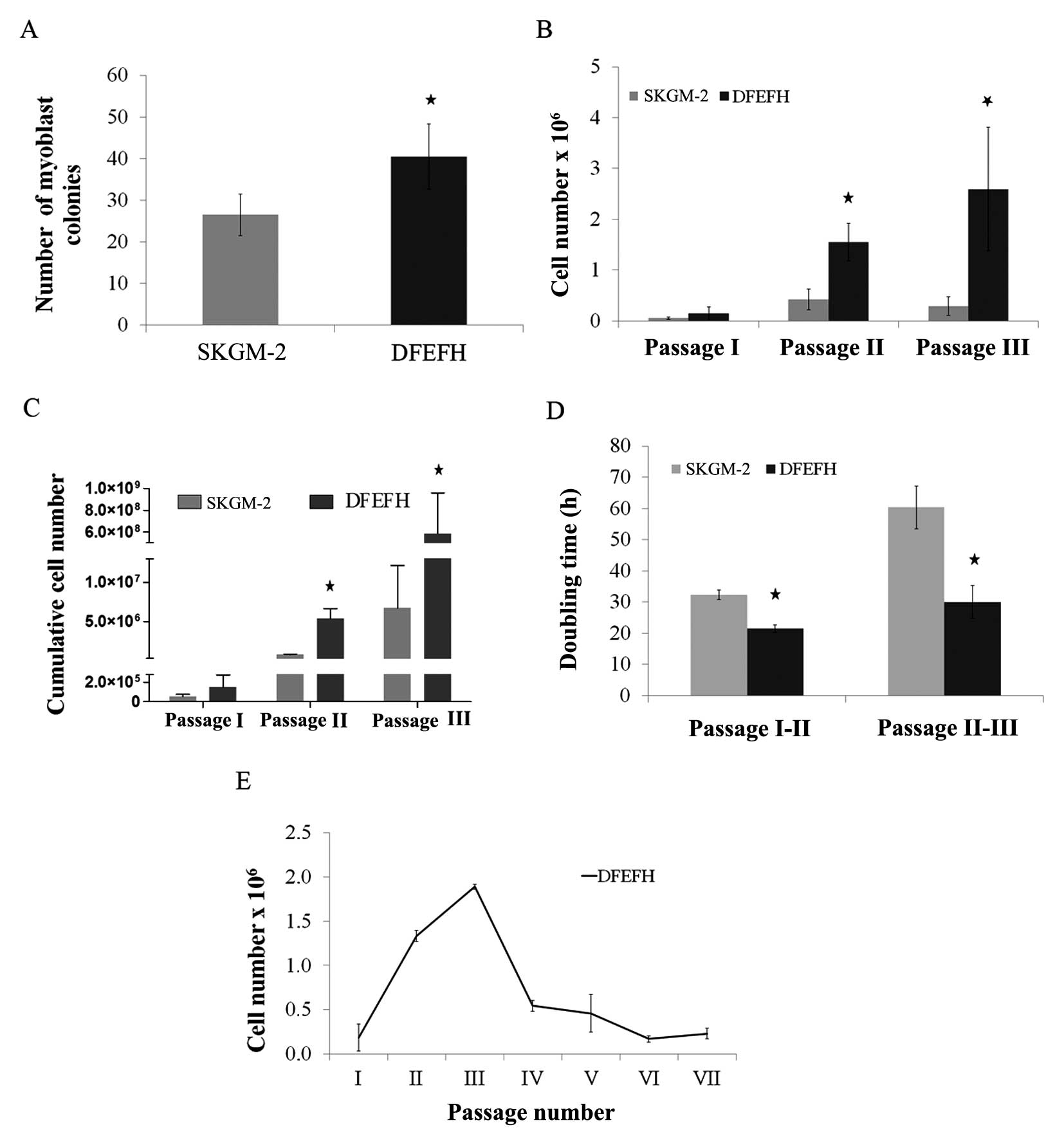
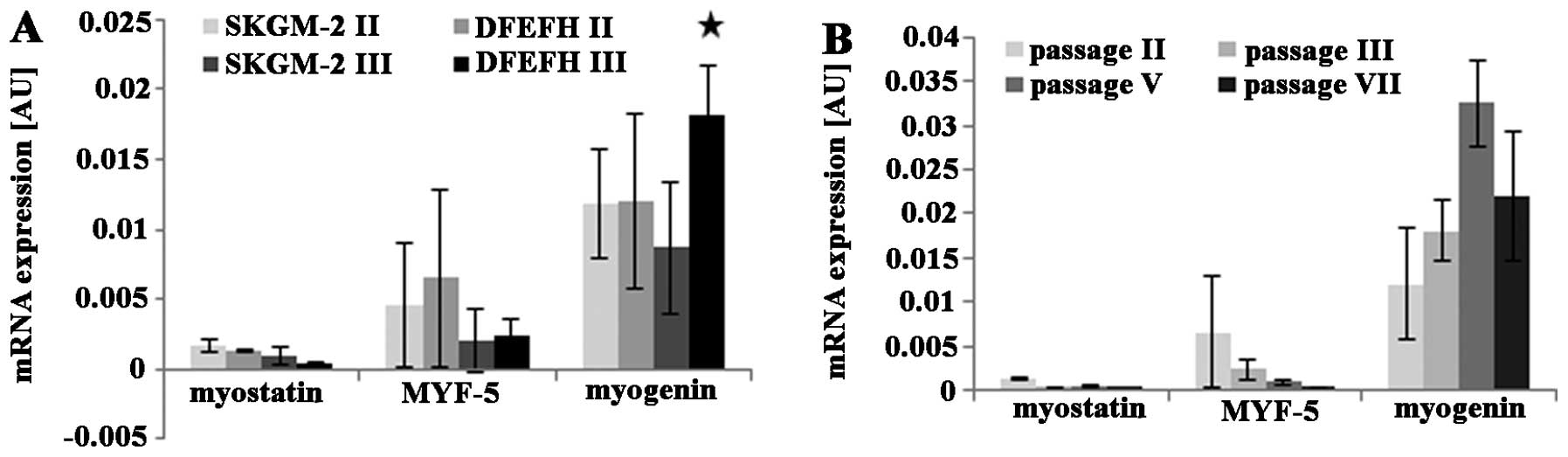
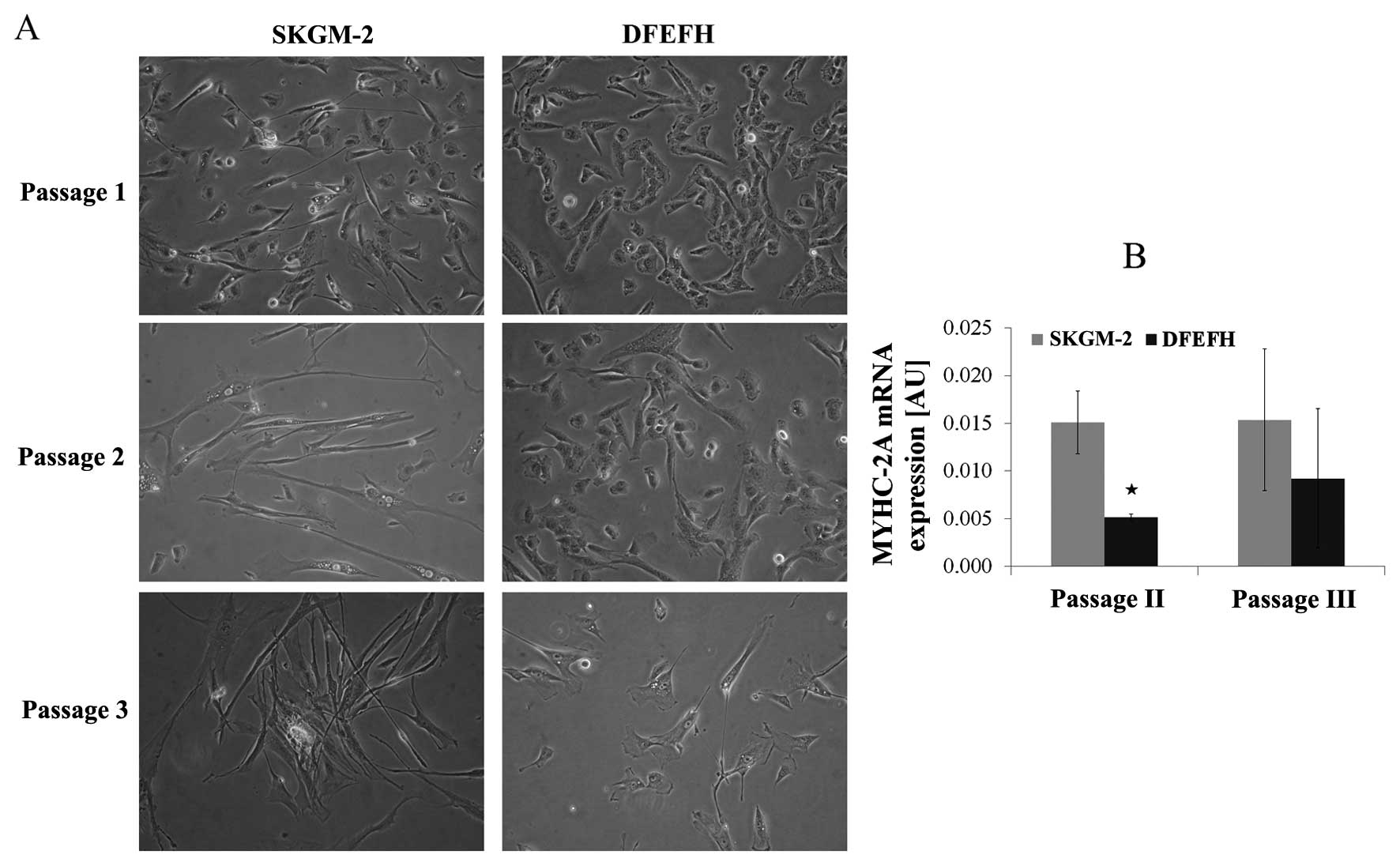
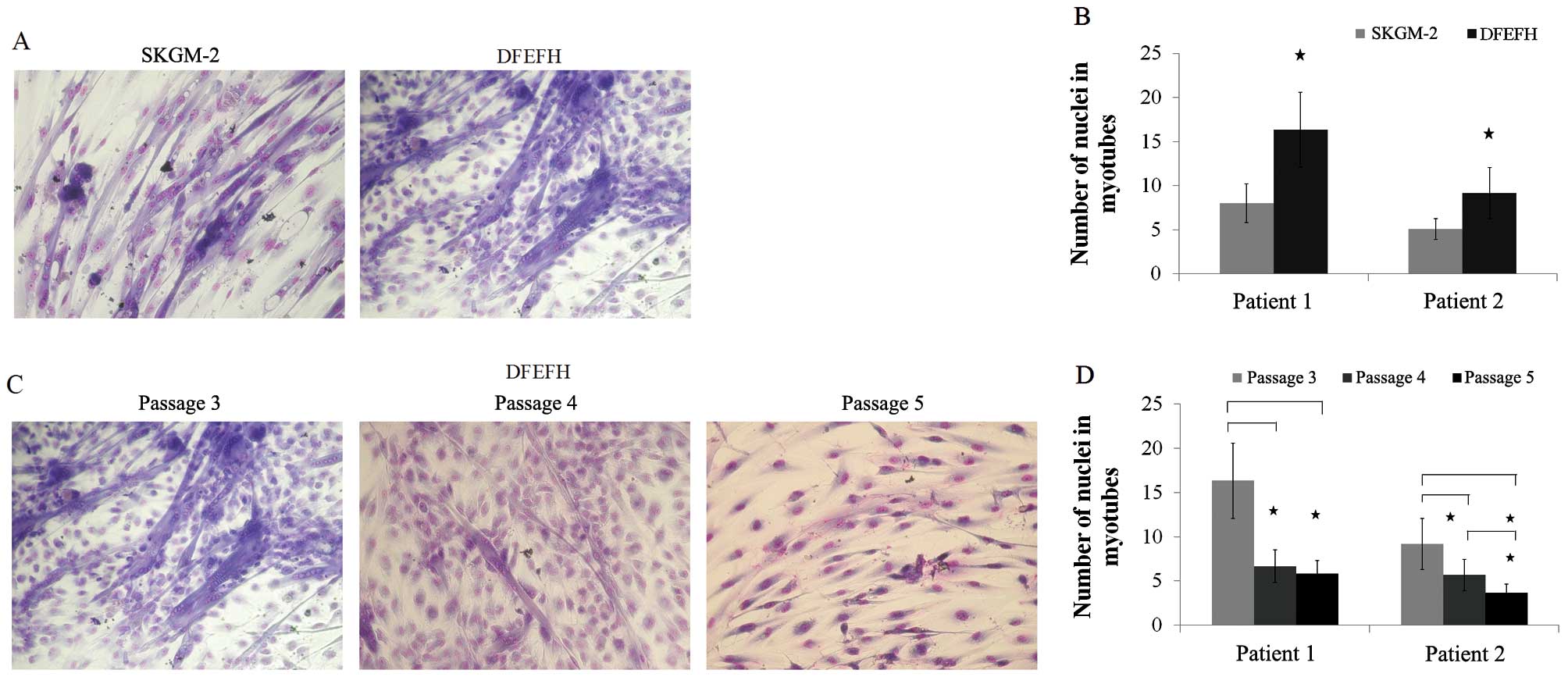
|
1
|
Peters K, Kaufman M, Dmochowski R, et al:
1340 Autologous muscle derived cell therapy for the treatment of
female stress urinary incontinence: A multi-center experience. J
Urol. 185:e535–e536. 2011. View Article : Google Scholar
|
|
2
|
Baj A, Bettaccini AA, Casalone R, et al:
Culture of skeletal myoblasts from human donors aged over 40 years:
dynamics of cell growth and expression of differentiation markers.
J Transl Med. 12:1–10. 2005.PubMed/NCBI
|
|
3
|
