Introduction
Diabetes is one of the major metabolic diseases
among adults worldwode. Type 1 diabetes, classified as
insulin-dependent diabetes mellitus, is caused by the loss of
insulin secretion due to the destruction of pancreatic β-cells
(1). This leads to an increase in
blood sugar levels and a decrease in the energy transfer to other
organs, which results in various secondary complications (2). Therefore, the protection of
pancreatic β-cells is crucial for preventing the onset of type 1
diabetes. Under homeostatic conditions, potentially toxic reactive
oxygen species (ROS) are primarily generated by mitochondrial
respiratory metabolism, and are subsequently neutralized
effectively by cellular antioxidant defense mechanisms. However,
the excessive generation of ROS, such as super oxide anions
(O2−) and hydrogen peroxide
(H2O2) due to environmental stress results in
significant oxidative damage to cell structures, such as DNA, the
mitochondria and cell membranes (3,4).
Recent studies have demonstrated that antioxidants
present in natural products protect cells by reducing
H2O2-induced oxidative stress in vitro
and in vivo (5–7). This indicates the importance of
isolating compounds with antioxidant activity and the
characterization of their antioxidant mechanisms. Mulberry fruit is
a well known medicinal plant and has long been used for the
treatment of different types of diseases (8–10).
Recent studies have suggested that anthocyanins isolated from
mulberry fruit exert anti-aging, anti-hyperlipidemic and
anti-cancer effects (11–13). In particular, a representative of
the anthocyanins isolated from mulberry fruit, cyanidin-3-glucoside
(C3G), is present in high quantities in mulberry fruit and is
considered a phytotherapeutic agent due to its anti-oxidant
properties (11,14).
In the present study, we investigated the protective
effects of C3G isolated from mulberry fruit against the
H2O2-induced apoptosis of MIN6N pancreatic
β-cells.
Materials and methods
Preparation of sample
The mulberry fruits (15 kg) were extracted in 70%
ethanol (36 liters) for 24 h at room temperature 3 times. The
solvent was filtered, concentrated using an evaporator, redissolved
in distilled water and lyophilized (yield, 14.6%). The powder
obtained from the mulberry extract was soaked in n-hexane
for 24 h to remove fats and oils. C3G in the powder was extracted
with acidified methanol (methanol/1.0 N HCl, 85:15, v/v),
centrifuged at 12,000 × g for 15 min to remove the precipitate and
then filtered through a 0.45-μm filter. The purified
anthocyanin extract was evaporated at 46°C to dryness and stored
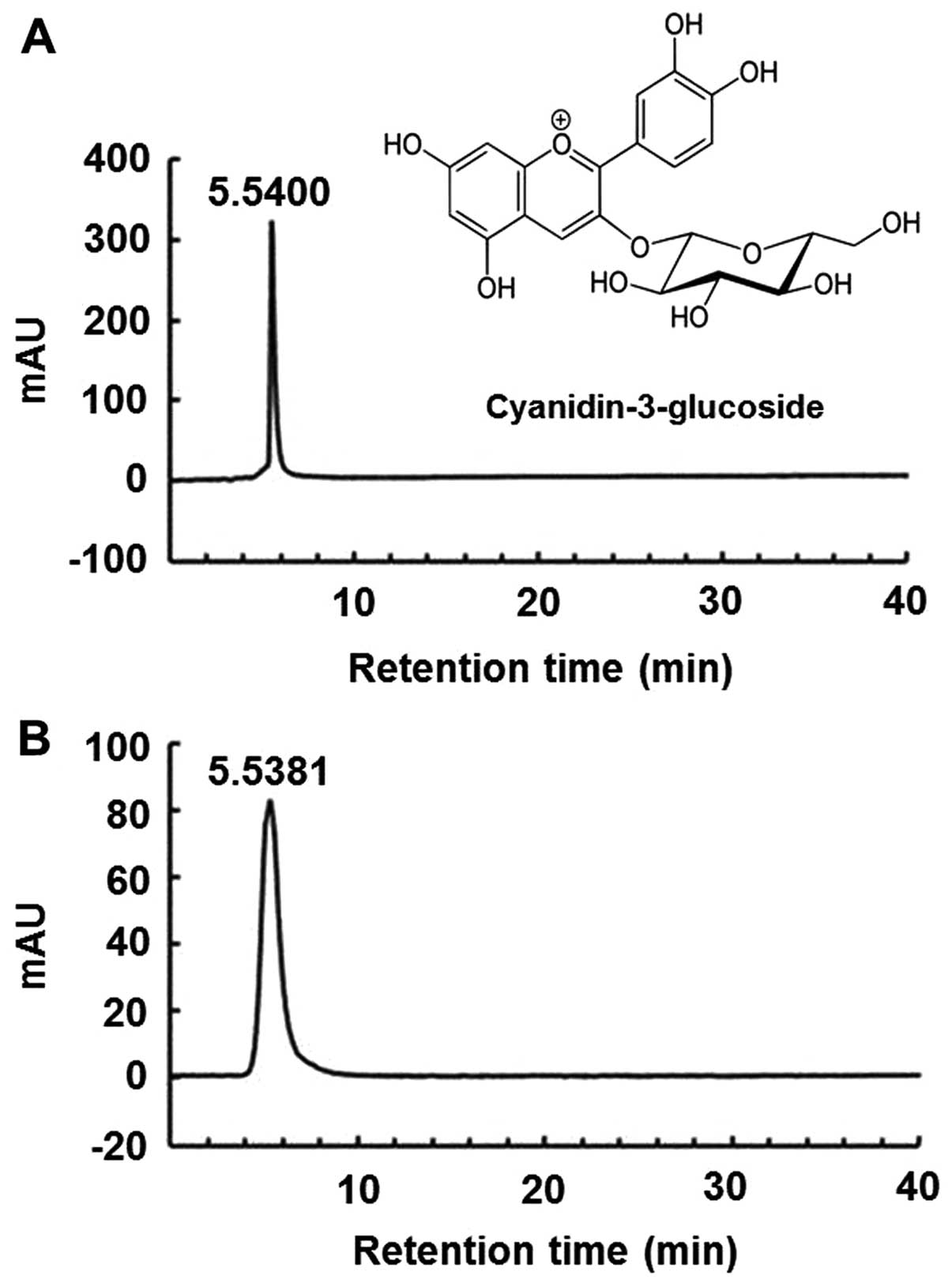
4°C. The sample was identified by diluting the purified anthocyanin
powder with the solvent (methanol/H2O/formic acid,
75:20:5) and performing high-performance liquid chromatography
(HPLC). The pure C3G standard (kuromanin chloride) and purified
anthocyanin isolated from the mulberry fruit were identified using
the HPLC retention time (Fig. 1),
and their purity was >99%, as shown by HPLC. Reversed-phase HPLC
was performed using a Waters 486 detector (Water, Milford, MA, USA)
under the following conditions: column, μBondapak C18 (Waters,
3.9×300 mm); flow rate, 0.5 ml/min; injection volume, 10 μl;
column temperature, 46°C. The absorbance was recorded at 520 nm
using a UV/Vis spectrophotometer (Spectronic Instruments; Thermo
Fisher Scientific, Waltham, MA, USA). The identified C3G powder was
redissolved in medium and filtered using a 0.22-μm filter
before using it in cell culture.
