Introduction
Atherosclerosis, a progressive disease characterized
by excessive cholesterol deposition and persistent inflammation
within the artery wall. Epidemiological and experimental evidence
indicates that the deregulation of cholesterol metabolism is the
most important risk factor for the development of atherosclerosis
(1). Monocytes play an important
role in the progression of the disease (2,3).
Modified lipoproteins recruit monocytes to the vascular intima,
where monocytes differentiate into macrophages to engulf these
lipoprotein molecules and then become foam cells (4,5).
The accumulation of macrophage-derived foam cells in the
subendothelial space is the crucial step in the initiation and
progression of atherosclerosis (6).
The balance between lipids entering into and moving
out of the macrophage is necessary to avoid lipid overload, and
ultimately, atheroma development (7). Silent information regulator T1
(sirtuin 1 or SIRT1), a NAD+-dependent histone
deacetylase, is a fundamental factor in sensing caloric
restriction, improving insulin secretion in pancreatic β cells, and
reducing the accumulation of fatty acids in white adipose tissue
(8). SIRT1 has various targets,
including nuclear peroxisome proliferator-activated receptor-γ
(PPAR-γ), PPAR-γ activator 1α (PGC-1α) and p53, many of which have
also been shown to play a role in atherogenesis (9,10);
however, little is known about the association between SIRT1 and
atherosclerosis. In atherogenesis, SIRT1 overexpression has been
shown to prevent atherosclerosis by improving vascular function
(11). In particular, the role of
SIRT1 in monocyte adhesion, macrophage infiltration, lipid uptake
and foam cell formation remains to be determined.
Berberine (BBR) is a botanical alkaloid isolated
from traditional Chinese medicinal herbs, such as Coptidis Rhizoma
(Huanglian) and Cortex Phellodendri (Huangbai) (12). BBR is known to have protective
effects in cardiovascular diseases. For example, BBR decreases
plasma cholesterol and glucose levels (13,14). It has been used as an
anti-inflammatory agent, antimicrobial agent, antihypertensive
agent, anti-arrhythmic agent, antitumor agent and an antidiabetic
agent (15). In addition, BBR
exerts antioxidant activity by upregulating the expression of the
cellular survival-associated factor, SIRT1 (16). However, to the best of our
knowledge, the effects of BBR on macrophage foam cell formation and
intracellular cholesterol metabolism remain unexplored.
In the present study, we aimed to investigate the
molecular mechanisms underlying the anti-atherogenic effects of BBR
on foam cell formation and the role of SIRT1 in these
processes.
Materials and methods
Cell culture
The human monocytic cells, THP-1 (ATCC, Rockville,
MD, USA), were maintained in RPMI-1640 medium containing 10% fetal
bovine serum (FBS), 0.05 mM 2-mercaptoethanol, 10 mM HEPES, 1 mM
sodium pyruvate, 4.5 g/l glucose and 1.5 g/l bicarbonate in a
humidified atmosphere of 5% CO2 and 95% air at 37°C. The
differentiation of THP-1 monocytes into macrophages was induced by
exposure to 100 nM phorbol 12-myristate 13-acetate (PMA)
(Sigma-Aldrich, St. Louis, MO, USA) for 48 h. The differentiated
THP-1 macrophages were extensively washed in PBS before being used
in the experiments.
Extraction and oxidation of low density
lipoprotein (LDL)
The extraction and oxidation of LDL were performed
according to a previously performed method (17). For the production of oxidized LDL
(ox-LDL), CuSO4 was added to LDL at a final
concentration of 10 μM for 24 h at 37°C and the mixture was then
dialyzed for 24 h at 4°C. Finally, the bacteria in the solution
were removed through ultrafiltration.
Treatment with BBR
The THP-1-derived macrophages were divided into an
ox-LDL (50 mg/l) group and BBR (5, 10 and 20 mg/l; Wuhan Fortuna
Chemical Co., Ltd., Wuhan, China) groups. The experiments were
performed in serum-free (SF) experimental medium. The BBR groups
were co-cultured with BBR for 2 h prior to the addition of ox-LDL,
which was added at a concentration of 50 mg/l. These cells were
then collected after having been incubated for 24 h.
