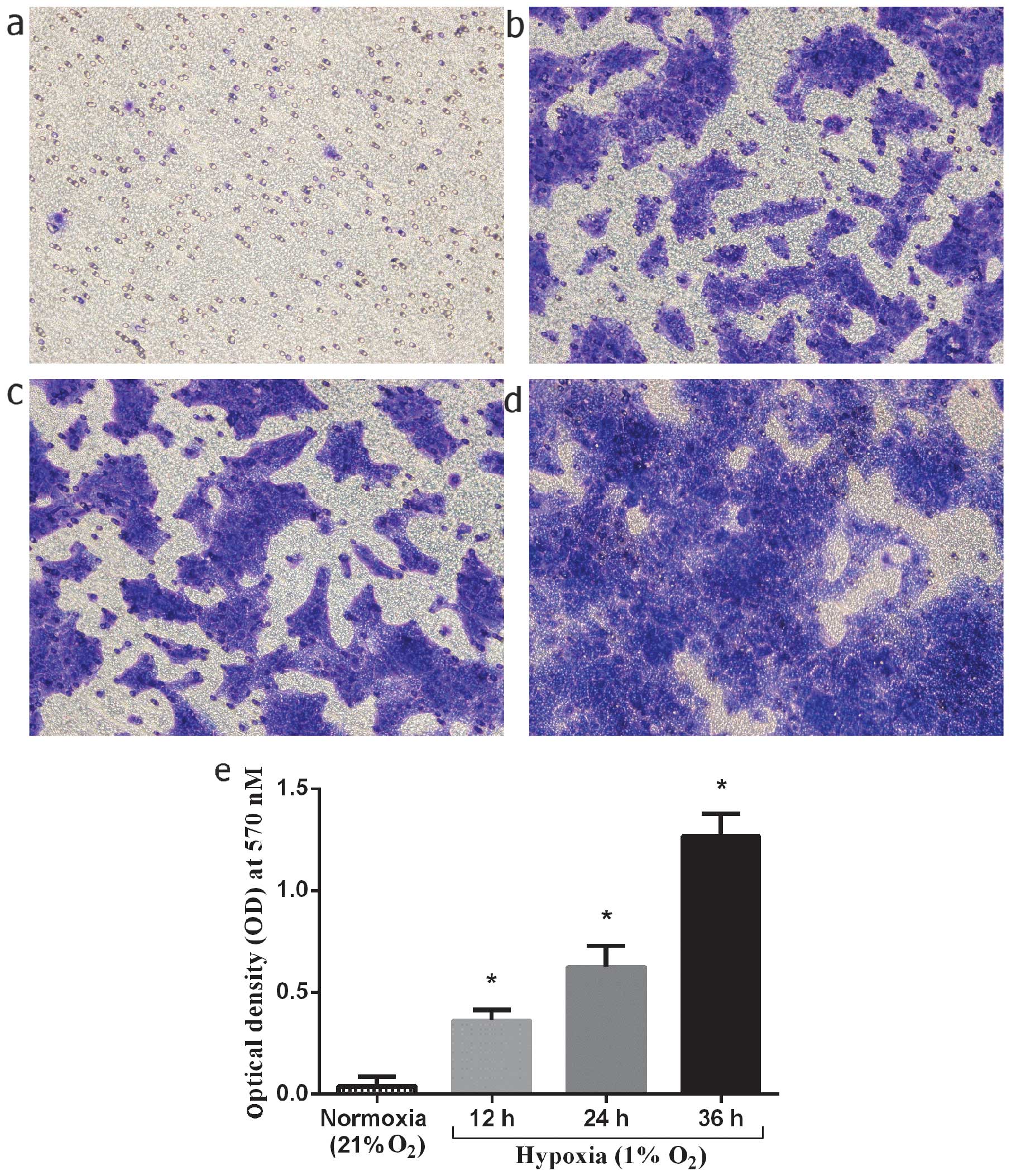
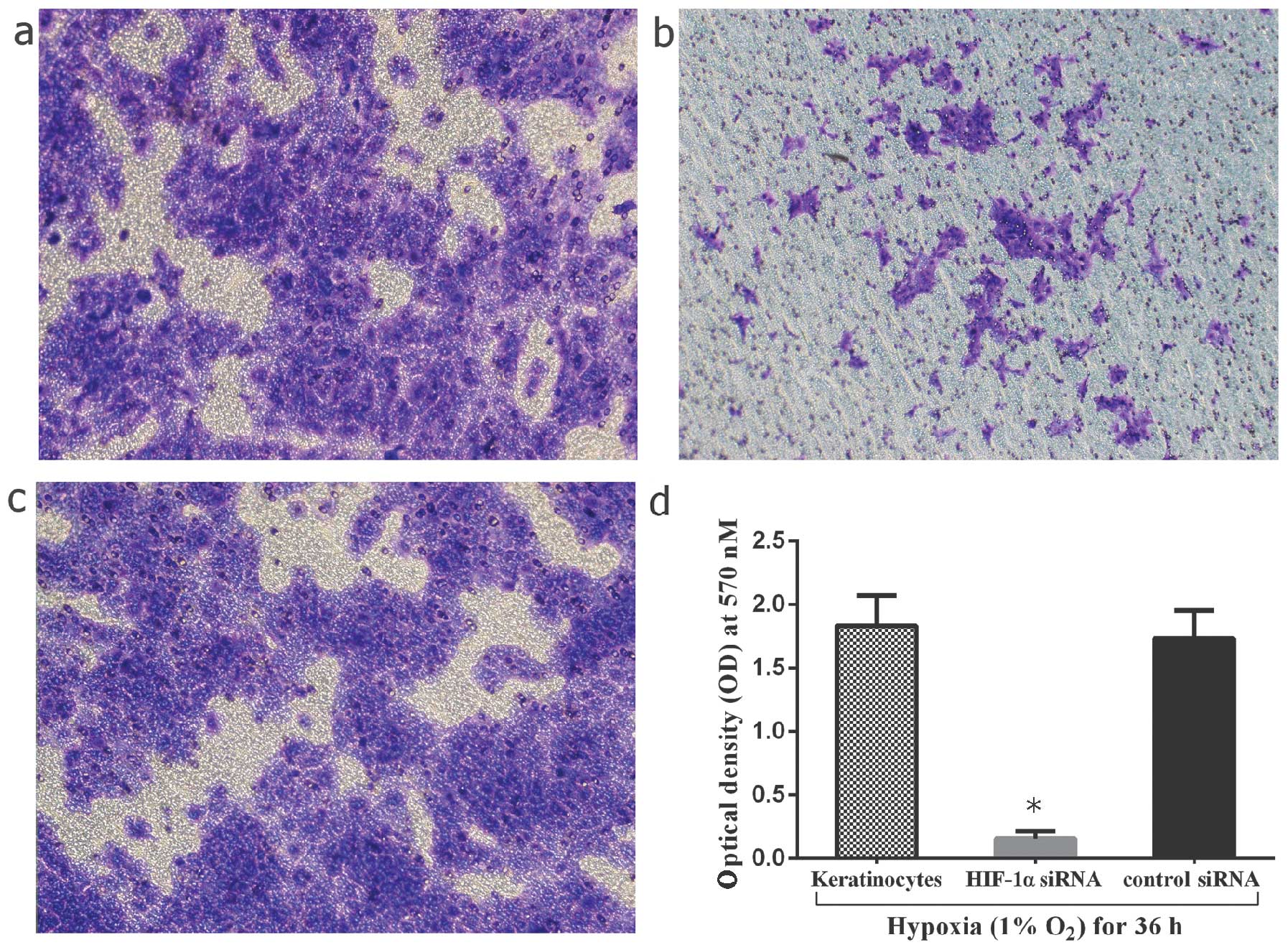
|
1
|
Zhang Z, Nie F, Kang C, Chen B, Qin Z, Ma
J, Ma Y and Zhao X: Increased periostin expression affects the
proliferation, collagen synthesis, migration and invasion of keloid
fibroblasts under hypoxic conditions. Int J Mol Med. 34:253–261.
