Introduction
Liver ischemia/reperfusion (I/R) is a common
physiopathological phenomenon that occurs during surgery. The
causes of liver I/R mainly include infection, shock,
cardiopulmonary dysfunction, repair of liver trauma, tumor
resection and transplantation. Liver I/R injury is of great
clinical significance as it not only affects the graft or remaining
liver following major hepatectomy, but also remote organs, such as
the intestines, kidneys, lungs and brain; it can even result in
multiple organ dysfunction syndrome (MODS) (1). In addition, approximately 10% of
transplant patients present with liver failure in the early
post-operative stage due to I/R. Indeed, I/R injury also
participates in acute and chronic reactions (2). Therefore, in recent years,
increasing attention has been paid to finding methods of
alleviating the injury caused by I/R in surgery as much as
possible.
Murry et al (3) reported that brief intermittent
periods of ischemia and reperfusion of the coronary artery did not
lead to irreversible damage of myocardial cells as did longer
periods of I/R; on the contrary, it surprisingly protected the
cardiomyocytes from the damage caused by subsequent long periods of
ischemia. They termed this phenomenon ischemic preconditioning
(IPC). Of note, brief periods of focal ischemia have also been
shown to induce ischemic tolerance in rat brains, and IPC has been
shown to exert profound protective effects on the liver and
intestines, and to enhance donor lung preservation (4–7).
The molecular mechanisms underlying IPC are not yet
fully understood. Indeed, the involvement of several signaling
pathways and molecules has been suggested, including protein kinase
C, nitric oxide (NO), cGMP-dependent protein kinase, Akt (protein
kinase B), extracellular signal-regulated kinase (ERK) and p38 MAP
kinases, AMP-dependent protein kinase, and the mitochondrial
ATP-sensitive potassium channel (8–10).
Compared to other organs, liver IPC has additional
features as it reduces inflammation and promotes hepatic
regeneration (11). Recently, it
was demonstrated that the inhibition of glycogen synthase kinase-3β
(GSK-3β) prior to hemorrhagic shock regulates the inflammatory
response, and improves hepatic microcirculation and hepatocellular
function (12). GSK-3β belongs to
a family of conserved serine/threonine kinases present in
eukaryotic groups and its activity is regulated by various pathways
in addition to the phosphoinositide 3-kinase
(PI3K)-PKB/Akt-dependent pathway, including Wnt signaling. The
phosphorylation of GSK-3β results in increased β-catenin levels and
its translocation to the nucleus (13). β-catenin has been shown to be a
critical mediator during development and angiogenesis (14); it is phosphorylated in a cytosolic
multiprotein complex containing the adenomatous polyposis coli
(APC) protein, axin and GSK-3β (15–17). When the phosphorylation of
β-catenin is blocked, the protein accumulates and translocates to
the nucleus, where it forms a complex with T-cell transcription
factor/lymphoid-enhancer binding factor (TCF/LEF) and activates or
represses several important target genes, including c-Myc, cyclin
D1, fibronectin, vascular endothelial growth factor (VEGF), Bcl-2
and survivin (18–20). However, whether IPC similarly
protects the liver against I/R injury by involving the
GSK-3β/β-catenin signaling pathway remains unknown.
The present study aimed to clarify the role of the
GSK-3β/β-catenin signaling pathway in the protective effects
induced by IPC agaisnt liver I/R injury. Liver morphology, and
serum alanine aminotransferase (ALT) and aspartate aminotransferase
(AST) levels, as well as liver maleic dialdehyde (MDA) and
superoxide dismutase (SOD) activity were assessed. In addition, the
levels of GSK-3β, phosphorylated (Ser9) GSK-3β (p-GSK-3β),
cytosolic and nuclear β-catenin, as well as those of VEGF were
quantified. Finally, the expression levels of the anti-apoptotic
markers, Bcl-2 and survivin, were also evaluated.
