Introduction
The majority of the molecular biology based
approaches developed for the experimental therapy of thalassemia
and sickle-cell anemia (SCA) are based on the induction of
defective globin (β-globin in β-thalassemia) (1–5) or
(also in association with this strategy) the induction of fetal
hemoglobin (HbF) (6–10). The de novo production of
adult hemoglobin (HbA) can be achieved in
β0-thalassemias by gene therapy (1–4)
and gene correction, by homologous recombination (11,12) and/or by the treatment of erythroid
cells with molecules causing read-through (13). The induction of HbF can be
obtained by using low molecular weight drugs causing the induction
of the γ-globin gene (6–8,14–17), artificial promoters (18,19), decoy molecules targeting
transcription factors involved in the transcriptional repression of
γ-globin genes (MYB, KLF-1 and BCL-11A) (20,21), or microRNAs targeting mRNAs coding
for these repressors (data are available for microRNAs miR-15a,
miR-16-1, miR-486-3p and miR-23a/27a) (22–24). On the other hand, clinical
complications in SCA and β-thalassemia are also related to the
production of defective proteins (β-globin in SCA) (25–27) or to the accumulation of free
globins which are not organized in a functional tetramer (such as
in the case of free α-globins in β-thalassemia) (28,29). It is well known that sickle
hemoglobin (HbS) has peculiar biochemical properties, leading to
polymerization when deoxygenated. HbS polymerization is associated
with a reduction in cell ions and water content (cell dehydration)
and increased red cell density, which further accelerates HbS
polymerization. Pathophysiological studies have indicated that the
dense, dehydrated red cells may play a central role in acute and
chronic clinical manifestations of sickle-cell disease, in which
intravascular sickling in capillaries and small vessels leads to
vaso-occlusion and impaired blood flow in a variety of organs and
tissues (30). Therefore, the
development of drug-based methods for the inhibition of the
accumulation of defective hemoglobins (HbS in SCA) or globin
produced in excess (α-globins in β-thalassemia) is required
(31,32).
In this field of investigation, peptide nucleic
acids (PNAs) may be of great interest (33). PNAs are DNA analogues in which the
sugarphosphate backbone is replaced by N-(2-aminoethyl)glycine
units (33). These very
interesting molecules were described for the first time by Nielsen
et al (34) and, despite a
radical structural change with respect to DNA and RNA, they are
capable of sequence-specific and efficient hybridization with
complementary DNA and RNA, forming Watson-Crick double helices
(35). In addition, they are able
to generate triple helix formation with double-stranded DNA and
perform strand invasion (34).
Accordingly, PNA-based analogues have been proposed as antisense
molecules targeting mRNAs and microRNAs, triple-helix forming
molecules targeting eukaryotic gene promoters, artificial promoters
and decoy molecules targeting transcription factors (36). To the best of our knowledge, PNAs
have not yet been employed to inhibit the expression of globin
genes in erythroid cells. Thus, the aim of this study was to verify
whether PNAs targeting globin mRNAs can be used to modulate globin
gene expression and to reduce the level of a given type of globin.
For this purpose, we produced one PNA targeting murine adult
β-globin mRNAs and another recognizing the human γ-globin and
β-globin mRNAs. These PNAs were tested on relevant target erythroid
cell lines, such as the murine erythroleukemia (MEL) cell line.
Erythroid differentiation and the high production of hemoglobins
were induced by treatment with dimethylsulfoxide (DMSO) and
hexamethylene bisacetamide (HMBA) (37–39).
Materials and methods
Synthesis and characterization of
PNAs
The synthesis of the two PNAs was performed using
standard automated Fmoc-based chemistry with HBTU/DIPEA coupling on
a ChemMatrix resin loaded with Fmoc-Gly-OH as first monomer
(loading 0.2 mmol/g, 5 μmol scale), on a Syro II peptide
synthesizer, using commercially available monomers (Link
Technologies, Bellshill, UK); Fmoc-Arg (Pbf)-OH (Sigma-Aldrich, St.
Louis, MO, USA) was used for octaarginine synthesis. PNA
purification was performed by reversed-phase high-performance
liquid chromatography (RP-HPLC) with UV detection at 260 nm using a
semi-prep column C18 (10 μm, 300×7.7 mm, Xterra Waters, 300 Å),
eluting with water containing 0.1% TFA (eluent A) and acetonitrile
containing 0.1% TFA (eluent B); elution gradient: from 100% A to
50% B in 30 min, flow: 4 ml/min. The purity and identity of the
purified PNA were examined by ultra-performance liquid
chromatography tandem mass-spectrometry (UPLC-MS; Waters Acquity
equipped with ESI-Q analizer) using an Acquity UPLC BEH C18; 2.1×50
MM, 1.7 μm column. Anti-M-βglob-PNA: yield, 6%; electrospray
ionization mass spectrometry (ESI-MS): m/z found (calculated):
1267.3 (1267.3) [MH55+], 1056.2 (1056.2)
[MH66+], 905.4 (905.5)
[MH77+], 792.2 (792.4)
[MH88+], 704.5 (704.5)
[MH99+]; calculated MW: 6331.39.
