Introduction
Clinical and epidemiological studies have
demonstrated an inverse correlation between high-density
lipoprotein (HDL) cholesterol (HDL-C) and the incidence of coronary
artery disease (CAD) (1). Studies
have also indicated that the quality of HDL can also influence the
risk of CAD (2,3), and that HDL function is more
important than the HDL-C plasma concentration (4). It is considered that HDL protects
against atherosclerosis in multiple ways, including both through
reverse cholesterol transport (RCT) and non-cholesterol-dependent
mechanisms (5).
RCT is a multistep process through which HDL
mobilizes excess cellular cholesterol from arterial-wall
lipid-laden macrophages (foam cells) to plasma lipid-poor
apolipoproteins, which are then transported to the liver, where
cholesterol is catabolized or excreted into bile. The transport
process is mediated by several transmembrane transporters,
including adenosine triphosphate binding cassette transporter A1
(ABCA1) and scavenger receptor class B type I (SR-BI) (6). ABCA1 is an ubiquitous protein
expressed abundantly in the liver, macrophages, brain and other
tissues. ABCA1 promotes the efflux of cellular phospholipids and
cholesterol to lipid-free apolipoprotein A (apoA)-I and other
apolipoproteins. This is further supported by data indicating that
the functional interactions between apoA-I and ABCA1 are necessary
for the initial lipidation of apoA-I (7). Further evidence indicates that ABCA1
plays a role in the liver and intestines in initiating HDL
formation and the RCT process (8). ABCA1 overexpression has been shown
to protect C57BL/6 mice from diet-induced atherosclerosis (9). Another primary transmembrane
receptor, SR-BI, is also highly expressed in the liver,
steroidogenic glands and other tissues and cells, including the
brain, the intestines, macrophages, endothelial cells and
astrocytes. In addition to mediating selective lipid uptake from
lipoproteins to cells, SR-BI mediates the bidirectional movement of
unesterified cholesterol between lipoproteins and cells (10). The hepatic overexpression of SR-BI
has been shown to be associated with decreased plasma levels of
HDL-C, increased HDL cholesteryl ester clearance, increased biliary
cholesterol content and the transport of cholesterol from the liver
to the bile (11,12). The transgene or
adenovirus-mediated hepatic overexpression of SR-BI has been found
to markedly reduce atherosclerosis in various murine models of the
disease (10).
Peroxisome proliferator-activated receptor (PPAR)γ
agonists, such as rosiglitazone, are extensively used in the
treatment of type 2 diabetes (13). These agonists have also been shown
to exert anti-atherogenic effects in subjects with or without
diabetes (14–17). PPARγ is primarily found in adipose
tissue and arterial wall cells, such as endothelial cells, vascular
smooth muscle cells and monocytes/macrophages where it modulates
lipid metabolism (18). Since
SR-BI, ABCA1 and PPARs are all expressed in the liver, it is
possible that the regulation of these proteins by PPARs may
modulate the atheroprotective effects. Malerød et al
(19) found that activated PPARγ
increased hepatic SR-BI levels in vitro, which may lead to
the increase in hepatic cholesterol uptake and the decrease in
lipid accumulation in peripheral tissues. Furthermore, Llaverias
et al (20) found that
treatment with rosiglitazone significantly induced the mRNA and
protein expression of ABCA1 and SR-BI and markedly reduced
intracellular free cholesterol levels. However, to the best of our
knowledge, few studies have evaluated the in vivo regulation
of SR-BI and ABCA1 by PPARs, and the effects of PPARγ agonists on
HDL quality remain unclear.
The benefit of rabbits as an animal model is that
they express cholesteryl ester transfer protein (CETP). The
objective of the present study was to investigate the effects of
rosiglitazone on the expression levels of ABCA1 and SR-BI, as well
as on the anti-atherosclerotic function of HDL in atherosclerotic
rabbits. The rate of HDL-mediated RCT, the antioxidant properties
of HDL and the pro-inflammatory state were also evaulated.
Materials and methods
Experimental animals
This study was carried out in strict accordance with
the recommendations in the Guide for the Care and Use of Laboratory
Animals of the National Institutes of Health. The experimental
procedures were in accordance with guidelines set by the Animal
Experiment Committee of Southern Medical University, Guangzhou,
China. All animal care and procedures were approved by the Animal
Experiment Committee of Southern Medical University. A total of 18
New Zealand white male rabbits (4 months old, weighing 2.0±0.1 kg)
were provided by the Laboratory Animal Center of Southern Medical
University. The rabbits were randomly divided into 3 groups (n=6 in
each group) as follows: the control group, the atherosclerosis
group and the rosiglitazone group. The animals in the control
group, atherosclerosis group and rosiglitazone group were fed a
regular diet, a high-fat diet supplemented with 1% (w/w)
cholesterol, 8% lard (w/w) and 0.05% cholate (w/w) and a high-fat
diet plus rosiglitazone (0.5 mg/kg body weight/day), respectively.
The doses were based on those indicated in previous studies
(21,22). Each rabbit consumed approximately
120 g of food daily. The rabbits were caged individually and had
access to water ad libitum for 12 weeks, and were maintained
under a 12-h day/night cycle. Fasting blood samples were collected
via the auricular vein in tubes without anticoagulant to obtain
serum. Following centrifugation (3,500 rpm, 15 min, 4°C), the blood
samples were aliquoted and stored at −70°C until the biochemical
measurements. Blood lipid analysis was performed at 0 and 12 weeks
(at the end of the experiment). Other laboratory analyses were
performed at the end of the study and all the experimental rabbits
were sacrificed by an overdose of 25 mg/kg pentobarbital at the end
of the 12-week experimental period, as previously described
(23).
Isolation of peritoneal macrophages and
hepatocytes
At the end of the 12-week experimental period, the
rabbits were anesthetized with 2% sodium pentobarbital. Under
sterile conditions, the peritoneal macrophages were collected by
peritoneal lavage with 200 ml phosphate buffer solution (PBS) and
purified using the adherent method. Subsequently, using a modified
method described by Zhao et al (23), the parenchymal hepatocytes were
isolated by classic in situ two-step perfusion of the liver,
with collagenase IV (0.05%) by enzyme digestion with collagenase II
(2 mg/ml).