Materials and methods
Animals
Male Sprague-Dawley, rats weighing 180–220 g, were
obtained from the Animal Center of Dalian Medical University
(Dalian, China) (Institutional protocol no. SCXK 2008-0002), and
maintained under standard laboratory conditions with free access to
food and water. The rats were housed in a barrier system kept at
25°C with 12/12 h light-dark cycles. They were allowed to
acclimatize for 1 week prior to the commencement of the
experiments. All procedures were conducted according to our
institutional animal care guidelines and approved by the
Institutional Ethics Committee (Peking University People’s
Hospital).
Surgical procedures and experimental
groups
A total of 30 rats were randomly divided into 3
groups, including the sham-operated, the liver I/R and the IPC
groups. The rats in the sham-operated group underwent surgery, with
the portal vein and artery isolated without occlusion. In the I/R
group, the animals were subjected to 70% liver ischemia for 45 min,
followed by 3 h of reperfusion, as previously described (21). In the IPC group, the rats were
subjected to 10 min of ischemia and 10 min of reperfusion prior to
the sustained ischemia, as previously described (22). At the end of reperfusion, blood
and liver samples were collected and preserved for the subsequent
procedures.
The animal experiments were approved by the ethics
committee of our institution. The animals were kept under
pathogen-free conditions under a 12-h light/dark cycle (4–6 animals
per cage) and allowed free access to food and water. Care was taken
to minimize the suffering of the animals as much as possible. The
outcomes of the preliminary experiment were taken into
consideration when designing the sample size and operation
standard. A daily observation was performed to examine the
physiological and mental state of the animals, to make sure the
animals were kept at a normal state. At the endpoint of the
experiment, the mice were quickly sacrificed by an intraperitoneal
antesthetic injection. After the mice were sacrificed, blood and
liver samples were collected.
Liver morphological assessment
The liver tissues were harvested and fixed in 10%
formalin. Consecutive 5-μm-thick sections from
paraffin-embedded liver tissues were prepared for hematoxylin and
eosin staining and subsequently evaluated as previously described
(23). Briefly, the liver
specimens were evaluated at ×200 magnification by a point-counting
method for the severity of liver injury with an ordinal scale as
follows: grade 0, minimal or no evidence of injury; grade 1, mild
injury consisting of cytoplasmic vacuolation and focal nuclear
pyknosis; grade 2, moderate to severe injury with extensive nuclear
pyknosis, cytoplasmic hypereosinophilia, loss of intercellular
borders and mild to moderate neutrophil infiltration; and grade 3,
severe injury with disintegration of hepatic cords, hemorrhaging
and severe polymorphonuclear (PMN) cell infiltration. An average of
100 adjacent points on a 1-mm2 grid was graded for each
specimen.
Serum ALT and AST levels
Blood samples were drawn from the abdominal aorta
and centrifuged at 3,000 rpm for 15 min to yield serum.
Subsequently, serum ALT and AST levels, which are generally
considered the most sensitive indexes of acute liver injury, were
measured using an Olympus AU1000 automatic analyzer (Olympus
Optical, Tokyo, Japan) according to the manufacturer’s instructions
(Nanjing Jiancheng, Nanjing, China).
Liver MDA and SOD activity assay
Liver tissues were harvested and homogenized
immediately on ice in 5 volumes of normal saline. The homogenates
were centrifuged at 3,000 rpm for 5 min. MDA and SOD activity in
the supernatants was determined using specific assay kits (Nanjing
Jiancheng), according to the manufacturer’s recommendations. MDA
and SOD activity was expressed in nmol/mg protein and U/mg protein,
respectively.
