Introduction
Obesity involves the adjustment of metabolism for
energy storage and the accumulation of fat (1). The accumulation of excess fat is
accompanied by inflammatory reactions that can induce chronic
diseases, including diabetes, hypertension and cardiovascular
disease (2,3). Accordingly, there has been active
research on stimulating metabolism or preventing the inflammatory
response and excessive lipid accumulation in cells through lipid
degradation.
Obesity has a variety of causes, including endocrine
disorders and genetic factors. Recently, with the increase in the
consumption of high-fat diets and the availability of ‘fast food’,
increases in the obesity rate and, consequently, in the numbers of
adult patients with various chronic diseases have been observed.
With obesity, major concerns include the increased serum
triglyceride and low-density lipoprotein (LDL)-cholesterol levels
which then lead to increased cardiovascular diseases.
Hyperlipidemia can lead to a number of
complications, including respiratory dysfunction, infertility and
even certain thypes of cancer due to the accumulation of neutral
lipids in peripheral tissues and the abdomen that can induce
insulin resistance (4). Thus,
avoiding this cascade of metabolic events has become a major
concern worldwide, and functional foods with anti-obesity effects
have been developed (5).
Tuna is a nutritionally superior high-protein food
with various positive effects, including lowering the blood
cholesterol levels and preventing arteriosclerosis, and is known to
possess anticancer properties (6,7).
Boiled tuna extract contains various ingredients, including useful
proteins derived from collagen and free amino acids (8). In this study, we confirmed the
anti-obesity effects and the inhibitory effects on adipogenesis of
a tuna-derived peptide in 3T3-L1 cells.
Generally, adipocytes play important roles in energy
homeostasis and lipid metabolism. The differentiation of adipocytes
from preadipocytes is indicative of clear morphological and
biochemical changes (9). In
vitro, 3T3-L1 cells, preadipocytes or matrix-derived precursor
cells from adipose tissue can be used to demonstrate the
differentiation process of adipocytes. The differentiation of
adipocytes can be induced by the glucocorticoid, dexamethasone, and
the phosphodiesterase inhibitor, methylisobutylxanthine. During
this process, changes in cell shape occur, together with cell
division and an adipocyte phenotype can be observed within 5–7 days
(10). Adipocyte differentiation
involves changes in the expression of various proteins. In
particular, as previously demonstrated, the expression levels of
proteins involved in the transport of lipids and hormonal responses
related to lipid metabolism are affected (11). In addition, lipids have been shown
to induce the accumulation of triglycerides (TG) and the
upregulation of the transcription factors, CCAAT/enhancer-binding
protein (C/EBP)-α and peroxisome proliferator-activated receptor-γ
(PPAR-γ) (12). IBMX and
dexamethasone induce the upregulation of C/EBP-β and C/EBP-δ during
the early stages of adipocyte differentiation (13). Subsequently, during the later
stages, C/EBP-α and PPAR-γ induce the activation of
adipocyte-specific mRNAs (14).
In this study, we used various assays to assess the anti-obesity
effects of a tuna peptide derived from desalinated boiled tuna
extract on 3T3-L1 cells.
Materials and methods
Preparation of desalinated boiled tuna
extract
The desalinated boiled tuna extract used in this
study was prepared in Korea in 2014. First, boiled tuna extract was
centrifuged to remove any suspended solids that may interfere with
the desalting step. This process involves a change from 55 Brix,
13% salinity to 45 Brix, 12% salinity. We performed membrane
filtration (membrane 2319/size 200 kDa) on the desalinated boiled
tuna extract. We finally obtained a tuna extract of 30 Brix, 1%
salinity, which was subjected to heat exchanger-type momentary
sterilization (conditions: 110°C, 10 sec). The tuna extract sample
was then divided into 1.5-ml tubes and stored at −70°C until
use.
Preparation of soluble/insoluble boiled
tuna proteins and synthesis of tuna peptides
First, we reacted the desalinated boiled tuna
extract with Tween-20 and -60 extraction buffer by overnight
incubation at room temperature. The boiled tuna extract in
extraction buffer was centrifuged (5,000 rpm, 10 min, 4°C) and the
upper phase was collected and mixed with cold methanol and
chloroform to separate the proteins. The mixture was centrifuged
again (12,000 rpm, 5 min, 4°C), and the aqueous layer was removed
and cold methanol was added, followed by further centrifugation
(12,000 rpm, 10 min, 4°C). Finally, we removed the supernatant and
dried the pellet.
We assessed the molecular weight of the tuna
proteins by Coomassie blue staining. After subjecting the tuna
extract to sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE), the protein bands were observed on the
gel using staining solution (7% acetic acid, 40% methanol, 0.1%
Coomassie blue) and destaining solution (7% acetic acid, 20%
methanol). Tuna extract proteins of ~10 kDa were selected for
analysis by quadrupole time-of-flight mass spectrometry (Q-TOF
MS/MS). Each protein sample was subjected to in-gel trypsin
digestion according to the method described in the study by
Shevchenko et al (15).
The digested peptide mixture was desalted and concentrated, then
prepared using a C18 nano column and POROS R2 reverse-phase
material (20–30-μm bead size; PerSeptive Biosystems,
Framingham, MA, USA). MS/MS analysis was performed using a nano-ESI
and MicrOTOF-Q mass spectrometer (Bruker Daltonics, Bremen,
Germany). Following their identification, 3 peptides were
synthesized by Peptron (Daejeon, Korea): tuna-derived peptide,
D-I-V-D-K-I-E-I (TP-D), tuna-derived peptide, I-D-T-I-I-E-T-I-M-E
(TP-I) and tuna-derived peptide, N-I-N-E-D-P-Y-E-N-W-I-V (TP-N).