2014.PubMed/NCBI
|
|
2
|
Zhang Q, Yamaza T, Kelly AP, Shi S, Wang
S, Brown J, Wang L, French SW, Shi S and Le AD: Tumor–like stem
cells derived from human keloid are governed by the inflammatory
niche driven by IL–17/IL–6 axis. PLoS One. 4:e77982009. View Article : Google Scholar
|
|
3
|
Vincent AS, Phan TT, Mukhopadhyay A, Lim
HY, Halliwell B and Wong KP: Human skin keloid fibroblasts display
bioener–getics of cancer cells. J Invest Dermatol. 128:702–709.
2008. View Article : Google Scholar
|
|
4
|
Kischer CW and Brody GS: Structure of the
collagen nodule from hypertrophic scars and keloids. Scan Electron
Microsc. (Pt 3): 371–376. 1981.PubMed/NCBI
|
|
5
|
Balestri R, Misciali C, Zampatti C,
Odorici G and Balestri JA: Keloidal basal cell carcinoma: Should it
be considered a distinct entity? J Dtsch Dermatol Ges.
11:1196–1198. 2013.PubMed/NCBI
|
|
6
|
Lewis JE: Keloidal basal cell carcinoma.
Am J Dermatopathol. 29:4852007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Steinbrech DS, Mehrara BJ, Chau D, Rowe
NM, Chin G, Lee T, Saadeh PB, Gittes GK and Longaker MT: Hypoxia
upregulates VEGF production in keloid fibroblasts. Ann Plast Surg.
42:514–519; discussion 519–520. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Q, Wu Y, Ann DK, Messadi DV, Tuan
TL, Kelly AP, Bertolami CN and Le AD: Mechanisms of hypoxic
regulation of plasminogen activator inhibitor–1 gene expression in
keloid fibroblasts. J Invest Dermatol. 121:1005–1012. 2003.
View Article : Google Scholar
|
|
9
|
Zhang Q, Wu Y, Chau CH, Ann DK, Bertolami
CN and Le AD: Crosstalk of hypoxia–mediated signaling pathways in
upregulating plasminogen activator inhibitor–1 expression in keloid
fibroblasts. J Cell Physiol. 199:89–97. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu Y, Zhang Q, Ann DK, Akhondzadeh A,
Duong HS, Messadi DV and Le AD: Increased vascular endothelial
growth factor may account for elevated level of plasminogen
activator inhibitor–1 via activating ERK1/2 in keloid fibroblasts.
Am J Physiol Cell Physiol. 286:C905–C912. 2004. View Article : Google Scholar
|
|
11
|
Du R, Xia L, Ning X, Liu L, Sun W, Huang
C, Wang H and Sun S: Hypoxia-induced Bmi1 promotes renal tubular
epithelial cell–mesenchymal transition and renal fibrosis via
PI3K/Akt signal. Mol Biol Cell. 25:2650–2659. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun S, Ning X, Zhang Y, Lu Y, Nie Y, Han
S, Liu L, Du R, Xia L, He L and Fan D: Hypoxia–inducible
factor–1alpha induces Twist expression in tubular epithelial cells
subjected to hypoxia, leading to epithelial–to–mesenchymal
transition. Kidney Int. 75:1278–1287. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Higgins DF, Kimura K, Bernhardt WM,
Shrimanker N, Akai Y, Hohenstein B, Saito Y, Johnson RS, Kretzler
M, Cohen CD, et al: Hypoxia promotes fibrogenesis in vivo via HIF–1
stimulation of epithelial–to–mesenchymal transition. J Clin Invest.
117:3810–3820. 2007.PubMed/NCBI
|
|
14
|
Jiang J, Tang YL and Liang XH: EMT: A new
vision of hypoxia promoting cancer progression. Cancer Biol Ther.
11:714–723. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chalamalasetty RB, Garriock RJ, Dunty WC
Jr, Kennedy MW, Jailwala P, Si H and Yamaguchi TP: Mesogenin 1 is a
master regulator of paraxial presomitic mesoderm differentiation.
Development. 141:4285–4297. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bronner ME: Formation and migration of
neural crest cells in the vertebrate embryo. Histochem Cell Biol.