Quantification of liver GSK-3β, p-GSK-3β,
cytosolic and nuclear β-catenin, VEGF, Bcl-2 and survivin protein
levels by western blot analysis
Cytosolic, nuclear and total protein samples were
obtained from snap-frozen tissues using a protein extraction kit
(Beyotime Institute of Biotechnology, Nantong, China). The proteins
were separated by sodium dodecyl sulfate polyacrylamide gel
electrophoresis (SDS-PAGE) with appropriate gel concentrations (10%
for β-catenin, GSK-3β, p-GSK-3β and VEGF; 15% for Bcl-2) and then
electroblotted onto polyvinylidene fluoride (PVDF) membranes
(Millipore, Bedford, MA, USA) for 2 h. The membranes were then
incubated overnight at 4°C with antibodies raised against β-actin
(sc-47778; 1:1,000), GSK-3β (sc-9166; 1:800), p-GSK-3β (sc-11757;
1:600), β-catenin (sc-7963; 1:500), VEGF (sc-7269; 1:600), Bcl-2
(sc-7382; 1:600) and survivin (sc-17779; 1:500; all from Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA). Secondary antibodies
(ZDR-5308, ZDR-5306 and ZDR-5307) conjugated to horseradish
peroxidase (HRP; 1:2,000) were from Beijing Zhongshan Golden Bridge
Biological Technology (Beijing, China). The signals were visualized
using a chemiluminescent substrate kit (Thermo Fisher Scientific,
Rockford, IL, USA) and analyzed using a gel imaging system (Kodak
System EDAS120; Kodak, Tokyo, Japan). Gray values were normalized
to those of β-actin.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from the liver tissues using
TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the
manufacturer’s instructions. RNA was quantified using the Quant-iT™
RiboGreen® RNA Assay kit (Invitrogen, Eugene, OR, USA).
Equal amounts of mRNA were reverse transcribed into single-stranded
cDNA using the High Capacity cDNA Reverse Transcription kit
(Applied Biosystems, Framingham, MA, USA). The expression of the
target genes was measured by quantitative PCR using Power
SYBR-Green PCR Master mix, on an ABI PRISM 7300 sequence detection
