Introduction
Pulmonary arterial hypertension (PAH) is a common
and multifactorial disease characterized by the progressive
remodeling of the small pulmonary arterial, and with the
progression of the disease, this can finally lead to an elevation
in pulmonary vascular resistance, right ventricular dysfunction and
even death (1,2).
Wang et al reported that pulmonary arterial
collagen accumulation plays an important role in hypoxic pulmonary
hypertension (HPH)-induced pulmonary vascular remodeling in distal
arteries and large proximal arteries (3). It can also prevent normal
hemodynamic recovery which may have severe consequences for right
ventricular function (4–6). In hypoxia-induced pulmonary vascular
adventitial remodeling, Zhang et al (7) discovered that hypoxia increases the
expression of type I collagen in cultured fibroblasts. Changes in
the levels of extracellular matrix (ECM) proteins, particularly
those of collagen have been proven to contribute to arterial
stiffening, and the content of collagen in mice remains at a higher
level even after being removed from the hypoxic environment due to
impaired type I collagen degradation (4).
Baicalin is a flavonoid compound purified from the
dry roots of Scutellaria baicalensis, which has been
demonstrated to possess multiple pharmacological activities,
including antioxidant, antitumor, anti-inflammatory and
anti-proliferative activities (8,9).
Baicalin has been shown to exert certain therapeutic effects on
rats with hepatic fibrosis induced by carbon tetrachloride and to
significantly attenuate the degree of hepatic fibrosis, and
decrease the collagen area and the collagen area percentage in
liver tissue (10). Baicalin has
been demonstrated to have anti-fibrotic activity by suppressing
collagen I expression at both the mRNA and protein level (11). Yet, there are only few reports
concerning the effects of baicalin on hypoxia-induced pulmonary
hypertension.
A disintegrin and metalloprotease with
thrombospondin motif (ADAMTS) proteinases were first described in
mice by Kuno et al in 1997 (12) and have subsequently been
identified in mammals and Caenorhabditis elegans. Thus far,
19 distinctive human ADAMTS gene products have been identified.
ADAMTS proteases are secreted enzymes that act on a wide variety of
ECM substrates, including pro-collagen, proteoglycans, hyalectans
and cartilage oligomeric matrix protein (13–16). It has been reported that the
expression of ADAMTS-1 contributes to tissue destruction and has
been implicated in vascular diseases, such as atherosclerosis. It
may also destroy the aortic wall by degrading other ECM components
(17–19). In addition, ADAMTS-1 levels in
chronic viral myocarditis (CVMC) have been found to negatively
correlate with type I collagen levels. Hence, it was concluded that
ADAMTS-1 contributes to the anti-fibrotic effect by accelerating
the degradation of type I collagen in CVMC (20).
Based on the possible links among HPH, collagen,
baicalin and ADAMTS-1, in the present study, we created a rat model
of HPH, and measured the expression levels of collagen and ADAMTS-1
in order to determine the effects of baicalin on the synthesis of
collagen in rats with HPH.
Materials and methods
Materials
The polyclonal rabbit anti-rat type I collagen
(ab34710) and type III collagen (ab7778) antibodies were obtained
from Abcam (Cambridge, UK). The polyclonal goat anti-rabbit
antibody (sc-2004) and polyclonal rabbit anti-rat ADAMTS-1 antibody
(sc-25581) were purchased from Santa Cruz Biotechnology (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA). Secondary HRP-linked
goat anti-rabbit antibody (sv-0002) was obtained from Boster
Biotech Co., Ltd. (Wuhan, China). Baicalin was obtained from
Sigma-Aldrich (St. Louis, MO, USA). The hybridization kit in
situ was purchased from Boster Biotech Co., Ltd. Adult male
Sprague-Dawley (SD) rats (obtained from the Laboratory Animal
Centre of Wenzhou Medical University, Wenzhou, China) weighing
250–300 g were used in our experiments in accordance with the
guidelines for animal procedures provided by Wenzhou Medical
College and the National Institutes of Health standards for animal
care. An effort was made to reduce the number of experimental
animals used and to minimize their suffering. This study was
approved by the Animal Ethics Committee of Wenzhou Medical College
under permit number SCXK (Shanghai 2010–0002).
Treatment of animals
The animal models and experimental groups were as
follows: 24 male SPF SD rats weighing 250–300 g provided by the
Experimental Animal Centre of Wenzhou Medical University were
randomly assigned to the following 3 groups (8 rats/group): the
control group (C), the hypoxic group (H) and the hypoxia + baicalin
group (B). The rats in groups H and B were kept in a normobaric
hypoxic (80–110 ml/l O2, 8 h/day) chamber for 4 weeks.
The rats in group C were exposed to room air. Anhydrous calcium
chloride and sodium hydroxide were used for water and carbon
dioxide absorption, respectively. Baicalin was injected
intraperitoneally into the rats in group B at a daily dose of 30
mg/kg, 30 min before they were placed in the chamber.
Measurement of hemodynamic indexes: mean
pulmonary artery pressure (mPAP), mean systemic (carotid) artery
pressure (mSAP) and mass ratio of right ventricle to left ventricle
plus septum [RV/(LV + S)]
The rats were anesthetized by an intraperitoneal
injection of 5% chloral hydrate (40 mg/kg body weight). A
polyethylene catheter (inside diameter, 0.9 mm; outside diameter,
1.1 mm) was gradually inserted into the pulmonary artery through an
incision in the right external jugular vein and the mPAP was
recorded. The rats were then anatomised after blood samples were
obtained. We collected and weighed the left ventricle plus the
interventricular septum (LV + S) and the right ventricle (RV)
tissue by cutting along the edge of the ventricle and the
interventricular septum. The mass ratio of the RV to the (LV + S)
was used as the index for RV hypertrophy.
Hematoxylin and eosin staining Following
deparaffinization and dehydration, the sections of lungs were
incubated in hematoxylin solusion for 10–30 min and then washed
with running water for 15 min. The sections were then placed into
1% hydrochloric acid ethanol for 2–10 sec before being dehydrated
by a graded ethanol series. Finally, they were dehydrated again
immediately following counterstaining with 0.5% eosin solution for
1–3 min.