Transfection with small interfering RNA
(siRNA)
The transient transfection of siRNA into
THP-1-derived macrophages was performed using Lipofectamine RNAi
MAX (Sigma-Aldrich). The oligos used for SIRT1-siRNA have been
previously described (9). The
negative control (NC) group was transfected with a siRNA sequence
which had no effect on gene expression. Subsequently, the cells
were exposed to BBR (10 mg/l) with or without ox-LDL (50 mg/l) for
12 h.
Treatment with inhibitor
Compound C (Sigma-Aldrich) is a specific adenosine
5′-monophosphate (AMP)-activated protein kinase (AMPK) inhibitor.
Compound C (10 μM, in DMSO) was added 15 min prior to treatment
with BBR. For treatment, the cells were treated only with BBR (10
mg/l) for 24 h or BBR was added to the cultures to a final
concentration of 10 mg/l for 2 h prior to the addition of ox-LDL,
which was added at a concentration of 50 mg/l. These cells were
then collected after 12 h of incubation.
Treatment with BBR and atorvastatin
The THP-1-derived macrophages were divided into
atorvastatin (20 and 40 μM; Sigma-Aldrich), BBR (10 mg/l) and
atorvastatin plus BBR (atorvastatin 20 μM, BBR 10 mg/l) groups. The
experiments were performed in SF experimental medium. The treatment
groups were co-cultured with the drugs for 2 h and were then
treated with ox-LDL (50 mg/l) or lipopolysaccharide (LPS; 10 μM;
Sigma-Aldrich) for 12 h.
Foam cell formation assay and cytoplasmic
lipid detection
The formation of foam cells was evaluated using Oil
Red O staining. The THP-1-derived macrophages were cultured with
ox-LDL for 24 h and then washed 3 times with PBS. Following
fixation with 4% formaldehyde for 15 min, the cells were stained
with Oil Red O (3 mg/ml in 60% isopropanol; Sigma-Aldrich) for 10
min at 37°C to evaluate the characteristic lipid accumulation in
macrophage-derived foam cells. The cells were then rinsed with
water, and hematoxylin was introduced to label the cell nuclei.
Foam cell formation was observed under a microscope, and Oil Red O
staining was assessed by a color density assay using iVision
Software. The density of lipid content was evaluated by alcohol
extraction after staining. The absorbance at 540 nm was measured
using a microplate reader (BioTek Instruments, Winooski, VT,
USA).
Lipid analysis by high-performance liquid
chromatography (HPLC)
Cellular total triglyceride content and cholesterol
levels were analyzed by HPLC. Briefly, the cells were rinsed in PBS
3 times and lysed by the addition of a 0.9% NaOH solution. Masterol
was used as a standard, and the samples were dissolved in 100 μl of
isopropanol-acetonitrile (v/v, 20:80), followed by incubation in
ultrasound water at room temperature for 5 min. Finally, the
samples were placed in the Agilent 1100 series HPLC system (Agilent
Technologies, Santa Clara, CA, USA).
Analysis of mRNA and protein
expression
Total RNA was extracted using TRIzol (Sigma-Aldrich)
reagent and reverse transcribed into cDNA. The cDNA was quantified
by SYBR-Green qPCR using the SYBR Premix Ex Taq™ II kit (Takara Bio
Inc., Otsu, Japan). The reaction conditions followed the
instructions provided by the manufacturers. Gene-specific primers
for monocyte chemotactic protein-1 (MCP-1), SIRT1, PPAR-γ and
β-actin (Table I) were used in
the reactions.
 | Table IOligonucleotides used in RT-qPCR. |
Table I
Oligonucleotides used in RT-qPCR.
| Genes | Primers |
|---|
| β-actin | Forward:
5′-GATCATTGCTCCTCCTGAGC-3′
Reverse: 5′-ACTCCTGCTTGCTGATCCAC-3′ |
| SIRT1 | Forward:
5′-GAGTGGCAAAGGAGCAGA-3′
Reverse: 5′-TCTGGCATGTCCCACTATC-3′ |
| PPAR-γ | Forward:
5′-GCAGTGGGGATGTCTCATAATGC-3′
Reverse: 5′-CAGGGGGGTGATGTGTTTGAA-3′ |
| MCP-1 | Forward:
5′-AGCCACCTTCATTCCCCAAG-3′
Reverse: 5′-CTCCTTGGCCACAATGGTCT-3′ |
Protein expression was evaluated by western blot
analysis. Specific antibodies for phospho-AMPKα (Thr172), AMPK,
SIRT1, PPAR-γ and β-actin were purchased from Cell Signaling
Technology (Danvers, MA, USA) and Sigma-Aldrich. Protein
concentrations were determined using the Bio-Rad protein assay kit
(Bio-Rad Laboratories, Hercules, CA, USA). Equal amounts of protein
(20 μg) from each lysate were subjected to SDS-PAGE. The proteins
were transferred onto nitrocellulose membranes at 80 V for 1 h and
blocked for 4 h in 5% skim milk, then incubated overnight at 4°C
with a 1:500 dilution of primary antibody (produced in rabbit and
mouse) followed by incubation with the corresponding secondary
antibody (goat anti-rabbit or anti-mouse IgG). The enhanced
chemiluminescence system (Amersham Pharmacia Biotech Inc.,
Piscataway, NJ, USA) was used for detection. Filters were
subsequently exposed to Kodak BioMax light-1films (Eastman Kodak,
Rochester, NY, USA) and the intensity of the western blot signals
was quantified by densitometry.
