Introduction
Keratinocytes are the main cells found in the
epidermal layer of the skin and are exposed to a variety of stress
stimuli such as ultraviolet (UV) radiation (1,2).
Solar UV radiation is divided into three main wavelength ranges:
UVC (λ=100–290 nm), ultraviolet B (UVB) (λ=290–320 nm), and UVA
(λ=320–400 nm) (3,4). Although UVC is completely absorbed
by the ozone layer, UVA and UVB both reach the Earth’s surface in
sufficient amounts to have damaging effects on skin (5). As an environmental factor, UVB
radiation has numerous effects on human health. UVB exposure is
necessary to produce vitamin D in the skin. As vitamin D deficiency
causes immune dysfunction, nervous system, bone growth, cell
proliferation, insulin secretion, and blood pressure, regular and
minimum exposure of skin to UVB is required for maintaining optimal
health (6). However, UVB in
particular has a wide spectrum of biological effects on the skin,
and its acute exposure causes a variety of adverse skin reactions
such as erythema, edema, sunburn, hyperplasia, inflammation, and
immunosuppression (7).
UV-irradiated cells in inflammatory infiltration and various
non-epidermal skin cells also produce cytokines, many of which may
act on keratinocytes. The regulation and function of cytokines in
keratinocytes have been extensively studied and reviewed. These
pro-inflammatory cytokines are considered to be closely associated
with the progression of photodamage. Among keratinocyte-derived
cytokines, the pro-inflammatory cytokines IL-1β, IL-6, TNF-α, and
IL-8 have been well characterized (8,9).
IL-6 and IL-8 induce an acute-phase response and stimulate
leukocyte infiltration in the skin (10).
Cyclooxygenase-2 (COX-2) is the rate-limiting enzyme
involved in the conversion of arachidonic acid to prostaglandins.
COX-2 expression is induced by inflammatory cues in various tissues
such as the epidermis (11,12). Previous findings have shown that
chronic exposure to UVB radiation increases COX-2 expression
through various cell signaling pathways, resulting in the induction
of skin cancer (13,14). Mitogen-activated protein kinases
(MAPKs) are a family of proline-directed Ser/Thr kinases comprising
extracellular signal-regulated kinase (ERK), c-Jun NH2-terminal
kinase (JNK), and p38 MAPK. The activation of ERK, JNK, and p38
MAPK was found to be strongly correlated with acute inflammation
and development of skin cancer through an increased expression of
COX-2 (15–17).
Ixeris dentata family Compositae
(Asteraceae), a typical medicinal herb, has been used for the
treatment of indigestion, pneumonia, hepatitis, contusions, and
tumors (18). Ixerisoside A (IXA)
is an active constituent isolated from the extract of Ixeris
dentata and contains aliphatic compounds, triterpenoids, and
sesquiterpene glycosides (19).
Pharmacological studies on Ixeris dentata
showed that water or organic extracts of whole herbal medicine
decreased lipid concentrations and acted as an antioxidant,
antiallergic, monamine oxidase, anti-inflammatory, antimutagenic,
and anticancer activity (20–24). In particular, previous findings
showed that Ixeris dentata exerted a protective effect
against UVB-induced skin inflammation (25,26). Therefore, in this study, we
examined the potential anti-inflammatory action of IXA. We examined
its effect on UVB-induced pro-inflammatory cytokine production in
human keratinocytes (HaCaT cells) by evaluating UVB-stimulated
cells in the presence or absence of IXA.
Materials and methods
Plant material
Whole plants of Ixeris dentata family
Compositae (Asteraceae) were collected in May 2006 from the
herbarium at the Korea Research Institute of Chemical Technology
(KRICT) and were authenticated by Dr Young Sup Kim. A voucher
specimen (KR0472) was deposited into the herbarium at KRICT
(27).
Extraction and isolation
The air-dried whole plants (6 kg) of Ixeris
dentata were soaked in methanol (MeOH) (2×40 l) at room
temperature for 7 days. The MeOH extract was filtered and
evaporated to dryness under reduced pressure. The concentrated
extract (840 g) was suspended in 20 l of water and then extracted
successively with an equal volume of dichloromethane (MC), ethyl
acetate (EtOAc), and n-butanol (n-BuOH) to afford 160, 15 and 60 g
of the compound in MC, EtOAc, and n-BuOH fractions, respectively
(27). The suggested chemical
structure of IXA is shown in Fig.
1.
Reagents
RPMI-1640, penicillin, and streptomycin were
obtained from HyClone Laboratories, Inc. (Logan, UT, USA). Bovine
serum albumin and
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
(MTS) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Antibodies for ERK, phosphorylated ERK, JNK, phosphorylated JNK,
p38, phosphorylated p38, and β-actin, and peroxidase-conjugated
secondary antibodies were purchased from Santa Cruz Biotechnology,
Inc. (Santa Cruz, CA, USA). Antibodies for human IL-6 and IL-8 and
biotinylated antibodies for human IL-6 and IL-8 were purchased from
BD Biosciences (San Jose, CA, USA). The RNeasy Mini kit and
QuantiTect Reverse Transcription kit were purchased from Qiagen
(Hilden, Germany). IL-6, IL-8, COX-2, and β-actin oligonucleotide
primers were purchased from Bioneer Corp. (Daejeon, Korea).