Analysis of the HDL-mediated cholesterol
efflux from peritoneal macrophages and hepatocytes
Experiments were performed as previously described
(23,24) with minor modifications. Oxidized
low-density lipoprotein (Ox-LDL) was obtained from human
low-density lipoprotein (LDL), as previously described by Havel
et al (25) and Pirillo
et al (26). In this
study, the concentration of Ox-LDL was 30 μg/ml. Peritoneal
macrophages and hepatocytes which were isolated as previously
described, were planted at a density of 2×105 cells/ml
in 24-well culture dishes, and incubated with Dulbecco’s modified
Eagle’s medium (DMEM)/F12 supplemented with 0.2% bovine serum
albumin, 1 μCi/ml [3H] cholesterol (PerkinElmer
Life Sciences, Inc., Boston, MA, USA) and 30 μg/ml of
Ox-LDL. Twenty-four hours later, to equilibrate cellular free
cholesterol pools, the cells were washed once with serum-free
medium, and then incubated for a further 12 h with DMEM/F12
supplemented with 0.3 mmol/l cAMP (Sigma, St. Louis, MO, USA). For
free cholesterol efflux experiments, 10 μg/ml apoA1 (Sigma)
were added and the cells were incubated for 4 h. The incubation
medium was collected and centrifuged before assessing the
radioactivity using a counter. Cell monolayers were washed with PBS
and lysed with 1 ml of 0.1 M NaOH. The radioactivity of the medium
and cell lysates was measured by liquid scintillation spectrometry
(Beckman Instruments, Inc., Fullerton, CA, USA). The cholesterol
efflux was measured as the medium [3H]cholesterol
radioactivity, representing a percentage of total
[3H]cholesterol radioactivity (cells plus medium).
Individual efflux values were calculated as averages of 3
determinations in each well.
Measurement of ABCA1 and SR-B1 protein
expression by flow cytometry
The measurement of the protein expression of ABCA1
and SR-B1 in the peritoneal macrophages and hepatocytes was
performed as previously described in the study by Pirillo et
al (27). Specifically the
suspension of peritoneal macrophages and hepatocytes
(2×105 cells/ml) was mixed with either mouse anti-ABCA1
antibody (CB11308030) or mouse anti-SR-B1 antibody (CB41343199;
Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) for 60 min at
room temperature in test tubes. Each suspension was then washed
twice with PBS and subsequently added to a labeled PE fluorescence
antibody (sc-53749; Seretec Inc., Charlotte, NC, USA). Thirty
minutes later, the cells were collected and subjected to
fluorescence flow cytometry using a FACSCalibur and FACScan flow
cytometer (Becton Dickinson, San Jose, CA, USA). The values were
expressed by the ABCA1 and SR-B1 average protein contents per 100
detected cells.
Measurement of ABCA1 and SR-B1 mRNA
expression by reverse transcription quantitative
(real-time)-polymerase chain reaction (RT-qPCR)
A total of 200 mg of liver tissue was powdered in
liquid nitrogen. Total RNA was isolated from the rabbit livers
using TRIzol reagent (Gibco-BRL, Gaithersburg, MD, USA).
First-strand complementary deoxyribonucleic acid (cDNA) was
synthesized with random primers and the First Strand cDNA Synthesis
kit (Cat. no. C0210A; GeneCopoeia, Rockville, MD, USA). All primer
sets were subjected to rigorous database searches to avoid
potential conflicting transcript matches to pseudogenes or
homologous domains within related genes. The sequences of the
primers used for quantitative PCR (qPCR) were as previously
described (28): ABCA1 forward,
5′-GAT GGC AAT CAT GGT CAA TGG-3′ and reverse, 5′-AGC TGG TAT TGT
AGC ATG TTC CG-3′, yielding a 201-bp size product; SR-BI forward,
5′-CAG TGG GCA TTG TGT CCT GTC-3′ and reverse, 5′-GGC TCA GTG CAG
GCT GAT GTC-3′, yielding a 286-bp size product; and GAPDH forward,
5′-GGA GCC AAA AGG GTC ATC-3′ and reverse, 5′-CCA GTG AGT TTC CCG
TTC-3′, yielding a 346-bp size product. The qPCR chain reaction was
carried out on a MX3000P thermocycler (Stratagene, La Jolla, CA,
USA) and was used for detecting the products from the
reverse-transcribed cDNA samples. The abundance of ABCA1 and SR-BI
messenger ribonucleic acid (mRNA) was determined by SYBRI assay
with GAPDH as the normalizer. The PCR reactions for each sample
were performed in duplicate, and the relative gene expression was
analyzed, as previously described (28).
Quantification of aortic atherosclerosis
by histological analysis and immunohistochemistry
After the animals were sacrificed, the entire aorta
was removed and fixed in a 10% neutral buffered formaldehyde
solution for 48 h. For the microscopic quantification of the lesion
area, 3 segments were obtained from the aortic arch, the thoracic
aorta and the abdominal aorta. All segments were embedded in
paraffin, cut into 4-μm-thick cross sections and stained
with hematoxylin and eosin (H&E) for the histological
examination. The percentage of plaque area, which was defined as
the surface area of plaque/surface area of the whole intima, as
well as the aortic intima-media thickness (IMT) were calculated.
For the microscopic evaluation of ABCA1 and SR-B1 protein
expression in the lesions of the aorta, immunohistochemistry was
performed as previously described (29). Immunostaining for ABCA1 (Boster
Biotechnology Co. Ltd., Wuhan, China) and SR-BI (Abcam Co. Ltd.,
Cambridge, MA, USA) was performed on paraffin-embedded aortic
atherosclerotic sections using the specific antibody and a
streptavidin-biotin peroxidase-complex (SABC). Antibody binding was
visualized using SABC kits (Boster Biotechnology Co. Ltd.),
diaminobenzidine (DAB) and 3-amino-9-ethylcarbazole (AEC) were used
as the chromogen and Mayer’s hematoxylin as the nuclear
counterstain. The sections were dehydrated, cleared, mounted and
subjected to morphometric analysis. Images (H&E-stained and
immunostained) were captured using an Olympus BX51 light microscope
equipped with a DP70 digital camera (Olympus, Tokyo, Japan). Image
Pro Plus 6.0 special image analysis software (Media Cybernetics
Inc., Rockville, MD, USA) was used to quantify the images.