Pagani FD, DerSimonian H, Zawadzka A, et
al: Autologous skeletal myoblasts transplanted to ischemia-damaged
myocardium in humans. J Am Coll Cardiol. 41:879–888. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bischoff R: A satellite cell mitogen from
crushed adult muscle. Dev Biol. 115:140–147. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Clarke MS, Khakee R and McNeil PL: Loss of
cytoplasmic basic fibroblast growth factor from physiologically
wounded myofibers of normal and dystrophic muscle. J Cell Sci.
106:121–133. 1993.PubMed/NCBI
|
|
6
|
Allen RE, Sheehan SM, Taylor RG, et al:
Hepatocyte growth factor activates quiescent skeletal muscle
satellite cells in vitro. J Cell Physiol. 165:307–312. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tatsumi R, Anderson JE, Nevoret CJ, et al:
HGF/SF is present in normal adult skeletal muscle and is capable of
activating satellite cells. Dev Biol. 194:114–128. 1998. View Article : Google Scholar
|
|
8
|
Johnson SE and Allen RE: Activation of
skeletal muscle satellite cells and the role of fibroblast growth
factor receptors. Exp Cell Res. 219:449–453. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rao SS and Kohtz S: Positive and negative
regulation of d-type cyclin expression in skeletal myoblasts by
basic Fibroblasts growth factor and Transforming growth Factor
beta. J Biol Chem. 270:4093–4100. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sheehan SM and Allen RE: Skeletal muscle
satellite cell proliferation in response to members of the
fibroblast growth factor family and hepatocyte growth factor. J
Cell Physiol. 181:499–506. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Clegg CH, Linkhart TA, Olwin BB, et al:
Growth factor control of skeletal muscle differentiation:
commitment to terminal differentiation occurs in G1 phase and is
repressed by fibroblast growth factor. J Cell Biol. 105:949–956.