Immunohistochemistry
Following blocking, the sections were incubated with
rabbit anti-rat type I collagen polyclonal antibody [diluted 1:500
with phosphate-buffered saline (PBS)], type III collagen antibody
(diluted 1:500 with PBS), ADAMTS-1 antibody (diluted 1:25 with
PBS). They were then incubated in secondary HRP-linked goat
anti-rabbit antibody. Lung specimens incubated with 10% goat serum
in place of the specific primary antibody served as the negative
controls. Immunoreactivity was visualized using
3,30-diaminobenzidine (DAB). All antibodies used for
immunohistochemistry were diluted in PBS, and 5 fields of vision
were selected randomly from each section for quantitative analyses.
Using Image-Pro Plus software, we measured the area of tunica media
of pulmonary arterioles and the integrated optical density of
positive products, then we calculated the ratio of the integrated
optical density and area and named the average optical density (OD
value) to reflect the expression of positive products.
Hybridization in situ
The sections of each case were deparaffinized and
dehydrated through a graded ethanol series, then in 3% hydrogen
peroxide solution for 10 min. Subsequently, each section was
digested by pepsin (1.3 mg/ml; Dako, Glostrup, Denmark) for 30 min,
washed in 0.1 M PBS (pH 7.4) buffer and fixed with 4%
polyformaldehyde-PBS liquor for 10 min, then washed 3 times with
PBS buffer for 5 min. The probes and tissue RNA were co-denatured
at 40°C overnight. They were then washed with hybridization buffer
(2X SSC for 5 min 2 times, 0.5X SSC and 0.2X SSC for 15 min only
once, respectively). This was followed by incubation with blocking
buffer at 37°C for 30 min, and the sections were checked with a
digoxin reagent box. Subsequently, they were color-produced by
chromogenic reagent and were monitored visually and this reaction
was terminated by placing the slides in water. The slides were
counterstained with nucleus fixed red reagent to visualize the
nuclei, then washed again with water, dehydrated, vitrified by
dimethylbenzene, and mounted with neuter balata. The negative
control was hybridized with no-probe liquor in parallel with the
experimental reactions and 5 fields of vision were selected
randomly from each section for quantitative analyses.
Western blot analysis
Fresh lung tissue weighing 50 mg was homogenized
with a glass homogenizer on ice, then lysed with chilled lysis
buffer followed by centrifugation at 12,000 × g for 30 min at 4°C.
The supernatant was collected as total protein and transferred to a
new cooled 1.5 ml Eppendorf tube. The protein concentration was
measured by the Bradford method and the homogenate was diluted to 2