Laboratory analyses
Serum lipid analysis
The serum triglyceride (TG), total cholesterol (TC),
HDL-C and LDL cholesterol (LDL-C) concentrations were measured
using an automated biochemical analyzer (Type AU5421; Olympus).
Assessment of serum paraoxonase (PON)1
activity
Serum PON1 activity was assayed according to the
method described in the study by Beltowski et al (30), using the synthetic substrate
phenyl acetate (Sigma). PON1 activity towards phenyl acetate was
determined by measuring the initial rate of substrate hydrolysis
within an assay mixture (3 ml) containing 2 mM phenyl acetate, 2 mM
CaCl2 and 10 μl of plasma in 100 mM Tris-HCl (pH
8.0). The absorbance was monitored for 90 sec at 270 nm and the
enzymatic activity was calculated from the E270 of
phenyl acetate (1,310/M/cm) and expressed in U/ml (where 1 U of
arylesterase hydrolyzes 1 μmol of phenyl acetate/min).
Assessment of serum myeloperoxidase
(MPO) activity
MPO activity was determined using a MPO activity kit
(Jiancheng Bioengineering Co, Nanjing, China) using commercially
available reagents, according to the manufacturer’s instructions.
Briefly, the serum samples were incubated in a 50 mM sodium
phosphate buffer containing 1.5 M hydrogen peroxide and 0.167 mmol
o-dianisidine dihydrochloride for 30 min. The increase in
absorbance at 460 nm was recorded with the use of a
spectrophotometer and the enzymatic activity was calculated from
E460 = 11,300/M/cm. A unit of MPO activity is defined as
the amount of enzyme degrading 1 μmol
H2O2 per minute at 37°C.
Statistical analysis
Data are presented as the means ± SEM. One-way ANOVA
was used for analyzing differences in variables between groups at
the same time point. When the value was P≤0.05, the least
significant difference method was used for comparison. An
independent sample t-test was used for analyzing the differences in
variables between 2 groups at the same time point. Coefficients of
correlation (r) were calculated by Pearson correlation analysis.
SPSS 13.0 software was used for statistical analysis with a value
of P<0.05 indicating a statistically significant difference.
Results
General animal characteristics
There were no significant differences in serum lipid
levels and body weight among the 3 groups at baseline (Table I). After 12 weeks of experiments,
the atherosclerosis group had significant higher serum
concentrations of TC, TG, HDL-C and LDL-C than the control group,
while the rosiglitazone group had higher serum HDL-C concentrations
and a slightly lower serum level of TC than the atherosclerosis
group (Table I). There were no
significant differences in body weight among the 3 groups
throughout the experiment (Table
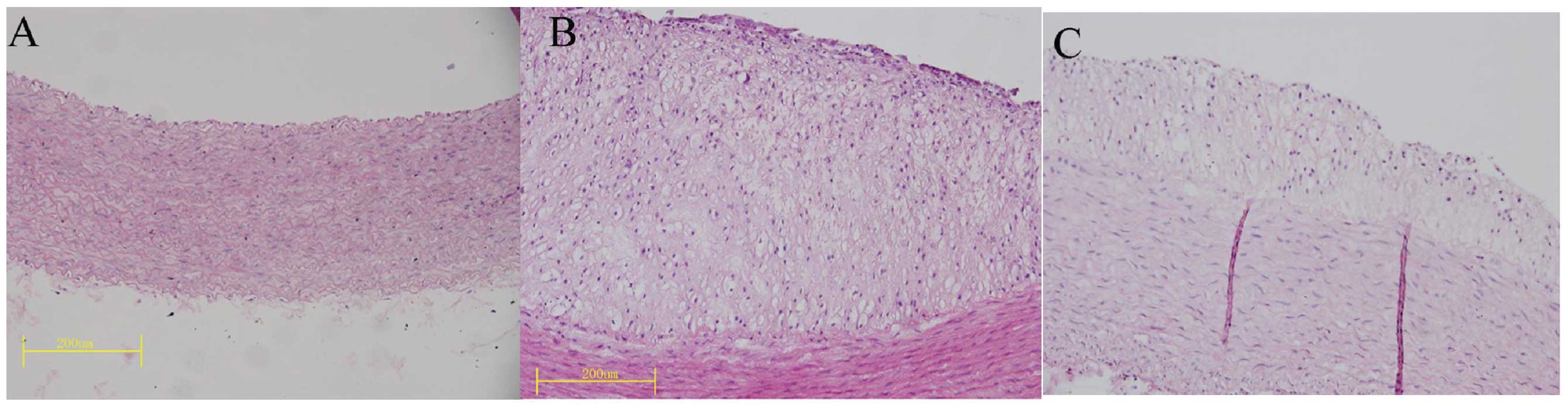
I). Furthermore, mild to moderate atherosclerosis,
characterized by the local thickening of the intima-media, was
observed in the thoracic aortic wall of the rabbits fed a high
cholesterol diet for 12 weeks (Fig.
1). The aortic IMT and the percentage of plaque area (surface
area of plaque/surface area of whole intima) were significantly
smaller in the rosiglitazone group compared with the
atherosclerosis group (Table
II). These results suggest that we successfully established a
hyperlipidemic and atherosclerotic animal model.
 | Table ISerum lipid and body weight profiles
in the control, atherosclerosis and rosiglitazone groups. |
Table I
Serum lipid and body weight profiles
in the control, atherosclerosis and rosiglitazone groups.
| Group | TC | TG | LDL-C | HDL-C | Body weight |
|---|
| Control |
| 0 weeks | 1.85±0.12 | 1.04±0.19 | 1.00±0.20 | 0.47±0.12 | 2.01±0.08 |
| 12 weeks | 1.79±0.21 | 1.02±0.15 | 1.09±0.23 | 0.45±0.12 | 2.77±0.04 |
| AS |
| 0 weeks | 1.77±0.15 | 1.10±0.14 | 1.00±0.20 | 0.48±0.12 | 2.07±0.06 |
| 12 weeks | 23.26±3.30a | 1.58±0.25a | 18.09±4.04a | 1.11±0.09a | 2.88±0.08 |
| Rosiglitazone |
| 0 weeks | 1.78±0.12 | 1.02±0.14 | 0.98±0.14 | 0.47±0.07 | 2.04±0.02 |
| 12 weeks | 19.78±1.68 | 1.54±0.15 | 15.57±1.81 | 1.94±0.30b | 2.78±0.12 |
 | Table IIQuantification of aortic
atherosclerotic lesions in the control, atherosclerotic and
rosiglitazone groups at 12 weeks. |
Table II
Quantification of aortic
atherosclerotic lesions in the control, atherosclerotic and
rosiglitazone groups at 12 weeks.