Anti-H-γglob-PNA: yield: 8%; ESI-MS: m/z found (calculated): 1346.0
(1345.4) [MH44+]; 1076.8 (1076.5)
[MH55+], 897.7 (897.3)
[MH66+], 769.5 (769.2)
[MH77+], 673.3 (673.2)
[MH88+], 598.8 (598.5)
[MH99+], 539.0 (538.8)
[MH1010+]; calculated MW: 5377.54.
MEL and K562 cell lines and culture
conditions
MEL cells (37–39) were cultured in humidified
atmosphere of 5% CO2/air in RPMI-1640 medium
(Sigma-Aldrich) supplemented with 10% fetal bovine serum (FBS;
Biowest, Nuaillé, France), 50 U/ml penicillin and 50 μg/ml
streptomycin (39). DMSO and HMBA
were from Sigma-Aldrich. Stock solutions of HMBA were stored at
−20°C in the dark and diluted immediately before use. Treatment of
the MEL cells with HMBA and DMSO was carried out by adding the
appropriate drug concentrations at the beginning of the cultures
(30,000 cells/ml were seeded). To determine the effects of the
treatments on the proliferation of the MEL cells, cell growth was
monitored by determining the cell number/ml using a Z1 Coulter
Counter (Coulter Electronics, Hialeah, FL, USA). Erythroid
differentiated MEL cells containing hemoglobin were detected by
specific reaction with a benzidine/hydrogen peroxide solution (0.2%
in 5 M glacial acetic acid and 10% H2O2). The
K562-D5 cell line was employed as it produces, in addition to
hemoglobin (Hb) Gower1 and hemoglobin (Hb) Portland, HbF and HbA
(40).
RNA extraction
The cells were isolated by centrifugation at 1,500
rpm for 10 min at 4°C, washed with phosphate-buffered saline (PBS)
and lysed with TRI-reagent™ (Sigma-Aldrich) according to the
manufacturer’s instructions. The isolated RNA was washed once with
cold 75% ethanol, dried and dissolved in nuclease-free pure water
prior to use.
Quantitative reverse transcription
polymerase chain reaction (RT-qPCR)
For gene expression analysis, 500 ng of total RNA
were reverse transcribed using random hexamers. Quantitative PCR
assays were carried out using gene-specific double-quenched probes
containing a 5′-FAM fluorophore, a 3′-IBFQ quencher and an internal
ZEN quencher. The nucleotide sequences used for the RT-qPCR
analysis of mouse globin mRNAs were α-globin forward, 5′-CTG ACC
TCC AAG TAC CGT TAA G-3′ and reverse primer, 5′-GCT TCT TCC TAC TCA
GGC TTT AT-3′ and α-globin probe, 5′-/56-FAM/TCT CTC CCT/ZEN/TGC
ACC TGT ACC TCT/3IABkFQ/-3′; β-globin forward, 5′-GGA AAG GTG AAC
TCC GAT GAA-3′ and reverse primer, 5′-TGA TAG CAG AGG CAG AGG ATA
G-3′ and β-globin probe, 5′-/56-FAM/CCT TGG ACC/ZEN/CAG CGG TAC TTT
GAT/3IABkFQ/-3′. The primers and probes used to assay mouse globins
were purchased from Integrated DNA Technologies (IDT; Coralville,
IA, USA). The relative expression was calculated using the
comparative cycle threshold method and the endogenous control mouse
gene, GAPDH, was used as a reference gene (PrimeTime Mm.PT.39a.1,
IDT).
HPLC analysis
The cells were harvested, washed once with PBS and
the pellets were lysed in lysis buffer (sodium dodecyl sulphate
0.01%). After spinning for 1 min in a microcentrifuge, the
supernatant was collected and stored at 4°C. Hemoglobins in the
lysates were separated by cation-exchange HPLC (Pharmacia LKB
Gradient Pump 2249, VWM 2141), using a Synchropak CM300 (250×4.6
mm) column (Eichrom Technologies, Inc., Darien, IL, USA) and
BisTris (30 mM) buffer. Standard HbA and HbF (Sigma-Aldrich,
Milwaukee, WI, USA; Helena Laboratories, Beaumont, TX, USA)
solutions were used as a reference.
Bioinformatics analysis
The secondary structure of the mouse
βmajor- and βminor-globin mRNA sequences, 5′
untranslated region (UTR), coding sequence (CDS) and 3′UTR, was
predicted using the program available online TBI ViennaRNA Web
Services (http://rna.tbi.univie.ac.at). The
mouse β-globin reference sequences analyzed were obtained from the
NCBI website.
Statistical analysis
The results are expressed as the means ± standard
error of the mean (SEM). Comparisons between groups were made using
a paired Student’s t-test and one-way analysis of variance (ANOVA).
Statistical significance was defined with p<0.01.