Chemicals
Dulbecco’s modified Eagle’s medium (DMEM), fetal
bovine serum (FBS), penicillin/streptomycin (PS) and trypsin-EDTA
were purchased from Gibco (Grand Island, NY, USA).
Dichlorodihydrofluorescein-diacetate (H2DCF-DA) and the
apoptosis assay kit were obtained from Molecular Probes (Carlsbad,
CA, USA). The pure C3G standard (kuromanin chloride),
H2O2, Hoechst 33342 and the mitochondria
isolation kit were purchased from Sigma-Aldrich (St. Louis, MO,
USA). The rat/mouse insulin enzyme-linked immunosorbent assay
(ELISA) kit was obtained from Linco Research Inc. (St. Charles, MO,
USA). Antibodies against c-Jun NH2-terminal kinase (JNK;
#9252), phosphorylated (p-)JNK (#9255S), extracellular
signal-regulated kinase (ERK; #4695), p-ERK (#9101S), p38 (#9212),
p-p38 (#4631S) and horseradish peroxidase (HRP)-linked anti-rabbit
IgG (#7074) were purchased from Cell Signaling Technology (Beverly,
MA, USA). Antibodies against β-actin (sc-47778), Bax (sc-493),
Bcl-2 (sc-7382), caspase-3 (sc-7272) and HRP-linked goat anti-mouse
IgG (sc-2005) were purchased from Santa Cruz Biotechnology, Inc.
(Santa Cruz, CA, USA). The mitogen-activated protein kinase (MAPK)
inhibitors, PD98059 and SB203580, were obtained from Calbiochem (La
Jolla, CA, USA). All other chemicals were of analytical grade.
Cell culture and treatment
The MIN6N pancreatic β-cells were derived from a
mouse pancreatic islet cell line. The cells were provided by
Professor H.Y. Kwon (College of Medicine, Hallym University,
Chuncheon, Korea). The MIN6N β-cells were cultured in DMEM (11 mM
glucose; Gibco) supplemented with 10% inactivated FBS and 1%
penicillin-streptomycin (PS) at 37°C in a humidified 5%
CO2 incubator. The cells were cultured to ~90%
confluence and were harvested with 0.25% trypsin-EDTA. The cells
were harvested and subcultured for an additional 48 h in DMEM. The
cells were maintained under these culture conditions for all the
experiments. The cells were seeded in 6- or 12-well plates,
pre-incubated with the indicated concentrations of C3G for 20 h at
37°C, and were then treated with H2O2.
Cell viability assay
The MIN6N β-cells were seeded into 12-well plates
and were incubated for 20 h. The cells were exposed to the drug
treatments (C3G, H2O2) at the indicated
concentrations for the indicated periods of time. Cell viability
was evaluated using the
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT)
assay, which is based on the reduction of a tetrazolium salt by
mitochondrial dehydrogenase in viable cells. After the treatments,
500 μg/ml MTT solution were added to each well followed by
incubation for 3 h at 37°C. The formazan crystals in each well were
dissolved in isopropyl alcohol and the absorbance of each well was
measured at 595 nm using an ELISA microplate reader (model 550;
Bio-Rad, Hercules, CA, USA).
Measurement of lipid peroxidation
Lipid peroxidation was assayed using the
thiobarbituric acid (TBA) reaction, as previously described
(15). Briefly, the treated cells
were washed with cold phosphate-buffered saline (PBS), harvested
with 0.25% trypsin-EDTA and homogenized in cold 1.15% KCl.
Subsequently, a 100 μl aliquot of the homogenized cells was
mixed with 0.2 ml of 8.1% sodium dodecyl sulfate (SDS), 1.5 ml of
20% acetic acid (pH 3.6) and 1.5 ml of 0.8% TBA. The mixture was
then heated to 95°C for 2 h. After cooling to room temperature, an
n-butanol/pyridine mixture (15:1, v/v) was added and the mixture
was then shaken for 5 min prior to centrifugation at 1,000 × g for
10 min. The supernatant was isolated, and the absorbance was
measured at 535 nm.
Measurement of intracellular ROS
scavenging activity and image analysis
To determine the effects of C3G on oxidative
stress-induced ROS generation, we added 5 μM of
H2DCF-DA in PBS (pH 7.38) to the treated cells, and
measured the fluorescence at excitation and emission wavelengths of
485 and 535 nm, respectively, using a microplate spectrofluorometer
(Molecular Devices Corp., Sunnyvale, CA, USA). Image analysis to
determine the production of intracellular ROS was performed by
seeding the MIN6N β-cells in coverslip-loaded 12-well plates. The
cells were treated with the indicated concentrations of C3G for 20
h followed by the addition of 0.7 mM H2O2.
Following incubation for 4 h, H2DCF-DA solution was
added to each well of the plate, and the cells were incubated
further for 2 h at 37°C. Images of the stained cells were acquired
using a fluorescence microscope (Nikon, Melville, NY, USA).
Determination of apoptotic cells
The ditermination of apoptotic cells was carried out
as perviously described (16).
Cells undergoing apoptosis were examined using a fluorescein
isothiocyanate (FITC)-labeled Annexin V/propidium iodide (PI)
apoptosis detection kit (Molecular Probes, Eugene, OR, USA)
according to the manufacturer’s instructions. In brief, the cells
were harvested, washed with PBS and centrifuged to collect the cell
pellet. The number of cells was adjusted to 1×106
cells/ml. Subsequently, the cells were resuspended in binding
buffer [10 mM HEPES, 140 mM NaCl and 2.5 mM CaCl2 (pH
7.4)] and stained with FITC-labeled Annexin V/PI at room
temperature for 15 min under light-protected conditions. Flow
cytometric analysis was performed using a FACSCalibur flow
cytometer (Becton-Dickinson, Mountain View, CA, USA). The
percentages of apoptotic cells were calculated using CellQuest
software (Becton-Dickinson). Cells in the early phase of apoptosis
were Annexin V-positive and PI-negative; however, cells in the late
phase of apoptosis were positive for both Annexin V and PI. The
apoptotic index (%) was calculated as the sum of cells in the early
and late phases of apoptosis divided by the total number of
events.
To determine the effects of C3G on
H2O2-induced DNA fragmentation, the MIN6N
β-cells were labeled using the cell-permeable DNA-specific
fluorescent dye, Hoechst 33342. The cells that showed homogeneously
stained nuclei were considered viable, whereas the presence of
chromatin fragmentation indicated apoptosis. The MIN6N β-cells
treated with various concentrations of C3G were seeded in 12-well
plates; Hoechst 33342 (stock 10 mg/ml) was added to each well
followed by 15 min of incubation at room temperature. Images of the
stained cells were acquired using a fluorescence microscope (Nikon)
to examine the degree of DNA condensation and/or fragmentation.
