivo (25), a depression of the bronchial cilia
function is associated with the increased rate of pulmonary
complications. Sevoflurane anesthesia is reported to increase the
plasma levels of IL-6, TNF-α and IL-1β (26–28). In addition to the effects on the
inflammatory responses (cytokine/chemokine production), the
inhalation anesthetics such as sevoflurane and desflurane, which
comes directly into contact with airway epithelium and alveolar
macrophages, potently induce apoptosis and result in the decrease
of alveolar macrophages in blonchoalveolar fluid (BALF) (29). Propofol, however, which is
intravenously administered, exhibits anti-inflammatory and
anti-oxidant actions during mechanical ventilation, thus preventing
apoptosis of alveolar cells (30,31). Based on these findings, it can be
hypothesized that propofol exerts a more protective effect on the
local milieu of the airway compared with sevoflurane during
surgical stress with esopagectomy.
Damage of the alveolocapillary unit leads to an
increase in alveolar permeability and recruitment of neutrophils
and monocytes/macrophages into the alveolar space (32,33). During these processes,
pro-inflammatory cytokines are released from pulmonary epithelial
cells and inflammatory cells, resulting in an excessive
inflammatory response such as ARDS (34). By contrast, among cytokines, IL-10
has anti-inflammatory abilities and inhibits the synthesis of
pro-inflammatory cytokines (31).
Results of a previous study showed an increased anti-inflammatory
response with higher levels of IL-10 in patients receiving propofol
anesthesia compared with inhalation anesthesia with isoflurane in
abdominal surgery (32). In
addition, evidence suggests that the anti-inflammatory response
with the production of IL-10 is an important factor for reducing
the complications of major abdominal surgery, since the lower
IL-10/TNF-α ratio increases the occurrence of post-operative
complications (33). Similarly,
the present study results revealed that the IL-10 levels in the ELF
were increased in the ventilated DL and collapsed NDL in the
propofol group after OLV compared with the sevoflurane group
(Fig. 3C and D). Moreover, IL-6
and IL-8 levels were not essentially increased in the propofol
group following OLV compared with the sevoflurane group. These
observations suggest that propofol likely reduces the
post-operative complications by exhibiting anti-inflammatory action
at the airway through the induction of IL-10 production and
suppression of IL-6 and IL-8 production, although there was no
apparent difference in the clinical course such as durations of
ALI, ARDS and SIRDS after the esophagectomy between propofol and
sevoflurane anesthesia.
By analyzing the cytokine/chemokine levels in ELF,
we revealed that sevoflurane increased IL-6 and IL-8 but not IL-10
in ELF after OLV, whereas propofol increased IL-10 but not IL-6 and
IL-8 in ELF after OLV. By contrast, the levels of IL6 and IL-8 in
sera were almost the same between the sevoflurane and propofol
groups before and after OLV, IL6 and IL-8 similarly increased after
OLV, and decreased one day after the surgery in the sevoflurane and
propofol groups. These observations suggest that the inflammatory
response (the cytokine/chemokine production) at the airway cannot
be detected by the changes in the cytokine/chemokine levels in
sera. Of note, it has been reported that pro-inflammatory cytokines
such as IL-1β, IL-4, IL-6, TNF-α and IFN-γ are not significantly
increased in patients undergoing laparoscopic cholecystectomy
compared with open cholecystectomy (35). Moreover, the changes of serum
cytokoine/chemokine levels indicate the systemic inflammatory
response and depend on the invasive surgical procedure (36,37) such as esophagectomy. Thus, the
present study suggests that the cytokine/chemokine levels in ELF
may reflect the inflammatory response at the local milieu of the
airway more sensitively compared with those in sera during the
invasive esophageal surgery.
Previously, it has been shown that OLV more potently
induces the IL-6 production in the ventilated DL compared with the
collapsed NDL during lung resection with propofol anesthesia
(3), and that the inflammatory
response (cytokine production) in DL is significantly suppressed by
sevoflurane compared with propofol in patients undergoing lung
resection. By contrast, the present study revealed that there was
no essential difference in the IL-6 level in ELF between DL and NDL
during propofol anesthesia (Fig. 1C
and D), and that the cytokine production in DL was suppressed
by propofol but not sevoflurane in patients undergoing
esophagectomy (Figs. 1, and
2A and C). These discrepancies
remain to be elucidated. However, serum cytokine levels were not
significantly changed during lung resection (3,4),
but were markedly altered during esophagectomy (Fig. 4). These observations likely
suggest that esophagectomy with an invasive surgical procedure
(including, not only thoracotomy, but also laparotomy) induces a
more potent inflammatory response in the body, and modulates the
effects of anesthetic agents (sevoflurane and propofol) on the
inflammatory response at the airway, differently from lung
resection.