μg/μl with PBS. Equal amounts of proteins (20
μg) were separated by 12% sodium dodecyl
sulfate-polyacrylamide gel (SDS-PAGE) and transferred onto
polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA,
USA). The blots were blocked in Tris-buffered saline with Tween-20
(TBST) containing 5% bovine serum albumin (BSA) for 4 h.
Subsequently, the membranes were incubated overnight at 4°C with
specific primary antibodies: rabbit anti-rat collagen I antibody at
a dilution of 1:1,000, rabbit anti-rat collagen III antibody at a
dilution of 1:1,000. The membranes were incubated with
peroxidase-labeled affinity purified antibody to rabbit IgG (0.1
μg/ml) after washing with TBST buffer and visualized using
an enhanced chemiluminescence kit (Pierce, Rockford, IL, USA) with
an ECL Imaging System (Bio-Rad Laboratories, Hercules, CA, USA).
Band intensities were quantified using Image-Pro Plus software.
Statistical analysis
Values are presented as the means ± SD. All data
were analyzed using one-way ANOVA followed by a LSD-test. A value
of P<0.05 was considered to indicate a statistically significant
difference between groups.
Results
Baicalin improves hemodynamics,
attenuates right ventricular remodeling and morphological changes
in pulmonary arterioles in rats with HPH
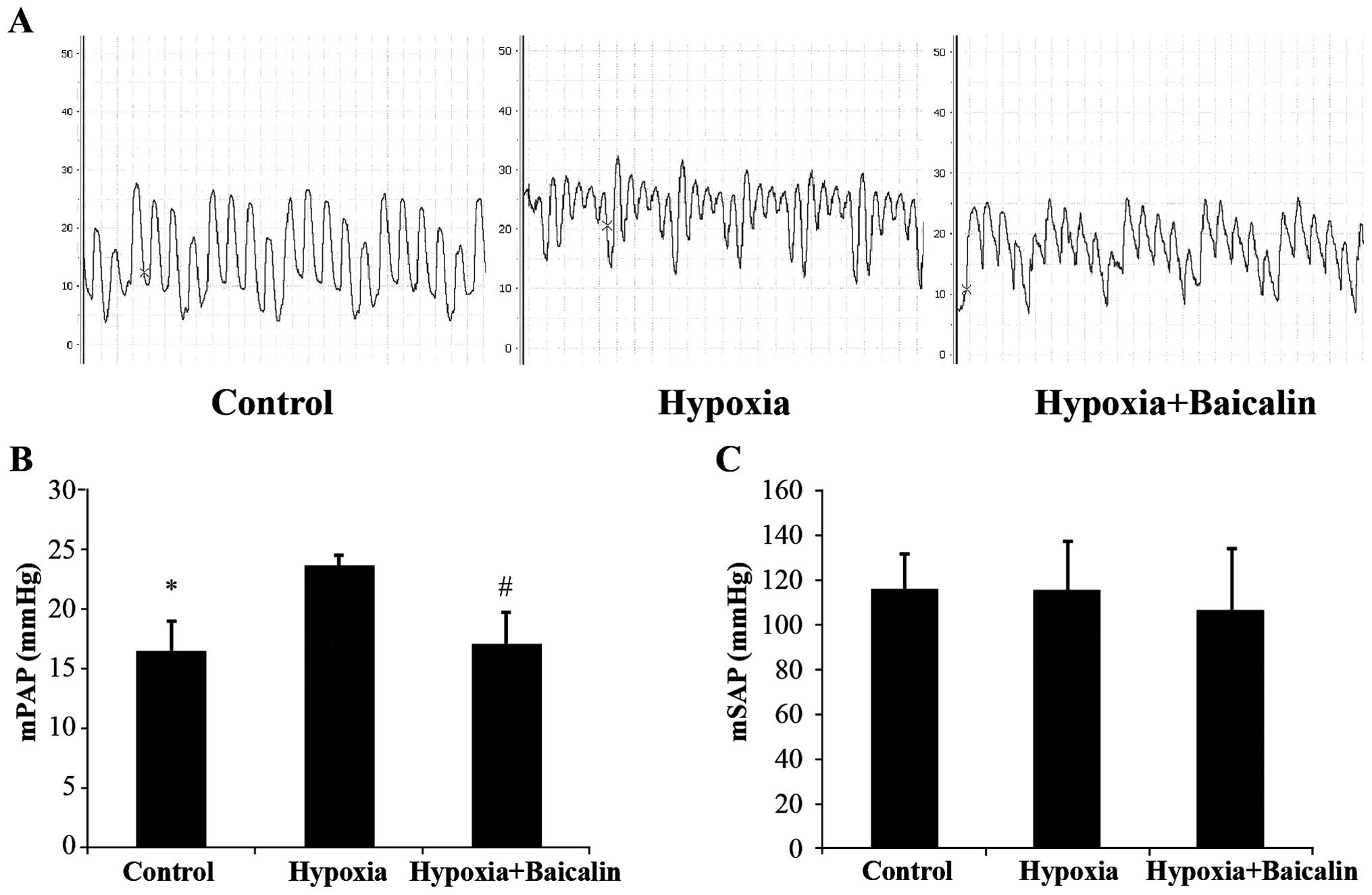
We measured mPAP and mSAP to reflect the hemodynamic
changes. Hematoxylin and eosin (H&E) staining of the pulmonary
artery tissue was carried out to calculate the ratio of the
pulmonary artery wall thickness to the total area of the artery
(WA/TA) for a comparison of the thickening of the arteries among
the 3 groups. The RV/(LV + S) was calculated to reflect the extent
of right ventricular hypertrophy. mPAP increased from 16.3±2.7 to
23.6±0.9 mmHg following the induction of chronic hypoxia
(P<0.01), and treatment with baicalin effectively attenuated
this increase, decreasing mPAP to 17.0±2.7 mmHg (P<0.01;
Fig. 1A and B). However, there
was no statistically significant difference in mSAP among the 3
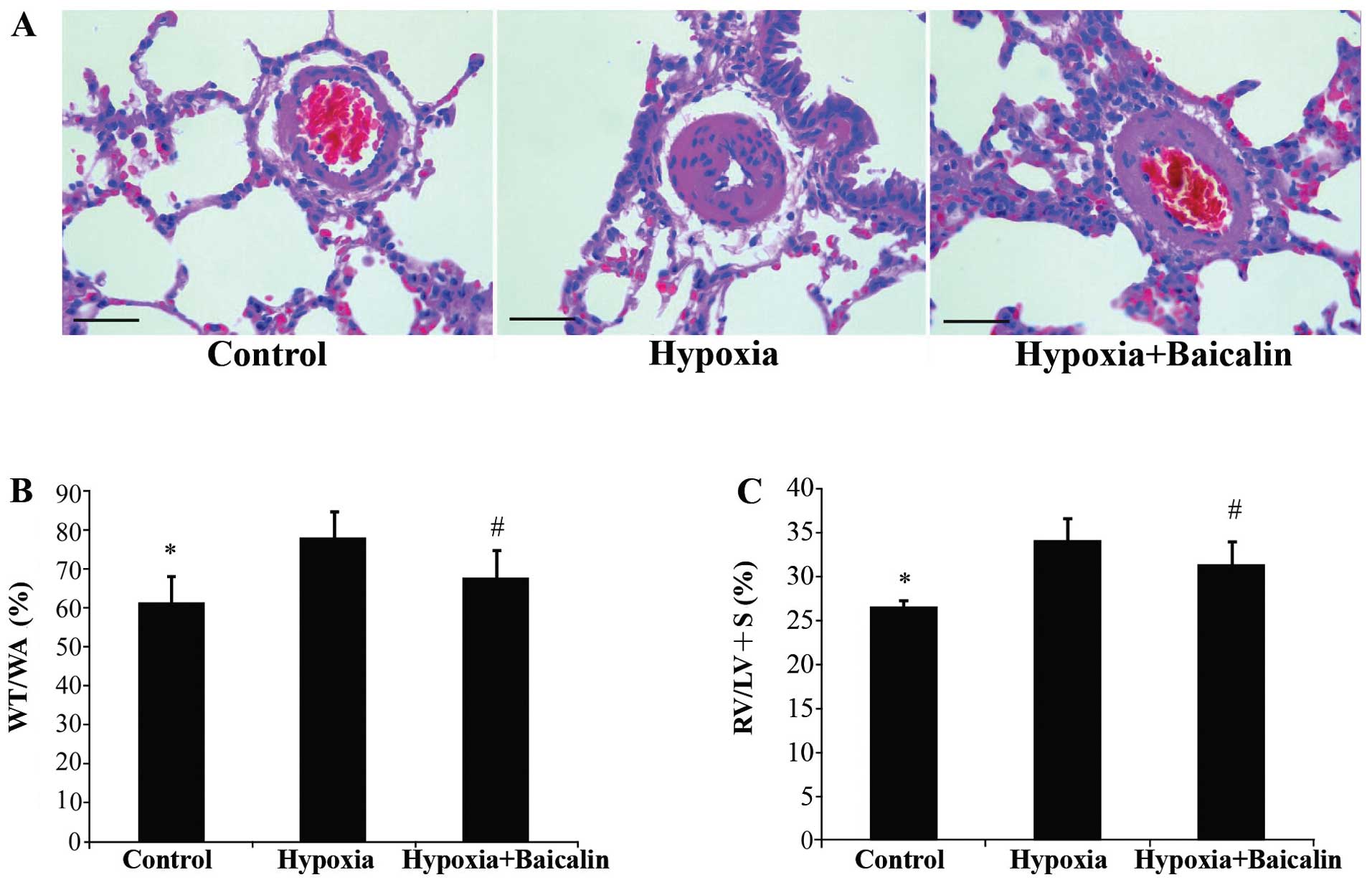
groups (Fig. 1C). The thickness
of the arteries which was induced by chronic hypoxia (77.75±6.79%)
was much more obvious than that of the rats in group C
(61.00±6.86%) (P<0.01). Bacalin markedly attenuated this effect,
decreasing the WA/TA and the WA/TA% to 67.72±6.76% (P<0.01;
Fig. 2A and B). The RV/(LV + S)%
was markedly elevated in the rats in group H (34.18±2.43%) compared
with the rats in group C (26.57±0.77%) (P<0.01). Following the
injection of bacalin, the RV/(LV + S)% decreased to 31.36±2.70% and
right ventricular hypertrophy was significantly attenuated
(P<0.05; Fig. 2C).