system (both from Applied Biosystems). The following primer sets
were used in quantitative PCR: Bcl-2 forward,
5′-AGCCCTGTGCCACCTGTGGT-3′ and reverse,
5′-ACTGGACATCTCTGCAAAGTCGCG-3′; survivin forward,
5′-AGGACCACCGGATCTACACCTTCA-3′ and reverse,
5′-CTCGGTAGGGCAGTGGATGAAGC-3′. All results were normalized to
β-actin (forward, 5′-CCCATCTATGAGGGTTACGC-3′ and reverse,
5′-TTTAATGTCACGCACGATTTC-3′) and the values were calculated using
the 2−ΔCt method.
Statistical analysis
All data are presented as the means ± SD. One-way
analysis of variance (ANOVA) followed by LSD was used to compare
the differences between the 3 groups. A P-value <0.05 was
considered to indicate a statistically significant difference. All
statistical analyses were carried out using the Statistical Product
and Service Solutions (SPSS 16.0) statistical software package
(SPSS Inc., Chicago, IL, USA).
Results
IPC decreases the severity of
morphological and pathological changes induced by I/R injury
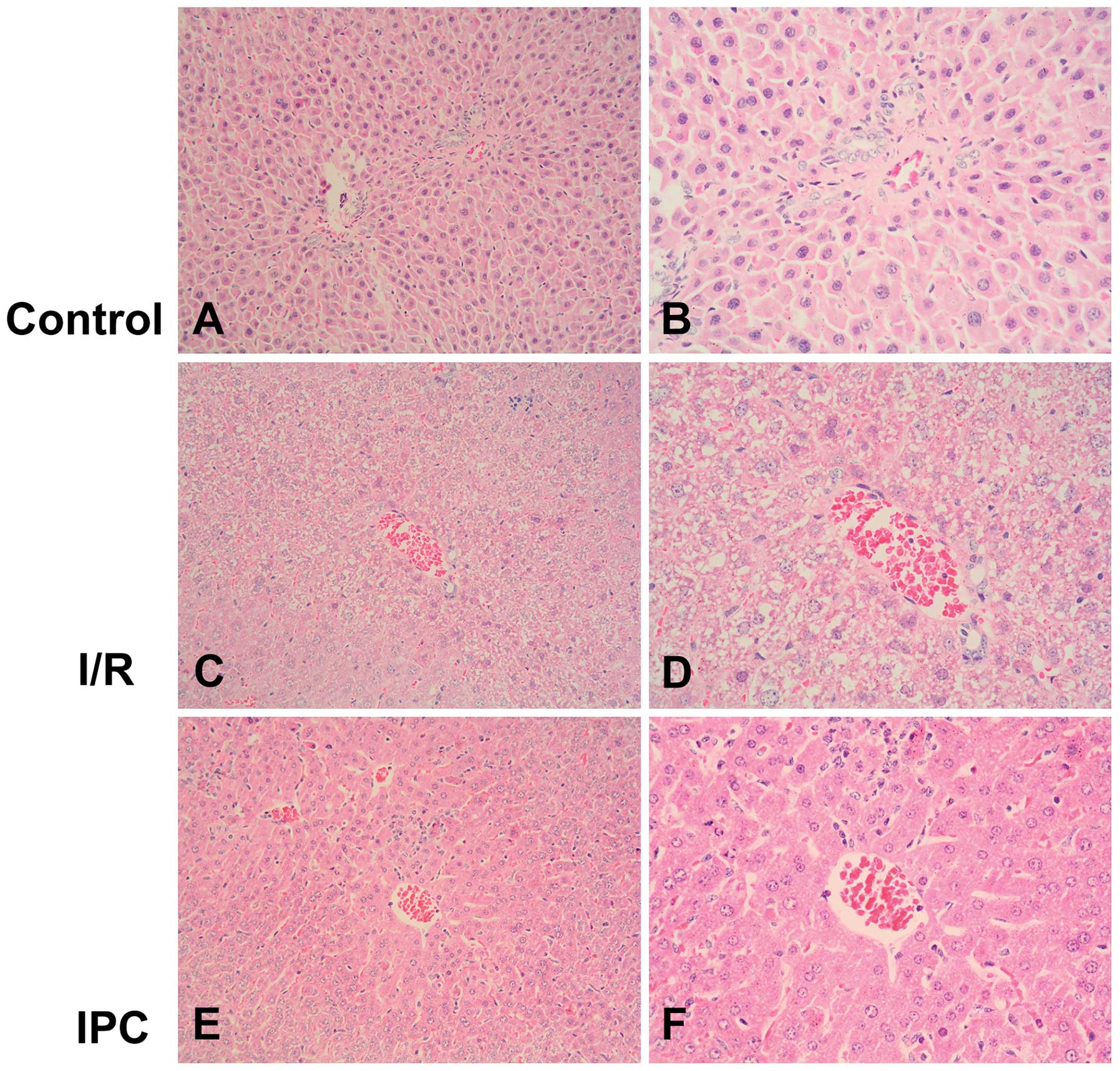
Morphological observations indicated that the liver
tissues from the rats in the sham-operated group were normal. On
the contrary, the liver tissues from the rats in the I/R group
appeared swollen and were dark red with mass effusion in the
abdominal cavity. In the rats from the IPC group, the liver tissues
presented only a mild increase in volume and slight effusion was
observed. In agreement with these results, light microscopy
revealed the presence of disorganized liver tissues in the I/R
group, with the disintegration of hepatic cords, blood stasis in
the central vein and small vessels within the portal area, edema
and hemorrhaging, as well as neutrophil infiltration. Compared with
the I/R group, IPC significantly decreased the severity of liver
injury; the organs showed more regularly arranged hepatocytes, and
less edema, hemorrhaging, blood stasis and neutrophil infiltration
(P<0.01) (Figs. 1 and 2).