Western blot analysis
The treated cells were washed in 1X PBS and lysed in
lysis buffer for 30 min on ice. The lysates were centrifuged at
12,000 × g for 30 min at 4°C. The supernatant was collected, and
the protein content in the supernatant was measured using a Bio-Rad
protein assay kit prior to analysis. The total or fractionated
protein samples were loaded and separated using SDS-polyacrylamide
gel electrophoresis (PAGE) and transferred onto nitrocellulose
membranes. The membranes were blocked with 5% non-fat powdered milk
in 1X Tris-buffered saline containing 0.1% Tween-20 (TBS-T) for 1
h, and were then incubated with primary antibodies at 4°C
overnight. Finally, the membranes were treated with HRP-linked
secondary antibodies for 1 h at room temperature. The membranes
were washed with TBS-T after each antibody binding reaction. The
detection of each protein was performed using an enhanced
chemiluminescence kit (Millipore Co., Billerica, MA, USA).
Measurement of caspase-3 activity
The treated MIN6N β-cells were lysed in 500
μl lysis buffer consisting of 10 mM Tris-HCl (pH 7.5), 10 mM
NaH2PO4/NaHPO4 (PH 7.5), 130 mM
NaCl, 1% Triton X-100 and 10 mM NaPPi. Caspase-3 activity in the
lysates was evaluated using a caspase assay kit (BD Biosciences,
San Diego, CA, USA) according to the manufacturer’s
instructions.
Measurement of insulin secretion
The cell culture medium was collected from the
treated cells, and the level of insulin released in the medium was
measured using a rat/mouse insulin ELISA kit according to the
manufacturer’s instructions.
Statistical analysis
All measurements were from at least 3 independent
experiments, and the values are expressed as the means ± standard
error (SE). Statistical analysis was performed using a Student’s
t-test. A value of P<0.05 was considered to indicate a
statistically significant difference.
Results
Optimal concentration of C3G and
H2O2 in MIN6N β-cells
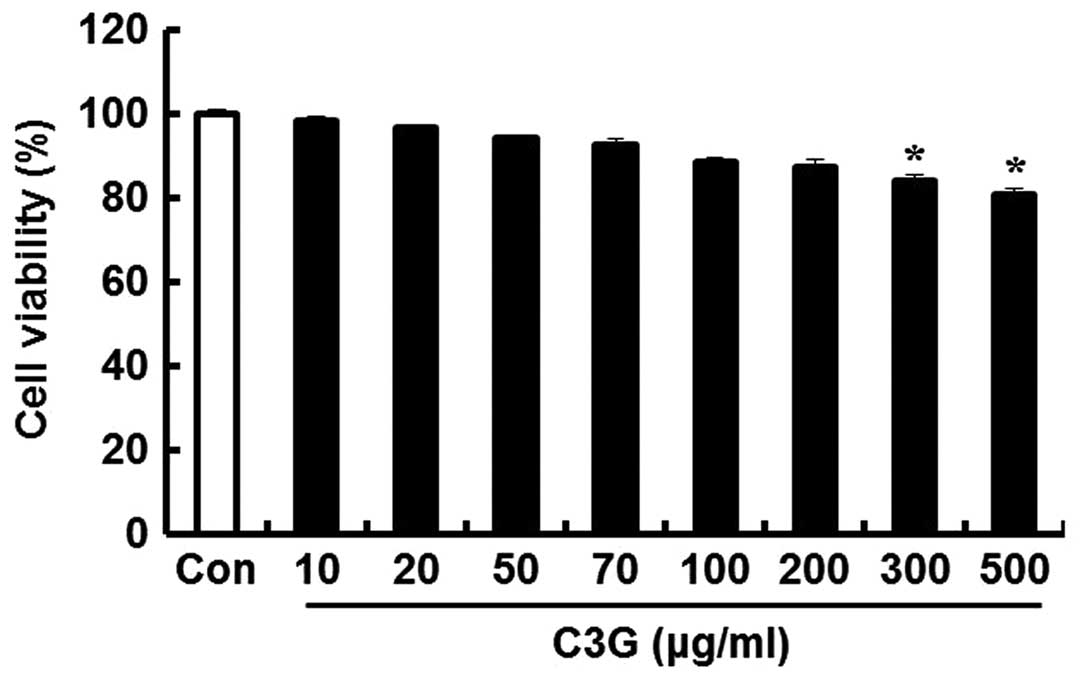
To determine the concentrations of C3G that could be
used without affecting the viability of the MIN6N β-cells, we
performed an MTT assay. The cells were seeded in 12-well plates and
incubated for 24 h; subsequently, various concentrations of C3G
were added to the cells followed by incubation for 20 h. C3G was
well tolerated (cell viability, 86.6%) up to a concentration of 200
μg/ml; subsequently, cell viability decreased in a
dose-dependent manner. At 300 and 500 μg/ml, C3G decreased
cell viability by 83.9 and 80.3%, respectively. Thus, we concluded
that the optimal concentration of C3G for use in further
experiments was up to 200 μg/ml (Fig. 2).
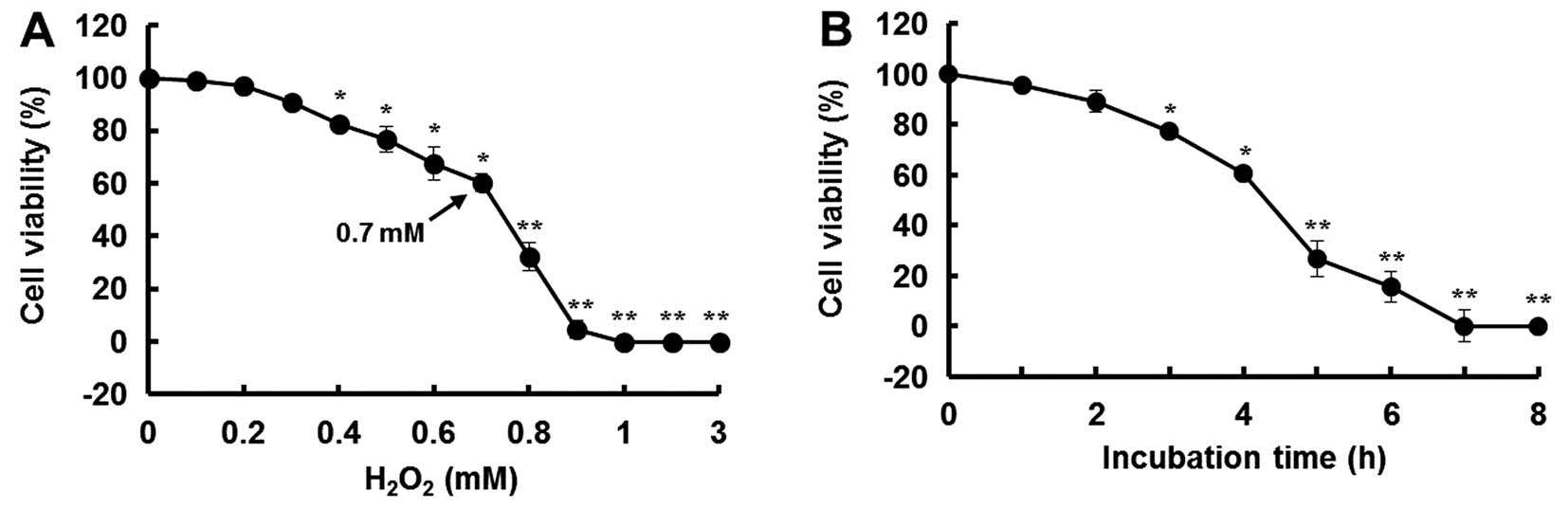
In this study, H2O2 was used
to induce oxidative stress in the MIN6N β-cells. After 4 h of
treatment with H2O2, the cell viability
decreased in a dose- and time-dependent manner. Compared to the
controls (untreated cells), the cells treated with 0.7 mM
H2O2 for 4 h showed a 40% decrease in
viability (Fig. 3).