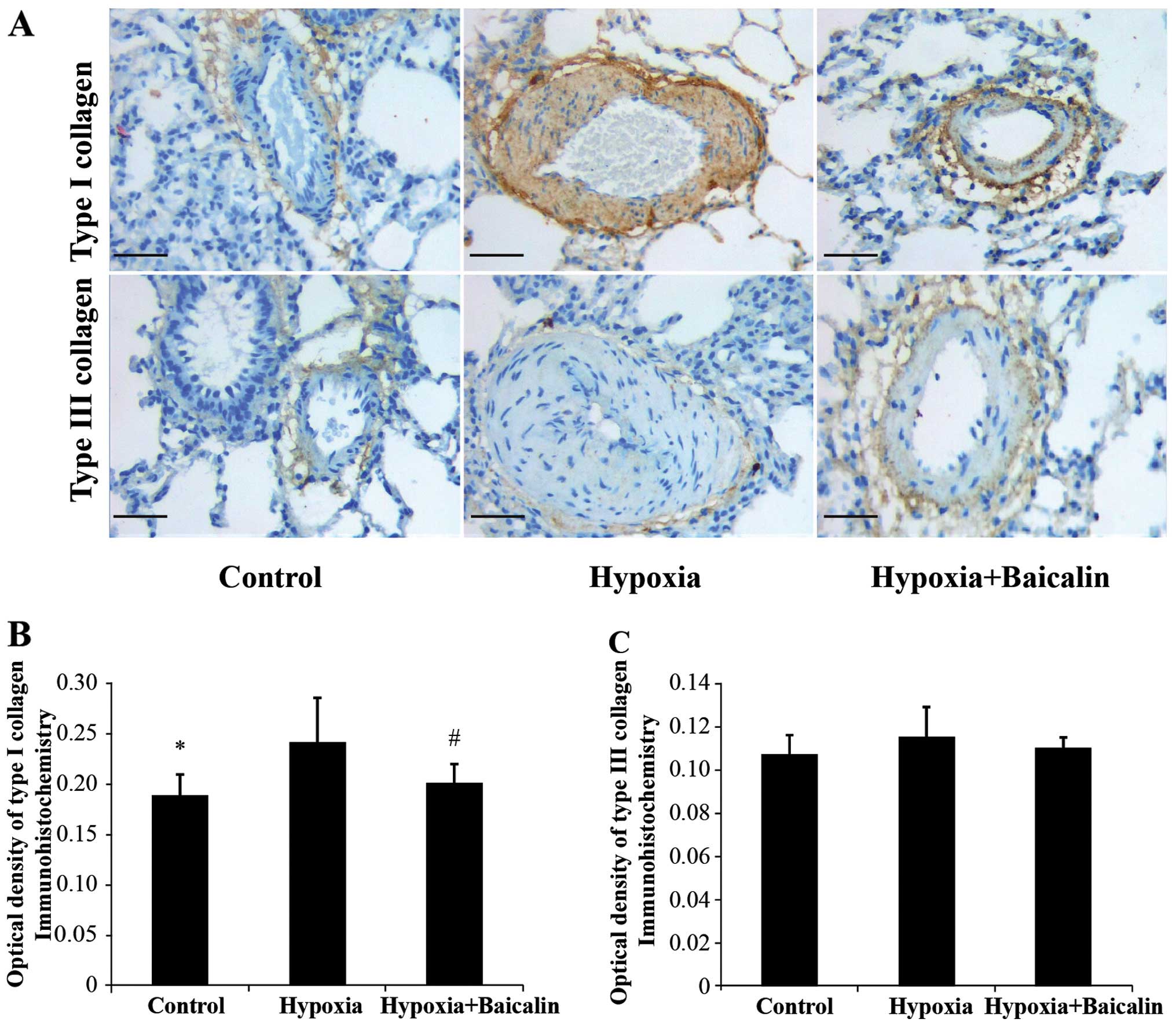
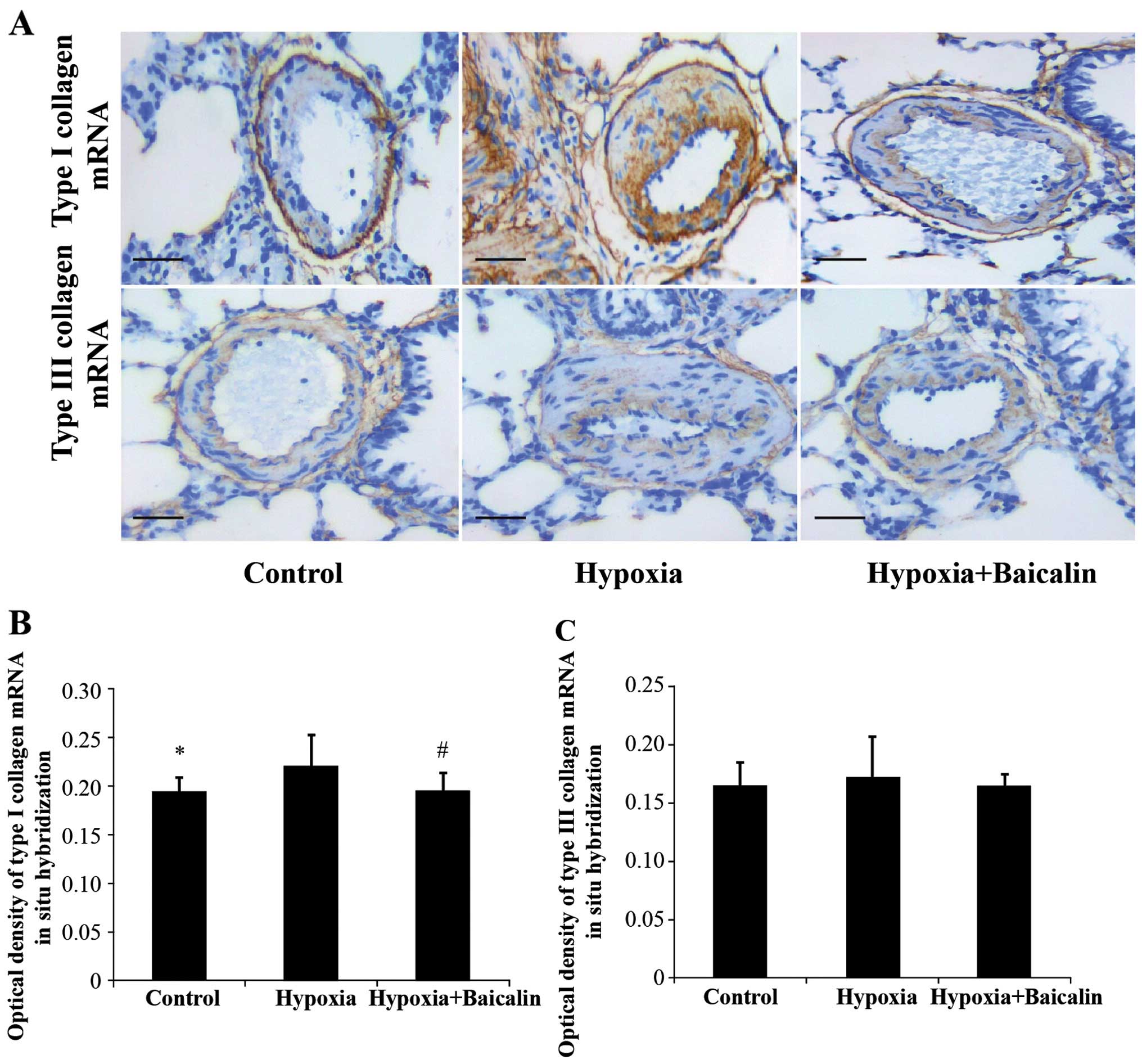
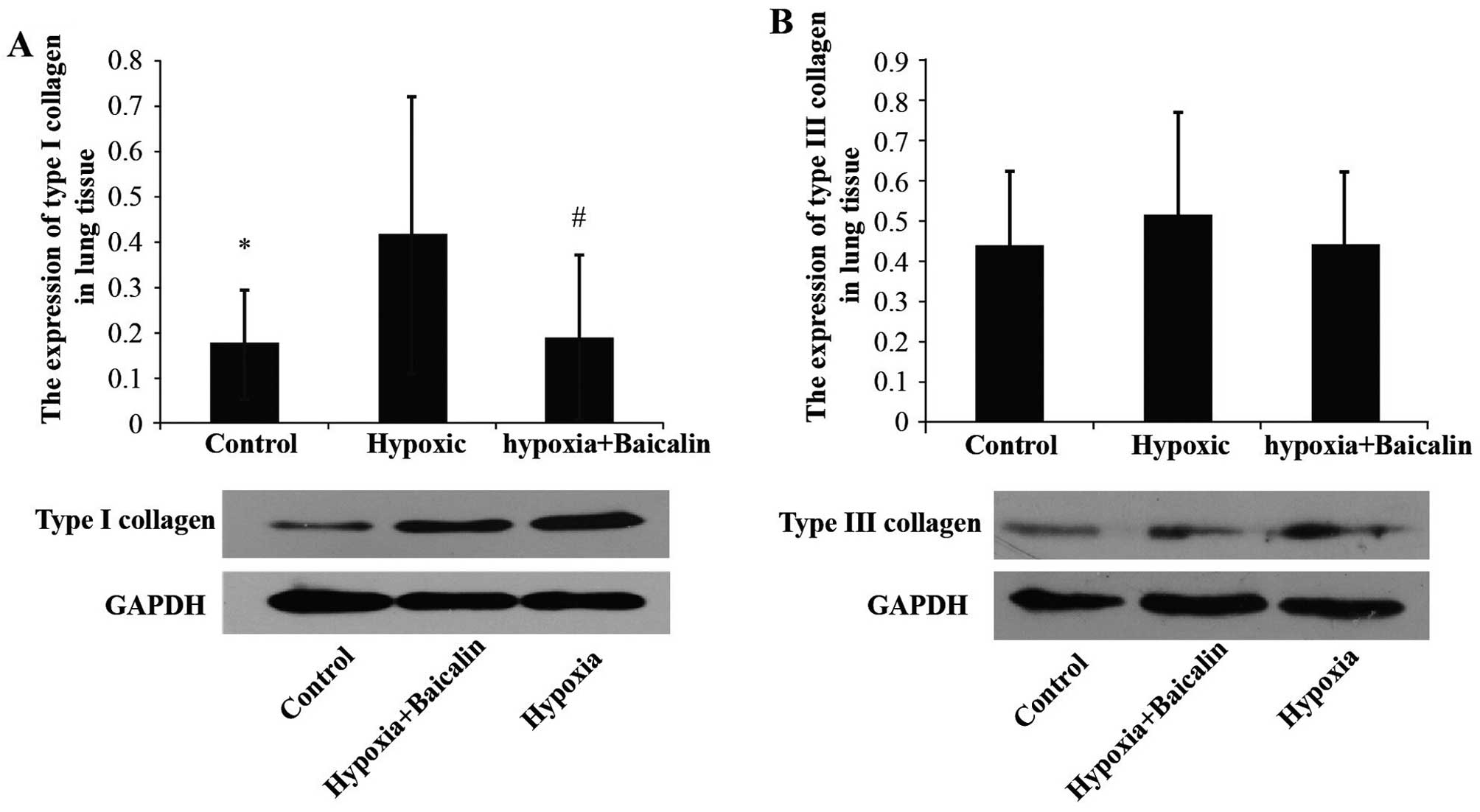
Baicalin inhibits the protein and mRNA
expression of collagen I induced by chromic hypoxia
Under the condition of chronic hypoxia, the OD value
of collagen I noticeably increased compared with the normal
condition in group C (0.242±0.043 vs. 0.188±0.021, P<0.01), and
its mRNA expression increased from 0.195±0.014 in group C to
0.220±0.033 in group H (Figs. 3
and 4). However, as expected,
treatment with baicalin decreased the OD value and mRNA expression
of collagen I to 0.201±0.019 (P<0.05) and 0.196±0.018
(P<0.05), respectively (Figs.
3 and 4). Western blot
analysis also revealed that chronic hypoxia markedly upregulated
the protein expression of collagen I from 0.175±0.119 to
0.417±0.305 (P<0.05), and treatment with baicalin markedly
decreased this expression (0.188±0.183; P<0.05; Fig. 5).