Enzyme-linked immunosorbent assay
(ELISA)
To evaluate the levels of MCP-1 produced, the
THP-1-derived macrophages were pre-treated with BBR or
atorvastatin, or a combination of both for 2 h then treated with
LPS for 12 h. Supernatants from the treated cells were collected
and analyzed for MCP-1 using a sandwich ELISA kit (R&D Systems,
Minneapolis, MN, USA) according to the manufacturer’s
instructions.
Statistical analysis
Data are presented as the means ± standard deviation
(SD, n=6). All data were evaluated using SPSS 11.0 software. Groups
were compared using analysis of variance (ANOVA) followed by the
Student’s t-test. P-values <0.05 or <0.01 were considered to
indicate statistically significant differences.
Results
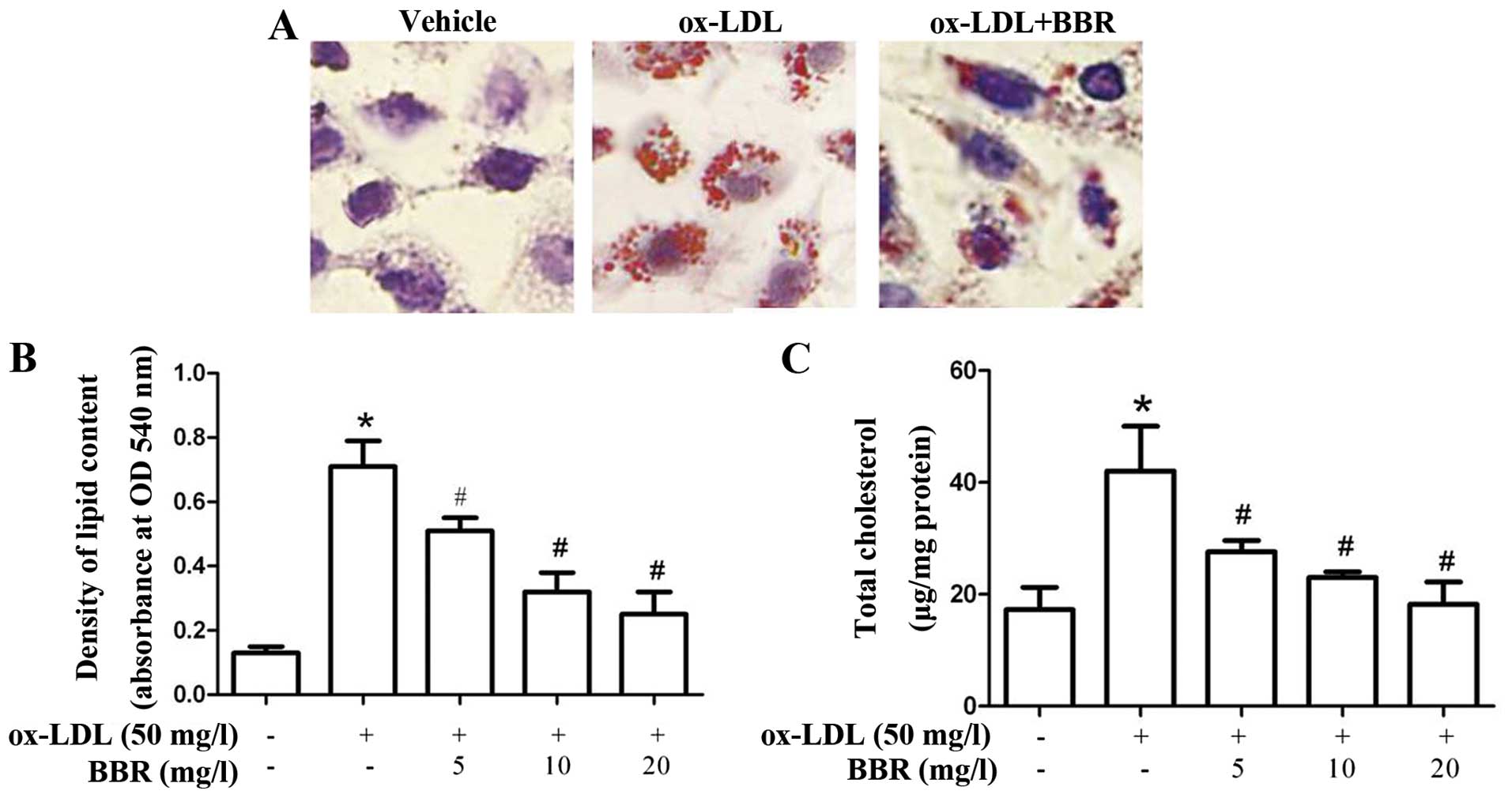
BBR inhibits ox-LDL-induced foam cell
formation and cholesterol accumulation in macrophages
To investigate the effects of BBR on ox-LDL-induced
foam cell formation, the THP-1-derived macrophages were treated
with ox-LDL in the presence or absence of BBR for 24 h. BBR
effectively suppressed ox-LDL-induced foam cell formation (Fig. 1A). The increase in lipid and
cholesterol accumulation was significantly attenuated by treatment
with BBR in a dose-dependent manner (Fig. 1B and C), which suggests that BBR
abrogates the formation of foam cells by regulating lipid
accumulation.
BBR inhibits lipid accumulation through
SIRT1 and PPAR-γ
SIRT1 can reduce the accumulation of fatty acids by
repressing PPAR-γ (18). In this
study, to explore the molecular mechanisms involved in the lipid
lowering effects of BBR, the THP-1-derived macrophages were treated
with ox-LDL in the presence or absence of BBR for 24 h and the
effects of BBR on the expression of SIRT1 and PPAR-γ were then
examined. Treatment with BBR increased the expression of SIRT1 and
decreased the expression of PPAR-γ at both the protein and mRNA
