Introduction
Sepsis, a systemic inflammatory response syndrome
(SIRS) caused by infection, is confirmed to be accompanied with the
presence of bacteria or highly suspicious focus of infection.
Sepsis can easily cause acute lung injury (ALI) (1,2).
When ALI occurs, cytokines, chemokines, adhesion molecules and
other inflammatory mediators are produced in the endothelial cells
within activated pulmonary vascular cells, which destroy the
integrity of pulmonary vascular endothelial cells and lead to the
increase of blood capillary permeability of the lung. This, in
turn, causes pulmonary edema (3),
resulting in acute respiratory distress syndrome, multiple organ
failure and high mortality, presenting issues for carers in the
Intensive Care Unit (ICU).
Toll-interacting protein (Tollip) is an interactor
of the Toll family of proteins, which are highly conserved in
evolution from Drosophila to humans, and include the
Toll-like and interleukin-1 (IL-1) receptors, which are involved in
the inflammatory response. Tollip is involved in two main
functions. The first, suggested by Burns and collaborators
(4), identifies Tollip as an
interactor of the IL-1 receptor TIR domain, mediates the binding of
the serine/threonine kinase IRAK-1 to the activated receptor
complex, making it an integral component of the IL-1RI signaling
cascade. In their study, Yamakami and Yokosawa (5) identified a negative regulatory role
of Tollip on the IL-1β and TNF-α signaling pathways, which is in
agreement with the inhibition of NF-κB activation observed
following Tollip overexpression (4). The second function, described by
Yamakami et al (6),
concerns the interaction of Tollip with Tom1, ubiquitin and
clathrin in a high molecular mass complex involved in protein
sorting. In agreement with findings of that study, an endosomal
function of the protein was suggested by Katoh et al
(6,7). Brissoni et al (8) showed that Tollip is required in the
sorting of the IL-1RI at late endosomes, further clarifying the
involvement of Tollip in the IL-1 inflammatory pathway. Zhang and
Ghosh (9) demonstrated that
Tollip is associated with IL-1RI and the TLR2 and TLR4 receptors
when activated by LPS stimulation. This interaction results in the
suppression of TLR-mediated cell responses through inhibition of
the phosphorylation and kinase activity of IRAK1. Active IRAK1
subsequently causes the dimerization and polyubiquitination of
TRAF6, ultimately leading to the production and release of multiple
cytokines via NF-κB activation (10). However, in vivo murine
knockout models have demonstrated that Tollip induced
proinflammatory pathways, in contrast to in vitro
experiments (11).
Xuebijing is a Chinese herb compound preparation
mainly comprising Chuanxiong (Rhizoma Chuanxiong), Chishao
(Radix Paeoniae Rubra), Danshen (Radix Salviae
Miltiorrhiae) and Honghua (Flos Carthami). Xuebijing can
clear toxic heat, cool blood, promote qi and blood circulation,
remove toxic substances and relieve pain (12). Furthermore, Xuebijing has been
used to treat systemic inflammatory response syndrome, pyemia and
multiple organ dysfunction syndrome. However, the underlying
mechanism of action of Xuebijing remains to be determined.
In the present study, we hypothesized that the
function of Xuebijing would reduce inflammatory-induced pulmonary
vascular permeability by upregulating Tollip expression. We
examined the expression of cytokines and Tollip in the lung
following treatment with Xuebijing in a rat model of CLP-induced
lung injury.
Materials and methods
Drug
The Xuebijing injection was purchased from Tianjin
Chase Sun Pharmaceutical Co., Ltd. (Tianjin, China; no. Z20040033)
and comprised Chuanxiong, Chishao, Danshen and Honghua. Chuanxiong,
Chishao, Danshen and Honghua were provided by Professor Li Shixia
of the Central South University and deposited in the pharmacy
centre.
Mice
Male Sprague-Dawley mice were purchased from the
Kunming Medical University Laboratory Animal Center (Kunming,
China). The mice were housed in the Kunming Medical University
Animal Care Facility and were maintained in pathogen-free
conditions. The mice were 8–9 weeks of age at the initiation of the
experiment and were maintained on standard laboratory chow and
water ad libitum. The experimental protocols were approved
by the Committee of Animal Experimentation of the Kunming Medical
University, and by the Animal Experimentation Ethics Committee,
Kunming Medical University (approval no. 09/060/MIS).
Reagents
A reverse transcription reaction kit was purchased
from Takara Biotechnology Co., Ltd. (Dalian, China). TRIzol and
electrophoresis reagents were from ProMag Co., Ltd. (Ningbo,
China). The RT reaction kit was obtained from Takara Biotechnology
Co., Ltd. The PCR amplification reagent kit and DNA ladder/marker
were obtained from Shanghai Sangon Biological Engineering Co., Ltd.
(Shanghai, China). GAPDH was obtained from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). Rabbit anti-mouse
Tollip, TLR4, TRAF6, p-IRAK1, VEGF-α, HO-1 and NF-κB polyclonal
antibodies were purchased from Wuhan Boster Biological Technology,
Ltd. (Wuhan, China). Rabbit anti-mouse IRAK1 and phosphorylated
IRAK1 were purchased from Cell Signaling, Technology (Beverly, MA,
USA). FITC-albumin, hexadecyl-trimethyl-ammonium bromide were
purchased from Sigma-Aldrich (St. Louis, MO, USA). SYBR-Green I was
obtained from Biotium (Hayward, CA, USA). The Oligo(dT)18 and
primers were synthesized by Shanghai Invitrogen Biotechnology Co.,
Ltd (Shanghai, China). The dNTP was obtained from Promega (Madison,
WI, USA).
Animal model of sepsis
Studies were performed on rats with an average
weight of 40.4 g. To induce sepsis, the rats were anesthetized with
isoflurane (4% induction and 2% maintenance) and placed on a
warming pad. Following laparotomy, the cecum was exteriorized, and
the membrane between the cecum and the mesentery was carefully cut
to release the cecum. The cecum was ligated 1.5 cm from the tip or
just below the ileocecal valve with 4-0 silk. Two punctures were
made with an 18-gauge needle, and 1 mm of fecal material was
expressed from the punctures. The incision was sutured in two
layers with 4-0 silk. In sham pups, the cecum was located but not
ligated or punctured. The animals were resuscitated with 3 ml/100 g
body wt normal saline subcutaneously immediately after surgery.
Grouping and treatment
According to a random number table, 88 rats were
randomly divided into 4 groups (n=22 rats per group): normal
control group, sham operation group (sham group), sepsis model
group (model group) and melilotus treatment group (treatment
group). The model and treatment groups were induced by cecal
ligation and puncture (CLP) administered via tube into the
melilotus extract at a volume of 25 mg/kg every 8 h. The normal
control, sham and control groups were administered the same volume
of normal saline. Twenty-two rats in each group were anesthetized
using ether at specific time-points 24 h post-injection. Blood was
collected via the orbital sinus. Ethylenediaminetetraaceticacid
(EDTA) was used as an anti-coagulant, and the plasma was isolated
by centrifugation at 10,000 × g for 5 min. Lung tissues were washed
with saline solution, dried with filter paper and weighed. The
plasma and tissues were stored at −20°C for subsequent
experiments.
RNA extraction
For the isolation of RNA tissue, rats were humanely
sacrificed under ether anesthesia and aseptic conditions. The lung
tissues were removed and immediately frozen in liquid nitrogen.
Prior to RNA extraction, the lung samples were homogenized in
TRIzol™ reagent (Invitrogen) using Mixer 301. Total RNA was
extracted according to the manufacturer’s instructions. RNA samples
were electrophoresed in agarose gels and visualized with ethidium
bromide for quality control.
cDNA synthesis and real-time quantitative
PCR
RNA (3 μg) was reverse-transcribed with reverse
transcriptase for 1 h at 37°C for synthesis of cDNA. Quantitative
changes in mRNA expression were assessed with real-time
quantitative PCR (Bio-Rad CFX) using SYBR-Green detection
consisting of SYBR-Green PCR Master Mix (Aria-tous, Iran). The PCR
Master Mix was comprised 0.5 units of Taq polymerase, 2 μl of each
primer and 3 μl of each cDNA samples in a final volume of 20 μl.
Amplifications were repeated three times. Oligonucleotide primer
sequences are provided in Table
I. β-actin was used as an endogenous control and each sample
was normalized on the basis of its β-actin content. The relative
quantification of the mRNA expression levels of target genes was
calculated using the 2−ΔΔCt method [Celik et al
(13)] (Table II). ΔΔCt = (Ct
gene studied − Ct β-actin) treated − (Ct gene studied − Ct β-actin)
control.
 | Table IPrimer sequences for the genes to
validate the microarray analysis by RT-PCR. |
Table I
Primer sequences for the genes to
validate the microarray analysis by RT-PCR.
| Gene | Primer | Product (bp) |
|---|
| Tollip | mRNA F:
5′-GGACAACGGTCAGCGACGCA-3′
R: 5′-CATAGCCCAGACGCAGGCGG-3′ | 272 |
| NF-κB mRNA | F:
5′-GCACGGATGACAGAGGCGTGTATAAGG-3′
R: 5′-GGCGGATGATCTCCTTCTCTCTGTCTG-3′ | 420 |
| IRAK1 mRNA | F:
5′-ATGCCTATGTTCATCGTGAAC-3′
R: 5′-GGCTACGACGAAGGTGGAAC-3′ | 341 |
| TLR4 mRNA | F:
5′-CCAGGAAGGCTTCCACAAGA-3′
R: 5′-AATTCGACCTGCTGCCTCAG-3′ | 351 |
| TRAF6 mRNA | F:
5′-AAGATTGGCAACTTTGGGATG-3′
R: 5′-GTGGGATTGTGGGTCGCTG-3′ | 331 |
| β-actin | F:
5′-TGGAATCCTGTGGCAGTCCAGT-3′
R: 5′-TAAAACGCAGCTCAGTAACAG-3′ | 349 |
Western blot analysis
Lung tissues were snap frozen in liquid nitrogen,
pulverized and resuspended in ice-cold lysis buffer (Solarbio,
Beijing, China). Protein concentrations were determined with the
Bradford method. Lysates were allowed to solubilize on ice for 30
min, and particulate mass was removed by centrifugation at 15,000 ×
g for 15 min at 4°C. Supernatants were analyzed by SDS-PAGE.