Purification of the tuna-derived peptides was performed using a C18
column (Shiseido CAPCELL PAK; Shiseido, Tokyo, Japan) and a
Shimadzu Prominence high-performance liquid chromatography (HPLC)
apparatus in 0.1% trifluoroacetic acid (TFA)/water and a gradient
of 0–90% acetonitrile in 0.1% TFA, with a flow rate of 1 ml/min and
UV detection at 220 nm. The TP-D, TP-I and TP-N molecular weights
were determined as 944, 1,177 and 1,505 kDa, respectively, by mass
analysis (HP 1100 series LC/MSD).
Cell culture and adipocyte
differentiation
The 3T3-L1 mouse fibroblasts (American Type Culture
Collection, Manassas, VA, USA) were maintained at 37°C in a 5%
CO2, humidified atmosphere. The cells were cultured in
Dulbecco’s modified Eagle’s medium (DMEM) with 10% bovine calf
serum (BCS; HyClone, Logan, UT, USA) and 100 U/ml penicillin/100
mg/ml streptomycin. The 3T3-L1 cells were cultured to ~60–80%
confluence in a 6-well plate, and upon confluence, were allowed to
grow for 2–4 days in DMEM medium containing 10% fetal bovine serum
(FBS; HyClone). Cell differentiation was initiated by treatment
with MDI (0.5 mM IBMX, 0.25 μM dexamethasone and 10 mg/l
insulin) for 48 h. The medium was then replaced with DMEM
supplemented with 10 mg/l insulin and changed once every 2
days.
Glucose uptake assay
The 3T3-L1 preadipocytes were incubated with DMEM
containing 10% BCS. Cell differentiation was induced by treatment
with MDI in fresh DMEM containing 10% FBS. Following
differentiation, the medium was changed to serum-free medium (SFM),
and the cells were treated with TP-D, TP-I or TP-N at 500 ng/ml for
48 h before the glucose uptake assay. After collecting the cell
culture medium, we confirmed glucose uptake using a kit according
to the manufacturer’s instructions (Asan Pharmaceutical Co., Ltd.,
Gyeonggi, Korea). Enzyme solution was added to the culture medium
and maintained at 37°C for 5 min in a 5% CO2, humidified
atmosphere. The absorbance at 500 nm was measured within 40 min.
The absorbance of the solution in each well was measured at 490 nm
using a microplate reader (Benchmark microplate reader; Bio-Rad
Laboratories, Hercules, CA, USA).
TG component assay
The 3T3-L1 preadipocytes were incubated with DMEM
containing 10% BCS. Cell differentiation was induced by MDI
treatment in fresh DMEM containing 10% FBS. After differentiation,
the medium was changed to SFM supplemented with 500 ng/ml TP-D,
TP-I and TP-N for 48 h prior to the TG assay. We performed the TG
assay according to the kit protocol (Cleantech TG-S kit; Asan Pharm
Co., Ltd., Seoul, Korea). The enzyme solution was added to the cell
lysate and maintained at 37°C for 10 min in a 5% CO2,
humidified atmosphere. The absorbance at 550 nm was measured within
60 min. The absorbance of the solution in each well was measured at
490 nm using a microplate reader (Benchmark microplate reader;
Bio-Rad Laboratories).
Oil Red O staining
The 3T3-L1 cells were washed carefully with
phosphate-buffered saline (PBS) and then fixed with 10% formalin
for 5 min. The formalin was then refreshed followed by incubation
for 1 h. After removal of the formalin, 60% isopropanol was added
to each well and dried. Subsequently, 60% Oil Red O staining
solution was added to each well for 1 h. In the final step, the
wells were washed 3 times with PBS, and the cell morphology and
staining of the lipid droplets were observed using an inverted
microscope (ECLIPSE TS100-F; Nikon, Tokyo, Japan).
Reverse transcription-polymerase chain
reaction (RT-PCR) for the expression of mRNAs
The 3T3-L1 preadipocytes were seeded into 6-well
plates at 2×104 cells/well in 2 ml medium. Cell
differentiation was induced by treatment with MDI in fresh DMEM
containing 10% FBS. Following differentiation, the medium was
changed to SFM supplemented with 500 or 1,000 ng/ml TP-D for 48 h.
The cells were then treated with TRIzol reagent (Invitrogen,
Carlsbad, CA, USA), and the extracted RNA was used as a template
for cDNA synthesis using an oligo(dT) primer (Intron Co., Gyeonggi,
Korea). The synthesized cDNA was mixed with 2X TOPsimple
DyeMIX-nTaq (Enzynomics Inc., Daejeon, Korea) and primers (Table I) in 0.1% diethylpyrocarbonate
(DEPC)-treated water for PCR. Using a 1% agarose gel, the PCR
products were separated and stained with Red safe nucleic acid
staining solution (Intron Co.).
 | Table IOligonucleotide sequences of the
primer pairs used for RT-PCR. |
Table I
Oligonucleotide sequences of the
primer pairs used for RT-PCR.
| Name | Sequences of
primers |
|---|
| C/EBP-α |
| Sense |
5′-CTG-CCC-CTC-AGT-CCC-TGT-C-3′ |
| Antisense |
5′-GTT-CCT-TCA-GCA-ACA-GCG-G-3′ |
| PPAR-γ |
| Sense |
5′-CCT-GTT-GAC-CCA-GAG-CAT-GG-3′ |
| Antisense |
5′-CGA-GTG-GTC-TTC-CAT-CAC-GC-3′ |
| GAPDH |
| Sense |
5′-AAC-TTT-GGC-ATT-GTG-AAG-G3′ |
| Antisense |
5′-ACA-CAT-TGG-GGG-TAG-GAA-CA-3′ |
Western blot analysis
The 3T3-L1 preadipocytes were seeded into 6-well
plates at 2×104 cells/well in 2 ml medium. Cell
differentiation was induced by treatment with MDI in fresh DMEM
containing 10% FBS. Following differentiation, the medium was
changed to SFM supplemented with 500 or 1,000 ng/ml TP-D for 48 h.