Results
Design of PNAs
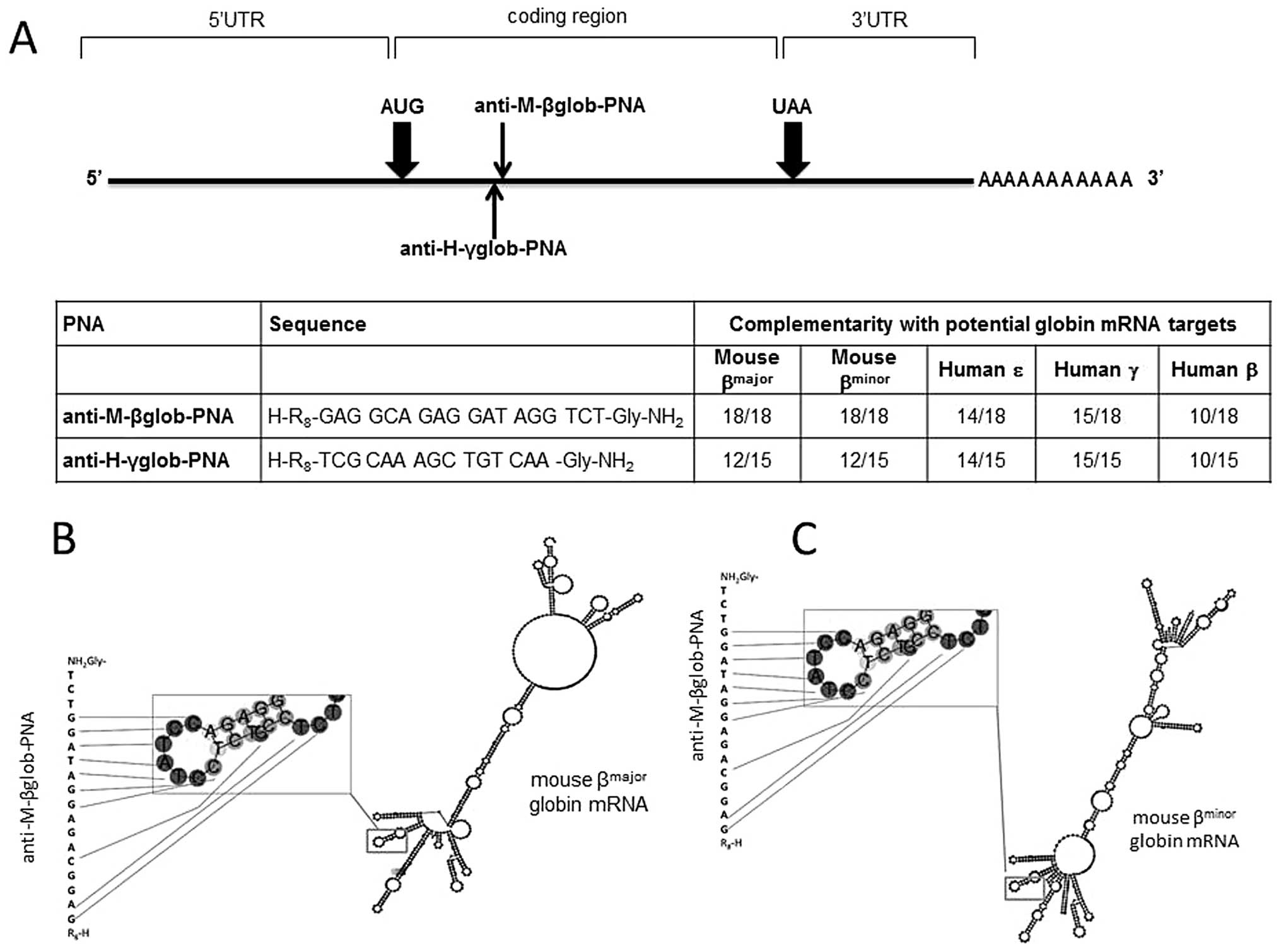
The location of the binding sites and the sequences
of the PNAs employed in this study are presented in Fig. 1. As clearly appreciable from the
analysis of the sequences recognized, the anti-M-βglob-PNA is able
to hybridize to a region of both mouse βmajor- and
βminor-globin mRNAs exhibiting similar predicted
secondary structures (Fig. 1B and
C). This feature allows us to study the effects of the PNAs
simply analyzing the proportion of erythroid differentiated MEL
cells.
These cells, upon the induction of erythroid
differentiation with HMBA or DMSO produce almost exclusively
Hbmajor (α2β2major) and
Hbminor (α2β2minor).
Moreover, these PNAs display 4 to 8 mismatches with human β-globin
(8 mismatches), γ-globin (3 mismatches) and ɛ-globin (4 mismatches)
(Fig. 1A, bottom panel). This
allows us to verify possible non-specific inhibitory effects when
used on K562 cell clones subjected to erythroid differentiation
which produce, upon treatment with mithramycin (MTH), high levels
of Hb Gower 1 (ζ2ɛ2), Hb Portland
(ζ2γ2), HbF (α2γ2) and
HbA (α2β2) (40). Conversely, as control PNA
molecules we used anti-H-γglob-PNA recognizing the evolutionarily
homologue human γ-globin mRNAs and displaying 3 mismatches with the
murine βmajor and βminor mRNAs (Fig. 1A). Both anti-M-βglob-PNA and
anti-H-γglob-PNA were linked with a R8 polyarginine
peptide, since it has been reported in several studies that the
uptake of PNAs by target cells is difficult (41,42). R8 was employed in order
to maximize PNA uptake, as reported by our group for several PNAs,
which, without exception, are internalized with very high
efficiency to target cells (43–45).