Baicalin has little effect on the protein
and mRNA expression of collagen III in rats suffering from HPH
The protein expression of collagen III was detected
by immunochemistry and western blot analysis. Hybridization in
situ was carried out to determine the changes in mRNA
expression. As shown in Figs. 3
and 4, there were no apparent
statistically significant differences in the mRNA expression of
collagen III among the 3 groups (P>0.05). A similar tendency was
detected by western blot analysis (Fig. 5).
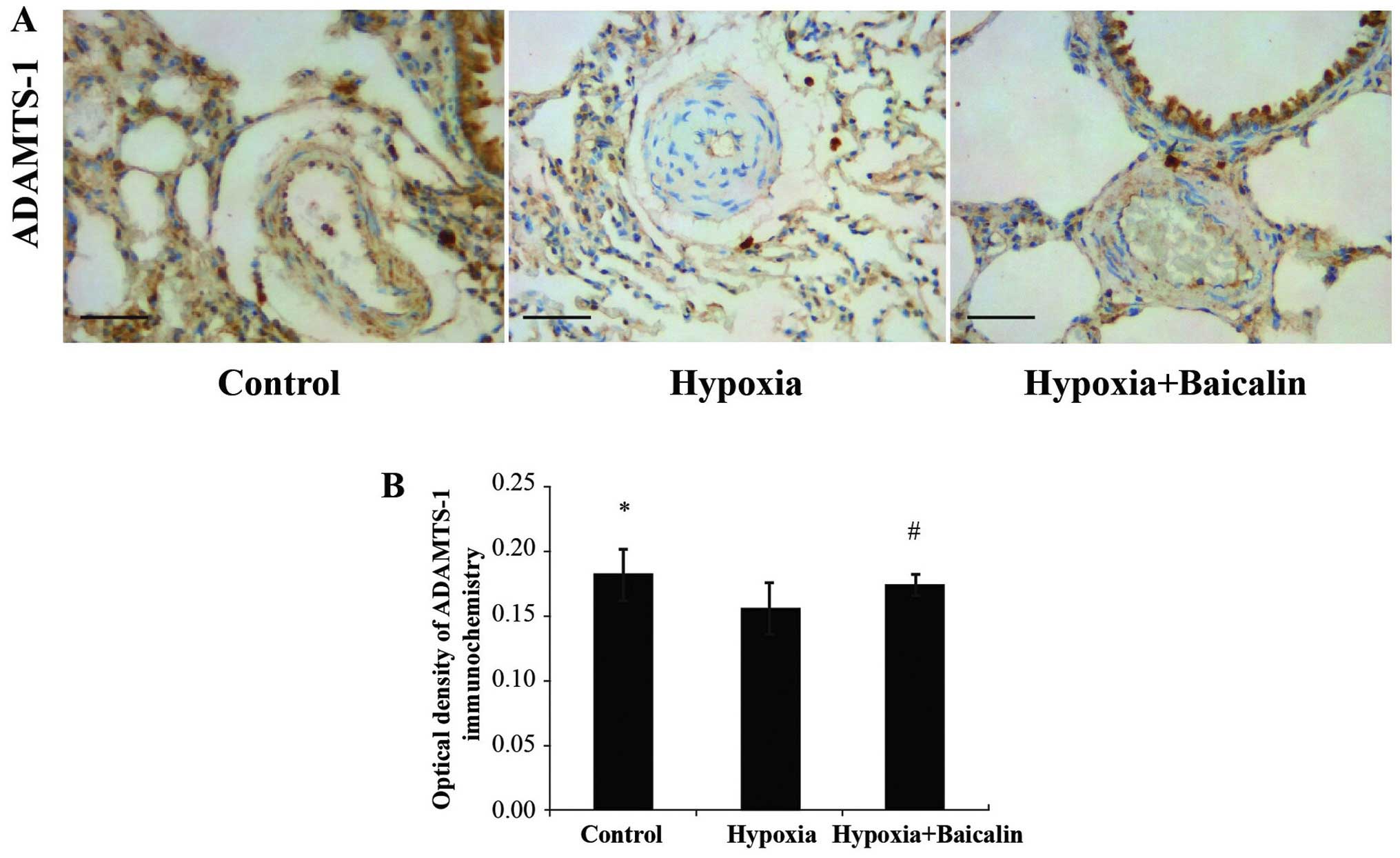
Baicalin increases the expression of
ADAMTS-1 in rats in response to chronic hypoxia
Immunohistochemistry was carried out to detect the
protein expression of ADAMTS-1 in the rats suffering from PAH. As
shown in Fig. 6, there was a
marked decrease in ADAMTS-1 expression in the pulmonary arterioles
of the rats under chronic hypoxic conditions (0.156±0.020) compared
to normal conditions (group C) (0.182±0.020; P<0.01); however,
treatment with baicalin significantly increased ADAMTS-1 expression
in the rats in group B (0.174±0.009) compared to the rats in group
H (P<0.05).
These results suggested that chronic hypoxia
stimulated the synthesis and deposition of collagen I, which played
a key role in the process of pulmonary vascular remodeling.
Simultaneously, a decrease in ADAMTS-1 expression was observed.
Baicalin was proven to effectively lower mPAP, which also reversed
pulmonary vascular remodeling partially by upregulating the
expression of ADAMTS-1, and thus inhibiting the expression of
collagen I, further exerting protective effects on rats suffering
from HPH.
Discussion
PAH is a fatal disease which progresses rapidly with
high mortality. HPH has been recognized as the most common
complication of some pulmonary diseases characterized by the
vasoconstriction and remodeling of the pulmonary vascular artery
which has been confirmed to play a role in the development of HPH
(21,22). Exposure to a hypoxic environment
for 4 weeks can be used to successfully establish a model of
chronic HPH (23). This study
demonstrated that mPAP was significantly higher in the hypoxic
group than in the control group. The thickening of the vascular
wall and stenosis of the lumen were obvious changes in the
morphology of the pulmonary arterioles. After calculating the
RV/(LV + S) ratio as the index of right ventricular hypertrophy and
the WA/TA%, we found that our data not only were consistent with
those of our previous studies, but also indicated success in
establishing the model of HPH (24,25).