The collected cells were washed with PBS, and extraction lysis
buffer (20 mM Tris-base, pH 8, 150 mM NaCl, 100 μM sodium
vanadate, 100 μM ammonium molybdate, 10% glycerol, 0.1%
Nonidet P-40, 0.1% SDS, 1 mM glycerophosphate, 1 μg/ml
aprotinin, 1 μg/ml leupeptin, 1 μg/ml pepstatin A and
1 mM PMSF) was added. The proteins were separated on 7–15% SDS-PAGE
gels and transferred onto polyvinylidene fluoride membranes
(Millipore, Billerica, MA, USA). The membranes were blocked at room
temperature with 1% bovine serum albumin in TBS-T (10 mM Tris-HCl,
pH 7.5, 150 mM NaCl and 0.1% Tween-20) and then incubated with
primary antibodies: anti-C/EBP-α (1:1,000; sc-9314), anti-PPAR-γ
(1:1,000; sc-1984) and anti-GAPDH (1:1,000; sc-25778) (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA). The secondary antibody
was a peroxidase-conjugated goat (sc-2420), mouse, or rabbit
antibody (sc-358920;f 1:10,000; GE Healthcare Bio-Sciences,
Piscataway, NJ, USA). The signal was developed using SuperSignal
West Pico Stable Peroxide Solution and the SuperSignal West Pico
Luminol/Enhancer solution (Thermo Fisher Scientific, Rockford, IL,
USA) prior to exposure to X-ray film (Kodak, Rochester, NY,
USA).
Statistical analysis
The results are expressed as the means ± SD. SPSS
software (version 10.0; SPSS, Inc., Chicago, IL, USA) was used.
Comparisons were made using ANOVA and Duncan’s multiple range test.
The level of significance was set at P<0.05.
Results
Soluble/insoluble boiled tuna protein and
synthetic tuna peptides
In this study, we performed a desalting process
before ultimately extracting the tuna peptides. We confirmed the
molecular weight of the tuna proteins by Coomassie blue staining.
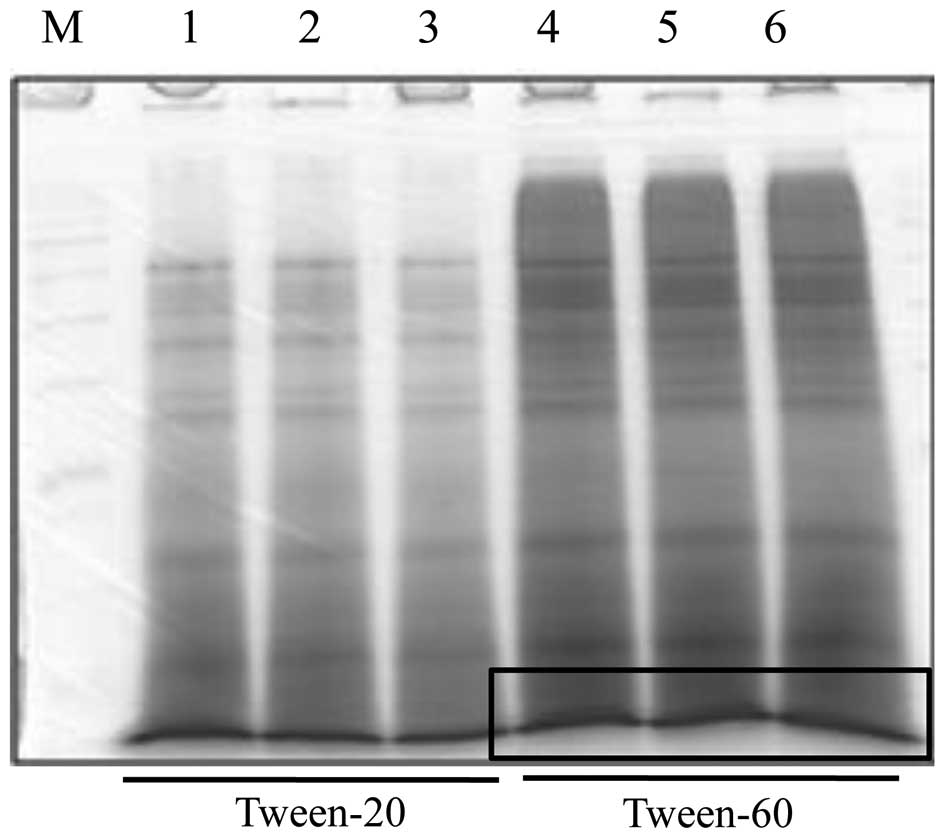
We observed a separated protein of boiled tuna extract at ~10 kDa
and performed protein extraction using Tween-60 extraction buffer,
as it yielded larger quantities of protein than did Tween-20 buffer
(Fig. 1). We then characterized
the ~10-kDa protein band by Q-TOF MS/MS. As shown in Fig. 2, we determined 3 peptide peaks and
sequenced these tuna-derived peptides. These samples were termed
tuna-derived peptide, D-I-V-D-K-I-E-I (TP-D), tuna peptide,
I-D-T-I-I-E-T-I-M-E (TP-I) and tuna peptide, N-I-N-E-D-P-YE-N-W-I-V
(TP-N); their molecular weights were determined to be 944, 1,177
and 1,505 kDa, respectively (Table
II).