Cell culture
HaCaT cells were grown in RPMI-1640 medium
containing 5% fetal bovine serum (FBS) and 100 U/ml
penicillin/streptomycin sulfate. The cells were incubated in 5%
humidified CO2 atmosphere at 37°C. To stimulate the
cells, the medium was replaced with fresh RPMI-1640 medium and
exposed to UVB in the presence or absence of IXA for the indicated
time points.
UVB source
UVB irradiation was delivered using a closely spaced
array of five sunlamps (G9T5E lamps; Sankyo Denki Co., Ltd.,
Hiratsuka, Japan). The distance between the sunlamps and the
surface of the cell cultures was fixed at 7.5 cm, and the distance
between the sunlamps and the surface of the cage was fixed at 30
cm. The energy output of the UVB (290–320 nm) lamps was measured
using a UV radiometer (UVX; UVP Inc., Upland, CA, USA).
MTS assay for cell viability
Cell viability was determined by the MTS assay.
HaCaT cells were plated at a density of 3×104 cells/well
in 96-well plates (Thermo Scientific Nunc®, Nunc AS,
Copenhagen, Denmark). Each experiment had a non-treated group as
the control. To determine the non-toxic concentration for the
cells, IXA (2.5, 5, 10, and 20 μM) was added to each well.
The plates were incubated for 24 h at 37°C under 5% CO2.
MTS solution (5 mg/ml) was added to each well, and the cells were
cultured for another 2 h, followed by measuring their optical
density at 490 nm. The cytotoxicity was calculated using the
formula: 1 – (mean absorbance value of treated cells/mean
absorbance value of untreated cells).
Enzyme-linked immunosorbent assay
Cells were seeded at a density of 3×104
cells/well in 48-well tissue culture plates and pre-treated with
two concentrations of IXA (5 and 10 μM) for 24 h prior to
UVB (100 mJ/cm2) stimulation. The culture supernatants
were analyzed by enzyme-linked immunosorbent assay (ELISA) for IL-6
and IL-8 levels. To measure the cytokines, a modified ELISA method
was used. First, a sandwich ELISA for IL-6 and IL-8 was conducted
in duplicate in 96-well immuno plates (Nunc 439454; Thermo
Scientific Nunc®). The supernatant was decanted into a
new microcentrifuge tube, and the cytokines were quantified by
ELISA. ELISA plates were coated overnight at 4°C with anti-human
IL-6 and IL-8 monoclonal antibodies diluted in coating buffer (0.1
M carbonate, pH 9.5), and then washed four times with
phosphate-buffered saline (PBS) containing 0.05% Tween-20. The
non-specific protein binding sites were blocked with assay diluent
(PBS containing 10% FBS at pH 7.0) for at least 1 h. After washing
the plates again, the test sample or recombinant IL-6 and IL-8
standards was added. After incubation for 2 h, a working detector
(biotinylated anti-human IL-6 and IL-8 monoclonal antibodies and
streptavidin-horseradish peroxidase reagent) was added, and the
mixture was incubated for 1 h. Subsequently, the substrate solution
(tetramethylbenzidine) was added to the wells and incubated for 30
min in the dark before the reaction was quenched by adding 1 M
H3PO4. The absorbance was read at 450 nm
using an ELISA reader (Infinite M200; Tecan, Männedorf,
Switzerland). Subsequent steps were conducted at room temperature,
and the standards and samples were assayed in duplicate.
Western blot analysis
Protein expression was assessed by western blot
analysis according to standard procedures. HaCaT cells were
cultured in 60-mm-diameter culture dishes (4×106
cells/well) and pre-treated with two concentrations of IXA (5 and
10 μM). After 30 min, 2 or 24 h, the cells were
UVB-irradiated (100 mJ/cm2) and then incubated at 37°C.
Following the incubation, the cells were washed twice in PBS (pH
7.4) and were resuspended in lysis buffer on ice for 20 min. The
cell debris was removed by centrifugation (10,000 × g, 10 min, and
4°C). The protein concentrations were determined using the Bio-Rad
protein assay reagent (Bio-Rad Laboratories, Hercules, CA, USA)
according to the manufacturer’s instructions. Equal amounts of
protein (20 μg) were subjected to sodium dodecyl
sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and then
transferred onto a polyvinylidene membrane (Millipore, Bedford, MA,
USA). The membrane was blocked using 5% non-fat milk in
Tris-buffered saline with Tween-20 buffer (150 mM NaCl, 20 mM
Tris-HCl, and 0.05% Tween-20, pH 7.4). After blocking, the membrane
was incubated with primary antibodies for 18 h. Antibodies against
ERK, phosphorylated ERK, JNK, phosphorylated JNK, p38,
phosphorylated p38 and β-actin, and peroxidase-conjugated secondary
antibodies were purchased from Santa Cruz Biotechnology, Inc. The
membrane was then washed with Tris-buffered saline containing
Tween-20 and incubated with anti-mouse or anti-rabbit
immunoglobulin G horseradish peroxidase-conjugated secondary
antibodies. The proteins were then supplemented with ECL Prime
Western Blotting Detection Reagents and an ImageQuant LAS 4000 mini
biomolecular imager (both from GE Healthcare, Cleveland, OH, USA)
was used to evaluate the bands.