Effects of IPC on I/R-induced acute liver
injury
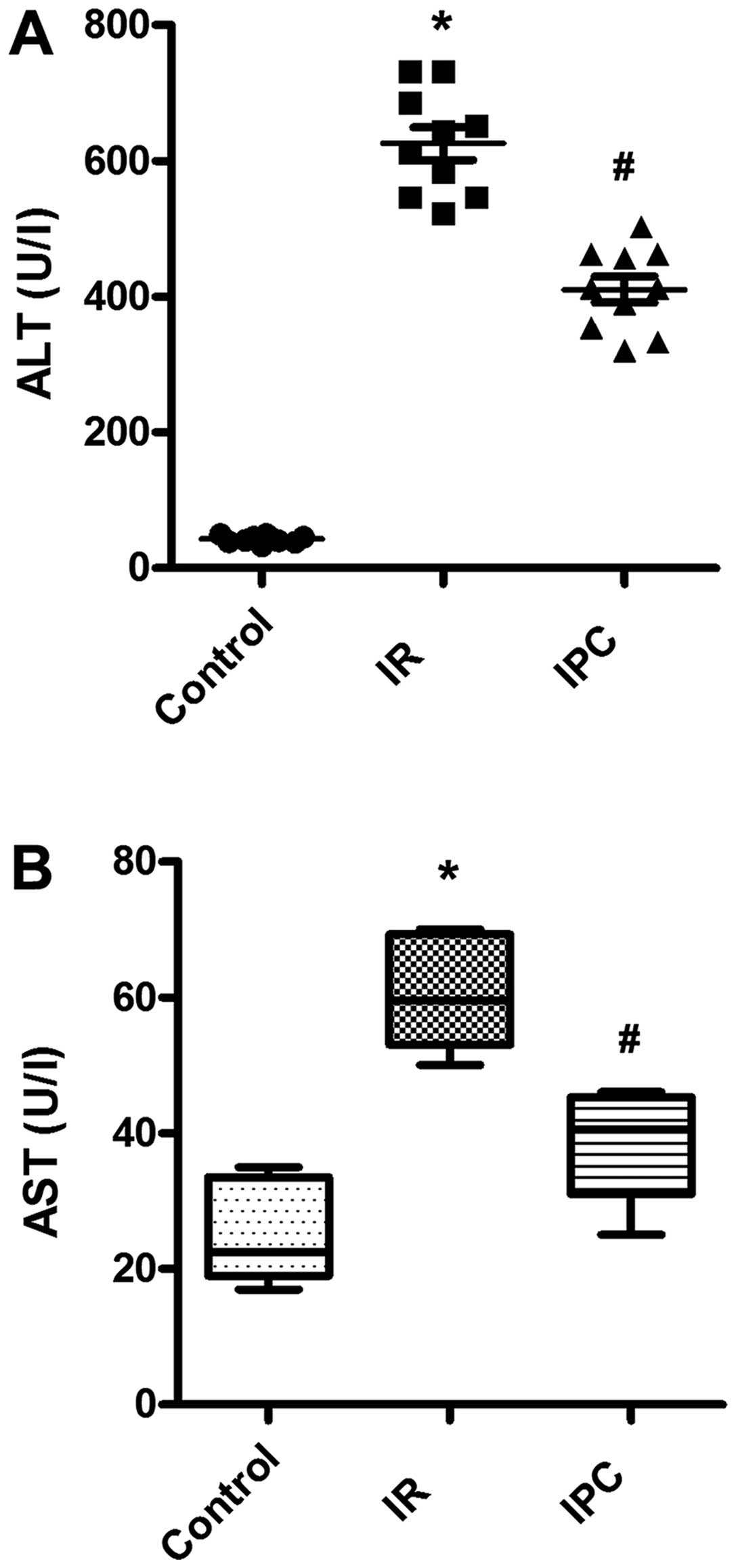
As general markers of acute liver injury, the serum
levels of ALT and AST were determined. As expected, there was a
significant increase in the serum ALT and AST levels in the I/R
group compared with sham-operated animals, indicating severe liver
damage caused by I/R. Of note, IPC exerted protective effects
against I/R-induced liver injury, which resulted in decreased ALT
and AST levels. The ALT levels were 42.80±5.51, 625.30±76.04 and
410.20±61.10 U/l in the sham-operated group (controls), the I/R
group and IPC group, respectively; the AST levels were 25.20±7.19,
60.20±7.48 and 38.20±7.97 U/l in the sham-operated group
(controls), the I/R group and IPC group, respectively (all
P<0.01) (Fig. 3).
Effects of IPC on liver MDA and SOD
activity
MDA is the degradation product of lipid peroxidation
caused by oxygen free radicals attacking polyunsaturated fatty
acids in biological membranes, which results in cross-linking and
the polymerization of macromolecules, such as proteins and nucleic
acids, leading to cell cytotoxicity (24). Generally, MDA levels indirectly
reflect the degree of cell membrane damage (24). Compared with the sham-operated
group, liver tissue MDA levels in the I/R group were significantly
increased (1.24±0.12 vs. 1.48±0.13 nmol/mg protein, P<0.01).