Primary antibodies used included rabbit anti-Tollip monoclonal
antibody (1:400), rabbit anti-NF-κB monoclonal antibody (1:400),
rabbit anti-IRAK1 monoclonal antibody (1:400), rabbit anti-TLR4
monoclonal antibody (1:400), rabbit anti-TRAF6 monoclonal antibody
(1:400), rabbit anti-VEGF-α monoclonal antibody (1:400), rabbit
anti-Nrf2 monoclonal antibody (1:400), mouse anti-GAPDH monoclonal
antibody (1:400) were purchased from Santa Cruz Biotechnology,
Inc.. Secondary antibodies were horseradish peroxidase-labeled
antibodies (Thermo Scientific Pierce, Rockford, IL, USA). Blots
were processed for enhanced chemifluorescence using a Pierce ECL
Western blotting substrate (Thermo Scientific Pierce).
Immunohistochemistry
Immunostaining was performed on lung sections after
antigen retrieval using Retrievagen A (Zymed Laboratories Inc.,
South San Francisco, CA, USA) at 100°C for 20 min, and quenching
endogenous peroxidases with 3% H2O2. Sections
were blocked with 2% BSA in PBS followed by staining with primary
anti-Tollip (BD Pharmingen, San Jose, CA, USA) at RT for 1 h. The
sections were washed, and after application of the secondary
antibody (R&D Systems) tissues were developed using Vectastain
ABC (Vector Laboratories Inc., Burlingame, CA, USA) and
3,3′-diaminobenzidine (Vector Laboratories). After staining, five
high-power fields (x200) were randomly selected on each slide, and
the average proportion of positive expression in each field was
counted using the true color multi-functional cell image analysis
management system (Image-Pro Plus; Media Cybernetics, Rockville,
MD, USA) and expressed as a positive unit (pu).
Cytokine and VEGF measurements in
bronchoalveolar lavage and plasma
Mice were sacrificed after 24 h of treatment, and
bronchoalveolar lavage (BAL) was performed via the tracheal
catheter in the right lung lobes using 0.8 ml of phosphate-buffered
saline. The withdrawn fluid was centrifuged at 15,000 × g, and the
supernatant was snap frozen and stored at −80°C for further use.
Aliquots of BAL fluid and plasma were detected in duplicate with an
enzyme-linked immunosorbent assay (ELISA kit offered by Glory
Science Co., Ltd. (Del Rio, TX, USA) kits for tumor necrosis
factor-α (TNF-α), interleukin (IL)-1β, IL-6 and IL-10 according to
the manufacturer’s instructions.
Measurement of O2−
in homogenates
Lung tissue (~200 mg of tissue) was placed in 1 ml
of ice-cold (4°C) Krebs HEPES buffer, cut with a pair of scissors
and homogenized using a pre-cooled Ultra-Turrax homogenizer (3×10
sec bursts). The crude homogenate was incubated for 30 min at 37°C
in Krebs HEPES buffer containing 1,000 mg F′ collagenase, 125 mg F′
elastase, 1,000 mg F′ aprotinin and 250 mg F′ trypsin inhibitor.
Crude homogenate was washed twice with Krebs HEPES buffer to remove
collagenase (centrifuged at 500 × g for 10 min). The pellet was
resuspended in 1 ml Krebs-HEPES buffer with a motor driven
glass-Teflon homogenizer (3×10 sec), and centrifuged at 12,000 ×g
for 20 min in order to remove mitochondria, after which the
supernatant was centrifuged at 100,000 × g for 60 min. The final
pellet was then resuspended in phosphate-EGTA buffer [mM: 50
phosphate buffer (pH 7.0); 1 EGTA and 100 sucrose]. In the
chemiluminescence experiments, 50 μl of resuspended pellet or
supernatant was added to 390 μl phosphate-EGTA buffer with 10 μl
lucigenin (0–23 mM) in the presence or absence of 50 μM NADH or
NADPH (100 μM). The protein content was measured according to the
method of Lowry et al (14).
MPO activity determination
MPO activities were determined using an MPO kit
produced by Jiancheng Bioengineering Institute (Nanjing, China)
according to the manufacturer’s instructions. Briefly, frozen lung
samples, were thawed and homongenized in ice-cold buffer provided
in the kit. The homogenates were centrifuged at 5,000 × g for 10
min. Pellets were suspended in 0.5% hexadecyl trimethyl ammonium
bromide in 50 mM PBS (pH 6.0) and incubated at 60°C for 2 h. After
another centrifugation (1,200 × g), supernatants were collected.
Their protein concentrations were measured using a protein assay
kit (A045; Jiancheng Bioengineering Institute). In a 96-well plate,
15 μg protein was incubated with 100 μl
3,3R,5,5R-tetramethylbenzidine for 3 min. After 100 μl sulphuric
acid (1 N) was added, absorbance was read in a spectrophotometer
(Metash Instruments Co., Ltd., Shanghai, China) using a wavelength
of 450 nm. Original MPO value was normalized with protein
contents.
Superoxide dismutase assay (SOD)
SOD activity was estimated by the method of Kakar
et al (15). The reaction
mixture of this method contained: 0.1 ml of phenazine methosulphate
(186 μmol), 1.2 ml of sodium pyrophosphate buffer (0.052 mmol; pH
7.0) and 0.3 ml of the supernatant after centrifugation (1,500 × g
for 10 min followed by 10,000 × g for 15 min) of the homogenate was
added to the reaction mixture. The enzyme reaction was initiated by
adding 0.2 ml of NADH (780 μmol) and stopped after 1 min by adding
1 ml of glacial acetic acid. The amount of chromogen formed was
measured by recording color intensity at 560 nm. Results are
expressed in U/mg protein.
Measurement of malondialdehyde (MDA)
MDA was quantified as thiobarbituric acid reactive
substances (TBARS) according to previously published methods
(16,17) as a measure of lipid peroxidation.
Briefly, the weighed samples were homogenized in 1 ml 5%
trichloroacetic acid. Samples were centrifuged and 250 ml of the
supernatant was reacted with the same volume of 20 mM
thiobarbituric acid for 35 min at 95°C, followed by 10 min at 4°C.
Sample fluorescence was read using a spectrophotometric plate
reader with an excitation wavelength of 515 nm and an emission
wavelength of 553 nm.
Determination of inflammatory cell count
in bronchoalveolar lavage (BAL)
BAL was performed by instilling 0.9% NaCl containing
0.6 mmol/l ethylenediaminetetraacetic acids in two separate 0.5 ml
aliquots, as previously described (18,19). The fluid was recovered by gentle
suction and placed on ice for immediate processing. An aliquot of
the BAL fluid was processed immediately for total and differential
cell counts. The remainder of the lavage fluid was centrifuged and
the supernatant was removed aseptically and stored in individual
aliquots at −70°C. Total cell counts in BAL fluid were determined
using a haemocytometer. A number of different inflammatory cells
was calculated as the percentage of various inflammatory cells
multiplied by the total number of cells in the BAL fluid sample.
All the analyses were performed in a blinded manner.
Albumin concentration of the BAL
The albumin content of the BAL supernatants was
assessed using an ELISA kit for Albumin (E91028Mu; Uscn Life
Science, Wuhan, China). Measurement of the absorbance at 450/540 nm
was performed with a microplate reader (Infinite 200; Tecan Group,
Männedorf, Switzerland).
Pulmonary vascular permeability
assays
Two hours prior to euthanasia, FITC-labeled albumin
(5 mg/kg body wt) was administered via a tail-vein injection at 6
and 24 h. Immediately after euthanasia, the lungs were lavaged
three times with phosphate-buffered saline (0.5 ml per lavage) and
the samples were combined. Fluid recovery was roughly 95%. The BAL
samples were centrifuged at 3,000 × g for 10 min. FITC fluorescence
in the BAL fluid was measured using a fluorescence
spectrophotometer with excitation at 484 nm and emission at 510
nm.
Wet/dry lung weight ratio and the water
content
A wet-to-dry weight ratio was used as an index of
tissue water content. After 24 h of melilotus extract, the animals
were anesthetized using ketamine (80 mg/kgip) and xylazine (20
mg/kgip), sacrificed and lungs were excised en bloc. The different
lung lobes were cut, blot dried and placed on preweighed glass
plates. The wet weight of the tissue was registered immediately.
The tray with the tissue was then baked in a hot air oven at 55°C
for 72 h to obtain a constant weight. After the dry weight of the
tissue was registered, the wet/dry lung weight ratio was calculated
as wet weight and dry weight ratio of lung tissue. Lung water
content was calculated as the wet weight minus dry weight and wet
weight ratio of lung tissue multiplied by 100%.