level in the macrophages in a dose-dependent manner (Fig. 2).
Inhibition of SIRT1 function abrogates
the lipid-lowering effects of BBR
To further investigate the role of SIRT1 in the
lipid-lowering effects of BBR, the THP-1-derived macrophages were
transfected with SIRT1 siRNA in the presence of BBR. Pre-treatment
of the macrophages with SIRT1 siRNA diminished the effects of BBR
on the expression of SIRT1 and PPAR-γ at both the protein and mRNA
level (Fig. 3A and B).
Additionally, pre-transfection with SIRT1 siRNA abolished the
BBR-mediated suppression of oxLDL-induced lipid accumulation
(Fig. 3C and D), suggesting that
the induction of SIRT1 is required for BBR-mediated protection
against foam cell formation.
Role of AMPK in the BBR-mediated increase
in SIRT1 expression
The activation of AMPK has been suggested to play an
important role in SIRT1 gene expression and to switch off a number
of processes that consume ATP, such as fatty acid, protein and
cholesterol synthesis (19–22). In this study, to determine whether
AMPK is involved in the BBR-induced upregulation of SIRT1, we
examined the expression of AMPK in response to BBR. The
phosphorylated isoform is the active AMPK form; thus, we determined
the phosphorylated AMPK/total AMPK protein ratio. BBR significantly
increased the phosphorylation of AMPK (Fig. 4A) suggesting that AMPK may
contribute to the enhancing effects of BBR on SIRT1 expression in
macrophages.
To verify this hypothesis, the THP-1-derived
macrophages were pre-treated with an AMPK inhibitor (compound C).
Pre-treatment of the macrophages with compound C diminished the
BBR-induced expression of SIRT1 at both the protein and mRNA level
(Fig. 4B). In addition, the
suppression of lipid accumulation induced by BBR was reversed when
the macrophages were pre-treated with compound C (Fig. 4C and D).