| Group | IMT
(μm) | Percentage of
plaque area (%) |
|---|
| Control | 203.21±30.61 | |
| AS |
527.42±85.16a | 27.78±12.00 |
| Rosiglitazone |
291.46±50.18b | 5.88±3.31c |
Rosiglitazone improves the HDL-induced
cholesterol efflux in peritoneal macrophages and hepatocytes
We measured the rate of the HDL-induced cholesterol
efflux in peritoneal macrophages and hepatocytes isolated from the
3 groups of rabbits and observed marked differences among the 3
groups. The cholesterol efflux rate in the peritoneal macrophages
and hepatocytes from the rabbits in the atherosclerosis group was
significantly lower than that in the control group rabbits
(peritoneal macrophages: 16.48±4.10 vs. 24.93±3.85%, P<0.01;
hepatocytes: 3.25±0.97 vs. 5.29±1.71%, P<0.05), while the
peritoneal macrophages and hepatocytes from the rabbits treated
with rosiglitazone showed a significantly enhanced HDL-induced
cholesterol efflux as compared with the atherosclerosis group
(peritoneal macrophages: 44.50±6.19 vs. 16.48±4.10%, P<0.01;
hepatocytes: 8.50±1.18 vs. 3.25±0.97%, P<0.01; Fig. 2).
Rosiglitazone increases ABCA1 and SR-B1
expression in peritoneal macrophages and hepatocytes
At the end of the 12-week experimental period, ABCA1
protein and mRNA expression in the peritoneal macrophages and
hepatocytes was significantly lower in the atherosclerosis group
compared with the control group (P<0.05; Figs. 3A and 4A). Compared with the atherosclerosis
group, the rosiglitazone group had a significantly higher level of
ABCA1 expression in the peritoneal macrophages and hepatocytes at
both the mRNA and protein level (P<0.01; Figs. 3A and 4A).
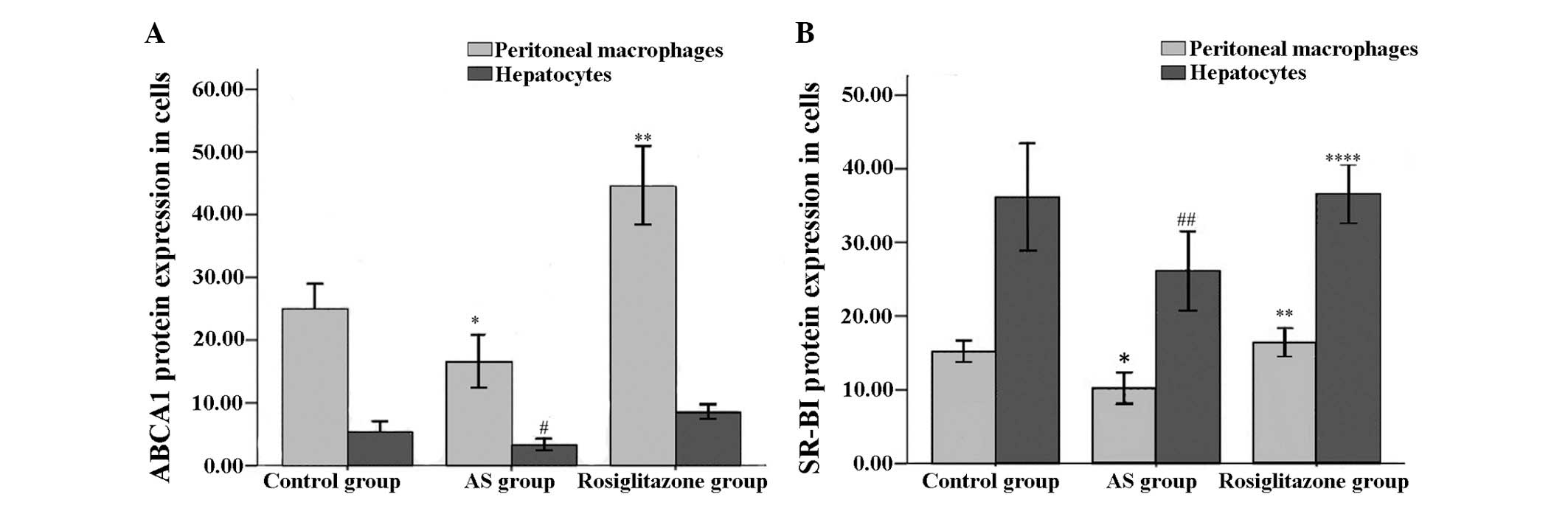 | Figure 3(A) ABCA1 protein expression in
hepatocytes and peritoneal macrophages determined by flow
cytometry. (B) SR-B1 protein expression in hepatocytes and
peritoneal macrophages determined by flow cytometry. Data are
presented as the means ± SEM, (n=6 in each group).
#P<0.05, *P<0.01,
##P<0.001, vs. control group; **P<0.01,
***P<0. 05, ****P<0. 001, vs. AS group
(ANOVA). ABCA1, adenosine triphosphate binding cassette transporter
A1; SR-B1, scavenger receptor class B type I; AS,
atherosclerosis. |
Compared with the control groups rabbits, the
atherosclerosis group showed a significant decrease in the
peritoneal macrophage and hepatocyte expression of SR-B1 at both
the mRNA and protein level (P<0.01; Figs. 3B and 4B). The mRNA and protein expression of
SR-B1 in the peritoneal macrophages and hepatocytes increased
significantly in the rosiglitazone group compared with the
atherosclerosis group (P<0.01; Figs. 3B and 4B).