Arterial blood gas analysis
Abdominal arterial blood samples (1.5 ml) were
obtained at the indicated time-points after 0.9% NaCl, LPS and 250
ppm CO challenge to analyze blood gas. COHb, serum lactate, partial
pressure of arterial oxygen (PaO2), and saturation of arterial
oxygen (SaO2) were measured using a blood gas analyzer (Roche OMNI
S6, USA).
Pathological observation of lung
tissues
The middle lobe of the right lung was fixed by
infusing 10% formaldehyde solution in the same pressure, and the
inflation of lung was kept uniform and then, embedded in paraffin
wax, cut into sections and stained with hematoxylin and eosin
(H&E). Pathological tissue changes in tissues were observed
using optical microscopy. Lung injury, based on the infiltration of
inflammatory cells, pulmonary interstitial and alveolar edema,
damage to alveolar structure and degree of fibrosis was assessed
using the grading system reported by Szapiel et al (20). And ALI was scored as follows
(18): i) alveolar congestion,
ii) hemorrhage, iii) infiltration or aggregation of neutrophils in
airspace or vessel wall, and iv) thickness of alveolar wall/hyaline
membrane formation. Each item was scored on a 5-point scale as
follows: 0, minimal damage, 1, mild damage, 2, moderate damage, 3,
severe damage, and 4, maximal damage. Repeated-measures data were
statistically analyzed using repeated-measures analysis of variance
(ANOVA).
Statistical analysis
Statistical analysis was performed with the
Statistical Package for the Social Sciences version 15.0 (IBM
Corp., Armonk, NY, USA). Data were analyzed for normality using the
Kolmogorov-Smirmov method, and the normally distributed data were
expressed as mean ± standard deviation. To compare normally
distributed data between each group, one-way analysis of variance
followed by the Student-Newman-Keuls post hoc test was employed.
P<0.05 was considered to indicate a significant result.
Results
XBJ administration upregulates the
expression of Tollip gene and protein, and blocks pro-inflammatory
gene and protein in lung tissue
To clarify the effect of XBJ on Tollip, TLR4, TRAF6,
p-IRAK1 and NF-κB expression induced by CLP, the expression of
Tollip, TLR4, TRAF6, p-IRAK1 and NF-κB was measured by RT-PCR and
western blotting, respectively. The mRNA and protein expression
levels of Tollip, TLR4, TRAF6, p-IRAK1 and NF-κB in rat lung showed
significant increases by CLP-induced ALI (P<0.05; Figs. 1–4). However, compared with those by
CLP-induced ALI, the mRNA and protein expression levels of Tollip
were significantly increased with the administration of XBJ, and
TLR4, TRAF6, p-IRAK1 and NF-κB gene and protein expression was
significantly decreased (P<0.05; Figs. 1–4).
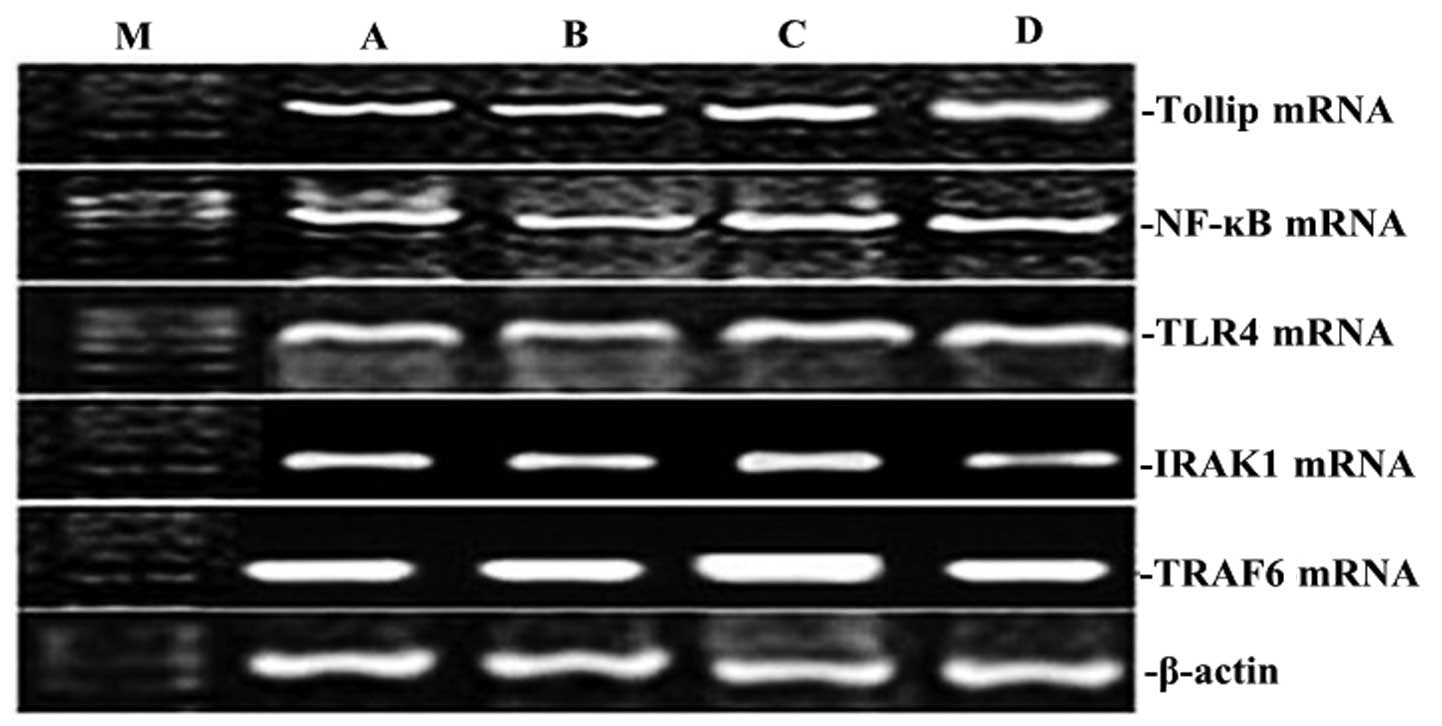 | Figure 1Effect of XBJ on the mRNA expression
of Tollip, IRAK1, TLR4, NF-κB65 and TRAF6 in lung tissue. Groups of
mice were challenged with CLP and treated with XBJ 24 h later. The
expression of Tollip, IRAK1, TLR4, NF-κB65 and TRAF6 in lung tissue
was determined by RT-PCR. Representative RT-PCR shows the level of
Tollip, IRAK1, TLR4, NF-κB65, and TRAF6 expression in the four rat
groups. M, marker; A, normal control group; B, sham operation
group; C, control group; D, treatment group. |
 | Figure 4Administration of XBJ enhanced the
expression of Tollip protein protein, and inhibition TLR4, NF-κB65,
p-IRAK1 and TRAF6 protein expression in lung tissue in CLP-ALI
mice. Groups of mice were challenged with LPS and treated with
salidroside 24 h later. Tollip, p-IRAK1, TLR4, NF-κB65 and TRAF6
were assayed by western blot analysis. Statistical summary of the
densitometric analysis of Tollip, p-IRAK1, TLR4, NF-κB65 and TRAF6
protein expression in the four rat groups. Data are presented as
mean ± standard deviation of one experiment consisting of three
replicates. Experiments were performed in triplicate;
**P<0.01 vs. the normal control group and sham
operation group. #P<0.05, ##P<0.01 vs.