Effects of anti-M-βglob-PNA on the growth
of MEL cells
Fig. 2 shows the
kinetics of differentiation (Fig.
2A) and cell growth (Fig. 2B)
obtained when the MEL cells are treated with 2% and 2.5 mM DMSO and
HMBA, respectively. The high level of induction (>80% after 3 or
4 days in all the experiments performed) confirms that this
cellular system is excellent to determine inhibitors of the
expression of adult β-globin genes, since, unlike other erythroid
cellular model systems (such as human K562 cells), these cells
mostly produce the adult-type Hbmajor and
Hbminor hemoglobins (39). As shown in Fig. 2C, the addition of anti-M-βglob-PNA
did not alter the proliferation rate of these cells, formally
demonstrating no cytotoxic effects of this PNA on the MEL cells,
either in the absence of differentiation inducers (data not shown)
or in the presence of DMSO or HMBA.
Anti-M-βglob-PNA inhibits the erythroid
differentiation of MEL cells induced by DMSO and HMBA
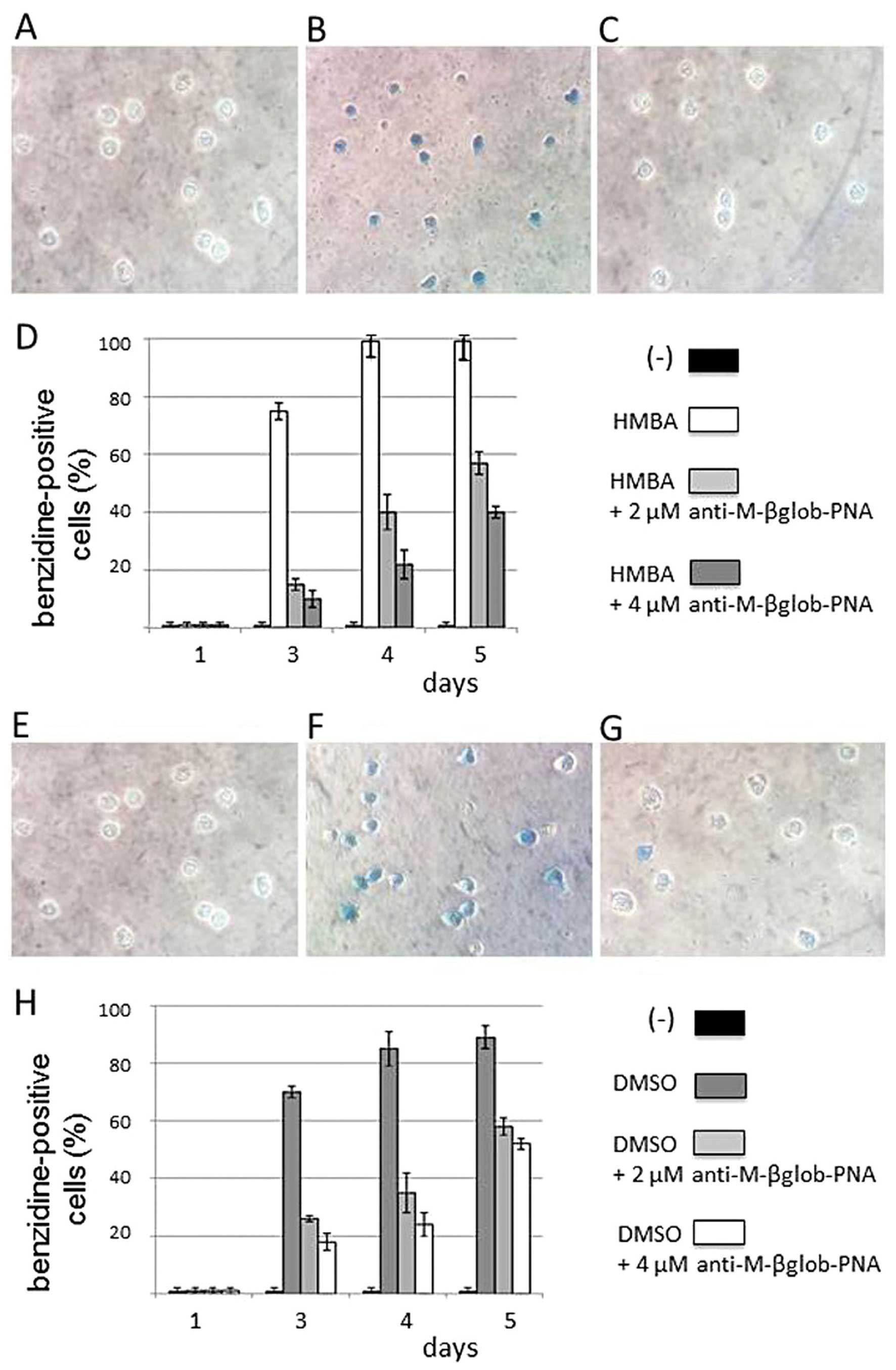
The effects of anti-M-βglob-PNA on the erythroid
differentiation of MEL cells were first analyzed by benzidine
staining of the treated cells. The results of this experiment are
presented in Fig. 3, which
clearly illustrates that the MEL cells treated with DMSO (Fig. 3G and H) and HMBA (Fig. 3C and D) do not efficiently
differentiate in the presence of anti-M-βglob-PNA. Fig. 3A–C and E–G shows representative
experiments, while Fig. 3D and H
shows the summary of 3 independent experiments, confirming a
decrease in the proportion of benzidine-positive
(hemoglobin-containing) PNA-treated cells.