 | Table IIIdentification analysis of the 10-kDa
peptide among the boiled tuna proteins. |
Table II
Identification analysis of the 10-kDa
peptide among the boiled tuna proteins.
| Identification | Peptide |
|---|
| Glycine cleavage
system H protein OS = Clostridium | TP-D:
DI(L)VDKI(L)EI(L) |
| Carboxidivorans
P7 | TP-I:
I(L)DTI(L)I(L)ETI(L)ME |
| TP-N:
NI(L)NEDPYENWI(L)V |
Effects of TP-D, TP-I and TP-N on glucose
uptake in 3T3-L1 adipocytes
Glucose consumption is a prerequisite for 3T3-L1
cell differentiation. In this study, we compared glucose
consumption, measured by a glucose uptake assay, between the
differentiated cell group (MDI treatment) and the groups of cells
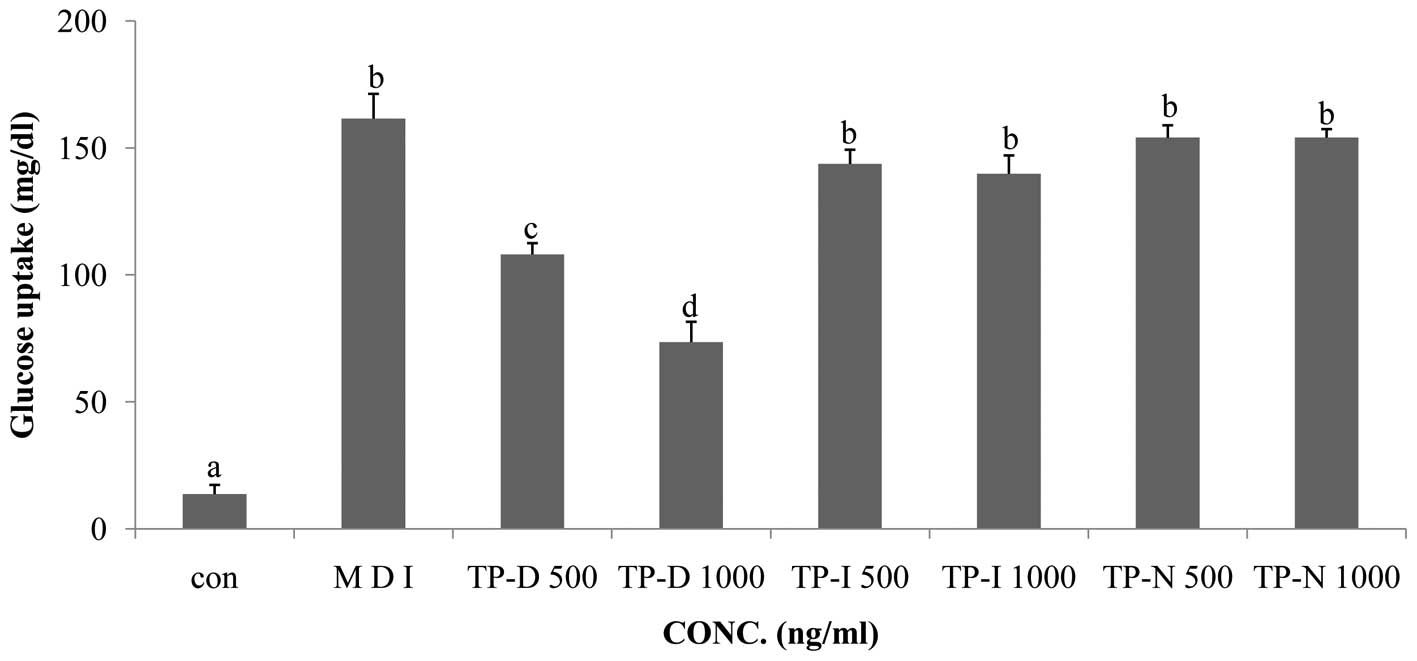
treated with TP-D, TP-I or TP-N. All 3 tuna peptides were applied
at concentrations of 500 and 1,000 ng/ml for 48 h. The TP-D-treated
cells showed decreased glucose uptake compared with the MDI-treated
group. TP-D significantly attenuated glucose uptake following
treatment for 48 h at both 500 and 1,000 ng/ml with a maximal
effect observed at the dose of 1,000 ng/ml. However, TP-I and TP-N
had no significant effect following treatment for 48 h at either of
the concentrations used (Fig.
3).