the control group. |
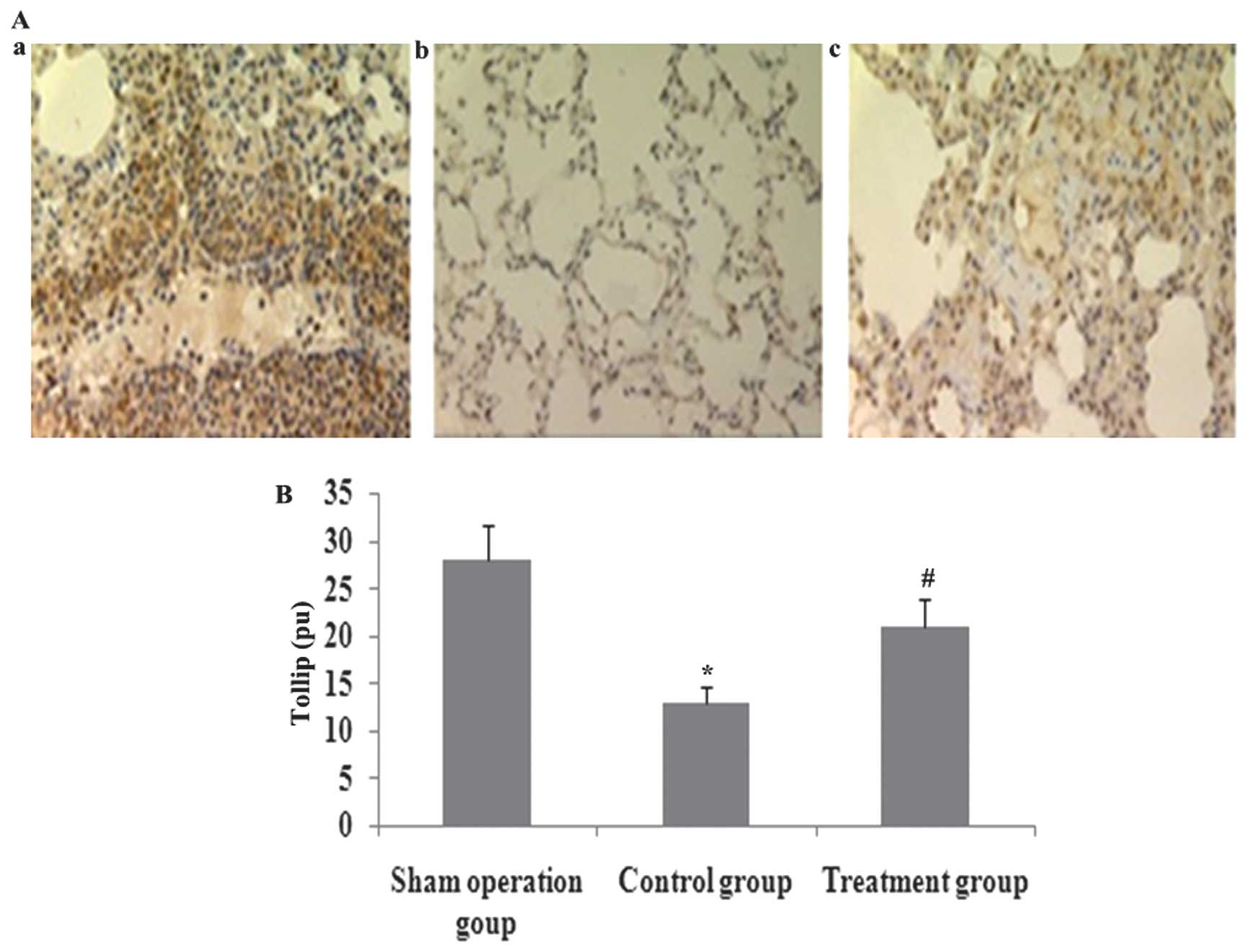
XBJ administration increases lung
localization of positive Tollip protein in CLP-induced acute lung
injury
Immunohistochemical analysis was used to determined
the lung distribution of positive Tollip protein in rat lung 24 h
after CLP or saline treatment. Positively immunostained cells
appeared brown. The expression of Tollip was specifically localized
to the alveolar epithelium. The number of cells expressing Tollip
was significantly decreased in CLP-induced acute lung injury, and
was markedly increased by XBJ treatment (Fig. 5).
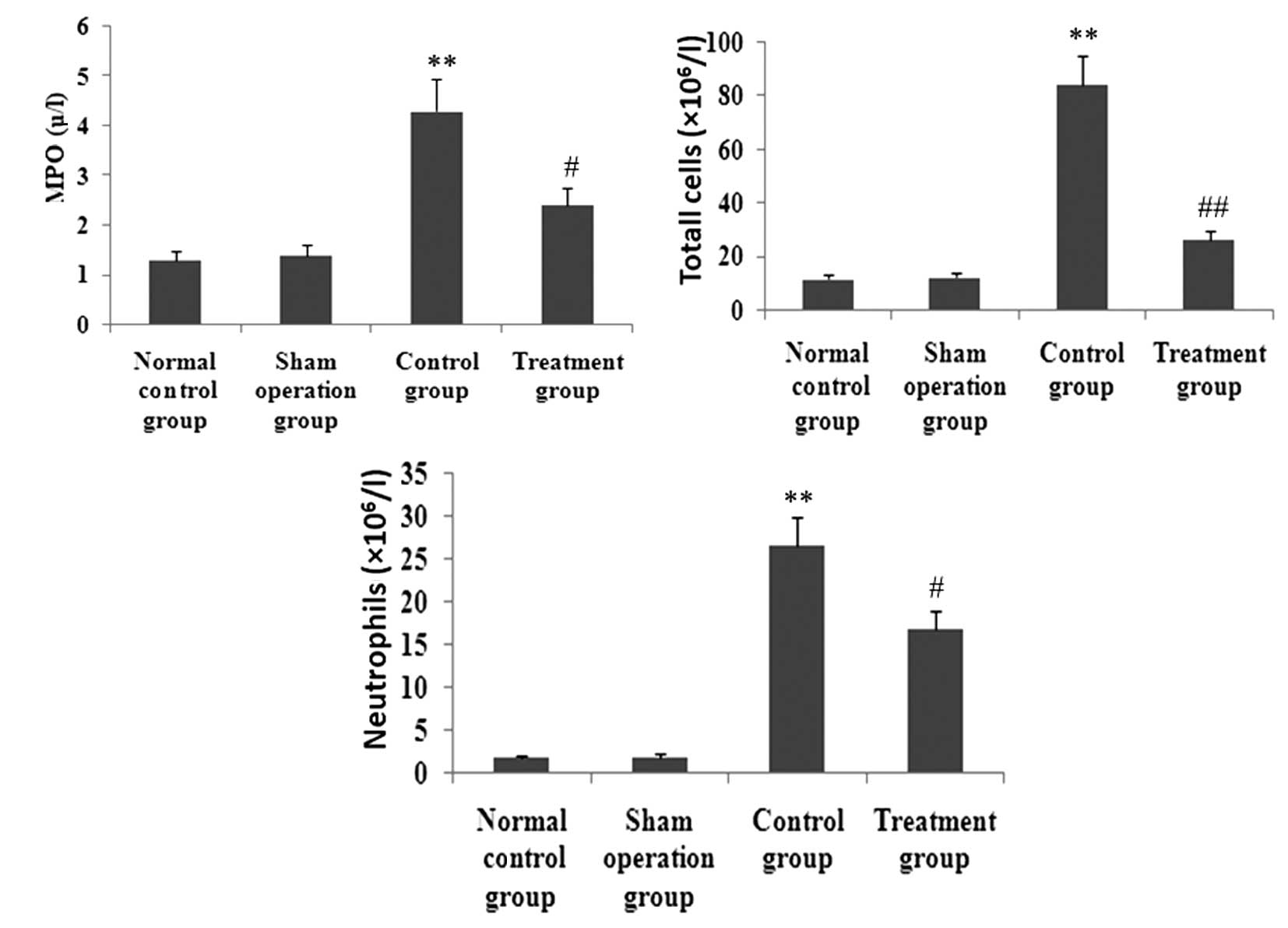
XBJ administration inhibits MPO activity
and inflammatory cell infiltration in lung tissues
To observe the effect of XBJ on MPO activity and
inflammatory cell infiltration in lung tissues, MPO activity in
lung tissue and inflammatory cells in BAL fluid was determined. As
shown in Fig. 6, after animals
received CLP, MPO activity in lung tissue and total cells and
neutrophils in BAL fluid was markedly enhanced. However, the
increase of MPO activity in lung tissue and total cells and
neutrophils in BAL fluid was markedly inhibited by XBJ.
XBJ administration decreases CLP-induced
lung inflammatory response
CLP caused a significant acute systemic inflammatory
response as evidenced by the increased BAL concentrations of the
pro-inflammatory mediators TNF-α, IL-1β and IL-6. The presence of
XBJ reduced these three pro-inflammatory cytokines. CLP also caused
an increase of the BAL concentration of the anti-inflammatory
cytokine IL-10. This change in IL-10 and IL-4 concentration was
relatively increased by the administration of XBJ (Fig. 7).
XBJ administration suppresses oxidative
stress response
To investigate the effect of XBJ on the oxidative
stress response in septic rat lung, the activity of MDA,
O2− and SOD, and Nrf2 protein expression in
lung tissues were measured (Fig.
8). After CLP operation, the expression of Nrf2 protein and the
activity of MDA and O2− in lung tissues was
increased significantly, while the activity of SOD was markedly
decreased. However, after the administration of XBJ, the protein
expression of Nrf2 and activity of SOD was markedly enhanced, and
the activity of MDA and O2− in lung tissues
was inhibited.
XBJ administration suppresses VEGF-α
expression and secretion
The increase of VEGF-α expression exacerbated
pulmonary permeability leakage in septic rats. To analyze the
effect of XBJ treatment on VEGF-α expression in lung tissue and
secretion in serum and BAL, VEGF-α expression and secretion were
measured (Fig. 9). After CLP
operation, the expression of VEGF-α in lung tissue and VEGF-α
levels in serum and BAL were elevated significantly. However, after
XBJ treatment, the expression of VEGF-α in lung tissue and VEGF-α
levels in serum and BAL was blocked significantly.