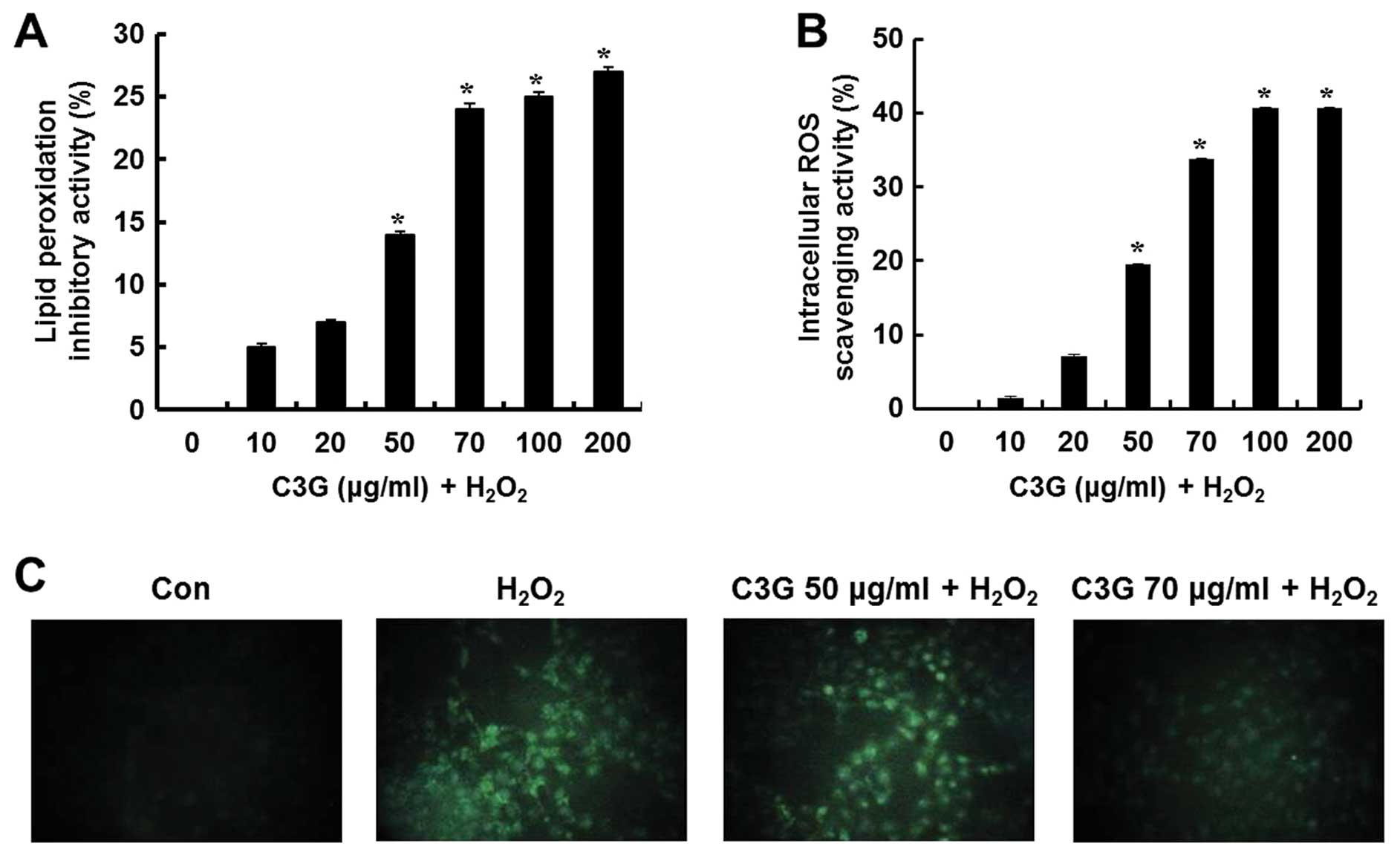
Effects of C3G on lipid peroxidation and
ROS generation
The inhibitory effects of C3G on lipid peroxidation
in the H2O2-treated MIN6N β-cells were
determined by measuring the levels of thiobarbituric acid reactive
substance (TBARS), a lipid peroxidation product. Lipid peroxidation
was increased in the cells exposed to H2O2,
whereas C3G inhibited lipid peroxidation. Compared to the untreated
cells, the cells treated with 50 and 70 μg/ml of C3G showed
a 14.1 and 23.9% inhibition of lipid peroxidation, respectively
(Fig. 4A). Intracellular ROS
levels in the H2O2-treated MIN6N β-cells were
determined using the ROS-sensitive fluorescent probe,
H2DCF-DA, a cell-permeable dye that is cleaved by
intracellular esterases into its non-fluorescent form,
2′,7′-dichlorofluorescin (DCFH). This form, which is no longer
membrane permeable, can be further oxidized by
H2O2 to form the fluorescent compound.
Compared to the untreated controls, the cells treated with C3G
showed a significant dose-dependent increase in intracellular ROS
scavenging activity (19.4 and 33.8% at 50 and 70 μg/ml C3G,
respectively; Fig. 4B). The
H2O2-dependent increase in the green
fluorescence of DCF markedly decreased following treatment with 70
μg/ml C3G and remained similar to the levels observed in the
control cells (Fig. 4C). These
data suggest that C3G inhibits lipid peroxidation and has ROS
scavenging activity. On the basis of these results, we hypothesized
that C3G inhibits oxidative stress-induced apoptosis in MIN6N
β-cells.