RNA extraction and reverse
transcription-polymerase chain reaction (RT-PCR)
Total cellular RNA was isolated using an easy-BLUE™
RNA extraction kit (Intron Biotechnology, Inc., Seongnam, Republic
of Korea) according to the manufacturer’s instructions. The total
RNA (2 μg) was then converted to cDNA by treating it with
200 units of reverse transcriptase and 500 ng of oligo (dT) primer
in 50 mM Tris-HCl (pH 8.3), 75 mM KCl, 3 mM MgCl2, 10 mM
dithiothreitol, and 1 mM deoxynucleotide triphosphates at 42°C for
1 h. The reaction was quenched by heating the samples at 70°C for
15 min, followed by enzymatic amplification of the cDNA mixture (3
μl). PCR was conducted in a reaction mixture containing 50
mM KCl, 10 mM Tris-HCl (pH 8.3), 1.5 mM MgCl2, 0.2 mM
deoxynucleotide triphosphates, 2.5 units of Taq DNA polymerase, and
0.1 μM each of IL-6, IL-8, COX-2, and GAPDH primers. PCR of
GAPDH was conducted by subjecting the reaction mixtures to an
initial denaturation of 92°C for 5 min, followed by 30 cycles of
92°C for 1 min, annealing at 50°C for 1 min, and extension at 72°C
for 2 min, with a final extension at 72°C for 5 min. PCR of IL-6
and IL-8 samples was conducted by heating the reaction mixtures to
95°C for 2 min, followed by 35 cycles at 95°C for 45 sec, 60°C for
45 sec, and 72°C for 90 sec, with a final extension at 72°C for 10
min. PCR of COX-2 samples was conducted by heating the reaction
mixtures to 94°C for 5 min, followed by 30 cycles at 94°C for 1
min, 55°C for 30 sec, and 72°C for 1 min, with a final extension at
72°C for 7 min. The PCR products were then electrophoresed on 1%
agarose gel and stained with ethidium bromide. The primer sequences
are listed in Table I.
 | Table ISequences of oligonucleotide primers
designed for PCR. |
Table I
Sequences of oligonucleotide primers
designed for PCR.
| cDNA | Sequences |
| IL-6 | F,
5′-ATGAACTCCTTCTCCACAAGCGC-3′ |
| R,
5′-GAAGAGCCCTCAGGCTGGACTG-3′ |
| IL-8 | F,
5′-ATGACTTCCAAGCTGGCCGTGGCT-3′ |
| R,
5′-TCTCAGCCCTCTTCAAAAACTTCTC-3′ |
| COX-2 | F,
5′-TTCAAATGAGATTGTGGGAAAATTGCT-3′ |
| R,
5′-AGATCATCTCTGCCTGAGTATCTT-3′ |
| GAPDH | F,
5′-CTGGCACCCAGCACAATGAAG-3′ |
| R,
5′-ACCGACTGCTGTCACCTTCA-3′ |
Statistical analysis
Statistical analysis was performed using one-way
analysis of variance (ANOVA) or the Student’s t-test for single
comparisons. Data are presented as the means ± standard error (SE),
and the number of individual experiments conducted is mentioned in
each figure legend. P<0.05 and P<0.005 were considered to
indicate statistically significant differences.
Results
Cell viability of UVB-irradiated HaCaT
cells
The effect of IXA on cell viability following UVB
irradiation was assessed on HaCaT cells. Cell viability was
evaluated using the MTS assay (Fig.
2). When the cultures were incubated after UVB irradiation,
UVB-induced toxicity increased compared to that in non-irradiated
cells. Cell viability decreased depending on the dose of UVB
irradiation and was markedly reduced at 24 h after UVB irradiation
of 150 mJ/cm2. Subsequently, an exposure dose of 100
mJ/cm2 was selected to study cell toxicity in HaCaT
cells treated with IXA 24 h after UVB irradiation (Fig. 3).