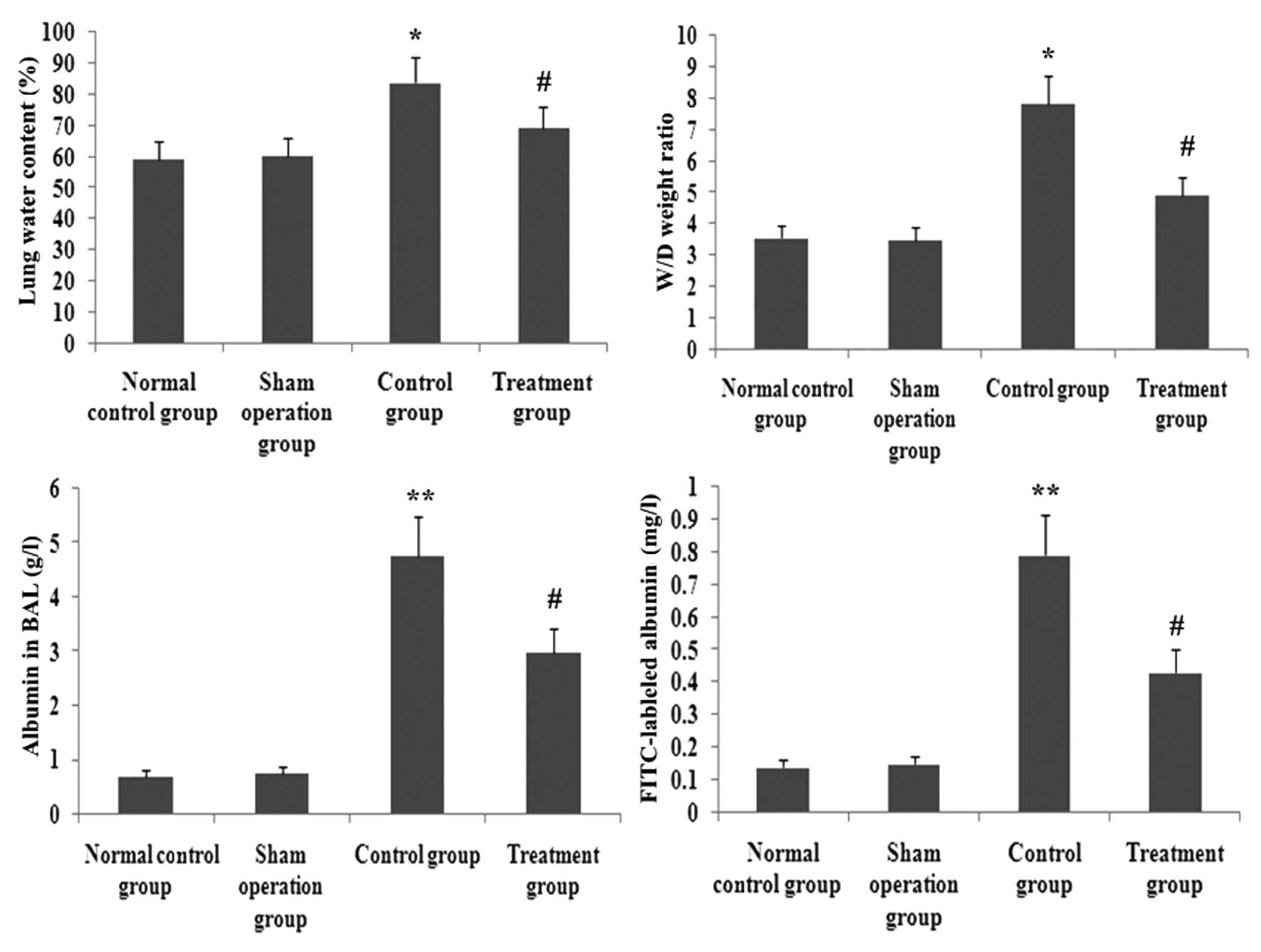
XBJ administration ameliorates pulmonary
permeability
FITC-labeled albumin in BAL, wet/dry lung weight
ratio and the water content in lung tissue were reliable parameters
of pulmonary permeability. To identify the effect of XBJ
administration on pulmonary permeability in septic rats,
FITC-labeled albumin in BAL, wet/dry lung weight ratio and the
water conten in lung tissue were assayed. As shown in Fig. 11, FITC-labeled albumin in BAL,
wet/dry lung weight ratio and the water conteny in lung tissue were
increased significantly in CLP-induced rats. However, the increase
of FITC-labeled albumin in BAL, wet/dry lung weight ratio and the
water content in lung tissue were decreased by XBJ. Therefore, the
effects of pulmonary permeability were significantly blocked by XBJ
(P<0.05; Fig. 11).
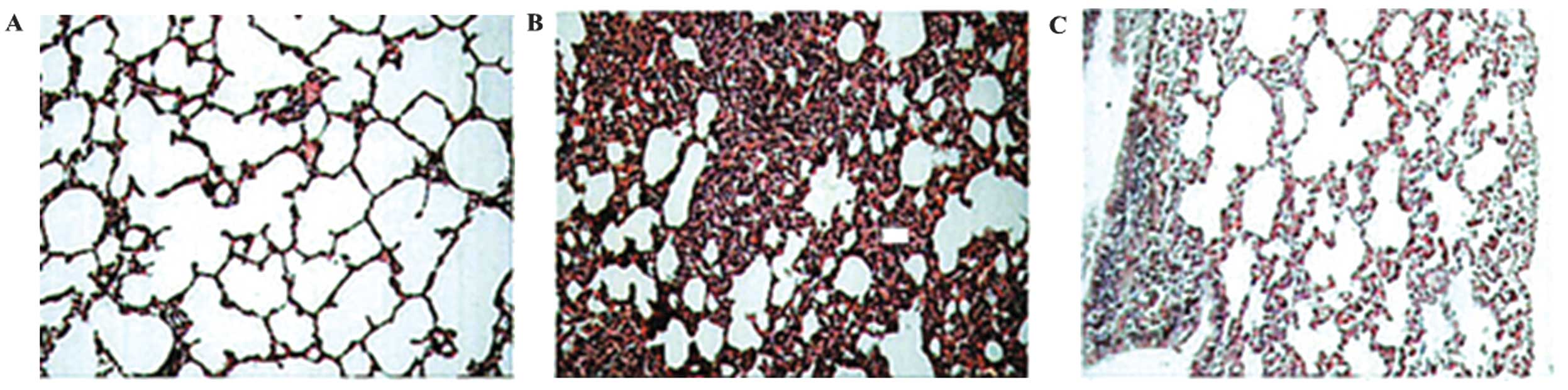
XBJ administration ameliorates
histological acute lung injury
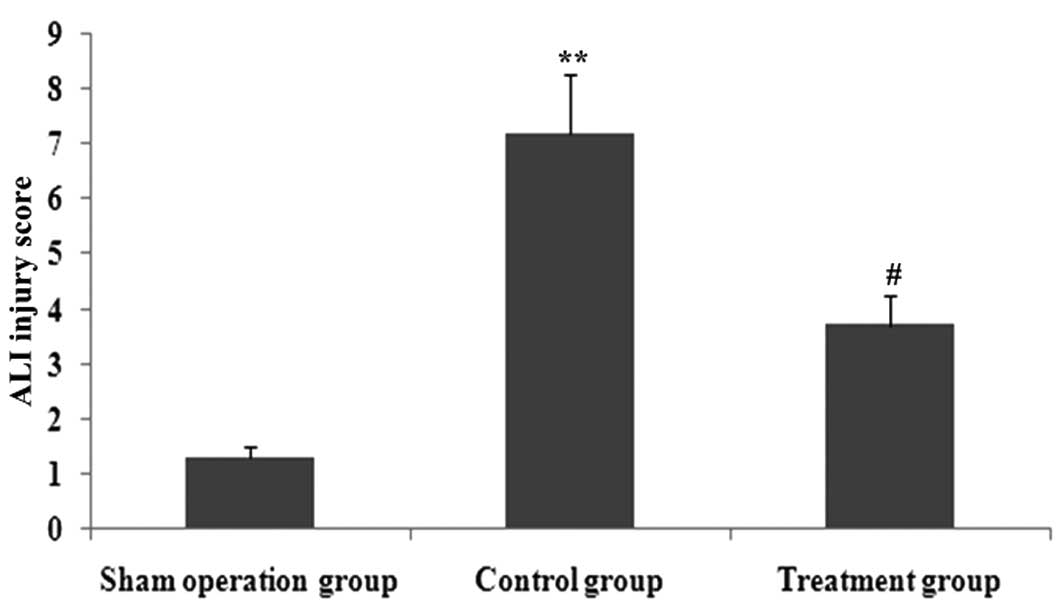
CLP significantly increased lung injury (Fig. 8). The lung tissue was
significantly injured with the presence of intra-alveolar exudate,
edema, and inflammatory cell infiltrationin the control group
compared with that in the treatment group, as evidenced by the
increase in the lung injury score (P<0.05; Fig. 7). XBJ significantly attenuated
CLP-induced histopathologic changes as evidenced by the decrease in
the lung injury score (P<0.05; Figs. 12 and 13).
XBJ administration ameliorates arterial
blood gas in rats with sepsis
The acute lung injury reduced PO2 and PO2/FiO2 in
arterial blood in rats with sepsis. To determine the effect of XBJ
administration on arterial blood gas in rats with sepsis. PO2 and
PO2/FiO2 in arterial blood were measured (Fig. 14). Compared with the CLP group,
XBJ administration significantly increased CLP-induced PO2 and
PO2/FiO2 (P<0.05). Therefore, the effects of arterial blood gas
were significantly improved by XBJ (P<0.05).
Discussion
The animal model with sepsis using the CLP method is
of high stability and repeatability as well as applicability
(21), and is currently regarded
as the ‘gold standard’ for studies of sepsis. Therefore, this
experiment utilized the CLP animal model with sepsis.