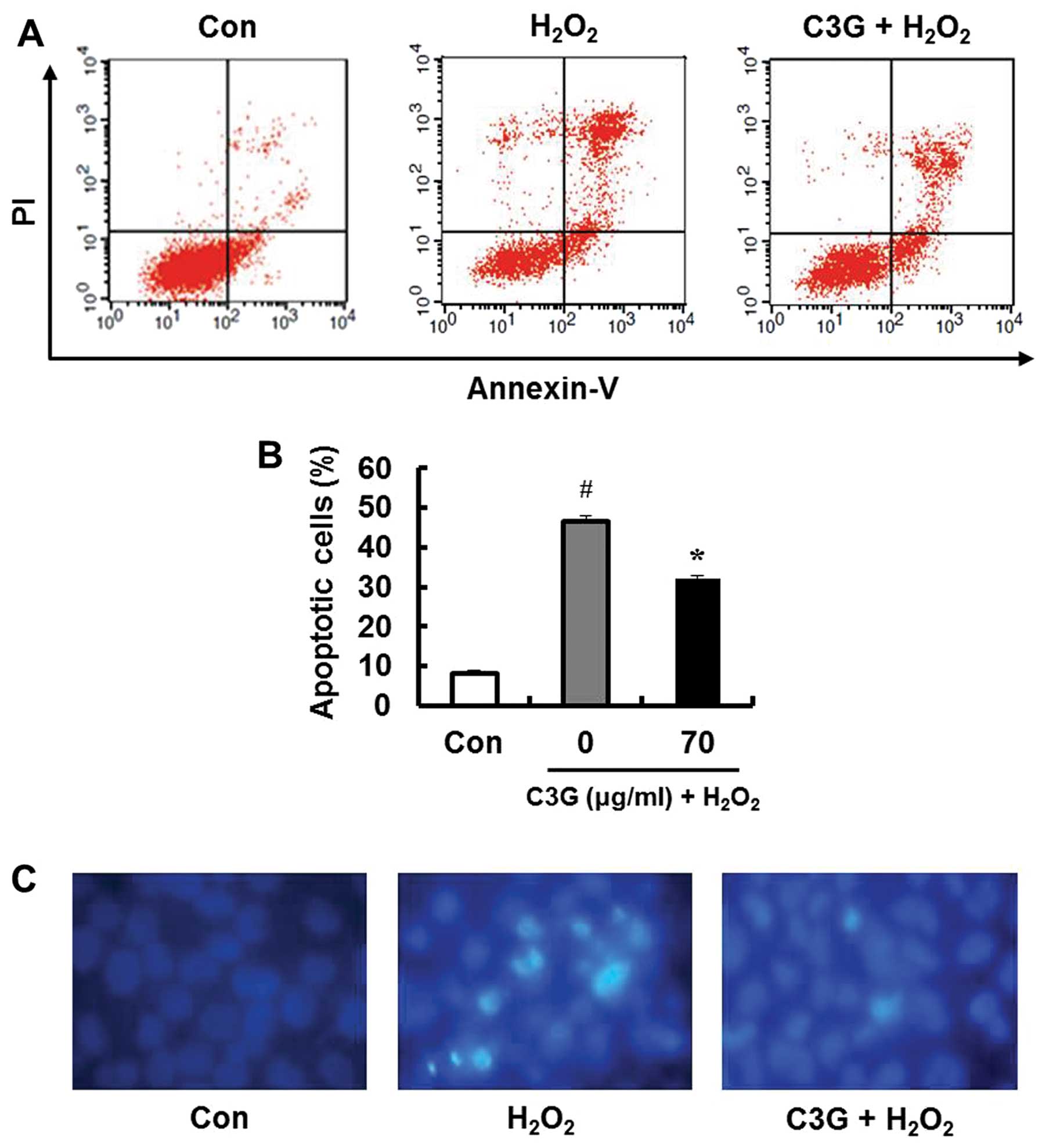
Determination of apoptotic cells
To determine whether the inhibitory effects of
H2O2 on MIN6N β-cells are associated with
apoptosis, we performed double staining using FITC-labeled Annexin
V and PI. Treatment with 0.7 mM H2O2 induced
apoptosis in 46.6% of the cells (Fig.
5A). However, pre-treatment with 70 μg/ml C3G markedly
inhibited the H2O2-induced apoptotic cell
death (32.2%; Fig. 5B).
Furthermore, DNA fragmentation upon apoptosis induced by
H2O2 was confirmed by staining the chromatin
of the MIN6N β-cells using Hoechst 33342. Only the
H2O2-treated MIN6N β-cells underwent DNA
fragmentation. The changes in chromatin condensation diminished
following pre-treatment with 70 μg/ml of C3G (Fig. 5C). These results indicate that C3G
exerts protective effects against oxidative stress-induced
apoptosis in MIN6N β-cells by inhibiting DNA fragmentation.
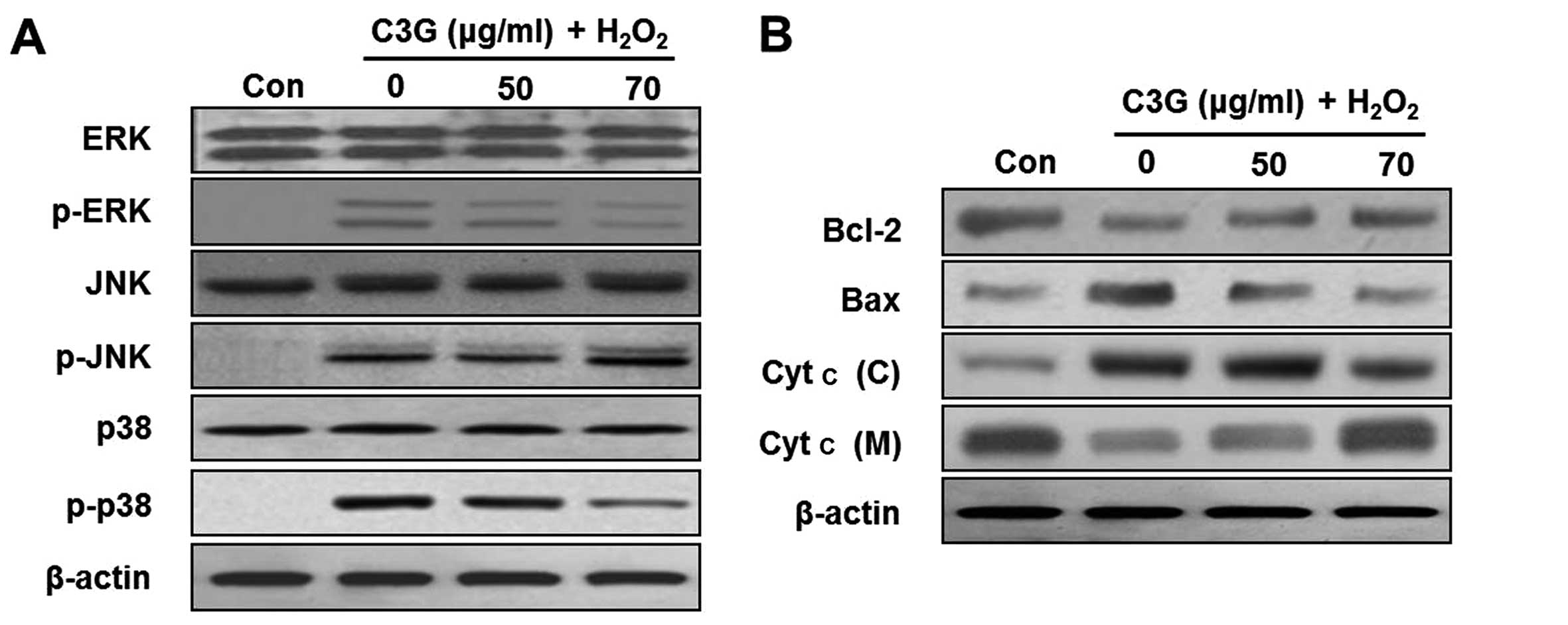
Effects of C3G on the phosphorylation of
ERK and p38 MAPK
In order to further examine the effects of C3G on
H2O2-induced MIN6N β-cell apoptosis, we
examined the effects of C3G on the phosphorylation of MAPKs (ERK,
JNK and p38) by western blot analysis. The levels of the
phosphorylated forms of ERK, JNK and p38 significantly increased
following treatment with H2O2 for 4 h. By
contrast, treatment with 70 μg/ml of C3G inhibited the
H2O2-induced phosphorylation of ERK and p38,
but C3G did not inhibit the phosphorylation of JNK (Fig. 6A).