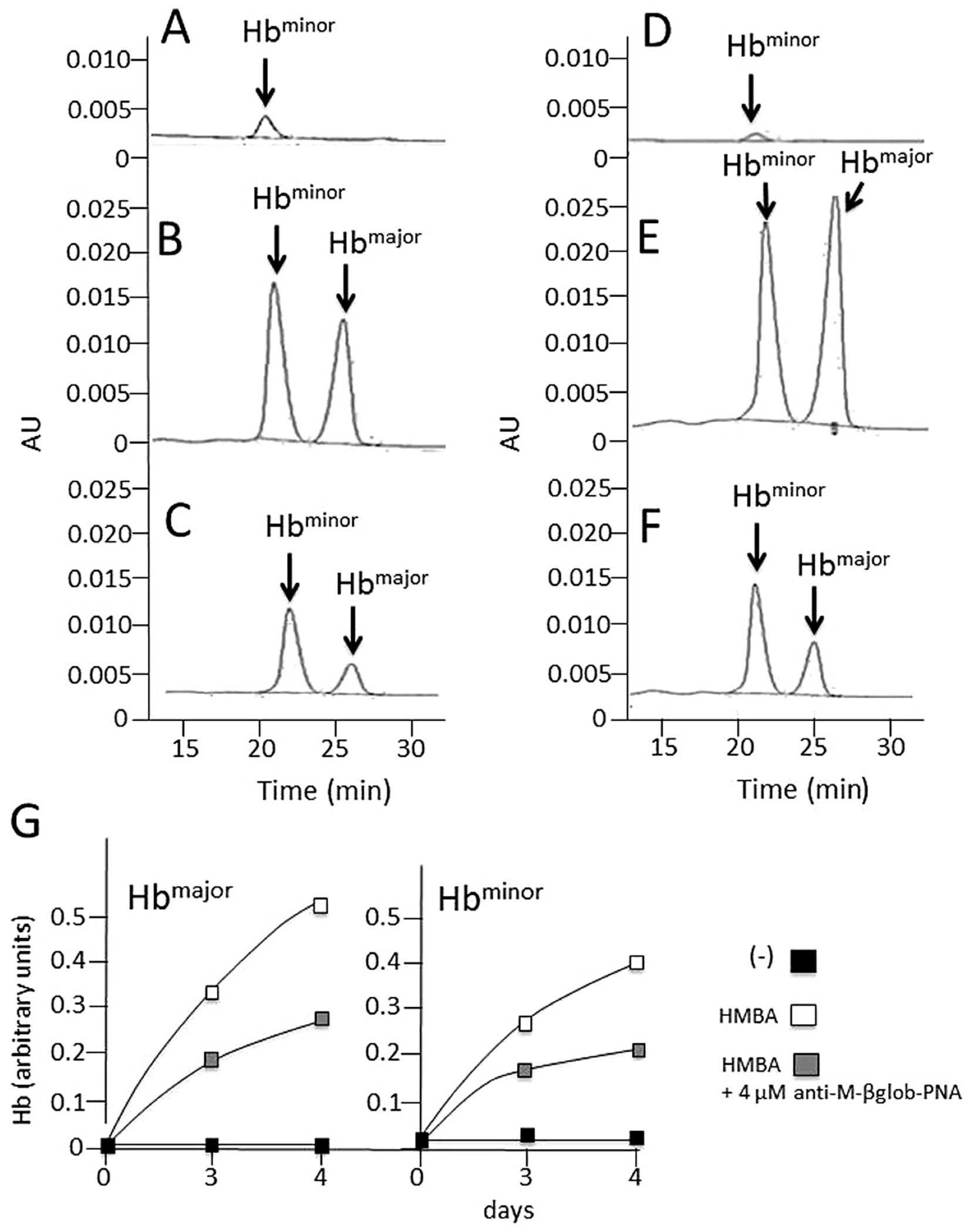
Effects of anti-M-βglob-PNA on hemoglobin
and β-globin mRNA accumulation in MEL cells treated with HMBA
The effects of anti-M-βglob-PNA on hemoglobin and
β-globin mRNA accumulation were examined in the HMBA-treated cells
by HPLC (for hemoglobin analysis) and RT-qPCR (for the analysis of
β-globin mRNA). The results are presented in Fig. 4, which clearly illustrates that
anti-M-βglob-PNA inhibited the accumulation of both
Hbmajor and Hbminor hemoglobins fully in
agreement with the data shown in Fig.