1987. View Article : Google Scholar
|
|
12
|
McGeachie JK and Grounds MD: Retarded
myogenic cell replication in regenerating skeletal muscles of old
mice: an autoradiographic study in young and old BALBc and SJL/J
mice. Cell Tissue Res. 280:277–282. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bischoff R: Proliferation of muscle
satellite cells on intact myofibers in culture. Dev Biol.
115:129–139. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Allen RE, Dodson MV and Luiten LS:
Regulation of skeletal muscle satellite cell proliferation by
bovine pituitary fibroblast growth factor. Exp Cell Res.
152:154–160. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Roe JA, Baba AS, Harper JM, et al: Effects
of growth factors and gut regulatory peptides on nutrient uptake in
ovine muscle cell cultures. Comp Biochem Physiol A Physiol.
110:107–114. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Alessandri G, Pagano S, Bez A, et al:
Isolation and culture of human muscle-derived stem cells able to
differentiate into myogenic and neurogenic cell lineages. Lancet.
364:1872–1883. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Boudreault P, Tremblay JP, Pépin MF, et
al: Scale-up of a myoblast culture process. J Biotechnol. 91:63–74.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Editor S, Mcinnes RR, La S, et al: The
molecular regulation of myogenesis. Clin Genet. 16:16–25. 2000.
|
|
19
|
Kitzmann M, Carnac G, Vandromme M, et al:
The muscle regulatory factors MyoD and myf-5 undergo distinct cell
cycle-specific expression in muscle cells. J Cell Biol.
142:1447–1459. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cooper RN, Tajbakhsh S, Mouly V, et al: In
vivo satellite cell activation via Myf5 and MyoD in regenerating
mouse skeletal muscle. J Cell Sci. 112:2895–2901. 1999.PubMed/NCBI
|
|
21
|
Hasty P, Bradley A, Morris JH, et al:
Muscle deficiency and neonatal death in mice with a targeted
mutation in the myogenin gene. Nature. 364:501–506. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rawls A, Valdez MR, Zhang W, et al:
Overlapping functions of the myogenic bHLH genes MRF4 and MyoD
revealed in double mutant mice. Development. 125:2349–2358.
1998.PubMed/NCBI
|
|
23
|
McPherron AC, Lawler AM and Lee SJ:
Regulation of skeletal muscle mass in mice by a new TGF-beta
superfamily member. Nature. 387:83–90. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
McCroskery S, Thomas M, Maxwell L, et al:
Myostatin negatively regulates satellite cell activation and
self-renewal. The J Cell Biol. 162:1135–1147. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Thomas M, Langley B, Berry C, et al:
Myostatin, a negative regulator of muscle growth, functions by
inhibiting myoblast proliferation. The J Biol Chem.
275:40235–40243. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Langley B, Thomas M, Bishop A, et al:
Myostatin inhibits myoblast differentiation by down-regulating MyoD
expression. The J Biol Chem. 277:49831–49840. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rios R, Carneiro I, Arce CM, et al:
Myostatin is an inhibitor of myogenic differentiation. Am J Physiol
Cell Physiol. 282:993–999. 2002. View Article : Google Scholar
|
|
28
|
Pette D and Staron RS: Myosin isoforms,
muscle fiber types, and transitions. Microsc Res Tech. 50:500–509.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chowdhury SR, Muneyuki Y, Takezawa Y, et
al: Growth and differentiation potentials in confluent state of
culture of human skeletal muscle myoblasts. J Biosci Bioeng.