C3G regulates the activation of
apoptosis-related proteins
Recent studies have demonstrated that the
phosphorylation of MAPKs is involved in the regulation of the
mitochondrial permeability-mediated activation of apoptotic
proteins, such as proteins of the Bcl-2 family and cytochrome
c (Cyt c) (17–19). In this study, we confirmed the
release of Cyt c and the induction of the expression of proteins of
the Bcl-2 family, including Bcl-2 and Bax, in the MIN6N β-cells.
The expression of Bcl-2 decreased and that of Bax increased in the
H2O2-treated cells (Fig. 6B). By contrast, the expression
levels of proteins of the Bcl-2 family in the group pre-treated
with 70 μg/ml of C3G were similar to those of the controls.
Subsequently, we investigated whether H2O2
induces the release of Cyt c from the mitochondria to the cytosol.
Western blot analysis of the cytosolic fraction revealed the
significant release of Cyt c from the mitochondria of the cells
cultured with H2O2. However, treatment with
C3G (50 and 70 μg/ml) induced a dose-dependent inhibition of
the release of Cyt c.
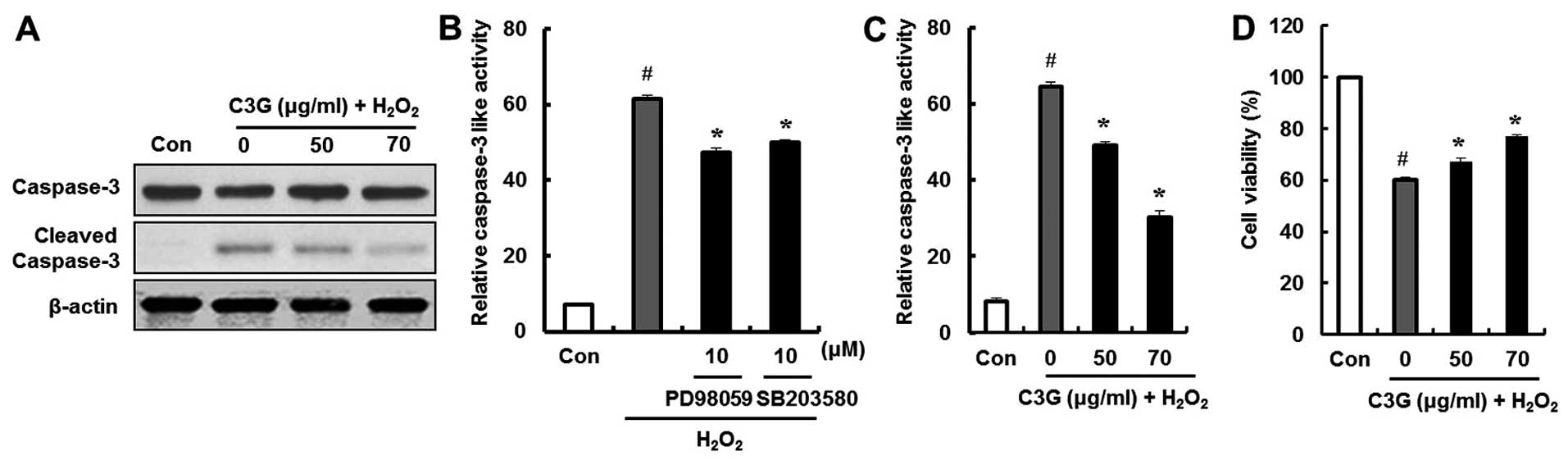
Effect of C3G on
H2O2-induced caspase-3 activity and cell
death
We examined the effects of C3G on the
H2O2-induced expression of cleaved caspase-3,
which is an essential trigger of apoptosis, by western blot
analysis. The cleaved form of caspase-3 was detected in the
H2O2-treated group. However, compared to the
group treated with H2O2, the group treated
with C3G (50 and 70 μg/ml) showed a decrease in the
expression of cleaved caspase-3 (Fig.
7A). In addition, the ERK and p38 inhibitors, PD98059 and
SB203580, were used to confirm that C3G inhibited the
H2O2-induced caspase-3 activation by
regulating the phosphorylation of ERK and p38. The
H2O2-induced increase in caspase-3 activity
was significantly decreased by the ERK and p38 inhibitors, PD98059
and SB203580 (Fig. 7B); by
contrast, pre-treatment with C3G significantly decreased the
H2O2-induced caspase-3 activity (Fig. 7C). Additionally, the
cytoprotective effects of C3G against the
H2O2-induced death of MIN6N β-cells were
examined by MTT assay. The cells were pre-treated with C3G (50 and
70 μg/ml) for 20 h followed by treatment with
H2O2. The cell viability decreased to 60%
following treatment with H2O2; however,
pre-treatment with 50 and 70 μg/ml of C3G restored the cell
viability to 67.8 and 76.1% of the controls, respectively (Fig. 7D). These results suggest that C3G
protects pancreatic β-cells against death by inactivating caspase-3
and regulating the phosphorylation of ERK and p38.