However, the MDA levels were markedly decreased in the IPC group
compared with the I/R group (1.48±0.13 vs. 1.35±0.15 nmol/mg
protein, P<0.01) (Fig. 4A). On
the contrary, SOD is an active substance that removes harmful
metabolic products and plays an important role in the balance
between oxidation and anti-oxidation (25). Liver tissue SOD levels in the I/R
group decreased significantly (46.70±3.83 vs. 37.80±3.52 U/mg
protein, P<0.01) compared with the sham-operated animals. Of
note, the SOD levels were increased in the IPC group compared with
the values obtained for the I/R group (3780±3.52 vs. 42.80±3.46
U/mg protein, P<0.01) (Fig.
4B).
IPC activates GSK-3 β/β -catenin
signaling during liver I/R
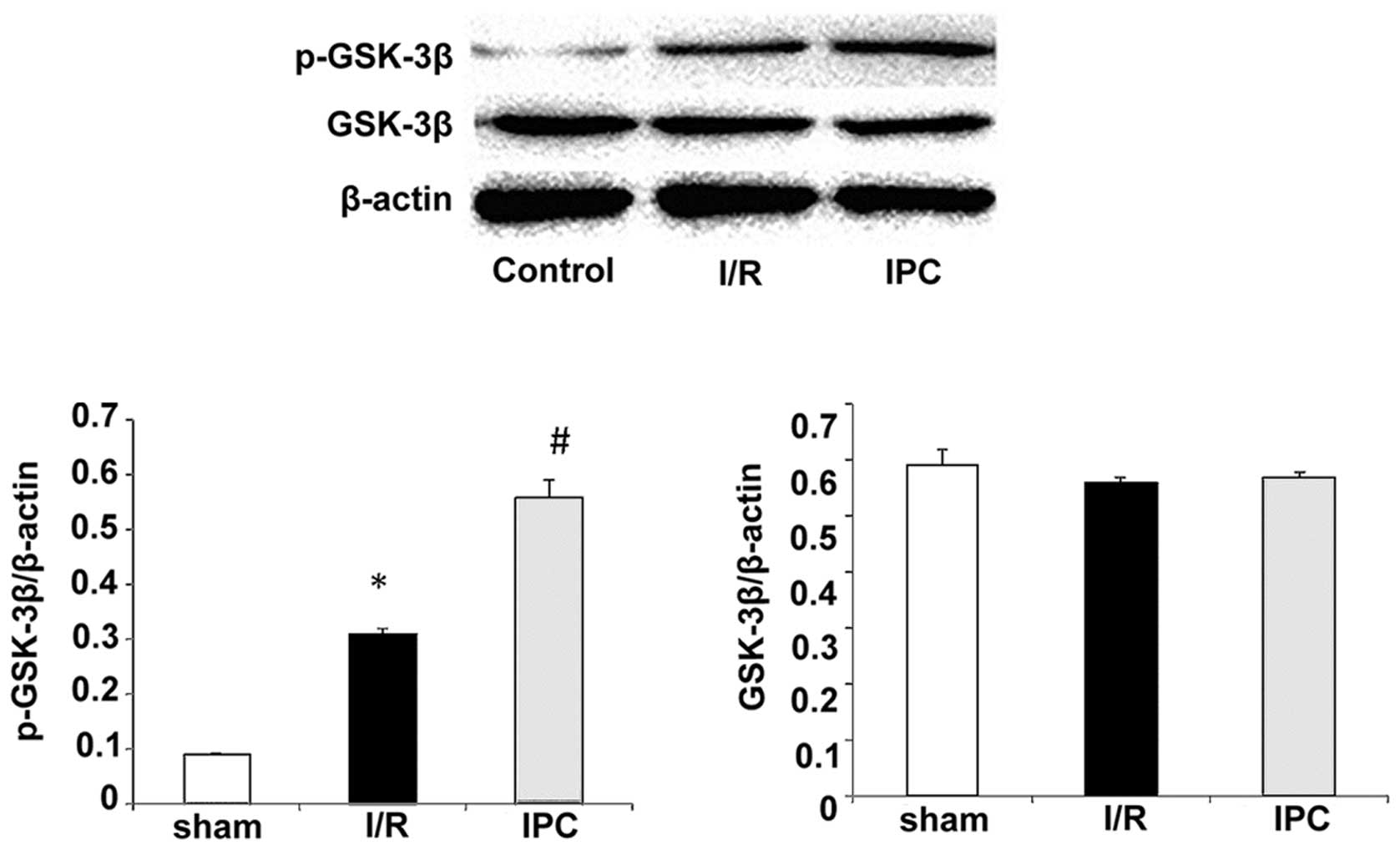
GSK-3β is an enzyme that is specifically inactivated
after phosphorylation. To further determine the effects of IPC on
I/R injury, we assessed the levels of GSK-3β phosphorylated at Ser9
(p-GSK-3β) by western blot analasis In the sham-operated group,
p-GSK-3β was barely detectable, while the expression of total
GSK-3β was relatively high. An increase in p-GSK-3β expression was
observed following I/R (Fig. 5).
Of note, IPC further increased the expression of p-GSK-3β compared
with the I/R group, while total GSK-3β expression remained