ABCA1 and SR-B1 expression in
atherosclerotic lesions
Immunohistochemical staining revealed substantial
ABCA1 and SR-B1 protein expression in the aortic plaques in both
the atherosclerosis and rosiglitazone groups (Figs. 5 and 6). However, plaques and
immunohistochemical staining in the aortic walls for ABCA1 and
SR-B1 were negative in the control group, and no lesions were
present. We further quantified ABCA1 and SR-B1 protein expression
(by the percentage of positively stained areas and the staining
intensity in the lesions by immunohistochemical staining) using
Image Pro Plus 6.0 special image analysis software (Media
Cybernetics Inc.). We found that ABCA1 protein expression was
significantly increased in the rosiglitazone group, compared with
the atherosclerosis group (Table
III). However, there was no significant difference in SR-B1
protein expression in the aortic plaques between the 2 groups
(Fig. 6).
 | Table IIIQuantification of immunohistochemical
staining for ABCA1 expression in aortic atherosclerotic lesions
among the control, atherosclerotic and rosiglitazone groups at 12
weeks. |
Table III
Quantification of immunohistochemical
staining for ABCA1 expression in aortic atherosclerotic lesions
among the control, atherosclerotic and rosiglitazone groups at 12
weeks.
| Group | Staining
intensity | Percentage of ABCA1
protein positive area (%) |
|---|
| AS | 0.17±0.03 | 24.13±9.85 |
| Rosiglitazone | 0.22±0.03a | 47.06±4.93b |
Rosiglitazone enhances HDL-associated
antioxidant enzyme PON1 activity and suppresses oxidation enzyme
MPO activity
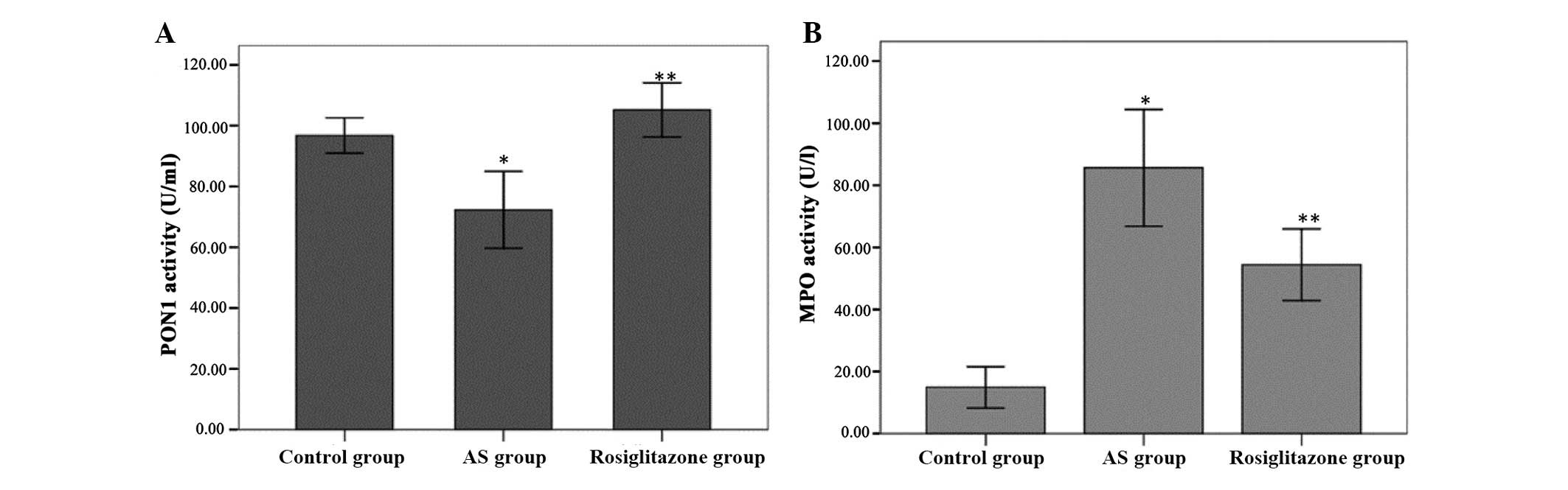
Serum PON1 activity (Fig. 7A) toward phenyl acetate was
significantly inhibited in the atherosclerosis group compared with
the control group (72.26±12.03 vs. 96.77±5.58 U/ml, P<0.001). In
accordance with the results of a previous study (21), serum PON1 activity was markedly
elevated in the rosiglitazone group compared with the
atherosclerosis group (105.18±8.49 vs. 72.26±12.03 U/ml,
P<0.01).
Serum MPO activity (Fig. 7B) was significantly higher in the
atherosclerosis group compared with the control group (85.67±17.92
vs. 14.94±6.36 U/l, P<0.001). However, MPO activity was
significantly attenuated in the rosiglitazone group compared with
the atherosclerosis group (54.45±10.99 vs. 85.67±17.92 U/l,
P<0.05).
Pearson correlation analysis was used to calculate
the coefficients of correlation. We found no correlation between
serum HDL-C levels and cellular cholesterol efflux, serum PON1
activity, serum MPO activity or IMT. However, the cellular
cholesterol efflux in the peritoneal macrophages and hepatocytes,
as induced by HDL, correlated with the protein expression level of
ABCA1 and SR-B1 (data not shown) (in peritoneal macrophages,
r=0.701, P=0.001; r=0.786, P<0.001, respectively; and in
hepatocytes, r=0.763, P<0.001; r=0.813, P<0.001;
respectively). In addition, IMT negatively correlated with serum
PON1 levels and cellular cholesterol efflux in the peritoneal
macrophages and hepatocytes (r=−0.675, P=0.002; r=−0.69, P=0.002;
r=−0.816, P<0.001; respectively) and positively correlated with
serum MPO activity (r=0.774, P<0.001) (data not shown).
Discussion
This study demonstrates that rosiglitazone, an
anti-diabetic medication with potential anti-atherogenic activity,
attenuates atherosclerosis, increases serum HDL-C levels and
improves the anti-atherogenic functions of HDL in atherosclerotic
rabbits. Treatment with rosiglitazone for 12 weeks significantly
decreased serum MPO activity and increased serum PON1 activity in
this model. This indicates that rosiglitazone improves the HDL
antioxidant and anti-inflammatory status. Moreover, we found that
the administration of rosiglitazone improved the rate of the
HDL-induced cholesterol efflux in peritoneal macrophages and
hepatocytes, which was due to the upregulated expression of ABCA1
and SR-B1 at both the mRNA and protein level. New Zealand white
rabbits were used as their lipoprotein profiles and lipid
metabolism patterns are similar to those of humans, with
differences in apoA-II and hepatic lipase levels (31).