Effects of TP-D, TP-I and TP-N on TG
levels in 3T3-L1 adipocytes
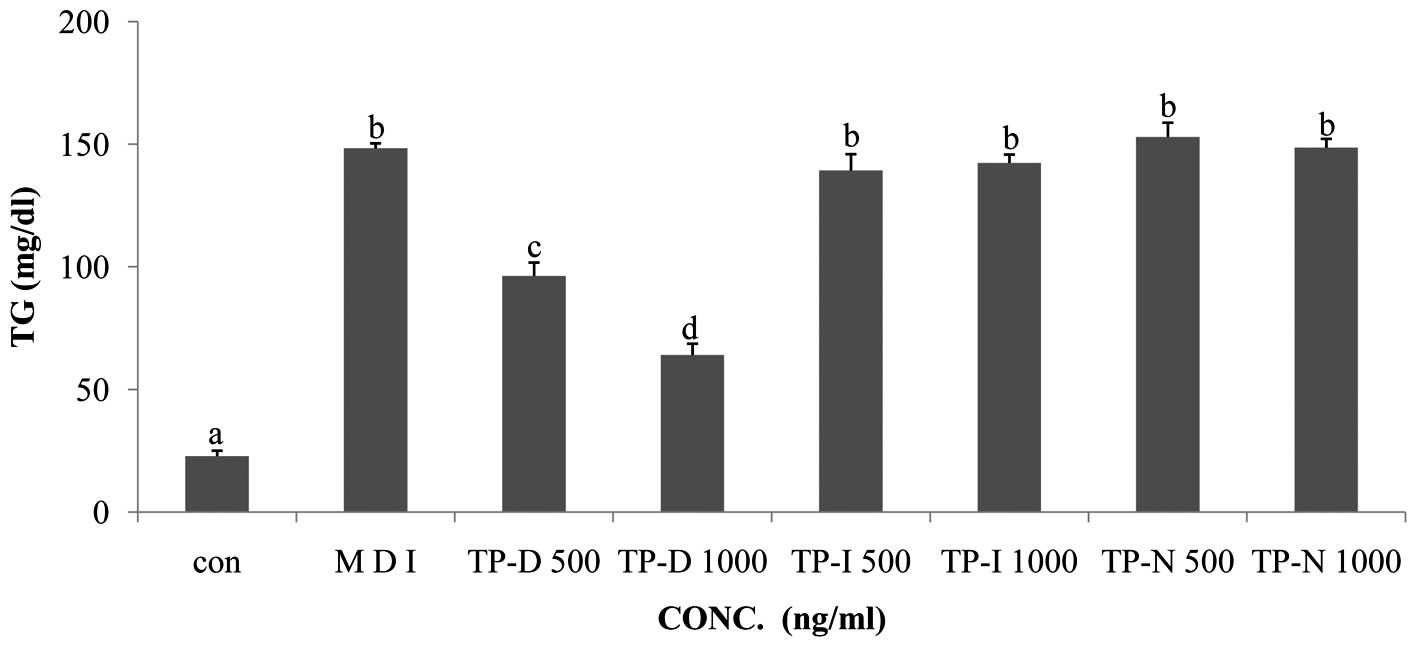
In order to evaluate the effects of TP-D, TP-N and
TP-I on TG levels in differentiating 3T3-L1 cells, the cells were
treated with the tuna-derived peptides at the dose of 500 and 1,000
ng/ml. Glucose consumption induced the active differentiation of
the 3T3-L1 cells and the accumulation of TG. In the glucose uptake
assay, we observed decreased glucose levels following treatment
with TP-D. Thus, we performed a TG assay under the same TP-D, TP-N
and TP-I treatment conditions. The TG levels in the 3T3-L1 cells
treated with TP-D (at 500 or 1,000 ng/ml), were significantly
deceased in a dose-dependent manner (Fig. 4). However, the TG levels in the
cells treated with TP-N and TP-I were not altered. Thus, all
further experiments were performed using treatment with TP-D at
concentrations of 500 and 1,000 ng/ml for 48 h.
Effect of TP-D on lipid accumulation in
3T3-L1 adipocytes
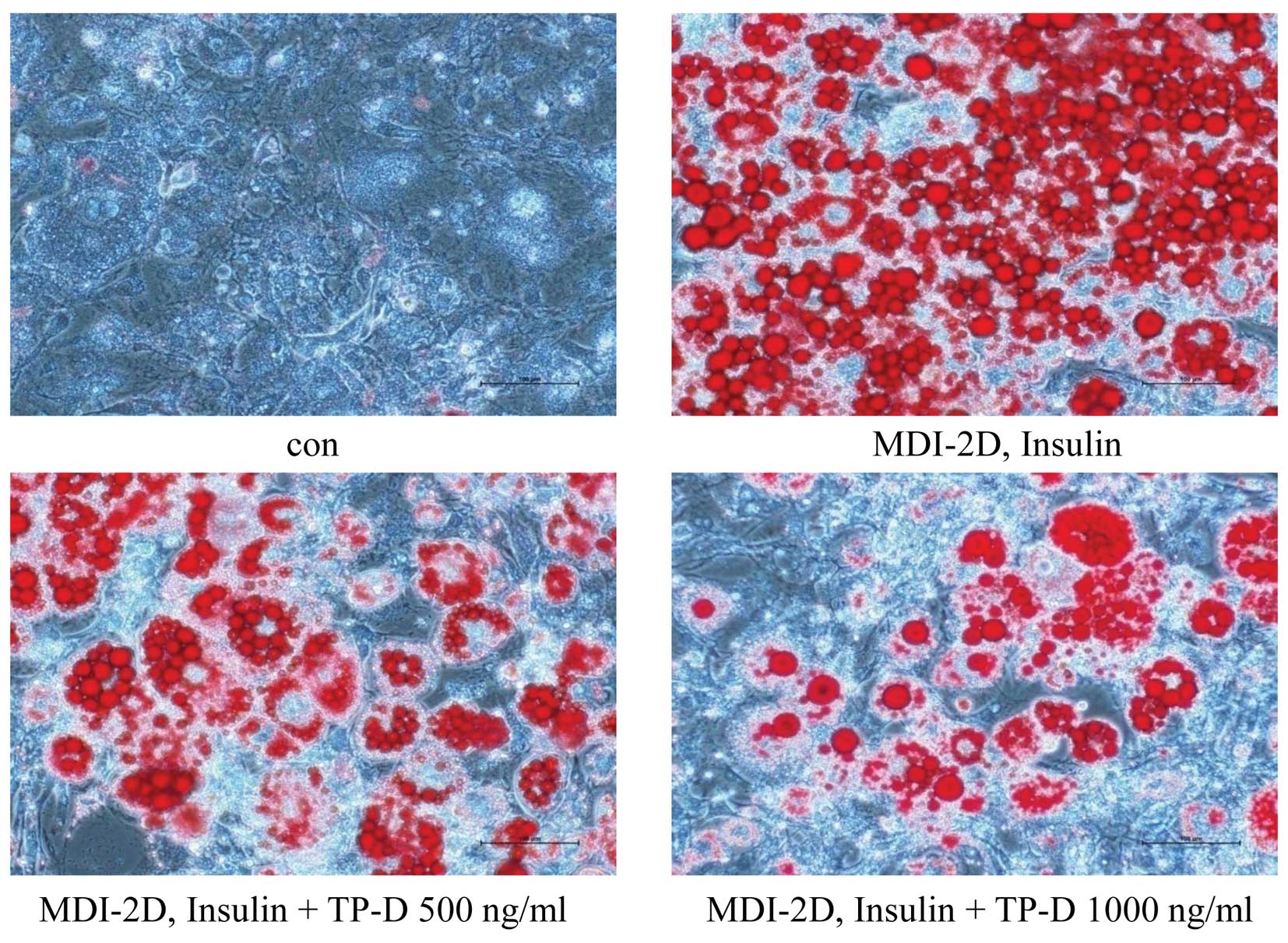
In the previous assays, we observed a decrease in
the glucose and TG levels by TP-D in a dose-dependent manner. Thus,
we then wished to confirm the reduced lipid accumulation by Oil Red
O staining, which targets lipid droplets and allows the
visualization of lipids (16). To
determine the effects of TP-D on lipid accumulation in
differentiating 3T3-L1 cells, the cells were treated with 500 and
1,000 ng/ml of TP-D. The cells were stained with Oil Red O solution
and examined under a microscope. The number of stained lipid