Effects of IXA on the production of
pro-inflammatory cytokines
Initially, the cytotoxicity of IXA on HaCaT cells
was examined using the MTS assay. The half maximal inhibitory
concentration (IC50) value of IXA was 50 μM (data
not shown), and IXA did not show any cytotoxic effects up to 10
μM (Fig. 3). To evaluate
the effect of IXA on the production of pro-inflammatory cytokines,
the cells were pre-treated with IXA (5 and 10 μM) prior to
stimulation by UVB (100 mJ/cm2) for 24 h and analyzed by
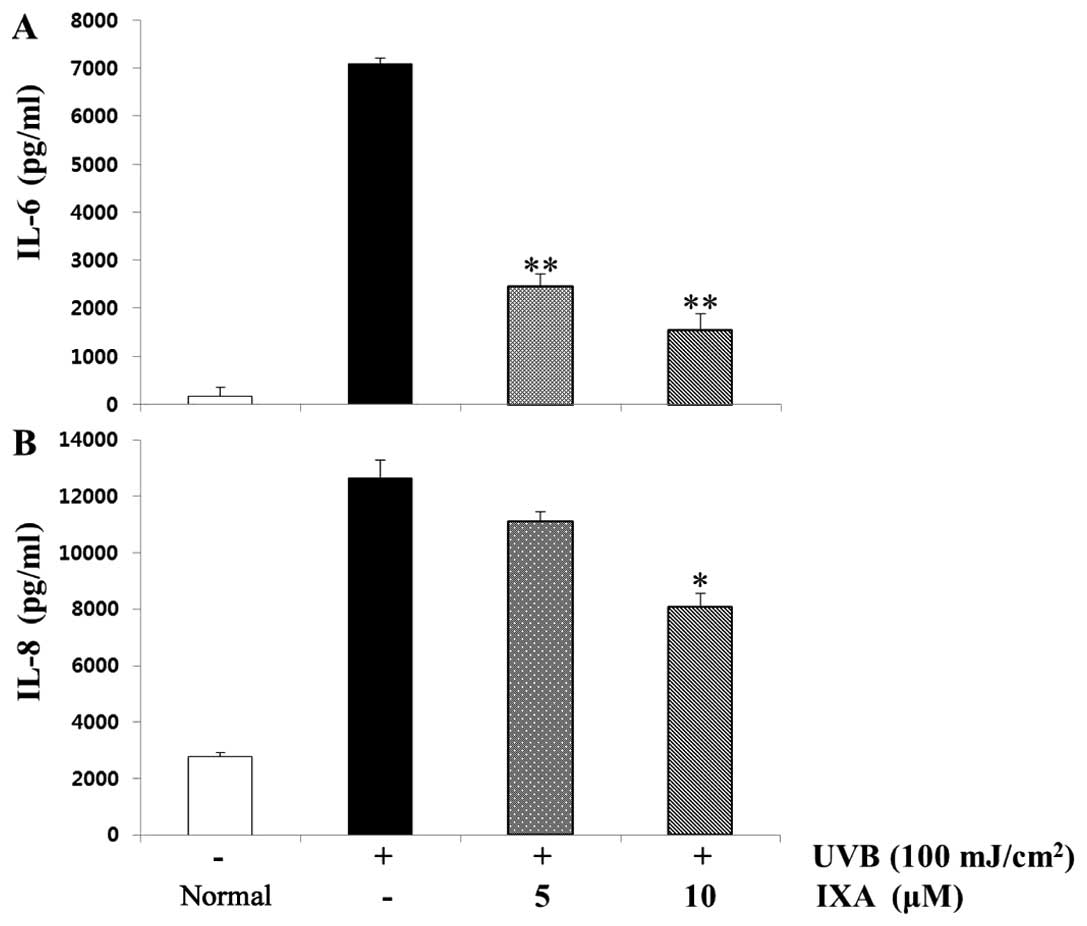
ELISA. As shown in Fig. 4, the
levels of IL-6 and IL-8 were considerably increased in HaCaT cells
after stimulation with UVB (100 mJ/cm2). Pre-treatment
of cells with IXA (5 or 10 μM) inhibited these increments in
a concentration-dependent and statistically significant manner.
Effects of IXA on pro-inflammatory
cytokine gene expression
The pro-inflammatory cytokine gene expression was
then analyzed using RT-PCR. Enhanced IL-6 and IL-8 mRNA expression
induced by UVB (100 mJ/cm2) was inhibited by
pre-treatment of the cells with IXA (Fig. 5). In particular, the pre-treatment
with IXA at a concentration of 10 μM inhibited the
UVB-induced gene expression of IL-6 and IL-8.
Effects of IXA on COX-2 protein and mRNA
expression
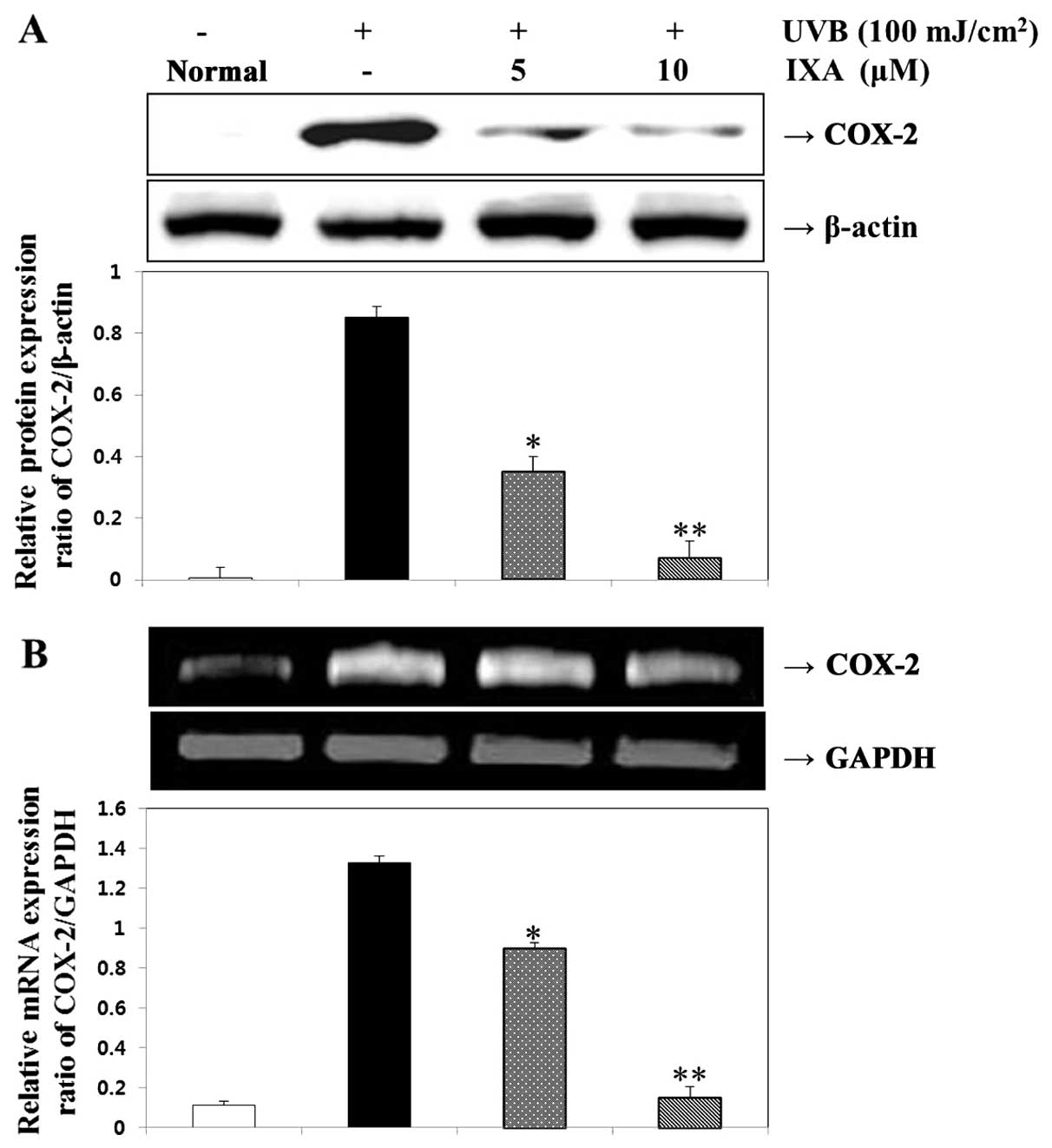
The effects of IXA on COX-2 expression were examined
in UVB-irradiated HaCaT cells. The expression levels of COX-2
protein and mRNA were measured in HaCaT cells exposed to UVB (100
mJ/cm2) for 24 h. IXA effectively suppressed UVB-induced
COX-2 expression. UVB (100 mJ/cm2) also increased COX-2
mRNA expression, which was inhibited in the presence of IXA
(Fig. 6). Thus, IXA suppressed
the expression of genes that were involved in the pathogenesis of
inflammatory responses.
Effects of IXA on the activation of
MAPKs
Inhibition of the ERK, JNK, and p38 MAPK pathways
was recently found to attenuate pro-inflammatory cytokine
secretion. The effect of IXA on UVB-induced MAPK phosphorylation in
HaCaT cells was examined by incubating the cells with IXA 24 h
prior to stimulation with UVB for 30 min, 1 or 2 h. UVB-induced
phosphorylation of ERK, JNK, and p38 MAPK was then determined by
western blot analysis. IXA pre-treatment significantly inhibited
the UVB-induced phosphorylation of ERK, JNK, and p38 MAPK in a