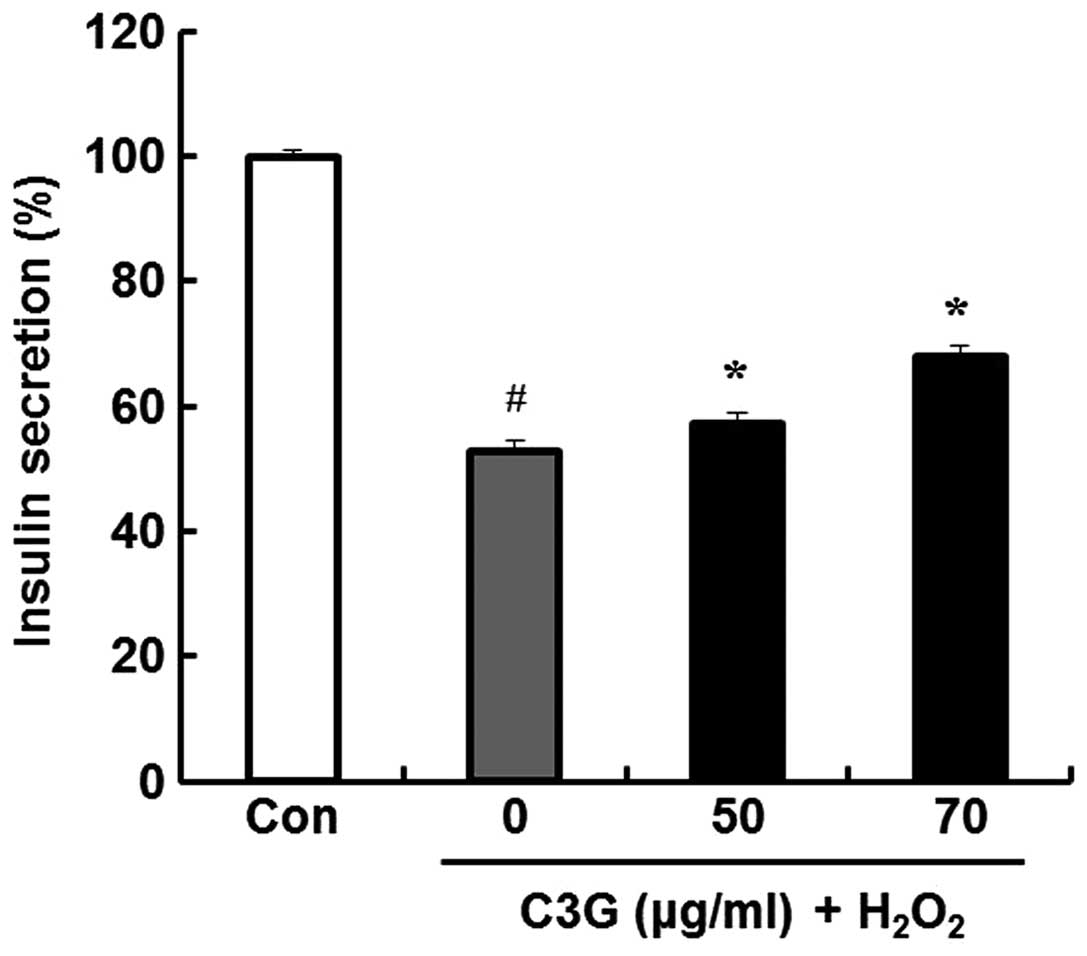
Effects of C3G on
H2O2-induced insulin secretion
The anti-diabetic efficacy of C3G was determined by
examining the effects of C3G on insulin release in MIN6N β-cells;
we measured insulin secretion using a rat/mouse insulin
enzyme-linked immunosorbent assay kit. Compared to the untreated
group, the group pre-treated with 70 μg/ml of C3G showed an
increase in insulin secretion (52.6 vs. 68%) (Fig. 8).
Discussion
Deficiency of insulin caused by the destruction of
pancreatic β-cells induces hyperglycemia, which leads to diabetes
and serious pathological effects in humans (20,21). ROS are heavily implicated in the
process of pancreatic β-cell destruction (22). ROS are routinely generated in the
human body, and their production can be exacerbated by lifestyle
choices, including eating, drinking and smoking habits (23). These factors can predispose
individuals to diseases associated with ROS production, including
cancer, stroke, cardiac disorders and diabetes (24–26). Thus, the inhibition of ROS
generation is an important therapeutic goal. In apoptosis-inducing
processes, H2O2 generation is attributed to
the uncoupling of the electron transport chain in the mitochondria
and to a decrease in mitochondrial membrane potential, leading the
mitochondrial translocation of Bax and the cytosolic mobilization
of Cyt c. Moreover, H2O2 has also been shown
as a strong signal for the activation of the MAPK family of
signaling proteins comprising of JNK, p38 and ERK (27). In this study, we used the
H2O2-induced toxicity of pancreatic β-cells
as a model to evaluate the antidiabetic efficacy of C3G isolated
from mulberry fruit. Pre-treatment with C3G reduced the levels of
intracellular ROS and lipid peroxidation in the MIN6N cells treated
with H2O2 (Fig.
4). In addition, the cells exposed to
H2O2 showed distinct characteristics of
apoptosis, such as DNA fragmentation and an increase in Annexin V
staining. However, the cells pre-treated with C3G showed a
significantly decreased percentage of apoptotic cells (Fig. 5). Studies have suggested that
oxidative stress causes pancreatic β-cell apoptosis through the
MAPK signaling pathway (28,29). MAPK proteins play a substantial
role in the regulation of cell viability, proliferation and
differentiation (30). The
excessive accumulation of intracellular ROS activates MAPKs, which
leads to apoptosis through the negative regulation of downstream
proteins, such as proteins belonging to the Bcl-2 family, Cyt c and
caspase-3 (31). Therefore, we
confirmed the phosphorylation of MAPKs, the activation of Bcl-2
family proteins and the release of Cyt c from the mitochondria to
the cytosol in the MIN6N β-cells damaged by
H2O2. All MAPKs were phosphorylated by
treatment with H2O2 for 4 h. The cells
treated with H2O2 showed an increase in the
expression of the pro-apoptotic protein, Bax, and a decrease in the
anti-apoptotic protein, Bcl-2. We confirmed the release of Cyt
cfrom the mitochondria to the cytosol in the cells treated with
H2O2; subsequently, cytosolic Cyt c activates
caspase-3, which plays an important role in the apoptotic pathway,
and leads to cell apoptosis. However, we demonstrated that
pre-treatment with C3G exerted protective effects against
H2O2-induced apoptosis by regulating the
phosphorylation of ERK and p38. In addition, C3G regulated the
proteins downstream of the MAPK signaling pathway, which resulted
in the inactivation of caspase-3 (Figs. 6 and 7). Insulin secretion was significantly
inhibited in the MIN6N β-cells exposed to
H2O2. By contrast, the cells treated with 70
μg/ml of C3G prior to exposure to H2O2
showed an increase in insulin secretion (Fig. 8).
In conclusion, our results demonstrate that C3G
isolated from mulberry fruit not only scavenges intracellular ROS
and inhibits lipid peroxidation, but also regulates the apoptotic
signaling pathways in pancreatic MIN6N β-cells following exposure
to H2O2, and thus results in a decrease in
the apoptotic cell rate. Thus, our findings indicate that the
anthocyanin isolated from mulberry fruit may prove to be a
potential therapeutic agent for the treatment of diabetes.
Acknowledgments
This study was supported by the High Value-added
Food Technology Development Program, Ministry of Agriculture, Food
and Rural Affairs, Republic of Korea.
References
|
1
|
Gray H and O’Rahilly S: Beta cell
dysfunction in non-insulin-dependent diabetes mellitus. Transplant
Proc. 26:366–370. 1994.PubMed/NCBI
|
|
2
|
Demirseren DD, Emre S, Akoglu G, Arpaci D,
Arman A, Metin A and Cakir B: Relationship between skin diseases
and extracutaneous complications of diabetes mellitus: clinical
analysis of 750 patients. Am J Clin Dermatol. 15:65–70. 2014.
View Article : Google Scholar
|
|
3
|
Lv L, Zheng L, Dong D, Xu L, Yin L, Xu Y,
Qi Y, Han X and Peng J: Dioscin, a natural steroid saponin, induces
apoptosis and DNA damage through reactive oxygen species: a
potential new drug for treatment of glioblastoma multiforme. Food
Chem Toxicol. 59:657–669. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ni Q, Xu G, Gao Q, Yang D and Zhang Y:
Evaluation of reactive oxygen species scavenging activities and DNA
damage prevention effect of Pleioblastus kongosanensis f.
aureostriatus leaf extract by chemiluminescence assay. J Photochem
Photobiol B. 128:115–121. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gutierrez RM: Effect of the hexane extract
of Piper auritum on insulin release from beta-cell and oxidative
stress in streptozotocin-induced diabetic rat. Pharmacogn Mag.