3. Of note, anti-M-βglob-PNA also inhibited, to a certain
extent, β-globin mRNA, suggesting that the inhibition of hemoglobin
production may be also associated with the lower stability of
β-globin mRNA (data not shown).
Specificity of the effects of
anti-M-βglob-PNA
The effects of anti-M-βglob-PNA on erythroid
differentiation were also examined using human K562 cells subjected
to erythroid differentiation by treatment with mithramycin (MTH).
As shown in Fig. 5 no inhibitory
effects of anti-M-βglob-PNA were observed on the MTH-stimulated
K562-D5 cell clones, indicating high levels of specificity of the
inhibitory effects of the anti-M-βglob-PNA. Furthermore, control
experiments were also performed using the anti-H-γglob-PNA on HMBA-
and DMSO-treated MEL cells. Of note, no inhibitory effects were
observed using the anti-H-γglob-PNA, suggesting that the effects of
treatment of the erythroid cells with PNAs were sequence-specific
(Fig. 6).
Discussion
In the treatment of SCA, HbS appears to be an
important therapeutic target, since its polymerization is
responsible for the sickling of SCA red-blood cells and important
adverse clinical parameters (25–27). For instance, intravascular
sickling in capillaries and small vessels leads to vaso-occlusion
and impaired blood flow in a variety of organs and tissues
(30). These conclusions are
sustained by strong evidence suggesting that the induction of HbF
following the treatment of SCA patients with hydroxyurea (HU)
appears to be beneficial, due to the intrinsic anti-sickling
properties of HbF (46,47). In any case, the inhibition of
β-globin may be beneficial, allowing further reduction of sickling
properties. Therefore the proof-of-principle of PNA-based methods
enabling the effective inhibition of the accumulation of β-globin
is of interest and of potential biomedical application.
Acknowledgements
This study was supported by a grant from the Italian
Ministry of University and Research (MIUR) and from TOP-THAL
(R&S project for PMI Lazio-Cup code F57I-12000010007). R.G.
received a grant from Fondazione Cariparo (Cassa di Risparmio di
Padova e Rovigo), CIB, from UE FP7 THALAMOSS Project (THALAssaemia
Modular Stratification System for personalized therapy of
β-thalassemia; 306201), from Telethon GGP10124 and from AIRC
IG13575. This study was also supported by Associazione Veneta per
la Lotta alla Talassemia (AVLT), Rovigo.
References
|
1
|
Cavazzana-Calvo M, Payen E, Negre O, Wang
G, Hehir K, Fusil F, Down J, Denaro M, Brady T, Westerman K,
Cavallesco R, Gillet-Legrand B, Caccavelli L, Sgarra R,
Maouche-Chrétien L, Bernaudin F, Girot R, Dorazio R, Mulder GJ,
Polack A, Bank A, Soulier J, Larghero J, Kabbara N, Dalle B,
Gourmel B, Socie G, Chrétien S, Cartier N, Aubourg P, Fischer A,
Cornetta K, Galacteros F, Beuzard Y, Gluckman E, Bushman F,
Hacein-Bey-Abina S and Leboulch P: Transfusion independence and
HMGA2 activation after gene therapy of human β-thalassaemia.
Nature. 467:318–322. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kaiser J: Gene therapy. Beta-thalassemia
treatment succeeds, with a caveat. Science. 326:1468–1469. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dong A, Rivella S and Breda L: Gene
therapy for hemoglobinopathies: progress and challenges. Transl
Res. 161:293–306. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Breda L, Casu C, Gardenghi S, Bianchi N,
Cartegni L, Narla M, Yazdanbakhsh K, Musso M, Manwani D, Little J,
Gardner LB, Kleinert DA, Prus E, Fibach E, Grady RW, Giardina PJ,
Gambari R and Rivella S: Therapeutic hemoglobin levels after gene
transfer in β-thalassemia mice and in hematopoietic cells of
β-thalassemia and sickle cells disease patients. PLoS One.
7:e323452012. View Article : Google Scholar
|
|
5
|
Zou J, Mali P, Huang X, Dowey SN and Cheng
L: Site-specific gene correction of a point mutation in human iPS
cells derived from an adult patient with sickle cell disease.
Blood. 118:4599–4608. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gambari R and Fibach E: Medicinal
chemistry of fetal hemoglobin inducers for treatment of
beta-thalassemia. Curr Med Chem. 14:199–212. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Reid ME, El Beshlawy A, Inati A, Kutlar A,
Abboud MR, Haynes J Jr, Ward R, Sharon B, Taher AT, Smith W,
Manwani D and Ghalie RG: A double-blind, placebo-controlled phase
II study of the efficacy and safety of 2,2-dimethylbutyrate
(HQK-1001), an oral fetal globin inducer, in sickle cell disease.