droplets detected decreased following treatment with TP-D in a
dose-dependent manner compared with the MDI-treated group (Fig. 5).
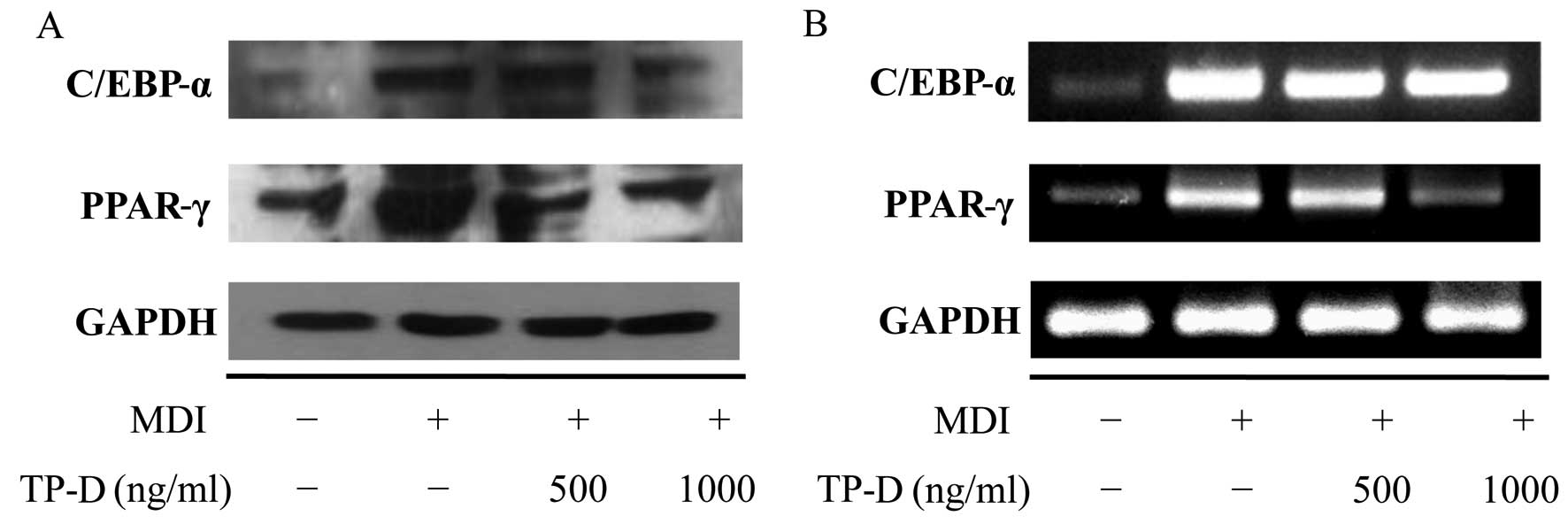
Effects of TP-D on the expression of
adipogenic genes (C/EBP-α and PPAR-γ) during the differentiation of
3T3-L1 adipocytes
The apparent morphological changes included changes
in the number of lipid droplets and lipid accumulation during the
differentiation of the cells into adipocytes. In addition,
biochemical changes were observed in terms of the expression of
adipocyte-specific protein markers. Generally, there is a
significantly increased expression of transcription factors and the
secretion of adipokines during adipocyte differentiation (17,18). Among these transcription factors,
C/EBP-α and PPAR-γ are considered important (19), with increased expression levels
observed in differentiated adipocytes. Their expression is induced
during early differentiation, and during the later stages of
differentiation, they induce the expression of various adipogenic
genes. In our previous experiment, we confirmed the inhibition of
lipid accumulation and cellular differentiation by treatment with
TP-D. Thus, we then measured adipocyte-related factors at the
protein and mRNA level by western blot analysis and RT-PCR,
respectively, and observed the inhibition of the activation of
C/EBP-α and PPAR-γ proteins. In turn, the controlled inhibition of
C/EBP-α and PPAR-γ by TP-D inhibited adipocyte formation in a
dose-dependent manner (Fig.