dose-dependent manner without affecting the total protein levels of
these kinases (Fig. 7). These
results indicated that the inhibitory effect of IXA on UVB-induced
MAPK phosphorylation may result in blockage of the cytokine
production and COX-2 expression in HaCaT cells.
Discussion
Naturally occurring chemical substances derived from
plants have been of much interest as therapeutics for several
diseases. The species Ixeris dentata is a perennial herb of
the Asteraceae family that is widely distributed and cultivated in
northeastern Asia. The young sprouts of this species have been used
as a famous bitter appetizing vegetable in Korea and also for the
treatment of indigestion, pneumonia, hepatitis, contusions, and
tumors (18).
Ixeris dentata is known to contain aliphatic
compounds, triterpenoids, and sesquiterpene glycosides (19). Chemical components such as
triterpenes, sesquiterpene glycosides, and flavonoids have been
isolated from the genus Ixeris, encompassing ~20 species
(28). IXA is an active
constituent isolated from the extract of Ixeris dentata.
Water and organic extracts of Ixeris dentata have shown
strong hypolipidemic effects with antioxidant (20), antiallergic (21), monamine oxidase (22), anti-inflammatory (23), antimutagenic, and anticancer
(24) activity. In particular,
our previous studies have shown that EtOAc and tectroside isolated
from Ixeris dentata showed protective effects against
UVB-induced skin inflammation (25,26).
IXA was examined for its inhibitory effects on the
human uterine carcinoma cell line, multidrug-resistant subline of
MES-SA, human colorectal adenocarcinoma cell line, and
multidrug-resistant subline of HCT15 according to the SRB assay
in vitro (27).
However, to the best of our knowledge, no
information is available concerning the effect of IXA on skin
inflammation. In this study, we investigated the protective effect
of IXA against UVB-induced damage in HaCaT cells. Keratinocytes are
the main cells present in the epidermal layer of the skin and are
exposed to a variety of stress stimuli such as UV radiation
(1,2). UVB, in particular, has a wide
spectrum of biological effects on the skin, and acute exposure
causes a variety of adverse skin reactions such as erythema, edema,
sunburn, hyperplasia, inflammation, and immunosuppression (7).