Atherosclerosis is a chronic inflammatory disease
that is initiated, in part, by the presence of Ox-LDL in the artery
wall (32). Rosiglitazone is an
orally active anti-diabetic drug in the thiazolidinedione drug
class. It functions by binding as an agonist to the PPARγ receptor,
where it inhibits the progression of atherosclerosis in patients
(16). However, its
anti-atherosclerotic mechanisms are not yet well understood. Since
multiple epidemiological studies have established a low level of
HDL-C as an independent risk factor for CAD (1), HDL has been under vigorous
investigation as a therapeutic target for atherosclerosis. It has
also been reported that PPARγ agonists increase HDL-C levels by 5
to 15% (33). However, there are
conflicting reports on the association between HDL-C and
atherosclerosis. Many data indicate that HDL-C levels and
atherosclerosis are not correlated, and it has been suggested that
the levels are associated with an increased risk, while others
suggest a reduced risk of atherosclerosis. For example, it has been
reported that the natural apo-A1 Milano mutation leads to low HDL-C
levels, but does not confer an increased risk of cardiovascular
events (34). Studies supporting
HDL-C as a protective factor, contribute its effects to being
mediated through multiple pathways, including RCT (particularly
macrophage-specific RCT), anti-inflammatory, antioxidant,
anti-aggregatory, anticoagulant and pro-fibrinolytic mechanisms
(35). In addition,
multifactorial actions, such as chronic inflammation and acute
phase responses, can lead to the loss of normal HDL biological
functions, resulting in dysfunctional HDL (4). Dysfunctional HDL exhibits
chameleon-like properties that can protect arteries or enhance
atherogenesis. For example, HDL isolated from some patients with
CAD has been found to be ineffective as an antioxidant and,
paradoxically appears to be pro-oxidant, as assessed by its lipid
peroxide content (36). Given
this complexity, it is not surprising that plasma HDL-C levels in a
single assay do not necessarily correlate with HDL functions.
Therefore, the evaluation of HDL function is more important than
the quantification of its levels when assessing its
atheroprotective properties. In this study, we investigated the
effects of rosiglitazone on the antiatherogenic function of HDL in
cholesterol-fed rabbits in order to obtain a better understanding
of the potential antiatherogenic mechanisms.
RCT mediates the transport of cholesterol from
peripheral cells back to the liver for excretion and is the most
important antiatherogenic function of HDL. In this process, HDL
mobilizes excess cellular cholesterol from arterial-wall
macrophages to lipid poor plasma apolipoproteins, a transportation
process primarily mediated by ABCA1 and SR-B1. It has been reported
that the overexpression of ABCA1 increases the cholesterol efflux
from cells (37). ABCA1-deficient
mice have been shown to have reduced cholesterol efflux from
macrophages to feces in vivo (38). The hepatic overexpression of SR-BI
has beens shown to markedly reduce plasma HDL-C levels (39,40) and reduce atherosclerosis (41) in mice. Conversely, the gene
deletion or attenuation of SR-BI in mice has been shown to result
in substantially increased HDL-C levels (41,42), but markedly increased
atherosclerosis (43).
Additionally, Zhang et al (44) demonstrated that the modulation of
hepatic SR-BI overexpression directly upregulates the rate of
macrophage RCT in vivo. Previous studies have demonstrated
that PPARγ agonists induce the expression of liver X receptor α
(LXRα) and thereby stimulate ABCA1-dependent cholesterol efflux to
apoA-1 or increase cholesterol efflux to HDL in an ABCG1-dependent
manner (45–47). Consistent with previous findings,
we found that rosiglitazone increased ABCA1 expression in
peritoneal macrophages and hepatocytes at both the mRNA and protein
level. We also provide the first demonstration, to the best of our
knowledge, that ABCA1 increases the mRNA and protein expression of
SR-B1 and enhances the HDL-induced cholesterol efflux in peritoneal
macrophages and hepatocytes. Moreover, we observed ABCA1 and SR-B1
protein in aortic lesions by immunohistochemistry staining. We
found that treatment with rosiglitazone was associated with
increased ABCA1 protein expression in aortic lesions; however,
there were no significant changes in SR-B1 expression. Furthermore,
statistical analysis indicated that the cellular cholesterol efflux
in peritoneal macrophages and hepatocytes was significantly
positively correlated with the protein expression level of ABCA1
and SR-B1. Our data suggest that rosiglitazone improves cellular
cholesterol efflux by upregulating ABCA1 and SR-B1 expression in
cells.
In addition, it has been reported that HDL is an
antioxidant and significantly reduces the oxidative modification of
LDL. PON1 is an enzyme associated with the antioxidant properties
of HDL (48). It has been
reported that human PON protects LDL against oxidative stress,
which helps explain its antiatherogenic mechanisms (49). A previous study provided direct
evidence of a mechanistic link between the genetic regulation of
PON and the resultant systemic oxidative stress (50). In our study, serum PON1 activity
increased significantly following treatment with rosiglitazone,
which is consistent with previous studies.
MPO, which is secreted by activated phagocytes, is
one of the pivotal factors involved in the initiation of lipid
oxidation of LDL (51). It has
been demonstrated that MPO interacts with apoA1 and impairs
cellular cholesterol efflux through ABCA1, leading to the formation
of pro-inflammatory HDL. Thus, MPO inhibition may also be
therapeutically valuable (52).
It has been demonstrated that PPARγ agonists strongly regulate MPO
gene expression through the Alu element encoding 4 hexamer repeats.
In this study, we also found that rosiglitazone reduced serum MPO
activity.
In conclusion, our in vivo study using
rabbits demonstrated that treatment with rosiglitazone increased
serum HDL-C levels, and improved the HDL-induced cholesterol efflux
in hepatic cells and macrophages by upregulating ABCA1 and SR-B1
mRNA and protein expression in an atherosclerotic rabbit model. The
anti-inflammatory and antioxidant effects of HDL may be promoted by
decreasing serum MPO activity and increasing PON1 activity, thus
deterring the development of atherosclerosis. These factors may
contribute to the anti-atherogenic potential of rosiglitazone.