138:179–186. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rogers CD, Saxena A and Bronner ME: Sip1
mediates an E–cadherin-to-N-cadherin switch during cranial neural
crest EMT. J Cell Biol. 203:835–847. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yin SY, Peng AP, Huang LT, Wang YT, Lan CW
and Yang NS: The phytochemical shikonin stimulates
epithelial-mesenchymal transition (EMT) in skin wound healing. Evid
Based Complement Alternat Med. 2013:2627962013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Savagner P and Arnoux V:
Epithelio–mesenchymal transition and cutaneous wound healing. Bull
Acad Natl Med. 193:1981–1991; discussion 1992, 2009 (In
French).
|
|
20
|
Yan C, Grimm WA, Garner WL, Qin L, Travis
T, Tan N and Han YP: Epithelial to mesenchymal transition in human
skin wound healing is induced by tumor necrosis factor–alpha
through bone morphogenic protein-2. Am J Pathol. 176:2247–2258.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tsai JH and Yang J: Epithelial-mesenchymal
plasticity in carcinoma metastasis. Genes Dev. 27:2192–2206. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun S and Qiu XS: Cancer stem cells and
tumor metastasis. J Cancer Res Ther. 9(Suppl): S150–S152. 2013.
View Article : Google Scholar
|
|
23
|
Seifert O and Mrowietz U: Keloid scarring:
Bench and bedside. Arch Dermatol Res. 301:259–272. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Robles DT, Moore E, Draznin M and Berg D:
Keloids: Pathophysiology and management. Dermatol Online J.
13:92007.
|
|
25
|
Ashcroft KJ, Syed F and Bayat A:
Site–specific keloid fibroblasts alter the behaviour of normal skin
and normal scar fibroblasts through paracrine signalling. PLoS One.
8:e756002013. View Article : Google Scholar
|
|
26
|
Muthusubramaniam L, Zaitseva T, Paukshto
M, Martin G and Desai T: Effect of collagen nanotopography on
keloid fibroblast proliferation and matrix synthesis: Implications
for dermal wound healing. Tissue Eng Part A. 20:2728–2736. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Suarez E, Syed F, Rasgado TA, Walmsley A,
Mandal P and Bayat A: Skin equivalent tensional force alters keloid
fibroblast behavior and phenotype. Wound Repair Regen. 22:557–568.
2014.PubMed/NCBI
|
|
28
|
Xia W, Phan TT, Lim IJ, Longaker MT and
Yang GP: Complex epithelial-mesenchymal interactions modulate
transforming growth factor–beta expression in keloid-derived cells.
Wound Repair Regen. 12:546–556. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Do DV, Ong CT, Khoo YT, Carbone A, Lim CP,
Wang S, Mukhopadhyay A, Cao X, Cho DH, Wei XQ, et al:
Interleukin–18 system plays an important role in keloid
pathogenesis via epithelial–mesenchymal interactions. Br J
Dermatol. 166:1275–1288. 2012. View Article : Google Scholar
|
|
30
|
Ong CT, Khoo YT, Tan EK, Mukhopadhyay A,
Do DV, Han HC, Lim IJ and Phan TT: Epithelial–mesenchymal
interactions in keloid pathogenesis modulate vascular endothelial
growth factor expression and secretion. J Pathol. 211:95–108. 2007.
View Article : Google Scholar
|
|
31
|
Phan TT, Lim IJ, Bay BH, Qi R, Huynh HT,
Lee ST and Longaker MT: Differences in collagen production between
normal and keloid–derived fibroblasts in serum–media co–culture
with keloid–derived keratinocytes. J Dermatol Sci. 29:26–34. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Distler JH, Jüngel A, Pileckyte M, Zwerina
J, Michel BA, Gay RE, Kowal–Bielecka O, Matucci–Cerinic M, Schett
G, Marti HH, et al: Hypoxia–induced increase in the production of
extracellular matrix proteins in systemic sclerosis. Arthritis
Rheum. 56:4203–4215. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hasmim M, Messai Y, Noman MZ and Chouaib
S: Tumor hypoxia: A key player in the regulation of stromal and
anti-tumor responses. Med Sci (Paris). 30:422–428. 2014.In French.
View Article : Google Scholar
|
|
34
|
Vaupel P and Mayer A: Hypoxia in tumors:
Pathogenesis–related classification, characterization of hypoxia
subtypes, and associated biological and clinical implications. Adv
Exp Med Biol. 812:19–24. 2014. View Article : Google Scholar
|