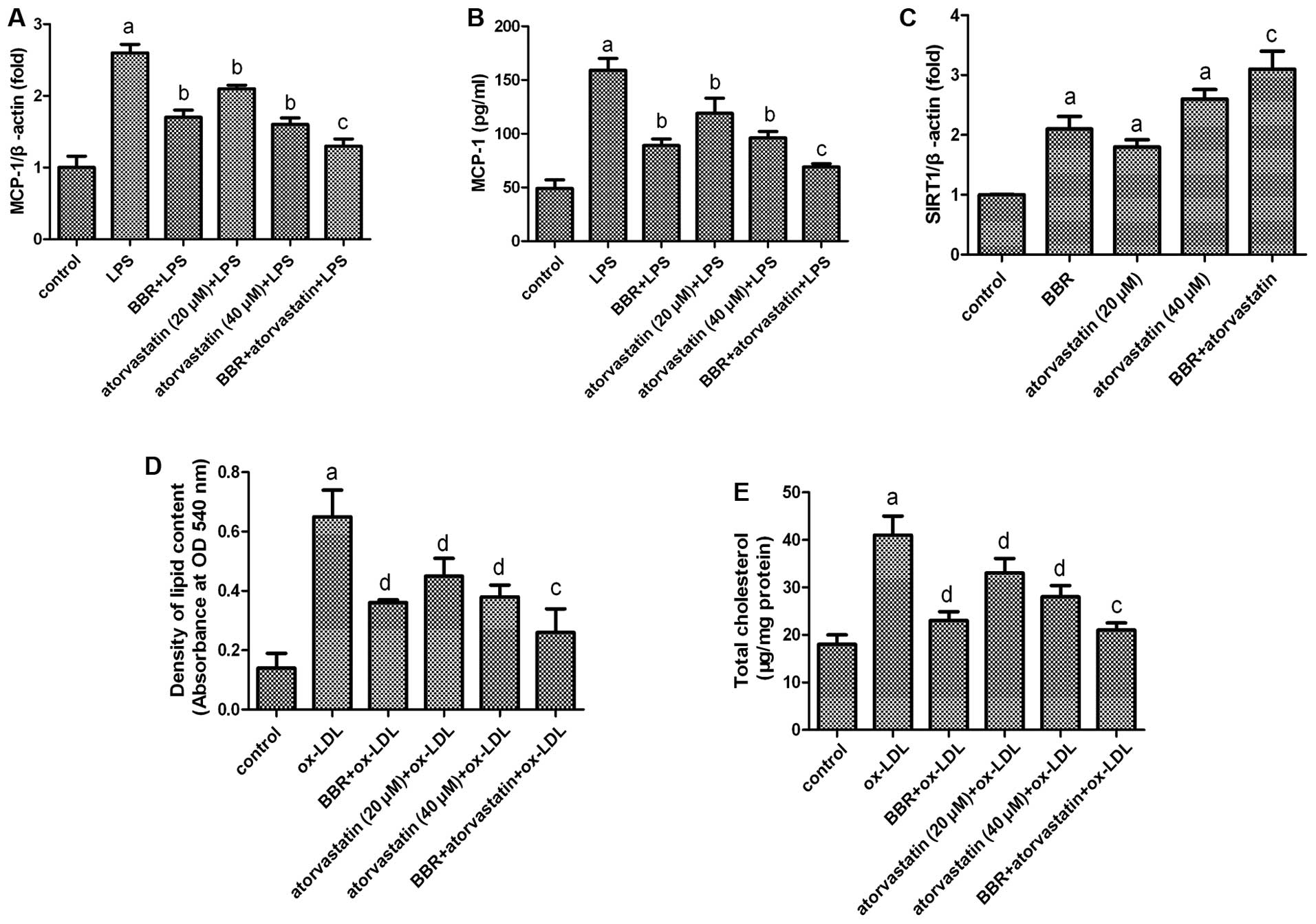
Effect of the combination of atorvastatin
and BBR on foam cell formation
MCP-1 is a potent chemoattractant for monocytes and
plays pivotal roles in the initiation and development of
atherosclerosis by promoting monocyte infiltration to lesion-prone
areas and penetration between endothelial cells into the inner
arterial space (23,24). BBR has been used as an
anti-inflammatory agent. It has been reported that BBR inhibits the
expression of MCP-1 in macrophages (25). Atorvastatin is a traditional
anti-atherosclerotic drug. The anti-atherosclerotic effects of
atorvastatin may be partly achieved by inhibiting the secretion of
MCP-1 and the expression of SIRT1 in foam cells (26,27).