In recent years, increasing attention has been paid
to the association between HPH and collagen accumulation. Studies
have discovered that collagen accumulation plays a key role in the
stiffening of the proximal pulmonary artery, which leads to
sustained increase in pulmonary artery pressure and finally the
failure of the right ventricle (3,26,30). Research on newborn Wistar rats
exposed to hypoxia has also revealed increased mPAP, right
ventricular hypertrophy, collagen deposition in the ECM and
pulmonary vascular remodeling (26). In the study by Wang et al,
it was suggested that the collagen total content was critical to
extralobar pulmonary artery stiffening during HPH (27). Type I collagen is a fibrillar
collagen subtype that plays a dominant role in the composition and
strength of the arteries (28–30). The expression of collagen,
including collagen I in rats exposed to hypoxia has been shown to
markedly increase pulmonary vascular remodeling. This increase may
be alleviated by inhibiting the collagen accumulation in pulmonary
arteries (31). In this study, we
also demonstrated a significantly increased protein and mRNA
expression of collagen I in the pulmonary arterial wall accompanied
by the stenosis of the lumen and the thickening of the pulmonary
arterial wall under hypoxic conditions, contributing to the
formation and progression of pulmonary vascular remodeling, which
is in accordance with what was observed in our previous study
(25).
Baicalin is a plant-derived flavonoid with a variety
of activities, including antioxidant and anti-inflammatory
properties, and is also known to alleviate ischemia-reperfusion
injury (8,9). It has been previoulsy demonstrated
that baicalin is essential for mesenchymal stem cell (MSC)
transplantation, exerting a therapeutic effect by reducing the
fibrotic area (32). Huang et
al (34) found that baicalin
attenuated human pulmonary artery smooth muscle cell proliferation
and the phenotypic switch induced by transforming growth factor-β1.
It has also been shown to prevent bleomycin-induced pulmonary
fibrosis in rats, and that the above effect of baicalin is related
to the blockage of the synthesis of type I collagen and the
decrease in the number of myofibroblasts in the lungs (33,34). However, studies on its effects on
HPH are limited. In this study, baicalin was found to be effective
in lowering mPAP and the expression of collagen I in the pulmonary
artery, thus partially reversing pulmonary vascular remodeling,
which may be a critical mechanism through which baicalin protects
rats with HPH.
Kaushal and Shah (35) previously demonstrated that
proteolytically active ADAMTS-1 participates in normal ECM turnover
and that its absence contributes to the fibrosis observed in mutant
mice. The upregulation of ADAMTS (ADAMTS-1, -4 and -15) has been
reported to be involved in disc ECM destruction in the human
degenerated intervertebral disc (36). Rehn et al found that
ADAMTS-1 induced collagen type I processing in bone in vitro
together with a positive influence on osteoblastic
three-dimensional growth by promoting collagen degradation directly
or indirectly (37). In this
study, we demonstrated that in rats with HPH, the expression of
ADAMTS-1 in the pulmonary arterioles decreased accompanied by an
increase in the expression of collagen I and not collagen III.
Following treatment with baicalin, there was a significant increase
in ADAMTS-1 expression and a decreased in collagen I expression. We
also observed a marked improvement in hemodynamics and in the
morphology of the pulmonary arterioles.
The results of the present study indicate that the
ADAMTS-1 participates in the inhibition of the synthesis of
collagen I by baicalin, suggesting that baicalin exerts protective
effects on rats with HPH and reserves pulmonary vascular remodeling
partially by increasing ADAMTS-1 expression, thus inhibiting the
overexpression of collagen I.
Taken together, our novel (to the very best of our
knowledge) findings suggest that ADAMTS-1 is involved in the
development of HPH, and that baicalin upregulates the expression of
ADAMTS-1 under chronic hypoxic conditions, thus inhibiting the
synthesis and expression of collagen I, contributing to the
decrease in pulmonary hypertension and pulmonary vessel
remodeling.
Acknowledgments
This study was supported by the Chinese National
Natural Science Foundation Grants (nos. 81473406, 81470250 and
81270110).
References
|
1
|
Safdar Z, Bartolome S and Sussman N:
Portopulmonary hypertension: an update. Liver Transpl. 18:881–891.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Morales-Blanhir JE, Carmona-Rubio AE,
Rosas-Romero MJ, Vergara de Marquez GS and Arbo-Oze-de-Morvil GA:
Pulmonary arterial hypertension, a rare entity. Rev Invest Clin.
66:65–78. 2014.In Spanish. PubMed/NCBI
|
|
3
|
Wang Z, Lakes RS, Eickhoff JC and Chesler
NC: Effects of collagen deposition on passive and active mechanical
properties of large pulmonary arteries in hypoxic pulmonary
hypertension. Biomech Model Mechanobiol. 12:1115–1125. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ooi CY, Wang Z, Tabima DM, et al: The role
of collagen in extralobar pulmonary artery stiffening in response
to hypoxia-induced pulmonary hypertension. Am J Physiol Heart Circ
Physiol. 299:H1823–H1831. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Estrada KD and Chesler NC:
Collagen-related gene and protein expression changes in the lung in
response to chronic hypoxia. Biomech Model Mechanobiol. 8:263–272.
2009. View Article : Google Scholar :
|
|
6
|
Schreier D, Hacker T, Song G and Chesler
N: The role of collagen synthesis in ventricular and vascular
adaptation to hypoxic pulmonary hypertension. J Biomech Eng.
135:0210182013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang L, Li Y, Chen M, et al:
15-LO/15-HETE mediated vascular adventitia fibrosis via p38
MAPK-dependent TGF-β. J Cell Physiol. 229:245–257. 2014. View Article : Google Scholar
|
|
8
|
Gao Z, Huang K and Xu H: Protective
effects of flavonoids in the roots of Scutellaria baicalensis
Georgi against hydrogen peroxide-induced oxidative stress in
HS-SY5Y cells. Pharmacol Res. 43:173–178. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dong LH, Wen JK, Miao SB, et al: Baicalin
inhibits PDGF-BB-stimulated vascular smooth muscle cell
proliferation through suppressing PDGFRβ-ERK signaling and increase
in p27 accumulation and prevents injury-induced neointimal
hyperplasia. Cell Res. 20:1252–1262. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Peng XD, Dai LL, Huang CQ, et al:
Correlation between anti-fibrotic effect of baicalin and serum
cytokines in rat hepatic fibrosis. World J Gastroenterol.