6).
Discussion
Recently, an increase in obesity has led to a
concomitant increase in serious disorders, such as metabolic
disease, hypertension, atherosclerosis and irregular eating habits
(20,21). Adipocytes play an important role
in the balance of energy and in the regulation lipid metabolism and
are associated with obesity and adipose tissue mass. Indeed, lipid
accumulation during adipogenesis and the programmed differentiation
of preadipocytes involve several steps related to obesity (22). A number of research studies have
focused on reducing obesity by inhibiting lipogenesis and the
differentiation of preadipocytes. Additionally, research on and the
development of ‘functional foods’ have been actively pursued to
address obesity and its complications.
Tuna is a high-protein and nutritionally superior
food. It exerts inhibitory effects on atherosclerosis by decreasing
the serum cholesterol concentrations. It also has a wide range of
bioactivities, including anticancer properties (23). The tuna catch is approximately 4
million tons per year worldwide, and 40% of that is processed into
canned tuna (24). Tuna canning
requires a boiling treatment, and 27,000 tons of boiled tuna
byproduct result from this process (25). Boiled tuna contains various active
ingredients, such as large amounts of free amino acids, collagen
protein, carnosine and taurine. However, the study of its
composition is incomplete, due to the difficulties in desalinating
it and removing the coloration.
In this study, we separated peptides from boiled
tuna and confirmed their anti-obesity properties on 3T3-L1 cells by
the inhibition of their differentiation. 3T3-L1 cells are commonly
used as a model for adipocyte differentiation to examine signaling
pathways in adipogenesis (26).
To examine the effects of the tuna peptides on the differentiation
of adipocytes, 3T3-L1 preadipocytes were treated with MDI to induce
differentiation and then cultured in medium containing insulin. The
3T3-L1 adipocytes were then treated with 500 or 1,000 ng/ml TP-D,
TP-I or TP-N for 2 days. We assessed the potentiating effects of
TP-D, TP-I and TP-N on glucose uptake in 3T3-L1 adipocytes.
Generally, a large amount of glucose consumption is required for
the differentiation of 3T3-L1 preadipocytes into adipocytes.
Treatment with 500 and 1,000 ng/ml TP-D led to a decrease in
glucose consumption, by ~33 and 63%, respectively, compared with
the MDI group. However, treatment with TP-I and TP-N did not have
any significant effects (Fig. 3).
Effects on lipid accumulation were also observed. When the
differentiation of the preadipocytes was induced, the consumption
of glucose was increased. Thus, we measured the TG levels under the
same treatment conditions used for the glucose assay. As a result,
treatment with 500 and 1,000 ng/ml TP-D led to a decrease in TG
levels by ~35 and 57%, compared with the MDI-treated group.
Treatment with TP-I and TP-N had no significant effects (Fig. 4). Based on these results,
treatment with TP-D decreased the amount of lipid droplets in a
dose-dependent manner compared with the MDI-treated group, as
revealed by Oil Red O staining and the microscopy examination of
the differentiated adipocytes. The stained lipid droplets showed a
significantly reduced lipid accumulation in the differentiated
adipocytes following treatment with TP-D in a dose-dependent manner
(Fig. 5). Subsequently, we
investigated the effects of TP-D on the differentiation of 3T3-L1
adipocytes by examining the expression levels of master adipogenic
transcription factors, such as C/EBP-α and PPAR-γ. Generally, the
differentiation of 3T3-L1 preadipocytes into adipocytes involves a
series of transcriptional processes. C/EBP-α plays important roles
during the early stages of preadipocyte differentiation, and
together with the induced upregulation of PPAR-γ, these
transcription factors activate a number of additional adipocyte
genes directly (27,28). PPAR-γ promotes the expression of
specific lipogenic and adipogenic genes during preadipocyte
differentiation and is necessary and sufficient for fat formation
(29). Thus, the downregulation
of C/EBP-α and PPAR-γ inhibits lipid accumulation and suppresses
adipocyte differentiation. The expression levels of C/EBP-α and
PPAR-γ were examined by western blot analysis and RT-PCR. As a
result, we found that TP-D induced the downregulation of C/EBP-α
and PPAR-γ (Fig. 6) and inhibited
the differentiation of 3T3-L1 cells, suggesting the potential use
of TP-D in anti-obesity functional foods and/or therapeutics.
Acknowledgments
This study was a part of the project entitled
‘Functional materials and foods using fisheries by-products’,
funded by the Ministry of Oceans and Fisheries, Korea
(20130279).