In the present study, we investigated the
anti-atherosclerotic effects of a combination of atorvastatin and
BBR. As shown in Fig. 5,
atorvastatin and BBR both suppressed the LPS-induced expression and
secretion of MCP-1, as well as lipid accumulation. In addition,
both atorvastatin and BBR upregulated the expression of SIRT1.
However, the combination of atorvastatin and BBR was more effective
than using atorvastatin alone. These data suggest a strong
synergistic benefit of combination therapy with BBR and
atorvastatin for preventing atherosclerotic processes.
Discussion
Atherosclerosis is a well-known multigenic,
progressive chronic inflammatory disease. A characteristic of the
atherosclerotic lesion is that macrophages change into foam cells
after phagocytizing cholesterol during the development of
atherosclerosis (28). The
accumulation of lipid-laden foam cells is a critical step in the
progression of atherosclerosis due to the augmented inflammation
and impaired cholesterol metabolism within vascular walls (6,29,30).
BBR is a traditional anti-inflammatory medicine used
in China. It has been shown to decrease the levels of LDL
cholesterol (LDL-C), prevent oxidative stress and inhibit the
migration and proliferation of vascular smooth muscle cells
(13,31–36). Therefore, BBR has
anti-atherosclerotic potency. In the present study, BBR
significantly reduced the accumulation of lipids and cholesterol in
THP-1-derived macrophages and suppressed foam cell formation. These
results are consistent with those of previous studies demonstrating
that treatment with BBR reduces serum cholesterol levels and
impedes the development of atherosclerosis (13). According to this observation, we
further elucidated the possible mechanisms underlying the
BBR-mediated inhibition of foam cell formation.
During the formation of foam cells, the intake of
cholesterol and reserve cholesterol transport play vital roles. It
has been confirmed that the intake of cholesterol is mediated by
certain proteins, such as PPAR-γ and SIRT1. SIRT1 is one of the
genes upstream of PPAR-γ. Our data demonstrated that treatment with
BBR upregulated the expression of SIRT1 at both the mRNA and
protein level and inhibited the expression of PPAR-γ. SIRT1 siRNA
reversed the effects of BBR on the expression of PPAR-γ and the
accumulation of lipid and cholesterol in THP-1-derived macrophages.
To investigate the mechanisms responsible for the effects of BBR on
SIRT1, we evaluated the activation of AMPK, a regulator of SIRT1,
which is an important serine/threonine kinase well known for
regulating cellular energy levels by balancing nutrient
availability and energy demand through its control of several
proteins involved in glucose and lipid metabolism (19,20). More importantly, we additionally
demonstrated that BBR promoted the activation of AMPK, and compound
C, a specific inhibitor of AMPK reversed the effects of BBR on the
expression of SIRT1 and PPAR-γ and the accumulation of lipid and
cholesterol in THP-1-derived macrophages.
It has been reported that atorvastatin exerts
pleiotropic effects in patients with atherosclerosis through the
inhibition of coronary atheroma progression, reducing clinical
events and by increasing the expression of genes involved in the
apoptosis of monocytes (37).
Furthermore, we investigated the effects of treatment with BBR or
atorvastatin alone, or a combination of both on foam cell
formation. We found that atorvastatin and BBR both suppressed the
LPS-induced expression and secretion of MCP-1 and lipid
accumulation, and upregulated the expression of SIRT1. However, the
combination of atorvastatin and BBR was more effective in than
using atorvastatin alone. These data suggest a strong synergistic
benefit of combination therapy with BBR and atorvastatin for
preventing atherosclerotic processes.
In conclusion, our results reveal a novel mechanism
through which BBR prevents atherogenesis: BBR suppresses foam cell
formation by activating the AMPK-SIRT1-PPAR-γ pathway and
diminishing the uptake of ox-LDL. Our findings provide a novel
explanation for the anti-atherosclerotic activity of BBR and
suggest that BBR may be a useful agent for the treatment of
atherosclerosis. Furthermore, the results obtained in the present
study demonstrate that a combination of atorvastatin and BBR is
more effective than atorvastatin alone in inhibiting foam cell
formation. Our data suggest a strong synergistic benefit of
combination therapy with BBR and atorvastatin for preventing
atherosclerotic processes.