Cytokines such as IL-6 and IL-8 undoubtedly play
pivotal roles in immunologic regulation in the human body and are
involved in the induction of proliferation, differentiation, and
cell death in many cell types such as leukocytes (29). These pro-inflammatory cytokines
are considered to be closely associated with the progression of
photodamage. In particular, IL-6 stimulates keratinocyte
proliferation and is therefore studied in diseases associated with
epidermal hyperplasia and in wound healing (30–34). IL-8, a powerful neutrophil
attractant, is produced by keratinocytes after external stimuli
such as arsenic, contact sensitizers, and irritants.
IL-8-stimulated keratinocyte proliferation has been observed in
auto-immune-mediated diseases such as pemphigus herpetiformis and
bullous pemphigoid (35–38). Therefore, the inhibitory effect of
IL-6 and IL-8 may have favorable anti-inflammatory effects on skin
diseases. To test this hypothesis, we determined the inhibitory
effect of IL-6 and IL-8 in UVB-irradiated HaCaT cells and
demonstrated the suppression of UVB-induced intracellular
production of IL-6 and IL-8. These results provide direct evidence
to prove that IXA acted as an inhibitory agent for IL-6 and
IL-8.
Moreover, COX-2 expression was induced by
inflammatory cues in various tissues such as the epidermis
(11,12). Previous studies have shown that
chronic exposure to UVB radiation increases COX-2 expression
through various cell signaling pathways, resulting in the induction
of skin cancer (13,14). Previous findings have shown that
UVB irradiation significantly increases COX-2 gene expression at
the mRNA and protein levels in the human skin and cultured
keratinocytes (14,39–41), and the resulting synthesis of
prostaglandins with their growth-promoting and anti-apoptotic
activities may be a contributory factor to UV radiation-induced
skin carcinogenesis (42). Our
results show that IXA inhibited UVB-induced COX-2 expression. Thus,
we determined the effect of IXA and regulation of COX-2 in
UVB-exposed keratinocytes.
UV irradiation activates the cell surface growth
factor and cytokine receptors in fibroblasts, stimulating MAPK
signal transduction pathways. ERK, JNK, and p38 are three families
of MAPKs existing in mammalian cells, each of which forms a
signaling module (43). MAPKs are
a large family of protein kinases that phosphorylate and
sequentially activate one another in a series of distinct cascades
in response to markedly diverse sets of stimuli involved in the
regulation of development, growth, differentiation, inflammation,
and cell death (44). The
expression of inflammatory cytokines (COX-2 and IL-6) has been
shown to be affected by various intracellular signaling MAPK
proteins such as ERK, JNK, and p38 (45). UVB exposure induces the rapid
activation of MAPK signals such as p38 leading to COX-2 expression
in HaCaT cells (15). Our results
indicate that IXA inhibited UVB-induced MAPKs phosphorylation.
Additionally, the anti-inflammatory effect of IXA inhibition of
MAPK phosphorylation in keratinocytes exposed to UVB irradiation
was confirmed.
We examined the effect of IXA on UVB-induced
pro-inflammatory cytokine production in HaCaT cells by evaluating
UVB-stimulated cells in the presence or absence of IXA.
Pro-inflammatory cytokine production was measured by ELISA and
RT-PCR, and the activation of MAPKs was determined by western blot
analysis. The inhibitory effects of IXA on the production of
inflammatory mediators were accompanied by a
concentration-dependent decrease in the protein and mRNA expression
levels of IL-6, IL-8, and COX-2. Therefore, IXA most probably acted
as an anti-inflammatory agent by mainly inhibiting COX-2 expression
in vivo. Furthermore, these effects were mediated by the
inhibition of COX-2 expression and ERK, JNK, and p38 MAPK
phosphorylation.
These results indicate that the inhibitory effect of
IXA on UVB-induced MAPK phosphorylation may result in blockage of
the cytokine production and COX-2 expression in HaCaT cells.
Therefore, this shows a potent anti-inflammatory effect of IXA
accomplished by blocking inflammatory mediators. Our data indicate
that IXA is a new source of potential drugs that may be used for
the treatment of inflammatory diseases.
In conclusion, we evaluated the effect of IXA on
skin inflammation in vitro and found that IXA has potential
to attenuate UVB-induced skin inflammation by suppressing MAPK
activation. Our findings provide new insight into the application
of IXA and its nutraceutical value. Further studies on IXA are
required to confirm its medicinal use.
Acknowledgments
This study was supported by the Basic Science
Research Program through the National Research Foundation (NRF) of
Korea funded by the Ministry of Education (2013060380), by the
Korea government (MSIP) (2008-0062484), and by the Ministry of
Knowledge Economy (MKE), Korea Institute for Advancement of
Technology (KIAT) through the Inter-ER Cooperation Projects
(R0002019).
References
|
1
|
Muthusamy V and Piva TJ: A comparative
study of UV-induced cell signalling pathways in human
keratinocyte-derived cell lines. Arch Dermatol Res. 305:817–833.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Muthusamy V and Piva TJ: The UV response
of the skin: a review of the MAPK, NFkappaB, and TNFalpha signal
transduction pathways. Arch Dermatol Res. 302:5–17. 2010.