In conclusion, we have demonstrated that the
administration of propofol suppresses the IL-6 and IL-8 production
but enhances the IL-10 production in ELF compared with sevoflurane
during esophagectomy. Thus, propofol anesthesia may more potently
suppress the surgical stress-induced inflammatory perturbation at
the local milieu of the airway compared with sevoflurane anesthesia
during esophagectomy. However, it is crucial that the role of these
anesthetic agents be given more attention in the clinic due to
their ability to induce an inflammatory response and possibly
affect post-operative pulmonary and systemic complications.
References
|
1
|
Yamaguchi K, Sugasawa Y, Takeuchi K, et
al: Effects of sivelestat on bronchial inflammatory responses after
esophagectomy. Int J Mol Med. 28:187–192. 2011.PubMed/NCBI
|
|
2
|
Sato N, Endo S, Kimura Y, et al: Influence
of a human protease inhibitor on surgical stress induced
immunosuppression. Dig Surg. 19:300–305. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sugasawa Y, Yamaguchi K, Kumakura S, et
al: The effect of one-lung ventilation upon pulmonary inflammatory
responses during lung resection. J Anesth. 25:170–177. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sugasawa Y, Yamaguchi K, Kumakura S, et
al: Effects of sevoflurane and propofol on pulmonary inflammatory
responses during lung resection. J Anesth. 26:62–69. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
De Conno E, Steurer MP, Wittlinger M, et
al: Anesthetic-induced improvement of the inflammatory response to
one-lung ventilation. Anesthesiology. 110:1316–1326.
2009.PubMed/NCBI
|
|
6
|
Schilling T, Kozian A, Kretzschmar M, et
al: Effects of propofol and desflurane anaesthesia on the alveolar
inflammatory response to one-lung ventilation. Br J Anaesth.
99:368–375. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schilling T, Kozian A, Senturk M, et al:
Effects of volatile and intravenous anesthesia on the alveolar and
systemic inflammatory response in thoracic surgical patients.
Anesthesiology. 115:65–74. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bernard GR, Artigas A, Brigham KL, et al:
The American-European Consensus Conference on ARDS. Definitions,
mechanisms, relevant outcomes, and clinical trial coordination. Am
J Respir Crit Care Med. 149:818–824. 1994. View Article : Google Scholar
|
|
9
|
American College of Chest
Physicians/Society of Critical Care Medicine Consensus Conference:
definitions for sepsis and organ failure and guidelines for the use
of innovative therapies in sepsis. Crit Care Med. 20:864–874. 1992.
View Article : Google Scholar
|
|
10
|
Nakayama H, Kitayama J, Muto T and Nagawa
H: Characterization of intracellular cytokine profile of
CD4+ T cells in peripheral blood and tumor-draining
lymph nodes of patients with gastrointestinal cancer. Jpn J Clin
Oncol. 30:301–305. 2000.PubMed/NCBI
|
|
11
|
Simon RH and Paine R III: Participation of
pulmonary alveolar epithelial cells in lung inflammation. J Lab
Clin Med. 126:108–118. 1995.PubMed/NCBI
|
|
12
|
Takizawa H: Airway epithelial cells as
regulators of airway inflammation (Review). Int J Mol Med.
1:367–378. 1998.PubMed/NCBI
|
|
13
|
Vozzelli MA, Mason SN, Whorton MH and
Auten RL Jr: Antimacrophage chemokine treatment prevents neutrophil
and macrophage influx in hyperoxia-exposed newborn rat lung. Am J
Physiol Lung Cell Mol Physiol. 286:L488–L493. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Beck-Schimmer B, Schwendener R, Pasch T,
Reyes L, Booy C and Schimmer RC: Alveolar macrophages regulate
neutrophil recruitment in endotoxin-induced lung injury. Respir
Res. 6:612005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Asadullah K, Sterry W and Volk HD:
Interleukin-10 therapy-review of a new approach. Pharmacol Rev.