Acknowledgements
The present study was supported by grants from the
Foundation of Shaanxi Province Natural Science Research Project
(2012JM4004) and the Foundation of Lanzhou Military Region Medical
Scientific Research Project (CLZ12JA24).
References
|
1
|
Zhao JF, Jim Leu SJ, Shyue SK, Su KH, Wei
J and Lee TS: Novel effect of paeonol on the formation of foam
cells: promotion of LXRα-ABCA1-dependent cholesterol efflux in
macrophages. Am J Chin Med. 41:1079–1096. 2013.PubMed/NCBI
|
|
2
|
Hansson GK: Inflammation, atherosclerosis,
and coronary artery disease. N Engl J Med. 352:1685–1695. 2005.
View Article : Google Scholar
|
|
3
|
Weber C, Zernecke A and Libby P: The
multifaceted contributions of leukocyte subsets to atherosclerosis:
lessons from mouse models. Nat Rev Immunol. 8:802–815. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Boring L, Gosling J, Cleary M and Charo
IF: Decreased lesion formation in CCR2−/− mice reveals a
role for chemokines in the initiation of atherosclerosis. Nature.
394:894–897. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Edfeldt K, Swedenborg J, Hansson GK and
Yan ZQ: Expression of toll-like receptors in human atherosclerotic
lesions: a possible pathway for plaque activation. Circulation.
105:1158–1161. 2002.PubMed/NCBI
|
|
6
|
Li AC and Glass CK: The macrophage foam
cell as a target for therapeutic intervention. Nat Med.
8:1235–1242. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Voloshyna I, Hai O, Littlefield MJ,
Carsons S and Reiss AB: Resveratrol mediates anti-atherogenic
effects on cholesterol flux in human macrophages and endothelium
via PPARγ and adenosine. Eur J Pharmacol. 698:299–309.
2013.PubMed/NCBI
|
|
8
|
Stein S, Lohmann C, Schafer N, et al:
SIRT1 decreases Lox-1-mediated foam cell formation in
atherogenesis. Eur Heart J. 31:2301–2309. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Picard F, Kurtev M, Chung N, et al: Sirt1
promotes fat mobilization in white adipocytes by repressing PPAR-γ.
Nature. 429:771–776. 2004.PubMed/NCBI
|
|
10
|
Vaziri H, Dessain SK, Ng Eaton E, et al:
hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell.
107:149–159. 2001.PubMed/NCBI
|
|
11
|
Zhang QJ, Wang Z, Chen HZ, et al:
Endothelium-specific overexpression of class III deacetylase SIRT1
decreases atherosclerosis in apolipoprotein E-deficient mice.
Cardiovasc Res. 80:191–199. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang Z, Dong F, Li S, et al:
Berberine-induced inhibition of adipocyte enhancer-binding protein
1 attenuates oxidized low-density lipoprotein accumulation and foam
cell formation in phorbol 12-myristate 13-acetate-induced
macrophages. Eur J Pharmacol. 690:164–169. 2012. View Article : Google Scholar
|
|
13
|
Kong W, Wei J, Abidi P, et al: Berberine
is a novel cholesterol-lowering drug working through a unique
mechanism distinct from statins. Nat Med. 10:1344–1351. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yin J, Xing H and Ye J: Efficacy of
berberine in patients with type 2 diabetes mellitus. Metabolism.
57:712–717. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu X, Li G, Zhu H, et al: Beneficial
effect of berberine on hepatic insulin resistance in diabetic
hamsters possibly involves in SREBPs, LXRα and PPARα
transcriptional programs. Endocr J. 57:881–893. 2010.PubMed/NCBI
|
|
16
|
Zhu X, Guo X, Mao G, et al:
Hepatoprotection of berberine against hydrogen peroxide-induced
apoptosis by upregulation of Sirtuin 1. Phytother Res. 27:417–421.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guan S and Wang B: Effects of fosinopril
and valsartan on expressions of ICAM-1 and NO in human umbilical
vein endothelial cells. Chin Med J (Engl). 116:923–927.
2003.PubMed/NCBI
|
|
18
|
Winnik S, Stein S and Matter CM: SIRT1 -
an anti-inflammatory pathway at the crossroads between metabolic
disease and atherosclerosis. Curr Vasc Pharmacol. 10:693–696. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vingtdeux V, Chandakkar P, Zhao H, Davies
P and Marambaud P: Small-molecule activators of AMP-activated
protein kinase (AMPK), RSVA314 and RSVA405, inhibit adipogenesis.