unaltered. The p-GSK-3β levels were 0.09±0.00, 0.31±0.01 and
0.56±0.03 in the sham-operated group, the I/R group and IPC group,
respectively, (all P<0.05); the total GSK-3β levels were
0.59±0.03, 0.56±0.01 and 0.57±0.01 in the sham-operated group, the
I/R group and IPC group, respectively (all P>0.05) (Fig. 5). As regards β-catenin, its
expression was detected at significantly greater levels in the
cytosolic and nuclear fractions in the IPC group in comparison with
the I/R group (cytosolic fraction, 0.17±0.01 vs. 0.40±0.01,
P<0.01; nuclear fraction, 0.36 ±0.01 vs. 0.57±0.03, P<0.01)
(Fig. 6A and B).
VEGF is a downstream effector of the
GSK-3β/β-catenin signaling pathway. When β-catenin accumulates in
the cytosol and translocates to the nucleus, it binds to TCF/LEF,
which results in the activation of VEGF and an increase in its
expression (37). To further
determine whether this pathway is activated, we measured the
expression of VEGF. Compared with the I/R group, VEGF expression in
the IPC group increased significantly (0.19±0.01 vs. 0.41±0.01,
P<0.01) (Fig. 6C), similar to
the expression of p-GSK-3β and β-catenin. These results indicate
that IPC activates the GSK-3β/β-catenin signaling pathway during
liver I/R.
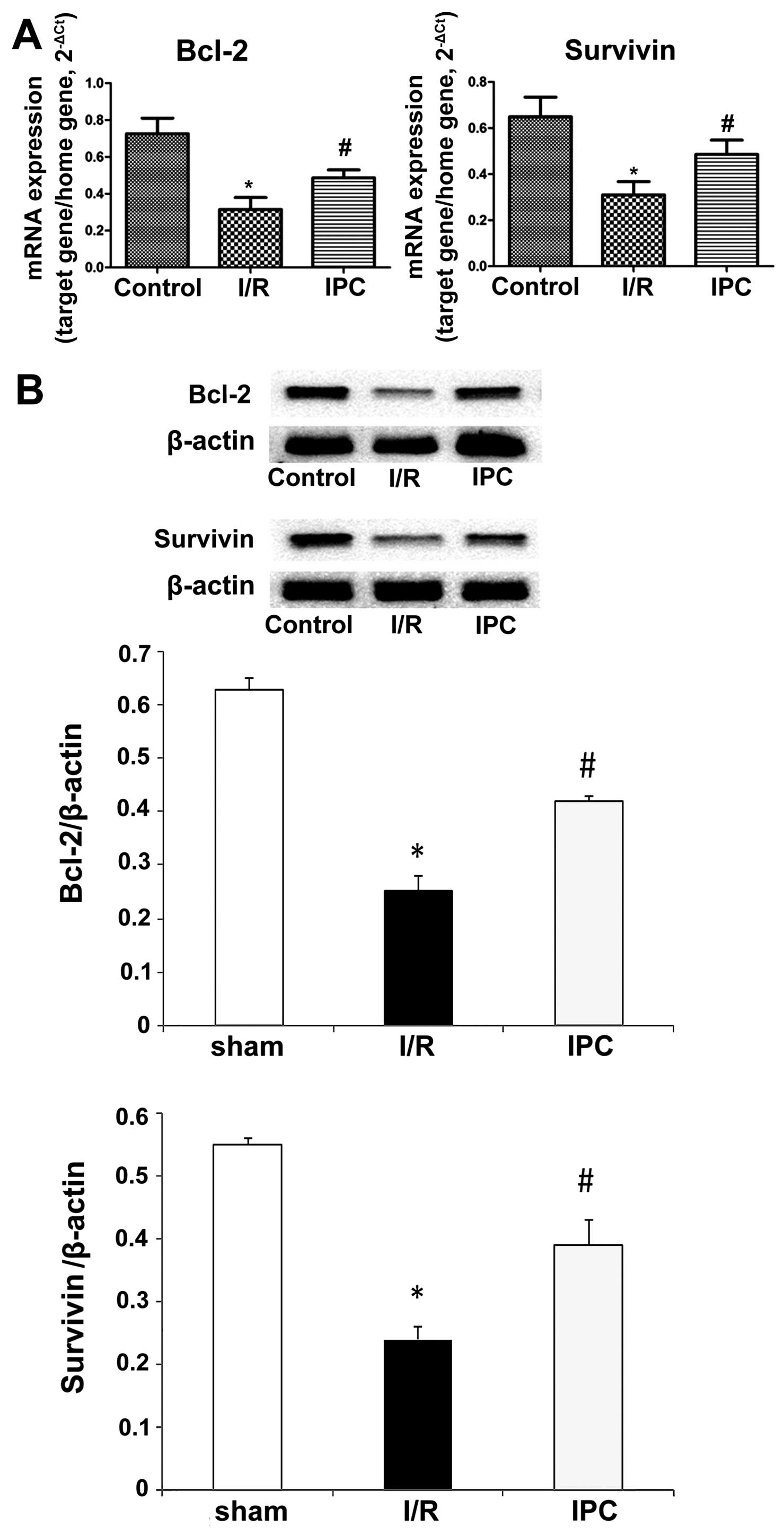
IPC upregulates Bcl-2 and survivin mRNA