View Article : Google Scholar
|
|
3
|
Duthie MS, Kimber I and Norval M: The
effects of ultraviolet radiation on the human immune system. Br J
Dermatol. 140:995–1009. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Svobodova A, Walterova D and Vostalova J:
Ultraviolet light induced alteration to the skin. Biomed Pap Med
Fac Univ Palacky Olomouc Czeck Repub. 150:25–38. 2006. View Article : Google Scholar
|
|
5
|
de Gruijl FR and Van der Leun JC:
Environment and health: 3. Ozone depletion and ultraviolet
radiation. CMAJ. 163:851–855. 2000.PubMed/NCBI
|
|
6
|
Holick MF: Vitamin D: a millenium
perspective. J Cell Biochem. 88:296–307. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Afaq F, Adhami VM and Mukhtar H:
Photochemoprevention of ultraviolet B signaling and
photocarcinogenesis. Mutat Res. 571:153–173. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Freedberg IM, Tomic-Canic M, Komine M and
Blumenberg M: Keratins and the keratinocyte activation cycle. J
Invest Dermatol. 116:633–640. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sauder DN: The role of epidermal cytokines
in inflammatory skin diseases. J Invest Dermatol. 95(Suppl 5):
S27–S28. 1990. View Article : Google Scholar
|
|
10
|
Ziegler A, Jonason AS, Leffell DJ, Simon
JA, Sharma HW, Kimmelman J, Remington L, Jacks T and Brash DE:
Sunburn and p53 in the onset of skin cancer. Nature. 372:773–776.
1994. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Masferrer JL, Leahy KM, Koki AT, Zweifel
BS, Settle SL, Woerner BM, Edwards DA, Flickinger AG, Moore RJ and
Seibert K: Antiangiogenic and antitumor activities of
cyclooxygenase-2 inhibitors. Cancer Res. 60:1306–1311.
2000.PubMed/NCBI
|
|
12
|
Higashi Y, Kanekura T and Kanzaki T:
Enhanced expression of cyclooxygenase (COX)-2 in human skin
epidermal cancer cells: evidence for growth suppression by
inhibiting COX-2 expression. Int J Cancer. 86:667–671. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rundhaug JE and Fischer SM:
Cyclo-oxygenase-2 plays a critical role in UV-induced skin
carcinogenesis. Photochem Photobiol. 84:322–329. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Buckman SY, Gresham A, Hale P, Hruza G,
Anast J, Masferrer J and Pentland AP: COX-2 expression is induced
by UVB exposure in human skin: implications for the development of
skin cancer. Carcinogenesis. 19:723–729. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen W, Tang Q, Gonzales MS and Bowden GT:
Role of p38 MAP kinases and ERK in mediating ultraviolet-B induced
cyclooxygenase-2 gene expression in human keratinocytes. Oncogene.
20:3921–3926. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin SK, Kok SH, Yeh FT, Kuo MY, Lin CC,
Wang CC, Goldring SR and Hong CY: MEK/ERK and signal transducer and
activator of transcription signaling pathways modulate oncostatin
M-stimulated CCL2 expression in human osteoblasts through a common
transcription factor. Arthritis Rheum. 50:785–793. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mahns A, Wolber R, Stäb F, Klotz LO and
Sies H: Contribution of UVB and UVA to UV-dependent stimulation of
cyclooxygenase-2 expression in artificial epidermis. Photochem
Photobiol Sci. 3:257–262. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yi JM, Hong SH, Lee HJ, Won JH, Kim JM,
Jeong DM, Baek SH, Lim JP and Kim HM: Ixeris dentata green sap
inhibits both compound 48/80-induced aanaphylaxis-like response and
IgE-mediated anaphylactic response in murine model. Biol Pharm
Bull. 25:5–9. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Seto M, Miyase T and Fukushima S:
Sesquiterpene lactones from Ixeris dentata Nakai. Chem Pharm Bull.
34:4170–4176. 1986. View Article : Google Scholar
|
|
20
|
Lee E: Effects of Ixeris dentata ext. on
lowering lipid and anti-oxidation. Korean J Plant Res. 24:55–60.
2011.In Korean. View Article : Google Scholar
|
|
21
|
Park EK, Sung JH, Trinh HT, Bae EA, Yun
HK, Hong SS and Kim DH: Lactic acid bacterial fermentation
increases the anti-allergic effects of Ixeris dentata. J Microbiol
Biotechnol. 18:308–313. 2008.PubMed/NCBI
|
|
22
|
Chung HS: Inhibition of monamine oxidase
by a flavone and its glycoside from Ixeris dentata Nakai. Nutraceut
Food. 8:141–144. 2003. View Article : Google Scholar
|
|
23
|
Chung HS, Jeong HJ, Han MJ, Park ST, Seong
KK, Baek SH, Jeong DM, Kim MJ and Kim HM: Nitric oxide and tumor
necrosis factor-alpha production by Ixeris dentata in mouse
peritoneal macrophages. J Ethnopharmacol. 82:217–222. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim MJ, Kim JS, Jeong DM, Ham SS and Yu
CY: Effect of antioxidant, antimutagenicity and anticancer of root
extract from Ixeris dentataNakai. Korean J Med Crop Sci.
10:222–229. 2002.In Korean.
|
|
25
|
Kim SB, Kang OH, Keum JH, Mun SH, An HJ,
Jung HJ, Hong SH, Jeong DM, Kweon KT and Kwon DY: Anti-inflammatory
effect of Ixeris dentata on ultraviolet B-induced HaCaT
keratinocytes. Nat Prod Sci. 18:60–66. 2012.
|
|
26
|
Kim SB, Kang OH, Joung DK, Mun SH, Seo YS,
Cha MR, Ryu SY, Shin DW and Kwon DY: Anti-inflammatory effects of
tectroside on UVB-induced HaCaT cells. Int J Mol Med. 31:1471–1476.