Acknowledgments
This study was supported by the Guangdong Scientific
and Technological Grant (no. 2008B030301158) and the Key Foundation
of Nanfang Hospital (no. 2008A003).
References
|
1
|
Rader DJ: Molecular regulation of HDL
metabolism and function: implications for novel therapies. J Clin
Invest. 116:3090–3100. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Frikke-Schmidt R, Nordestgaard BG, Stene
MC, et al: Association of loss-of-function mutations in the ABCA1
gene with high-density lipoprotein cholesterol levels and risk of
ischemic heart disease. JAMA. 299:2524–2532. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Roberts CK, Ng C, Hama S, Eliseo AJ and
Barnard RJ: Effect of a short-term diet and exercise intervention
on inflammatory/anti-inflammatory properties of HDL in
overweight/obese men with cardiovascular risk factors. J Appl
Physiol (1985). 101:1727–1732. 2006. View Article : Google Scholar
|
|
4
|
Dodani S, Grice DG and Joshi S: Is HDL
function as important as HDL quantity in the coronary artery
disease risk assessment? J Clin Lipidol. 3:70–77. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Assmann G and Gotto AM Jr: HDL cholesterol
and protective factors in atherosclerosis. Circulation.
109:III8–III14. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Singh IM, Shishehbor MH and Ansell BJ:
High-density lipoprotein as a therapeutic target: a systematic
review. JAMA. 298:786–798. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brewer HB Jr: High-density lipoproteins: a
new potential therapeutic target for the prevention of
cardiovascular disease. Arterioscler Thromb Vasc Biol. 24:387–391.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Attie AD, Kastelein JP and Hayden MR:
Pivotal role of ABCA1 in reverse cholesterol transport influencing
HDL levels and susceptibility to atherosclerosis. J Lipid Res.
42:1717–1726. 2001.PubMed/NCBI
|
|
9
|
Zannis VI, Chroni A and Krieger M: Role of
apoA-I, ABCA1, LCAT, and SR-BI in the biogenesis of HDL. J Mol Med
(Berl). 84:276–294. 2006. View Article : Google Scholar
|
|
10
|
Trigatti BL, Krieger M and Rigotti A:
Influence of the HDL receptor SR-BI on lipoprotein metabolism and
atherosclerosis. Arterioscler Thromb Vasc Biol. 23:1732–1738. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ji Y, Wang N, Ramakrishnan R, et al:
Hepatic scavenger receptor BI promotes rapid clearance of high
density lipoprotein free cholesterol and its transport into bile. J
Biol Chem. 274:33398–33402. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ueda Y, Royer L, Gong E, et al: Lower
plasma levels and accelerated clearance of high density lipoprotein
(HDL) and non-HDL cholesterol in scavenger receptor class B type I
transgenic mice. J Biol Chem. 274:7165–7171. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nolan JJ, Ludvik B, Beerdsen P, Joyce M
and Olefsky J: Improvement in glucose tolerance and insulin
resistance in obese subjects treated with troglitazone. N Engl J
Med. 331:1188–1193. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Choi D, Kim SK, Choi SH, et al:
Preventative effects of rosiglitazone on restenosis after coronary
stent implantation in patients with type 2 diabetes. Diabetes Care.
27:2654–2660. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gerstein HC, Ratner RE, Cannon CP, et al:
Effect of rosiglitazone on progression of coronary atherosclerosis
in patients with type 2 diabetes mellitus and coronary artery
disease: the assessment on the prevention of progression by
rosiglitazone on atherosclerosis in diabetes patients with
cardiovascular history trial. Circulation. 121:1176–1187. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kelly AS and Bank AJ: The cardiovascular
effects of the thiazolidinediones: a review of the clinical data. J
Diabetes Complications. 21:326–334. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sidhu JS, Kaposzta Z, Markus HS and Kaski
JC: Effect of rosiglitazone on common carotid intima-media
thickness progression in coronary artery disease patients without
diabetes mellitus. Arterioscler Thromb Vasc Biol. 24:930–934. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Marx N, Duez H, Fruchart JC and Staels B:
Peroxisome proliferator-activated receptors and atherogenesis:
regulators of gene expression in vascular cells. Circ Res.
94:1168–1178. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Malerød L, Sporstol M, Juvet LK, Mousavi
A, Gjøen T and Berg T: Hepatic scavenger receptor class B, type I
is stimulated by peroxisome proliferator-activated receptor gamma
and hepatocyte nuclear factor 4alpha. Biochem Biophys Res Commun.
305:557–565. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Llaverias G, Rebollo A, Pou J, et al:
Effects of rosiglitazone and atorvastatin on the expression of
genes that control cholesterol homeostasis in differentiating
monocytes. Biochem Pharmacol. 71:605–614. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Carreon-Torres E, Rendon-Sauer K,
Monter-Garrido M, et al: Rosiglitazone modifies HDL structure and
increases HDL-apoAI synthesis and catabolic rates. Clin Chim Acta.
401:37–41. 2009. View Article : Google Scholar
|
|
22
|
Fan JG, Chen LH, Xu ZJ and Zeng MD:
Overexpression of hepatic plasminogen activator inhibitor type 1
mRNA in rabbits with fatty liver. World J Gastroenterol. 7:710–712.
2001.
|
|
23
|
Zhao SP, Yang J, Li J, Dong SZ and Wu ZH:
Effect of niacin on LXRalpha and PPARgamma expression and
HDL-induced cholesterol efflux in adipocytes of
hypercholesterolemic rabbits. Int J Cardiol. 124:172–178. 2008.
View Article : Google Scholar
|
|
24
|
Troutt JS, Alborn WE, Mosior MK, et al: An
apolipoprotein A-I mimetic dose-dependently increases the formation
of prebeta1 HDL in human plasma. J Lipid Res. 49:581–587. 2008.
View Article : Google Scholar
|
|
25
|
Havel RJ, Eder HA and Bragdon JH: The
distribution and chemical composition of ultracentrifugally
separated lipoproteins in human serum. J Clin Invest. 34:1345–1353.