8:308–313. 2012. View Article : Google Scholar
|
|
6
|
Mane C, Loonis M, Juhel C, Dufour C and
Malien-Aubert C: Food grade lingonberry extract: polyphenolic
composition and in vivo protective effect against oxidative stress.
J Agric Food Chem. 59:3330–3339. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Martin MA, Fernández-Millán E, Ramos S,
Bravo L and Goya L: Cocoa flavonoid epicatechin protects pancreatic
beta cell viability and function against oxidative stress. Mol Nutr
Food Res. 58:447–456. 2014. View Article : Google Scholar
|
|
8
|
Aramwit P, Petcharat K and Supasyndh O:
Efficacy of mulberry leaf tablets in patients with mild
dyslipidemia. Phytother Res. 25:365–369. 2011.
|
|
9
|
Song N, Pang W, Yang H, Tan L, Fu J, Li H
and Jiang Y: Primary study on protective effect of mulberry
extracts on Abeta25-35-induced PC12 cells injury. Wei Sheng Yan
Jiu. 41:925–929. 2012.In Chinese.
|
|
10
|
Stefănuţ MN, Căta A, Pop R, Tanasie C, Boc
D, Ienascu I and Ordodi V: Anti-hyperglycemic effect of bilberry,
blackberry and mulberry ultrasonic extracts on diabetic rats. Plant
Foods Hum Nutr. 68:378–384. 2013. View Article : Google Scholar
|
|
11
|
Chang JJ, Hsu MJ, Huang HP, Chung DJ,
Chang YC and Wang CJ: Mulberry anthocyanins inhibit oleic acid
induced lipid accumulation by reduction of lipogenesis and
promotion of hepatic lipid clearance. J Agric Food Chem.
61:6069–6076. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen PN, Chu SC, Chiou HL, Kuo WH, Chiang
CL and Hsieh YS: Mulberry anthocyanins, cyanidin 3-rutinoside and
cyanidin 3-glucoside, exhibited an inhibitory effect on the
migration and invasion of a human lung cancer cell line. Cancer
Lett. 235:248–259. 2006. View Article : Google Scholar
|
|
13
|
Jiang Y: Effects of anthocyanins derived
from Xinjiang black mulberry fruit on delaying aging. Wei Sheng Yan
Jiu. 39:451–453. 2010.In Chinese. PubMed/NCBI
|
|
14
|
Liu LK, Lee HJ, Shih YW, Chyau CC and Wang
CJ: Mulberry anthocyanin extracts inhibit LDL oxidation and
macrophage-derived foam cell formation induced by oxidative LDL. J
Food Sci. 73:H113–H121. 2008. View Article : Google Scholar
|
|
15
|
Ohkawa H, Ohishi N and Yagi K: Assay for
lipid peroxides in animal tissues by thiobarbituric acid reaction.
Anal Biochem. 95:351–358. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee JH, Lee JS, Kim YR, Jung WC, Lee KE,
Lee SY and Hong EK: Hispidin isolated from Phellinus linteus
protects against hydrogen peroxide-induced oxidative stress in
pancreatic MIN6N β-cells. J Med Food. 14:1431–1438. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bian J, Wang K, Kong X, Liu H, Chen F, Hu
M, Zhang X, Jiao X, Ge B, Wu Y and Meng S: Caspase- and
p38-MAPK-dependent induction of apoptosis in A549 lung cancer cells
by Newcastle disease virus. Arch Virol. 156:1335–1344. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Haddad JJ: The role of Bax/Bcl-2 and
pro-caspase peptides in hypoxia/reperfusion-dependent regulation of
MAPK(ERK): discordant proteomic effect of MAPK(p38). Protein Pept
Lett. 14:361–371. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ravindran J, Gupta N, Agrawal M, Bala
Bhaskar AS and Lakshmana Rao PV: Modulation of ROS/MAPK signaling
pathways by okadaic acid leads to cell death via, mitochondrial
mediated caspase-dependent mechanism. Apoptosis. 16:145–161. 2011.
View Article : Google Scholar
|
|
20
|
Brayman KL, Nakai I, Field J, Lloveras JJ,
Farney A, Najarian JS and Sutherland DE: Intrathymic islet
allografts prevent hyperglycemia and autoimmune beta-cell
destruction in BB rats following transplantation in the prediabetic
period. Transplant Proc. 25:284–285. 1993.PubMed/NCBI
|
|
21
|
Nakanishi K and Watanabe C: 2008. Rate of
beta-cell destruction in type 1 diabetes influences the development
of diabetic retinopathy: protective effect of residual beta-cell
function for more than 10 years. J Clin Endocrinol Metab.
93:4759–4766. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pi J, Zhang Q, Fu J, Woods CG, Hou Y,
Corkey BE, Collins S and Andersen ME: ROS signaling, oxidative
stress and Nrf2 in pancreatic beta-cell function. Toxicol Appl
Pharmacol. 244:77–83. 2010. View Article : Google Scholar :
|
|
23
|
Sasaki T, Unno K, Tahara S and Kaneko T:
Age-related increase of reactive oxygen generation in the brains of
mammals and birds: is reactive oxygen a signaling molecule to
determine the aging process and life span? Geriatr Gerontol Int.
10(Suppl 1): S10–S24. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu Z, Celotto AM, Romero G, Wipf P and
Palladino MJ: Genetically encoded redox sensor identifies the role
of ROS in degenerative and mitochondrial disease pathogenesis.
Neurobiol Dis. 45:362–368. 2012. View Article : Google Scholar
|
|
25
|
Tsedensodnom O and Sadler KC: ROS: redux
and paradox in fatty liver disease. Hepatology. 58:1210–1212. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang C, Liu J, Pan H, Yang X and Bian K:
Mitochondrial dysfunction induced by excessive ROS/RNS-metabolic
cardiovascular disease and traditional Chinese medicines
intervention. Zhongguo Zhong Yao Za Zhi. 36:2423–2428. 2011.In
Chinese. PubMed/NCBI
|
|
27
|
Pervaiz S and Clément MV: Hydrogen
peroxide-induced apoptosis: oxidative or reductive stress? Methods
Enzymol. 352:150–159. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen CH, Chen SJ, Su CC, Yen CC, Tseng TJ,
Jinn TR, Tang FC, Chen KL, Su YC, Lee KI, Hung DZ and Huang CF:
Chloroacetic acid induced neuronal cells death through oxidative
stress-mediated p38-MAPK activation pathway regulated
mitochondria-dependent apoptotic signals. Toxicology. 303:72–82.
2013. View Article : Google Scholar
|
|
29
|
Supanji, Shimomachi M, Hasan MZ, Kawaichi
M and Oka C: HtrA1 is induced by oxidative stress and enhances cell
senescence through p38 MAPK pathway. Exp Eye Res. 112:79–92. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cargnello M and Roux PP: Activation and
function of the MAPKs and their substrates, the MAPK-activated
protein kinases. Microbiol Mol Biol Rev. 75:50–83. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fu YC, Yin SC, Chi CS, Hwang B and Hsu SL:
Norepinephrine induces apoptosis in neonatal rat endothelial cells
via a ROS-dependent JNK activation pathway. Apoptosis.
11:2053–2063. 2006. View Article : Google Scholar : PubMed/NCBI
|