2013.PubMed/NCBI
|
|
27
|
Cha MR, Choi YH, Choi CW, Yoo DS, Kim YS,
Choi SU, Kim YH and Ryu SY: New guaiane sesquiterpene lactones from
Ixeris dentata. Planta Med. 77:380–382. 2011. View Article : Google Scholar
|
|
28
|
Zidorn C: Sesquiterpene lactones and their
precursors as chemosystematic markers in the tribe Cichorieae of
the Asteraceae. Phytochemistry. 69:2270–2296. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yarilin AA and Belyakov IM: Cytokines in
the thymus: production and biological effects. Curr Med Chem.
11:447–464. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Grossman RM, Krueger J, Yourish D,
Granelli-Piperno A, Murphy DP, May LT, Kupper TS, Sehgal PB and
Gottlieb AB: Interleukin 6 is expressed in high levels in psoriatic
skin and stimulates proliferation of cultured human keratinocytes.
Proc Natl Acad Sci USA. 86:6367–6371. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Turksen K, Kupper TS, Degenstein L,
Williams I and Fuchs E: Interleukin 6: insights to its function in
skin by overexpression in transgenic mice. Proc Natl Acad Sci USA.
89:5068–5072. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sawamura D, Meng X, Ina S, Sato M, Tamai
K, Hanada K and Hashimoto I: Induction of keratinocyte
proliferation and lymphocytic infiltration by in vivo introduction
of the IL-6 gene into keratinocytes and possibility of keratinocyte
gene therapy for inflammatory skin diseases using IL-6 mutant
genes. J Immunol. 161:5633–5639. 1998.PubMed/NCBI
|
|
33
|
Sato M, Sawamura D, Ina S, Yaguchi T,
Hanada K and Hashimoto I: In vivo introduction of the interleukin 6
gene into human keratinocytes: induction of epidermal proliferation
by the fully spliced form of interleukin 6, but not by the
alternatively spliced form. Arch Dermatol Res. 291:400–404. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sugawara T, Gallucci RM, Simeonova PP and
Luster MI: Regulation and role of interleukin 6 in wounded human
epithilial keratinocytes. Cytokine. 15:328–336. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mohamadzadeh M, Müller M, Hultsch T, Enk
A, Saloga J and Knop J: Enhanced expression of IL-8 in normal human
kerati-nocytes and human keratinocyte cell line HaCaT in vitro
after stimulation with contact sensitizers, tolerogens and
irritants. Exp Dermatol. 3:298–303. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yen HT, Chiang LC, Wen KH, Chang SF, Tsai
CC, Yu CL and Yu HS: Arsenic induces interleukin-8 expression in
cultured keratinocytes. Arch Dermatol Res. 288:716–717. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
O’Toole AE, Mak LL, Guitart J, Woodley DT,
Hashimoto T, Amagai M and Chan LS: Induction of keratinocyte IL-8
expression and secretion by IgG autoantibodies as a novel mechanism
of epidermal neutrophil recruitment in a pemphigus variant. Clin
Exp Immunol. 119:217–224. 2000. View Article : Google Scholar
|
|
38
|
Schmidt E, Reimer S, Kruse N, Jainta S,
Bröcker EB, Marinkovich MP, Guidice GJ and Zillikens D:
Autoantibodies to BP180 associated with bullous pemphigoid release
interleukin-6 and interleukin-8 from cultured human keratinocytes.
J Invest Dermatol. 115:842–848. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pentland AP, Schoggins JW, Scott GA, Khan
KN and Han R: Reduction of UV-induced skin tumors in hairless mice
by selective COX-2 inhibition. Carcinogenesis. 20:1939–1944. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Athar M, An KP, Morel KD, Kim AL,
Aszterbaum M, Longley J, Epstein EH Jr and Bickers DR: Ultraviolet
B (UVB)-induced COX-2 expression in murine skin: an
immunohistochemical study. Biochem Biophys Res Commun.
280:1042–1047. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
An KP, Athar M, Tang X, Katiyar SK, Russo
J, Beech J, Aszterbaum M, Kopelovich L, Epstein EH Jr, Mukhtar H
and Bickers DR: Cyclooxygenase-2 expression in murine and human
nonmelanoma skin cancers: Implications for therapeutic approaches.
Photochem Photobiol. 76:73–80. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Grewe M, Trefzer U, Ballhorn A, Gyufko K,
Henninger H and Krutmann J: Analysis of the mechanism of
ultraviolet (UV) B radiation-induced prostaglandin E2 synthesis by
human epidermoid carcinoma cells. J Invest Dermatol. 101:528–531.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Robinson MJ and Cobb MH: Mitogen-activated
protein kinase pathways. Curr Opin Cell Biol. 9:180–186. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lewis TS, Shapiro PS and Ahn NG: Signal
transduction through MAP kinase cascades. Adv Cancer Res.
74:49–139. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ashida M, Bito T, Budiyanto A, Ichihashi M
and Ueda M: Involvement of EGF receptor activation in the induction
of cyclooxygenase-2 in HaCaT keratinocytes after UVB. Exp Dermatol.
12:445–452. 2003. View Article : Google Scholar : PubMed/NCBI
|