Xuebijing (XBJ) is a Chinese medicine compound
preparation, consisting of safflower yellow A, tetramethylpyrazine,
danshensu and ferulic acid. XBJ has been widely used to treat
sepsis and to protect specific organs, regulating the inflammatory
response and oxidative stress and improving coagulation and immune
function (22,23), all of which are involved in
sepsis. XBJ has been injected into patients for 8 years to treat
SIRS and MODS induced by infection or ischemia/reperfusion. XBJ has
been reported to significantly decrease the serum concentrations of
IL-1 and IL-8 in ICU patients (24). Moreover, XBJ injection was found
to reduce the secretion of TNF-α and IL-6 and to inhibit SIRS
during cardiopulmonary bypass (24). A meta-analysis evaluating the
efficacy and safety of XBJ injection in the treatment of sepsis
showed that XBJ injection may decrease 28-day mortality rates,
complication rates, average length of hospital stay and APACHE II
scores (25). The clinical
efficacy of XBJ in sepsis suggested that this agent can reduce the
secretion of inflammatory cytokines by LPS-activated mononuclear
cells/macrophages (26).
Therefore, we investigated the effects of XBJ pretreatment on lung
injury induced by CLP in rats, and whether the mechanisms
underlying these effects were correlated with pro-inflammatory
cytokines by induction of CLP.
Sepsis is caused by a systemic response to
infection, which is characterized by elevated levels of
proinflammatory cytokines such as TNF-α, IL-1β, IFNγ and IL-6 in
the circulation or in inflamed tissues (27,28). While the relevance of
proinflammatory cytokines to vascular leakage has previously been
established (28), the molecular
mechanism causing vascular leakage is not fully understood
(27). Moreover, no specific
therapy is available to treat this pathology. In the present study,
XBJ improved lung capillary leakage by inhibiting the generation of
inflammatory mediators, such as IL-6 and TNF-α by upregulating
Tollip expression. Evidence suggest that neutrophils play a
critical role in ALI. When ALI occurs, neutrophils adhere to the
injured capillary endothelium and migrate into the air spaces
(29). In the present study, mice
exposed to LPS exhibited massive recruitment of inflammatory cells,
including neutrophils and macrophages to the airways. By contrast,
pre-administration with XBJ significantly inhibited the LPS-induced
increase in the numbers of total cells, neutrophils and macrophages
in the BAL fluid. There was substantial infiltration of neutrophils
in mice with LPS-induced ALI, consistent with MPO activity analysis
of the lung, which reflects neutrophil and macrophage diapedesis
(30) and histological analysis
of the lung. XBJ successfully attenuated lung MPO activity and
reduced tissue neutrophilia. Additionally, the lung histological
examination revealed a decrease in the number of total cells and
neutrophils. These findings indicate that the protective effect of
XBJ on lung vascular leakage induced by LPS depends on the
attenuation of inflammatory cell sequestration and migration into
the lung tissue.
A limited number of transcription factors regulate
inflammatory pathways, with the most important transcriptional
regulator being NF-κB (31).
Following activation by a wide array of mediators, including
cytokines, bacterial toxins or oxidative stress, the signal
transduction cascade is initiated. Activated NF-κB can translocate
to the nucleus and bind to the promoters of proinflammatory genes,
leading to enhanced gene expression and amplification of the
inflammatory response. The NF-κB transcription factor belongs to a
family of closely related protein dimers that are considered to be
key mediators of inducible transcription in the immune system
(31). These mediators are
typically activated following the stimulation of cells with
pro-inflammatory ligands, including cytokines and bacterial
antigens (32). Additionally, the
NF-κB transcription factor simultaneously regulates the expression
of a great number of genes that have important functions in the
regulation of immune and inflammatory responses, including
cytokines, chemokines, adhesion molecules and other
immunoregulatory proteins to protect cells from the potentially
damaging effects of inflammation (32,33). Apart from these, a number of other
pathways for NF-κB activation such as p38MAPK and Toll-like
receptors signaling pathway have also been elucidated (34). Our results confirm XBJ was able to
suppress NF-κB transcriptional activation and led to the
concomitant attenuation of inflammatory cytokine expression in the
lung following experimental sepsis in the rats, while XBJ increased
Tollip expression and impaired NF-κB activation.
Negative regulators were identified at almost every
step of the TLR signaling cascade (35–37) and include Toll-interacting protein
(TOLLIP, also known as IL-1RAcPIP), A20 (38), single Ig IL-1-receptor (SIGIRR,
also termed TIR8) (39), and
interleukin-1 receptor-associated kinase 3 (IRAK-3, also known as
IRAK-M) (40). TOLLIP is a 274
amino acid protein with highly conserved C2 (amino acids 54–186,
similar to that found in PI-specific phospholipase C-d1I) and
C-terminal UBA (ubiquitin-associated) domains. Toll-interacting
protein (Tollip) is an adaptor protein that acts as an inhibitory
factor in TLR signaling cascades (35). When activated by IL-1 or
lipopolysaccharide stimulation, Tollip associates with the
cytoplasmic TIR domains of IL-1 receptor (IL-1R), as well as TLR2
and TLR4 (36,37). Tollip also interacts with IL-1
receptor-associated kinase 1 (IRAK1) and suppresses its kinase
activity (9). Therefore, in the
absence of infection, Tollip probably maintains immune cells in a
resting state and terminates IL-1R- and TLR-induced inflammatory
pathways via the suppression of IRAK1 activity (41). In this regard, Tollip resembles
IL-1R-associated kinase M, which also acts as a negative regulator
in IL-1β signaling. IL-1R-associated kinase M associates with IRAK1
by blocking IL-1R-associated kinase 4 recruitment, thereby
inhibiting IRAK1 phosphorylation and/or activation (42). IRAK1 is an adaptor for the
Toll/IL-1R receptor signaling complex. Following IL-1 stimulation,
formation of the heterodimeric receptor complex creates a scaffold
for the association of MyD88 and Tollip (40). IRAK1 is then recruited to the
active receptor complex. Concomitantly, IL-1R-associated kinase 4
is recruited to the receptor complex and may phosphorylate IRAK1,
thus, initiating further autophosphorylation of IRAK1.
Hyperphosphorylated IRAK1 dissociates from the receptor complex,
presumably dimerizes, and binds to TNF receptor-associated factor 6
(TRAF6). IRAK1 binding to TRAF6 functions together with Ubc13/Uev1A
to catalyze Lys63-polyubiquitination of IRAK1. Active IRAK1
subsequently causes the dimerization and polyubiquitination of
TRAF6, which activates the downstream component, transforming
growth factor-β activated kinase 1 (TAK1). TAK1 subsequently
phosphorylates several regulatory kinases in different downstream
signaling pathways, which ultimately leads to the production and
release of multiple cytokines via NF-κB activation (43). However, in vivo murine
knockout models demonstrated that TOLLIP induced proinflammatory
pathways, in contrast to in vitro experiments (11). In the experiment, CLP stimulated
TLR4, IRAK1, TRAF6 and NF-κB activation, and promoted Tollip
expression. However, the administration of XBJ increased Tollip
expression, blocked TLR4, IRAK1, TRAF6, while NF-κB activation
decreased inflammatory cytokine production. Therefore, XBJ
inhibited TLR4, IRAK1, TRAF6 and NF-κB activation by facilitating
Tollip overexpression.
Sepsis is associated with oxidative and nitrative
stress. It is well known that oxidative stress is a fundamental
component of pathogenesis of acute lung injury (44). MDA is the final product of
peroxidation (44). SOD and GSH
are important protective antioxidants against oxidants and
electrophilic compounds, which have been shown to be critical to
the antioxidant defences of the lung, particularly in protecting
epithelium and endothelium from oxidant injury and inflammation
(45,46). CLP results in marked oxidative
stress which is demonstrated by an increase of MDA and depletion of
SOD and GSH in the lungs. Oxidative stress increases the
permeability of the blood gas barrier and results in the formation
of lung oedema (46). Nuclear
factor-erythroid 2 related factor 2 (Nrf2) is a member of the
family of cap‘n’collar basic leucine zipper transcription factors
(47) and, although it is
ubiquitously expressed throughout the lung, it is found
predominantly in the epithelium and alveolar macrophages (AM)
(48). Activation of the majority
of antioxidant and defense genes are regulated by Nrf2 through
binding to antioxidant response elements (AREs) (47). It has been recently reported that
the antioxidant pathway controlled by Nrf2 is pivotal for
protection against the development of influenza virus-induced
pulmonary inflammation and lung injury in mice in vivo under
oxidative conditions (49). Our
results have shown that XBJ treatment enhances Nrf2 activity,
blocks and alleviates oxidative stress, and alleviates lung
permeability, which is partially contributed to the protection of
XBJ against lung injury by CLP through the upregulation of Tollip
activity.
VEGF is crucial in the known microvascular
permeability inducers, and histamines can have a 50,000-fold effect
when the concentration is <1 nmol/l. Such an effect cannot be
inhibited by antihistamines and PAF inhibitors as well as other
inflammatory inhibitors (48).
VEGF activates the Src-Vav2-Rac1-PAK pathway, leading to
phosphorylation and disassembly of VE cadherin (50). The latter is an adherens junction
protein that also contributes to endothelial barrier integrit.
Capillary permeability increase is a key factor in the development
of sepsis, while VEGF is the key molecule for controlling vascular
permeability, which is a potential factor that leads to
inflammation-related capillary permeability (51). In the present study, an increase
in the expression of proinflammatory media can promote the
expression of VEGF, and elevate lung capillary permeability,
however, the expression of VEGF was inhibited by XBJ by an increase
in Tollip expression, which ameliorates vascular permeability.
vWF is a substantially large plasma glycoprotein
synthesized in endothelial cells and megakaryocytes, the primary
function of which is to attach platelets to sites of blood vessel
injury (52). vWF is often
described as ‘a marker of endothelial activation’ (53). Although in many cases, this may be
true, is it only part of its function, especially given the effect
of inflammation and oxidation in preventing cleavage and enhancing
the function of important adhesive molecules, such as ICAM. In the
setting of sepsis, endothelial cells showed a marked increase in
surface ICAM-1 and expression of VCAM-1 on endothelial cells.