Am J Hematol. 89:709–713. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Perrine SP, Pace BS and Faller DV:
Targeted fetal hemoglobin induction for treatment of beta
hemoglobinopathies. Hematol Oncol Clin North Am. 28:233–248. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Breda L, Rivella S, Zuccato C and Gambari
R: Combining gene therapy and fetal hemoglobin induction for
treatment of β-thalassemia. Expert Rev Hematol. 6:255–264. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zuccato C, Breda L, Salvatori F,
Breveglieri G, Gardenghi S, Bianchi N, Brognara E, Lampronti I,
Borgatti M, Rivella S and Gambari R: A combined approach for
β-thalassemia based on gene therapy-mediated adult hemoglobin (HbA)
production and fetal hemoglobin (HbF) induction. Ann Hematol.
91:1201–1213. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Voit RA, Hendel A, Pruett-Miller SM and
Porteus MH: Nuclease-mediated gene editing by homologous
recombination of the human globin locus. Nucleic Acids Res.
42:1365–1378. 2014. View Article : Google Scholar :
|
|
12
|
Ma N, Liao B, Zhang H, Wang L, Shan Y, Xue
Y, Huang K, Chen S, Zhou X, Chen Y, Pei D and Pan G: Transcription
activator-like effector nuclease (TALEN)-mediated gene correction
in integration-free β-thalassemia induced pluripotent stem cells. J
Biol Chem. 288:34671–34679. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Salvatori F, Breveglieri G, Zuccato C,
Finotti A, Bianchi N, Borgatti M, Feriotto G, Destro F, Canella A,
Brognara E, Lampronti I, Breda L, Rivella S and Gambari R:
Production of beta-globin and adult hemoglobin following G418
treatment of erythroid precursor cells from homozygous beta039
thalassemia patients. Am J Hematol. 84:720–728. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ansari SH, Shamsi TS, Ashraf M, Perveen K,
Farzana T, Bohray M, Erum S and Mehboob T: Efficacy of hydroxyurea
in providing transfusion independence in β-thalassemia. J Pediatr
Hematol Oncol. 33:339–343. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fibach E, Bianchi N, Borgatti M, Prus E
and Gambari R: Mithramycin induces fetal hemoglobin production in
normal and thalassemic human erythroid precursor cells. Blood.
102:1276–1281. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Salvador A, Brognara E, Vedaldi D,
Castagliuolo I, Brun P, Zuccato C, Lampronti I and Gambari R:
Induction of erythroid differentiation and increased globin mRNA
production with furocoumarins and their photoproducts. J Photochem
Photobiol B. 121:57–66. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fibach E, Bianchi N, Borgatti M, Zuccato
C, Finotti A, Lampronti I, Prus E, Mischiati C and Gambari R:
Effects of rapamycin on accumulation of alpha-, beta- and
gamma-globin mRNAs in erythroid precursor cells from
beta-thalassaemia patients. Eur J Haematol. 77:437–441. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gräslund T, Li X, Magnenat L, Popkov M and
Barbas CF III: Exploring strategies for the design of artificial
transcription factors: targeting sites proximal to known regulatory
regions for the induction of gamma-globin expression and the
treatment of sickle cell disease. J Biol Chem. 280:3707–3714. 2005.
View Article : Google Scholar
|
|
19
|
Wilber A, Tschulena U, Hargrove PW, Kim
YS, Persons DA, Barbas CF III and Nienhuis AW: A zinc-finger
transcriptional activator designed to interact with the
gamma-globin gene promoters enhances fetal hemoglobin production in
primary human adult erythroblasts. Blood. 115:3033–3041. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu XS, Hong X and Wang G: Induction of
endogenous gamma-globin gene expression with decoy oligonucleotide
targeting Oct-1 transcription factor consensus sequence. J Hematol
Oncol. 2:152009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gambari R: Alternative options for
DNA-based experimental therapy of β-thalassemia. Expert Opin Biol
Ther. 12:443–462. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sankaran VG, Menne TF, Šćepanović D,
Vergilio JA, Ji P, Kim J, Thiru P, Orkin SH, Lander ES and Lodish
HF: MicroRNA-15a and -16-1 act via MYB to elevate fetal hemoglobin
expression in human trisomy 13. Proc Natl Acad Sci USA.
108:1519–1524. 2001. View Article : Google Scholar
|
|
23
|
Lulli V, Romania P, Morsilli O, Cianciulli
P, Gabbianelli M, Testa U, Giuliani A and Marziali G:
MicroRNA-486-3p regulates γ-globin expression in human erythroid
cells by directly modulating BCL11A. PLoS One. 8:e604362013.
View Article : Google Scholar
|
|
24
|
Ma Y, Wang B, Jiang F, Wang D, Liu H, Yan
Y, Dong H, Wang F, Gong B, Zhu Y, Dong L, Yin H, Zhang Z, Zhao H,
Wu Z, Zhang J, Zhou J and Yu J: A feedback loop consisting of
microRNA 23a/27a and the β-like globin suppressors KLF3 and SP1
regulates globin gene expression. Mol Cell Biol. 33:3994–4007.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kaul DK, Fabry ME and Nagel RL: The
pathophysiology of vascular obstruction in the sickle syndromes.
Blood Rev. 10:29–44. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rodgers GP: Overview of pathophysiology
and rationale for treatment of sickle cell anemia. Semin Hematol.