55:241–269. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kumakura S, Yamaguchi K, Sugasawa Y, et
al: Effects of nitrous oxide on the production of cytokines and
chemokines by the airway epithelium during anesthesia with
sevoflurane and propofol. Mol Med Rep. 8:1643–1648. 2013.PubMed/NCBI
|
|
17
|
Kotani N, Hashimoto H, Sessler DI, et al:
Intraoperative modulation of alveolar macrophage function during
isoflurane and propofol anesthesia. Anesthesiology. 89:1125–1132.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sato N, Koeda K, Kimura Y, et al: Cytokine
profile of serum and bronchoalveolar lavage fluids following
thoracic esophageal cancer surgery. Eur Surg Res. 33:279–284. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Abe T, Oka M, Tangoku A, et al:
Interleukin-6 production in lung tissue after transthoracic
esophagectomy. J Am Coll Surg. 192:322–329. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Galley HF, Dubbels AM and Webster NR: The
effect of midazolam and propofol on interleukin-8 from human
polymorphonuclear leukocytes. Anesth Analg. 86:1289–1293.
1998.PubMed/NCBI
|
|
21
|
O’Donnell NG, McSharry CP, Wilkinson PC
and Asbury AJ: Comparison of the inhibitory effect of propofol,
thiopentone and midazolam on neutrophil polarization in vitro in
the presence or absence of human serum albumin. Br J Anaesth.
69:70–74. 1992.
|
|
22
|
Ma L, Wu X, Chen W and Fujino Y: Propofol
has anti-inflammatory effects on alveolar type II epithelial cells.
Acta Anaesthesiol Scand. 54:362–369. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Murphy PG, Ogilvy AJ and Whiteley SM: The
effect of propofol on the neutrophil respiratory burst. Eur J
Anaesthesiol. 13:471–473. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mathy-Hartert M, Mouithys-Mickalad A,
Kohnen S, Deby-Dupont G, Lamy M and Hans P: Effects of propofol on
endothelial cells subjected to a peroxynitrite donor (SIN-1).
Anaesthesia. 55:1066–1071. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ledowski T, Paech MJ, Patel B and Schug
SA: Bronchial mucus transport velocity in patients receiving
propofol and remifentanil versus sevoflurane and remifentanil
anesthesia. Anesth Analg. 102:1427–1430. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Takala RS, Soukka HR, Salo MS, et al:
Pulmonary inflammatory mediators after sevoflurane and thiopentone
anaesthesia in pigs. Acta Anaesthesiol Scand. 48:40–45. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kotani N, Takahashi S, Sessler DI, et al:
Volatile anesthetics augment expression of proinflammatory
cytokines in rat alveolar macrophages during mechanical
ventilation. Anesthesiology. 91:187–197. 1999. View Article : Google Scholar
|
|
28
|
Koksal GM, Sayilgan C, Gungor G, et al:
Effects of sevoflurane and desflurane on cytokine response during
tympanoplasty surgery. Acta Anaesthesiol Scand. 49:835–839. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kalimeris K, Christodoulaki K, Karakitsos
P, et al: Influence of propofol and volatile anaesthetics on the
inflammatory response in the ventilated lung. Acta Anaesthesiol
Scand. 55:740–748. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wei H, Liang G, Yang H, et al: The common
inhalational anesthetic isoflurane induces apoptosis via activation
of inositol 1,4,5-trisphosphate receptors. Anesthesiology.
108:251–260. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Moore KW, de Waal Malefyt R, Coffman RL
and O’Garra A: Interleukin-10 and the interleukin-10 receptor. Annu
Rev Immunol. 19:683–765. 2001. View Article : Google Scholar
|
|
32
|
Gilliland HE, Armstrong MA, Carabine U and
McMurray TJ: The choice of anesthetic maintenance technique
influences the antiinflammatory cytokine response to abdominal
surgery. Anesth Analg. 85:1394–1398. 1997.PubMed/NCBI
|
|
33
|
Dimopoulou I, Armaganidis A, Douka E, et
al: Tumour necrosis factor-alpha (TNFα) and interleukin-10 are
crucial mediators in post-operative systemic inflammatory response
and determine the occurrence of complications after major abdominal
surgery. Cytokine. 37:55–61. 2007.
|
|
34
|
Hudson LD, Milberg JA, Anardi D and
Maunder RJ: Clinical risks for development of the acute respiratory
distress syndrome. Am J Respir Crit Care Med. 151:293–301. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Helmy SA, Wahby MA and El-Nawaway M: The
effect of anaesthesia and surgery on plasma cytokine production.
Anaesthesia. 54:733–738. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Michelet P, D’Journo XB, Roch A, et al:
Protective ventilation influences systemic inflammation after
esophagectomy: a randomized controlled study. Anesthesiology.
105:911–919. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
D’Journo XB, Michelet P, Marin V, et al:
An early inflammatory response to oesophagectomy predicts the
occurrence of pulmonary complications. Eur J Cardiothorac Surg.
37:1144–1151. 2010.PubMed/NCBI
|