Progressive increases in levels of vascular ICAM-1 occurred
following sepsis, and such changes were identified in numerous
organs (54). In this experiment,
we also found that CLP increased the secretion of vWF and ICAM-1 in
serum, and microvascular permeability in lung tissue deteriorated.
However, XBJ reduced the secretion of vWF and ICAM-1 in serum,
while the increase of microvascular permeability in lung tissue was
inhibited by XBJ (Fig. 10).
Vascular leakage is a critical pathological process
in sepsis-induced ALI (27). It
permits plasma protein and leukocyte extravasation, leading to
edema and inflammatory reactions in the inflamed tissues (27). Edema causes tissue hypoxia.
Leukocytes, such as neutrophils, cause tissue damage through the
excessive production of free radicals and proteases. Vascular
leakage is thus a promising target for therapeutic treatment.
Vascular leakage in various organs is a characteristic pathological
change in sepsis (55). In the
present study, we first evaluated the W/D ratio of the lung. The
results show that salidroside treatment attenuates the development
of pulmonary edema, as determined by the significant decrease in
lung W/D ratio. As another index of ALI by LPS, we measured total
protein concentration in the BAL fluid, which indicates epithelial
permeability and pulmonary edema. FITC-labeled albumin, a
macromolecular marker, is widely used to evaluate pulmonary
microvascular permeability (50).
We also measured FITC-labeled albumin. As expected, LPS
instillation was found to cause a significant increase in BAL fluid
protein concentration, lung W/D ratio and FITC-labeled albumin.
LPS-induced increases in total protein in the BAL fluid, lung W/D
ratio and FITC-labeled albumin were inhibited by XBJ.
An arterial blood gas (ABG) is a blood test
performed using blood from an artery. An ABG is a test that
measures the arterial oxygen tension (PaO2), carbon dioxide tension
(PaCO2) and acidity (pH). In addition, arterial oxyhemoglobin
saturation (SaO2) can be determined. Such information is vital when
caring for patients with critical illness or respiratory disease.
As a result, the ABG is one of the most common tests performed on
patients in intensive care units (ICUs). The arterial oxygen
tension and oxygenation index(PaO2/FiO2) are two important
indicators to judge acute lung injury. In this experiment, the
results showed that the reduction of the arterial oxygen tension
and oxygenation index was enhanced by XBJ. We also found that XBJ
significantly attenuated CLP-induced pathologic changes as
evidenced by a decrease in the lung injury score.
In conclusion, although XBJ has been reported for
its decrease of the lung capillary leakage properties in clinical
practice, the inner molecular biological mechanisms have not been
sufficiently investigated. The study indicates XBJ may have an
impact on decreasing lung capillary leakage by upregulating Tollip
expression and inhibiting lung inflammatory reponse and oxidant
stress, and alleviating lung permeability, in order to induce
CLP-induced lung injury.
Acknowledgements
The authors would like to thank Professor Mei-Xian
Sun and Professor Lan-Fang Qin for their kind and excellent
technical assistance.
Abbreviations:
|
XBJ
|
Xuebijing injection
|
|
Tollip
|
Toll-interacting protein
|
|
IRAK1
|
interleukin-1 receptor-associated
kinase 1
|
|
TRAF6
|
TNF receptor-associated factor 6
|
|
NF-κB
|
nuclear factor κB
|
|
IL-6
|
interleukin-6
|
|
TNF-α
|
tumour necrosis factor-α
|
|
CLP
|
cecal ligation and puncture
|
|
RT-PCR
|
reverse transcription polymerase chain
reaction
|
|
BAL
|
bronchoalveolar lavage fluid
|
|
TLR4
|
Toll-like receptor 4
|
|
HO-1
|
heme oxygenase-1
|
|
MPO
|
myeloperoxidase
|
|
MDA
|
malondialdehyde
|
|
SOD
|
superoxide dismutase
|
References
|
1
|
Ware LB and Matthay MA: The acute
respiratory distress syndrome. N Engl J Med. 342:1334–1349. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rubenfeld GD, Caldwell E, Peabody E,
Weaver J, Martin DP, Neff M, Stern EJ and Hudson LD: Incidence and
outcomes of acute lung injury. N Engl J Med. 353:1685–1693. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Berthiaume Y, Folkesson HG and Matthay MA:
Lung edema clearance: 20 years of progress invited review: alveolar
edema fluid clearance in the injured lung. J Appl Physiol.
93:2207–2213. 2002.PubMed/NCBI
|
|
4
|
Burns K, Clatworthy J, Martin L, Martinon
F, Plumpton C, Maschera B, Lewis A, Ray K, Tschopp J and Volpe F:
Tollip, a new component of the IL-1RI pathway, links IRAK to the
IL-1 receptor. Nat Cell Biol. 2:346–351. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yamakami M and Yokosawa H: Tom1 (target of
Myb 1) is a novel negative regulator of interleukin-1- and tumor
necrosis factor-induced signaling pathways. Biol Pharm Bull.
27:564–566. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yamakami M, Yoshimori T and Yokosawa H:
Tom1, a VHS domain containing protein, interacts with Tollip,
ubiquitin, and clathrin. J Biol Chem. 278:52865–52872. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Katoh Y, Shiba Y, Mitsuhashi H, Yanagida
Y, Takatsu H and Nakayama K: Tollip and Tom1 form a complex and
recruit ubiquitin conjugated proteins onto early endosomes. J Biol
Chem. 279:24435–24443. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brissoni B, Agostini L, Kropf M, Martinon
F, Swoboda Vl, et al: Intracellular trafficking of interleukin-1
receptor I requires Tollip. Curr Biol. 16:2265–2270. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang G and Ghosh S: Negative regulation
of Toll-like receptor mediated signaling by Tollip. J Biol Chem.
277:7059–7065. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Capelluto DG: Tollip: a multitasking
protein in innate immunity and protein trafficking. Microbes
Infect. 14:140–147. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sugi Y, Takahashi K, Nakano K, Hosono A
and Kaminogawa S: Transcription of the Tollip gene is elevated in
intestinal epithelial cells through impaired
O-GlcNAcylation-dependent nuclear translocation of the negative
regulator Elf-1. Biochem Biophys Res Commun. 412:704–709. 2011.
View Article : Google Scholar
|
|
12
|
Sun ML, Ma DH, Liu M, Yu YX, Cao DB, Ma C,
Wang X and Liu XL: Successful treatment of paraquat poisoning by
Xuebijing, an injection concocted from multiple Chinese medicinal
herbs: a case report. J Altern Complement Med. 15:1375–1378. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Celik S, Erdogan S and Tuzcu M: Caffeic
acid phenethyl ester (CAPE) exhibits significant potential as an
antidiabetic and liver-protective agent in streptozotocin-induced
diabetic rats. Pharmacol Res. 60:270–276. 2009. View Article : Google Scholar
|
|
14
|
Lowry OH, Rosebrough NJ, Farr AL and
Randall RJ: Protein measurement with the folin phenol reagent. J
Biol Chem. 193:265–275. 1951.PubMed/NCBI
|
|
15
|
Kakkar P, Das B and Viswanathan PN: A
modified spectrophotometric assay of superoxide dismutase. Indian J
Biochem Biophys. 21:130–132. 1984.PubMed/NCBI
|
|
16
|
Rokicki W, Zalejska-Fiolka J,
Mrukwa-Kominek E, Majewski W and Birkner E: Effect of selected
dietary compounds on extracellular superoxide dismutase in the
vitreous of chinchillas. Ophthalmic Res. 50:54–58. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
von Leitner EC, Klinke A, Atzler D, Slocum
JL, Lund N, Kielstein JT, Maas R, Schmidt-Haupt R, Pekarova M,
Hellwinkel O, Tsikas D, D’Alecy LG, Lau D, Willems S, Kubala L,
Ehmke H, Meinertz T, Blankenberg S, Schwedhelm E, Gadegbeku CA,
Böger RH, Baldus S and Sydow K: Pathogenic cycle between the
endogenous nitric oxide synthase inhibitor asymmetrical
dimethylarginine and the leukocyte-derived hemoprotein
myeloperoxidase. Circulation. 124:2735–2745. 2011.
|
|
18
|
Zhang B, Liu ZY, Li YY, Luo Y, Liu ML,
Dong HY, Wang YX, Liu Y, Zhao PT, Jin FG and Li ZC:
Antiinflammatory effects of matrine in LPS-induced acute lung
injury in mice. Eur J Pharm Sci. 44:573–579. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang L, Luo N, Liu J, Duan Z, Du G, Cheng
J, Lin H and Li Z: Emulsified isoflurane preconditioning protects
against liver and lung injury in rat model of hemorrhagic shock. J
Surg Res. 171:783–790. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Szapiel SV, Elson NA, Fulmer JD,
Hunninghake GW and Crystal RG: Bleomycin-induced interstitial
pulmonary disease in the nude, athymic mouse. Am Rev Respir Dis.