15:4720–4725. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hu Q, Noor M, Wong YF, et al: In vitro
anti-fibrotic activities of herbal compounds and herbs. Nephrol
Dial Transplant. 24:3033–3041. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kuno K, Kanada N, Nakashima E, et al:
Molecular cloning of a gene encoding a new type of
metalloproteinase-disintegrin family protein with thrombospondin
motifs as an inflammation associated gene. J Biol Chem.
272:556–562. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Salter RC, Ashlin TG, Kwan AP and Ramji
DP: ADAMTS proteases: key roles in atherosclerosis? Mol Med (Berl).
88:1203–1211. 2010. View Article : Google Scholar
|
|
14
|
Porter S, Clark IM, Kevorkian L and
Edwards DR: The ADAMTS metalloproteinases. Biochem J. 386:15–27.
2005. View Article : Google Scholar :
|
|
15
|
Tortorella MD, Malfait F, Barve RA, et al:
A review of the ADAMTS family, pharmaceutical targets of the
future. Curr Pharm Des. 15:2359–2374. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jones GC and Riley GP: ADAMTS proteinases:
a multi-domain, multi-functional family with roles in extracellular
matrix turnover and arthritis. Arthritis Res Ther. 7:160–169. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Taketani T, Imai Y, Morota T, et al:
Altered patterns of gene expression specific to thoracic aortic
aneurysms: microarray analysis of surgically resected specimens.
Int Heart J. 46:265–277. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jönsson-Rylander AC, Nilsson T,
Fritsche-Danielson R, et al: Role of ADAMTS-1 in atherosclerosis:
remodeling of carotid artery, immunohistochemistry, and proteolysis
of versican. Arterioscler Thromb Vasc Biol. 25:180–185. 2005.
|
|
19
|
Sabatine MS, Ploughman L, Simonsen KL, et
al: Association between ADAMTS1 matrix metalloproteinase gene
variation, coronary heart disease, and benefit of statin therapy.
Arterioscler Thromb Vasc Biol. 28:562–567. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guo C, Wang Y, Liang H and Zhang J:
ADAMTS-1 contributes to the antifibrotic effect of captopril by
accelerating the degradation of type I collagen in chronic viral
myocarditis. Eur J Pharmacol. 629:104–110. 2010. View Article : Google Scholar
|
|
21
|
Duong-Quy S, Riviere S, Bei Y, et al:
Pulmonary hypertension: from molecular pathophysiology to
haemodynamic abnormalities. Rev Mal Respir. 29:956–970. 2012.In
French. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang LY and Gao BA: Relationship between
pulmonary artery smooth muscle cells and mechanism of
hypoxia-induced pulmonary vascular remodeling. Zhongguo Dong Mai
Ying Hua Za Zhi. 21:177–180. 2013.In Chinese.
|
|
23
|
Juan L, Xin S and Hui B: Establishment of
on animal model of hypobaric and hypoxia pulmonary hypertension. J
Clin Cardiol. 24:297–301. 2008.
|
|
24
|
Huang X, Fan R, Lu Y, et al: Regulatory
effect of AMP-activated protein kinase on pulmonary hypertension
induced by chronic hypoxia in rats: in vivo and in vitro studies.
Mol Biol Rep. 41:4031–4041. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qian G, Cao J, Chen C, et al: Paeoniflorin
inhibits pulmonary artery smooth muscle cells proliferation via
upregulating A2B adenosine receptor in rat. PLoS ONE. 8:e691412013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sang K, Zhou Y and Li MX: Pulmonary
vascular remodeling in neonatal rats with hypoxic pulmonary
hypertension. Zhongguo Dang Dai Er Ke Za Zhi. 14:210–214. 2012.In
Chinese. PubMed/NCBI
|
|
27
|
Wang Z and Chesler NC: Role of collagen
content and cross-linking in large pulmonary arterial stiffening
after chronic hypoxia. Biomech Model Mechanobiol. 11:279–289. 2012.
View Article : Google Scholar
|
|
28
|
Diez J: Arterial stiffness and
extracellular matrix. Adv Cardiol. 44:76–95. 2007. View Article : Google Scholar
|
|
29
|
Franco CD, Hou G and Bendeck MP:
Collagens, integrins, and the discoidin domain receptors in
arterial occlusive disease. Trends Cardiovasc Med. 12:143–148.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tabima DM, Roldan-Alzate A, Wang Z, et al:
Persistent vascular collagen accumulation alters hemodynamic
recovery from chronic hypoxia. J Biomech. 45:799–804. 2012.
View Article : Google Scholar :
|
|
31
|
Li XW, Du J and Li YJ: The effect of
calcitonin gene-related peptide on collagen accumulation in
pulmonary arteries of rats with hypoxic pulmonary arterial
hypertension. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 29:182–186.
1922013.In Chinese.
|
|
32
|
Qiao H, Tong Y, Han H, et al: A novel
therapeutic regimen for hepatic fibrosis using the combination of
mesenchymal stem cells and baicalin. Pharmazie. 66:37–43.
2011.PubMed/NCBI
|
|
33
|
Liu W, Chen XL, Liu JH, et al: The effect
of baicalein on bleomycin-induced fibrosis in lungs of rats.
Zhongguo Ying Yong Sheng Li Xue Za Zhi. 25:145–149. 2009.In
Chinese. PubMed/NCBI
|
|
34
|
Huang S, Chen P, Shui X, et al: Baicalin
attenuates transforming growth factor-β1-induced human pulmonary
artery smooth muscle cell proliferation and phenotypic switch by
inhibiting hypoxia inducible factor-1α and aryl hydrocarbon
receptor expression. J Pharm Pharmacol. 66:1469–1477. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kaushal GP and Shah SV: The new kids on
the block: ADAMTSs, potentially multifunctional metalloproteinases
of the ADAM family. J Clin Invest. 105:1335–1337. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vo NV, Hartman RA, Yurube T, et al:
Expression and regulation of metalloproteinases and their
inhibitors in intervertebral disc aging and degeneration. Spine J.
13:331–341. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rehn AP, Birch MA, Karlström E, et al:
ADAMTS-1 increases the three-dimensional growth of osteoblasts
through type I collagen processing. Bone. 41:231–238. 2007.
View Article : Google Scholar : PubMed/NCBI
|