References
|
1
|
Kim DM, Choi HR, Park A, Shin SM, Bae KH,
Lee SC, Kim IC and Kim WK: Retinoic acid inhibits adipogenesis via
activation of Wnt signaling pathway in 3T3-L1 preadipocytes.
Biochem Biophys Res Commun. 434:455–459. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ahn J, Lee H, Kim S and Ha T:
Curcumin-induced suppression of adipogenic differentiation is
accompanied by activation of Wnt/β-catenin signaling. Am J Physiol
Cell Physiol. 298:C1510–C1516. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kopelman PG: Obesity as a medical problem.
Nature. 404:635–643. 2000.PubMed/NCBI
|
|
4
|
Lew EA: Mortality and weight: Insured
lives and the American Cancer Society studies. Ann Intern Med.
103:1024–1029. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Visscher TL and Seidell JC: The public
health impact of obesity. Annu Rev Public Health. 22:355–375. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Carrill KK: Biological effects of fish
oils in relation to chronic disease. Lipis. 21:731–732. 1986.
|
|
7
|
Mehta J, Lopez LM and Wargovich T:
Eicosapentaenoic acid: Its relevance in atherosclerosis and
coronary artery disease. Am J Cardiol. 59:155–159. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee HS, Kim HJ, Choi JI, et al:
Antioxidant activity of the ethanol extract from cooking drips of
Thunnus thynnus by gamma irradiation. J Korean Soc Food Sci Nutr.
37:810–814. 2008. View Article : Google Scholar
|
|
9
|
Lee JA, Ahn EK, Hong SS and Oh JS:
Anti-obesity effect of ethyl acetate extracts from Agrimonia pilosa
Ledeb. in 3T3-L1 preadipocytes. J Korean Soc Food Sci Nutr.
41:161–167. 2012. View Article : Google Scholar
|
|
10
|
Smas CM and Sul HS: Control of adipocyte
differentiation. Biochem J. 309:697–710. 1995.PubMed/NCBI
|
|
11
|
Carey M: The enhanceosome and
transcriptional synergy. Cell. 92:5–8. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Darlington GJ, Ross SE and MacDougald OA:
The role of C/EBP genes in adipocyte differentiation. J Biol Chem.
273:30057–30060. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brun RP, Kim JB, Hu E, Altiok S and
Spiegelman BM: Adipocyte differentiation: A transcriptional
regulatory cascade. Curr Opin Cell Biol. 8:826–832. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hu E, Tontonoz P and Spiegelman BM:
Transdifferentiation of myoblasts by the adipogenic transcription
factors PPAR gamma and C/EBP alpha. Proc Natl Acad Sci USA.
92:9856–9860. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shevchenko A, Wilm M, Vorm O and Mann M:
Mass spectrometric sequencing of proteins silver-stained
polyacrylamide gels. Anal Chem. 68:850–858. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Beaudoin A: New technique for revealing
latent fingerprints on wet, porous surfaces: Oil Red O. J Forensic
Identif. 54:413–421. 2004.
|
|
17
|
Rosen ED, Walkey CJ, Puigserver P and
Spiegelman BM: Transcriptional regulation of adipogenesis. Genes
Dev. 14:1293–1307. 2000.PubMed/NCBI
|
|
18
|
Jeon T, Hwang SG, Hirai S, Matsui T, Yano
H, Kawada T, Lim BO and Park DK: Red yeast rice extracts suppress
adipo-genesis by down-regulating adipogenic transcription factors
and gene expression in 3T3-L1 cells. Life Sci. 75:3195–3203. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cornelius P, MacDougald OA and Lane MD:
Regulation of adipocyte development. Annu Rev Nutr. 14:99–129.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hursting SD and Hursting MJ: Growth
signals, inflammation, and vascular perturbations: Mechanistic
links between obesity, metabolic syndrome, and cancer. Arterioscler
Thromb Vasc Biol. 32:1766–1770. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kant R: Sweet proteins – potential
replacement for artificial low calorie sweeteners. Nutr J. 4:52005.
View Article : Google Scholar
|
|
22
|
Unger RH and Zhou YT: Lipotoxicity of
beta-cells in obesity and in other causes of fatty acid spillover.
Diabetes. 50(Suppl 1): S118–S121. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hunter E: PUFA and eicosanoid research.
JAOCS. 64:10881987.
|
|
24
|
Kang MK and Song KB: Quality
characteristics of Gochujang with the addition of skipjack cooking
broth as protein source. Korean J Food Preserv. 13:457–464.
2006.
|
|
25
|
Kim JS, Yeum DM, Kang HG, Kim IS, Kong CS,
Lee TG and Heu MS: Fundamentals and applications for canned foods.
95. Hyoil Publishing Co.; Seoul, Korea: pp. 351–360. 2002
|
|
26
|
Green H and Kehinde O: An established
preadipose cell line and its differentitation in culture. II.
Factors affecting the adipose conversion. Cell. 5:19–27. 1975.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee H, Bae S and Yoon Y: The
anti-adipogenic effects of (−)epigallocatechin gallate are
dependent on the WNT/β-catenin pathway. J Nutr Biochem.
24:1232–1240. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
He Y, Li Y, Zhao T, Wang Y and Sun C:
Ursolic acid inhibits adipogenesis in 3T3-L1 adipocytes through
LKB1/AMPK pathway. PLoS One. 8:e701352013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Farmer SR: Transcriptional control of
adipocyte formation. Cell Metab. 4:263–273. 2006. View Article : Google Scholar : PubMed/NCBI
|