Mol Med. 17:1022–1030. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fullerton MD, Steinberg GR and Schertzer
JD: Immunometabolism of AMPK in insulin resistance and
atherosclerosis. Mol Cell Endocrinol. 366:224–234. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Canto C, Jiang LQ, Deshmukh AS, et al:
Interdependence of AMPK and SIRT1 for metabolic adaptation to
fasting and exercise in skeletal muscle. Cell Metab. 11:213–219.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hardie DG: AMP-activated/SNF1 protein
kinases: conserved guardians of cellular energy. Nat Rev Mol Cell
Biol. 8:774–785. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Charo IF and Taubman MB: Chemokines in the
pathogenesis of vascular disease. Circ Res. 95:858–866. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
McLaren JE, Michael DR, Ashlin TG and
Ramji DP: Cytokines, macrophage lipid metabolism and foam cells:
implications for cardiovascular disease therapy. Prog Lipid Res.
50:331–347. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen FL, Yang ZH, Liu Y, et al: Berberine
inhibits the expression of TNFα, MCP-1, and IL-6 in
AcLDL-stimulated macrophages through PPARγ pathway. Endocrine.
33:331–337. 2008.
|
|
26
|
Zhu GY, Zhu XL, Li RT, Liu TB, Shang DY
and Zhang Y: Atorvastatin inhibits scavenger receptor A and
monocyte chemoattractant protein-1 expressions in foam cell.
Zhonghua Xin Xue Guan Bing Za Zhi. 35:666–669. 2007.(In
Chinese).
|
|
27
|
Kawai H, Kurata T, Deguchi K, et al:
Combination benefit of amlodipine plus atorvastatin treatment on
carotid atherosclerosis in Zucker metabolic rats. Neurol Res.
35:181–186. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guan S, Wang B, Li W, Guan J and Fang X:
Effects of berberine on expression of LOX-1 and SR-BI in human
macrophage-derived foam cells induced by ox-LDL. Am J Chin Med.
38:1161–1169. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kunjathoor VV, Febbraio M, Podrez EA, et
al: Scavenger receptors class A-I/II and CD36 are the principal
receptors responsible for the uptake of modified low density
lipoprotein leading to lipid loading in macrophages. J Biol Chem.
277:49982–49988. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rader DJ and Pure E: Lipoproteins,
macrophage function, and atherosclerosis: beyond the foam cell?
Cell Metab. 1:223–230. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Abidi P, Zhou Y, Jiang JD and Liu J:
Extracellular signal-regulated kinase-dependent stabilization of
hepatic low-density lipoprotein receptor mRNA by herbal medicine
berberine. Arterioscler Thromb Vasc Biol. 25:2170–2176. 2005.
View Article : Google Scholar
|
|
32
|
Brusq JM, Ancellin N, Grondin P, et al:
Inhibition of lipid synthesis through activation of AMP kinase: an
additional mechanism for the hypolipidemic effects of berberine. J
Lipid Res. 47:1281–1288. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li L, Sawamura T and Renier G: Glucose
enhances human macrophage LOX-1 expression: role for LOX-1 in
glucose-induced macrophage foam cell formation. Circ Res.
94:892–901. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liang KW, Ting CT, Yin SC, et al:
Berberine suppresses MEK/ERK-dependent Egr-1 signaling pathway and
inhibits vascular smooth muscle cell regrowth after in vitro
mechanical injury. Biochem Pharmacol. 71:806–817. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yokozawa T, Ishida A, Kashiwada Y, Cho EJ,
Kim HY and Ikeshiro Y: Coptidis Rhizoma: protective effects
against peroxynitrite-induced oxidative damage and elucidation of
its active components. J Pharm Pharmacol. 56:547–556. 2004.
View Article : Google Scholar
|
|
36
|
Cho BJ, Im EK, Kwon JH, et al: Berberine
inhibits the production of lysophosphatidylcholine-induced reactive
oxygen species and the ERK1/2 pathway in vascular smooth muscle
cells. Mol Cells. 20:429–434. 2005.PubMed/NCBI
|
|
37
|
Wang ZH, Liu XL, Zhong M, et al:
Pleiotropic effects of atorvastatin on monocytes in atherosclerotic
patients. J Clin Pharmacol. 50:311–319. 2010. View Article : Google Scholar : PubMed/NCBI
|