109:310–313. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ham RG, St Clair JA, Webster C, et al:
Improved media for normal human muscle satellite cells: serum-free
clonal growth and enhanced growth with low serum. In Vitro Cell Dev
Biol. 24:833–844. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Stern-Straeter J, Bran G, Riedel F, et al:
Characterization of human myoblast cultures for tissue engineering.
Int J Mol Med. 21:49–56. 2008.
|
|
32
|
Sheehan SM, Tatsumi R, Temm-Grove CJ, et
al: HGF is an autocrine growth factor for skeletal muscle satellite
cells in vitro. Muscle Nerve. 23:239–245. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yanagiuchi A, Miyake H, Nomi M, et al:
Modulation of the microenvironment by growth factors regulates the
in vivo growth of skeletal myoblasts. BJU Int. 103:1569–1573. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kühl U, Ocalan M, Timpl R, et al: Role of
laminin and fibronectin in selecting myogenic versus fibrogenic
cells from skeletal muscle cells in vitro. Dev Biol. 117:628–635.
1986.PubMed/NCBI
|
|
35
|
Ocalan M, Goodman SL, Kühl U, et al:
Laminin alters cell shape and stimulates motility and proliferation
of murine skeletal myoblasts. Dev Biol. 125:158–167. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Eberli D, Soker S, Atala A, et al:
Optimization of human skeletal muscle precursor cell culture and
myofiber formation in vitro. Methods. 47:98–103. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Martin SD, Collier FM, Kirkland MA, et al:
Enhanced proliferation of human skeletal muscle precursor cells
derived from elderly donors cultured in estimated physiological
(5%) oxygen. Cytotechnology. 61:93–107. 2009.PubMed/NCBI
|
|
38
|
Blau HM, Chiu C-P and Webster C:
Cytoplasmic activation of human nuclear genes in stable
heterocaryons. Cell. 32:1171–1180. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kino-Oka M, Chowdhury SR, Muneyuki Y, et
al: Automating the expansion process of human skeletal muscle
myoblasts with suppression of myotube formation. Tissue Eng Part C
Methods. 15:717–728. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nehlin JO, Just M, Rustan AC, et al: Human
myotubes from myoblast cultures undergoing senescence exhibit
defects in glucose and lipid metabolism. Biogerontology.
12:349–365. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Herschorn S, Carr L, Birch C, et al:
Autologous muscle-derived cells as therapy for stress urinary
incotinence: a randomized, blinded trial. Neurourol Urodyn.
29:3072010.
|
|
42
|
Zammit P: Kinetics of myoblast
proliferation show that resident satellite cells are competent to
fully regenerate skeletal muscle fibers. Exp Cell Res 2002.
281:39–49. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu H-Z, Li Q, Yang X-Y, et al: Expression
of basic fibroblast growth factor results in the decrease of
myostatin mRNA in murine C2C12 myoblasts. Acta Biochim Biophys Sin
(Shanghai). 38:697–703. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhu X, Topouzis S, Liang L-F, et al:
Myostatin signaling through Smad2, Smad3 and Smad4 is regulated by
the inhibitory Smad7 by a negative feedback mechanism. Cytokine.
26:262–272. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Forbes D, Jackman M, Bishop A, et al:
Myostatin auto-regulates its expression by feedback loop through
Smad7 dependent mechanism. J Cell Physiol. 206:264–272. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Cornelison DD and Wold BJ: Single-cell
analysis of regulatory gene expression in quiescent and activated
mouse skeletal muscle satellite cells. Dev Biol. 191:270–283. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bentzinger CF, Wang YX and Rudnicki M:
Building muscle: molecular regulation of myogenesis. Cold Spring
Harb Perspect Biol. 4:1–16. 2012. View Article : Google Scholar : PubMed/NCBI
|