120:893–899. 1979.PubMed/NCBI
|
|
21
|
Risøe PK, Ryg U, Wang YY, Rutkovskiy A,
Smedsrød B, Valen G and Dahle MK: Cecal ligation and puncture
sepsis is associated with attenuated expression of adenylyl cyclase
9 and increased miR142–3p. Shock. 36:390–395. 2011.PubMed/NCBI
|
|
22
|
Li HF, Sun MG, Yu YX and Liu XL: Xuebijing
alters tumor necrosis factor-alpha, interleukin-1beta and p38
mitogen activated protein kinase content in a rat model of cardiac
arrest following cardiopulmonary resuscitation. Neural Regen Res.
6:2573–2576. 2011.
|
|
23
|
Chen Y, Tong HS, Zhang XQ, Tang LQ, Pan
ZG, Liu ZF, Duan PK and Su L: Xuebijing injection alleviates liver
injury by inhibiting secretory function of Kupffer cells in heat
stroke rats. J Tradit Chin Med. 33:243–249. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qi F, Liang ZX, She DY, Yan GT and Chen
LA: A clinical study on the effects and mechanism of xuebijing
injection in severe pneumonia patients. J Tradit Chin Med.
31:46–49. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shao M, Liu B, Wang JQ, Tao XG, Zhou SS,
Jin K and Zhang CP: Effect of Xuebijing injection on T helper 17
and CD4+ CD25+ regulatory T cells in patients
with sepsis. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 23:430–434.
2011.(In Chinese).
|
|
26
|
Sun J, Xue Q, Guo L, Cui L and Wang J:
Xuebijing protects against lipopolysaccharide-induced lung injury
in rabbits. Exp Lung Res. 36:211–218. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou G, Kamenos G, Pendem S, Wilson JX and
Wu F: Ascorbate protects against vascular leakage in cecal ligation
and puncture-induced septic peritonitis. Am J Physiol Regul Integr
Comp Physiol. 302:R409–R416. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nan YH, Park IS, Hahm KS and Shin SY:
Antimicrobial activity, bactericidal mechanism and LPS-neutralizing
activity of the cell-penetrating peptide pVEC and its analogs. J
Pept Sci. 17:812–817. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hukkanen RR, Liggitt HD, Murnane RD and
Frevert CW: Systemic inflammatory response syndrome in nonhuman
primates culminating in multiple organ failure, acute lung injury,
and disseminated intravascular coagulation. Toxicol Pathol.
37:799–804. 2009. View Article : Google Scholar
|
|
30
|
Moncada-Pazos A, Obaya AJ, Llamazares M,
Heljasvaara R, Suárez MF, Colado E, Noël A, Cal S and López-Otín C:
ADAMTS-12 metalloprotease is necessary for normal inflammatory
response. J Biol Chem. 287:39554–39563. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ghosh S and Hayden MS: Newregulators of
NF-κB in inflammation. Nat Rev Immunol. 8:837–848. 2008.
|
|
32
|
Zhao D, Ding R, Mao Y, Wang L, Zhang Z and
Ma X: Heparin rescues sepsis-associated acute lung injury and
lethality through the suppression of inflammatory responses.
Inflammation. 35:1825–1832. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ji MH, Zhu XL, Liu FF, Li GM, Tian M, Wu
J, Fan YX, Li N and Yang JJ: Alpha 2A-adrenoreceptor blockade
improves sepsis-induced acute lung injury accompanied with
depressed high mobility group box-1 levels in rats. Cytokine.
60:639–645. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li X, Zheng Z, Li X and Ma X:
Unfractionated heparin inhibits lipopolysaccharide-induced
inflammatory response through blocking p38 MAPK and NF-κB
activation on endothelial cell. Cytokine. 60:114–121.
2012.PubMed/NCBI
|
|
35
|
Biswas A, Wilmanski J, Forsman H, Hrncir
T, Hao L, Tlaskalova-Hogenova H and Kobayashi KS: Negative
regulation of Toll-like receptor signaling plays an essential role
in homeostasis of the intestine. Eur J Immunol. 41:182–194. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ostuni R, Zanoni I and Granucci F:
Deciphering the complexity of Toll-like receptor signaling. Cell
Mol Life Sci. 67:4109–4134. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liew FY, Xu D, Brint EK and O’Neill LA:
Negative regulation of toll-like receptor-mediated immune
responses. Nat Rev Immunol. 5:446–458. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Oshima N, Ishihara S, Rumi MAK, Aziz MM,
Mishima Y, Kadota C, Moriyama I, Ishimura N, Amano Y and Kinoshita
Y: A20 is an early responding negative regulator of Toll-like
receptor 5 signalling in intestinal epithelial cells during
inflammation. Clin Exp Immunol. 159:185–198. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wald D, Qin J, Zhao Z, Qian Y, Naramura M,
Tian L, Towne J, Sims JE, Stark GR and Li X: SIGIRR, a negative
regulator of Toll-like receptor-interleukin 1 receptor signaling.
Nat Immunol. 4:920–927. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kobayashi K, Hernandez LD, Galan JE,
Janeway CA Jr, Medzhitov R and Flavell RA: IRAK-M is a negative
regulator of Toll-like receptor signaling. Cell. 110:191–202. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bulut Y, Faure E, Thomas L, Equils O and
Arditi M: Cooperation of toll-like receptor 2 and 6 for cellular
activation by soluble tuberculosis factor and Borrelia
burgdorferi outer surface protein a lipoprotein: role of toll
interacting protein and IL-1 receptor signaling molecules in
toll-like receptor 2 signaling. J Immunol. 167:987–994.
2001.PubMed/NCBI
|
|
42
|
Burns K, Janssens S, Brissoni B, Olivos N,
Beyaert R and Tschopp J: Inhibition of interleukin 1
receptor/toll-like receptor signaling through the alternatively
spliced, short form of MyD88 is due to its failure to recruit
IRAK-4. J Exp Med. 197:263–268. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Neumann D, Lienenklaus S, Rosati O and
Martin MU: IL-1β-induced phosphorylation of PKB/Akt depends on the
presence of IRAK-1. Eur J Immunol. 32:3689–3698. 2002.
|
|
44
|
Shimizu K, Taniyama Y, Sanada F, Azuma J,
Iwabayashi M, Iekushi K, Rakugi H and Morishita R: Hepatocyte
growth factor inhibits lipopolysaccharide-induced oxidative stress
via epithelial growth factor receptor degradation. Arterioscler
Thromb Vasc Biol. 32:2687–2693. 2012. View Article : Google Scholar
|
|
45
|
Bastarache JA, Sebag SC, Clune JK, Grove
BS, Lawson WE, Janz DR, Roberts LJ, Dworski R, Mackman N and Ware
LB: Low levels of tissue factor lead to alveolar haemorrhage,
potentiating murine acute lung injury and oxidative stress. Thorax.
67:1032–1039. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bedirli N, Demirtas CY, Akkaya T, Salman
B, Alper M, Bedirli A and Pasaoglu H: Volatile anesthetic
preconditioning attenuated sepsis induced lung inflammation. J Surg
Res. 178:e17–e23. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yageta Y, Ishii Y, Morishima Y, Masuko H,
Ano S, Yamadori T, Itoh K, Takeuchi K, Yamamoto M and Hizawa N:
Role of Nrf2 in host defense against influenza virus in cigarette
smoke-exposed mice. J Virol. 85:4679–4690. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Boutten A, Goven D, Boczkowski J and Bonay
M: Oxidative stress targets in pulmonary emphysema: focus on the
Nrf2 pathway. Expert Opin Ther Targets. 14:329–346. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Cho HY, Imani F, Miller-DeGraff L, Walters
D, Melendi GA, Yamamoto M, Polack FP and Kleeberger SR: Antiviral
activity of Nrf2 in a murine model of respiratory syncytial virus
disease. Am J Respir Crit Care Med. 179:138–150. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Jeong SJ, Han SH, Kim CO, Choi JY and Kim
JM: Anti-vascular endothelial growth factor antibody attenuates
inflammation and decreases mortality in an experimental model of
severe sepsis. Crit Care. 17:R97–R98. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Qiu Y, Ferguson J, Oltean S, Neal CR,
Kaura A, Bevan H, Wood E, Sage LM, Lanati S, Nowak DG, Salmon AH,
Bates D and Harper SJ: Overexpression of VEGF165b in podocytes
reduces glomerular permeability. J Am Soc Nephrol. 21:1498–1509.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Jezovnik MK and Poredos P: Idiopathic
venous thrombosis is related to systemic inflammatory response and
to increased levels of circulating markers of endothelial
dysfunction. Int Angiol. 29:226–231. 2010.PubMed/NCBI
|
|
53
|
Hack CE and Zeerleder S: The endothelium
in sepsis: source of and a target for inflammation. Crit Care Med.
29:S21–S27. 2001.PubMed/NCBI
|
|
54
|
Reinhart K, Bayer O, Brunkhorst F and
Meisner M: Markers of endothelial damage in organ dysfunction and
sepsis. Crit Care Med. 30:S302–S312. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lucas R, Sridhar S, Rick FG, Gorshkov B,
Umapathy NS, Yang G, Oseghale A, Verin AD, Chakraborty T, Matthay
MA, Zemskov EA, White R, Block NL and Schally AV: Agonist of growth
hormone-releasing hormone reduces pneumolysin-induced pulmonary
permeability edema. Am J Physiol Renal Physiol. 303:F1026–F1036.
2012.PubMed/NCBI
|