34:2–7. 1997.PubMed/NCBI
|
|
27
|
Vekilov PG: Sickle-cell haemoglobin
polymerization: is it the primary pathogenic event of sickle-cell
anaemia? Br J Haematol. 139:173–184. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vasseur C, Pissard S, Domingues-Hamdi E,
Marden MC, Galactéros F and Baudin-Creuza V: Evaluation of the free
α-hemoglobin pool in red blood cells: a new test providing a scale
of β-thalassemia severity. Am J Hematol. 86:199–202. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rivella S: Do not super-excess me! Blood.
119:5064–5065. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kassim AA and DeBaun MR: Sickle cell
disease, vasculopathy, and therapeutics. Annu Rev Med. 64:451–466.
2013. View Article : Google Scholar
|
|
31
|
Raghupathy R and Billett HH: Promising
therapies in sickle cell disease. Cardiovasc Hematol Disord Drug
Targets. 9:1–8. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Steinberg MH: Pathophysiologically based
drug treatment of sickle cell disease. Trends Pharmacol Sci.
27:204–210. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gambari R: Peptide nucleic acids: a review
on recent patents and technology transfer. Expert Opin Ther Pat.
24:267–294. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nielsen PE, Egholm M, Berg RH and Buchardt
O: Sequence-selective recognition of DNA by strand displacement
with a thymine-substituted polyamide. Science. 254:1497–1500. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Egholm M, Buchardt O, Christensen L,
Behrens C, Freier SM, Driver DA, Berg RH, Kim SK, Norden B and
Nielsen PE: PNA hybridizes to complementary oligonucleotides
obeying the Watson-Crick hydrogen-bonding rules. Nature.
365:566–568. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gambari R: Peptide-nucleic acids (PNAs): a
tool for the development of gene expression modifiers. Curr Pharm
Des. 7:1839–1862. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gambari R, Terada M, Bank A, Rifkind RA
and Marks PA: Synthesis of globin mRNA in relation to the cell
cycle during induced murine erythroleukemia differentiation. Proc
Natl Acad Sci USA. 75:3801–3804. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gambari R, Marks PA and Rifkind RA: Murine
erythroleukemia cell differentiation: relationship of globin gene
expression and of prolongation of G1 to inducer effects during
G1/early S. Proc Natl Acad Sci USA. 76:4511–4515. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rutherford TR and Harrison PR: Globin
synthesis and erythroid differentiation in a Friend cell variant
deficient in heme synthesis. Proc Natl Acad Sci USA. 76:5660–5664.
1979. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Salvatori F, Cantale V, Breveglieri G,
Zuccato C, Finotti A, Bianchi N, Borgatti M, Feriotto G, Destro F,
Canella A, Breda L, Rivella S and Gambari R: Development of K562
cell clones expressing beta-globin mRNA carrying the beta039
thalassaemia mutation for the screening of correctors of stop-codon
mutations. Biotechnol Appl Biochem. 54:41–52. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fabbri E, Manicardi A, Tedeschi T, Sforza
S, Bianchi N, Brognara E, Finotti A, Breveglieri G, Borgatti M,
Corradini R, Marchelli R and Gambari R: Modulation of the
biological activity of microRNA-210 with peptide nucleic acids
(PNAs). ChemMedChem. 6:2192–2202. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nastruzzi C, Cortesi R, Esposito E,
Gambari R, Borgatti M, Bianchi N, Feriotto G and Mischiati C:
Liposomes as carriers for DNA-PNA hybrids. J Control Release.
68:237–249. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Borgatti M, Breda L, Cortesi R, Nastruzzi
C, Romanelli A, Saviano M, Bianchi N, Mischiati C, Pedone C and
Gambari R: Cationic liposomes as delivery systems for
double-stranded PNA-DNA chimeras exhibiting decoy activity against
NF-kappaB transcription factors. Biochem Pharmacol. 64:609–616.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Brognara E, Fabbri E, Bianchi N, Finotti
A, Corradini R and Gambari R: Molecular methods for validation of
the biological activity of peptide nucleic acids targeting
microRNAs. Methods Mol Biol. 1095:165–176. 2014. View Article : Google Scholar
|
|
45
|
Brognara E, Fabbri E, Aimi F, Manicardi A,
Bianchi N, Finotti A, Breveglieri G, Borgatti M, Corradini R,
Marchelli R and Gambari R: Peptide nucleic acids targeting miR-221
modulate p27Kip1 expression in breast cancer MDA-MB-231
cells. Int J Oncol. 41:2119–2127. 2012.PubMed/NCBI
|
|
46
|
Bhatnagar P, Keefer JR, Casella JF,
Barron-Casella EA, Bean CJ, Hooper CW, Payne AB, Arking DE and
Debaun MR: Association between baseline fetal hemoglobin levels and
incidence of severe vaso-occlusive pain episodes in children with
sickle cell anemia. Pediatr Blood Cancer. 60:E125–E127. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Rodrigue CM, Arous N, Bachir D,
Smith-Ravin J, Romeo PH, Galacteros F and Garel MC: Resveratrol, a
natural dietary phytoalexin, possesses similar properties to
hydroxyurea towards erythroid differentiation. Br J Haematol.
113:500–507. 2001. View Article : Google Scholar : PubMed/NCBI
|