1955. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pirillo A, Norata GD, Zanelli T and
Catapano AL: Overexpression of inducible heat shock protein 70 in
Cos-1 cells fails to protect from cytotoxicity of oxidized ldls.
Arterioscler Thromb Vasc Biol. 21:348–354. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pirillo A, Uboldi P, Kuhn H and Catapano
AL: 15-Lipoxygenase-mediated modification of high-density
lipoproteins impairs SR-BI- and ABCA1-dependent cholesterol efflux
from macrophages. Biochim Biophys Acta. 1761:292–300. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhong JK, Guo ZG, Li C, et al: Probucol
alleviates atherosclerosis and improves high density lipoprotein
function. Lipids Health Dis. 10(210): 2011
|
|
29
|
Speidl WS, Cimmino G, Ibanez B, et al:
Recombinant apolipoprotein A-I Milano rapidly reverses aortic valve
stenosis and decreases leaflet inflammation in an experimental
rabbit model. Eur Heart J. 31:2049–2057. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Beltowski J, Jamroz-Wisniewska A,
Borkowska E and Wojcicka G: Differential effect of antioxidant
treatment on plasma and tissue paraoxonase activity in
hyperleptinemic rats. Pharmacol Res. 51:523–532. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fan J and Watanabe T: Transgenic rabbits
as therapeutic protein bioreactors and human disease models.
Pharmacol Ther. 99:261–282. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ansell BJ, Fonarow GC and Fogelman AM:
High-density lipoprotein: is it always atheroprotective? Curr
Atheroscler Rep. 8:405–411. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Szapary PO, Bloedon LT, Samaha FF, et al:
Effects of pioglitazone on lipoproteins, inflammatory markers, and
adipokines in nondiabetic patients with metabolic syndrome.
Arterioscler Thromb Vasc Biol. 26:182–188. 2006. View Article : Google Scholar
|
|
34
|
Chiesa G and Sirtori CR: Apolipoprotein
A-I(Milano): current perspectives. Curr Opin Lipidol. 14:159–163.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hiroshi N: Latest insights into
high-density lipoprotein functions. Endocrinologist. 19:179–186.
2009. View Article : Google Scholar
|
|
36
|
Fogelman AM: When good cholesterol goes
bad. Nat Med. 10:902–903. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
von Eckardstein A, Nofer JR and Assmann G:
High density lipoproteins and arteriosclerosis. Role of cholesterol
efflux and reverse cholesterol transport. Arterioscler Thromb Vasc
Biol. 21:13–27. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Calpe-Berdiel L, Rotllan N, Palomer X,
Ribas V, Blanco-Vaca F and Escola-Gil JC: Direct evidence in vivo
of impaired macrophage-specific reverse cholesterol transport in
ATP-binding cassette transporter A1-deficient mice. Biochim Biophys
Acta. 1738:6–9. 2005. View Article : Google Scholar
|
|
39
|
Kozarsky KF, Donahee MH, Rigotti A, Iqbal
SN, Edelman ER and Krieger M: Overexpression of the HDL receptor
SR-BI alters plasma HDL and bile cholesterol levels. Nature.
387:414–417. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang N, Arai T, Ji Y, Rinninger F and Tall
AR: Liver-specific overexpression of scavenger receptor BI
decreases levels of very low density lipoprotein ApoB, low density
lipoprotein ApoB, and high density lipoprotein in transgenic mice.
J Biol Chem. 273:32920–32926. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kozarsky KF, Donahee MH, Glick JM, Krieger
M and Rader DJ: Gene transfer and hepatic overexpression of the HDL
receptor SR-BI reduces atherosclerosis in the cholesterol-fed LDL
receptor-deficient mouse. Arterioscler Thromb Vasc Biol.
20:721–727. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Varban ML, Rinninger F, Wang N, et al:
Targeted mutation reveals a central role for SR-BI in hepatic
selective uptake of high density lipoprotein cholesterol. Proc Natl
Acad Sci USA. 95:4619–4624. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Braun A, Trigatti BL, Post MJ, et al: Loss
of SR-BI expression leads to the early onset of occlusive
atherosclerotic coronary artery disease, spontaneous myocardial
infarctions, severe cardiac dysfunction, and premature death in
apolipoprotein E-deficient mice. Circ Res. 90:270–276. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang Y, Da Silva JR, Reilly M, Billheimer
JT, Rothblat GH and Rader DJ: Hepatic expression of scavenger
receptor class B type I (SR-BI) is a positive regulator of
macrophage reverse cholesterol transport in vivo. J Clin Invest.
115:2870–2874. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chawla A, Boisvert WA, Lee CH, et al: A
PPAR gamma-LXR-ABCA1 pathway in macrophages is involved in
cholesterol efflux and atherogenesis. Mol Cell. 7:161–171. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chinetti G, Lestavel S, Bocher V, et al:
PPAR-alpha and PPAR-gamma activators induce cholesterol removal
from human macrophage foam cells through stimulation of the ABCA1
pathway. Nat Med. 7:53–58. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang N, Ranalletta M, Matsuura F, Peng F
and Tall AR: LXR-induced redistribution of ABCG1 to plasma membrane
in macrophages enhances cholesterol mass efflux to HDL.
Arterioscler Thromb Vasc Biol. 26:1310–1316. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sviridov D, Mukhamedova N, Remaley AT,
Chin-Dusting J and Nestel P: Antiatherogenic functionality of high
density lipoprotein: how much versus how good. J Atheroscler
Thromb. 15:52–62. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Tward A, Xia YR, Wang XP, et al: Decreased
atherosclerotic lesion formation in human serum paraoxonase
transgenic mice. Circulation. 106:484–490. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Bhattacharyya T, Nicholls SJ, Topol EJ, et
al: Relationship of paraoxonase 1 (PON1) gene polymorphisms and
functional activity with systemic oxidative stress and
cardiovascular risk. JAMA. 299:1265–1276. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Holvoet P: Oxidized LDL and coronary heart
disease. Acta Cardiol. 59:479–484. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Shao B, Oda MN, Oram JF and Heinecke JW:
Myeloperoxidase: an inflammatory enzyme for generating
dysfunctional high density lipoprotein. Curr Opin Cardiol.
21:322–328. 2006. View Article : Google Scholar : PubMed/NCBI
|