expression and attenuates apoptosis
Bcl-2 and survivin are anti-apoptotic proteins that
inhibit the release of cytochrome c and the induction of
subsequent apoptosis by various inducers (37). RT-qPCR revealed that both Bcl-2
and survivin were detected in the 3 groups. I/R conspicuously
downregulated Bcl-2 and survivin expression compared with the
sham-operated animals, while IPC significantly increased the mRNA
expression of Bcl-2 and survivin in comparison with the I/R group.
For Bcl-2, relative mRNA amounts of 0.73±0.08, 0.31±0.06 and
0.49±0.04 were obtained in the sham-operated group, I/R group and
IPC group, respectively (all P<0.01); the relative mRNA levels
for survivin were 0.65±0.09, 0.31±0.06 and 0.49±0.06 in the
sham-operated group, I/R group and IPC group, respectively (all
P<0.05) (Fig. 7A). The results
from western blot analysis corroborated these findings. Indeed, the
relative Bcl-2 protein levels were 0.63±0.02, 0.25±0.03 and
0.42±0.01 in the sham-operated group, I/R group and IPC group,
respectively (all P<0.01); the survivin levels obtained in the
sham-operated, I/R group and IPC group were 0.55±0.01, 0.24±0.02
and 0.39±0.04, respectively (all P<0.05) (Fig. 7B).
Discussion
In this study, we demonstrated that IPC ameliorates
I/R-induced-liver injury at the morphological and pathological
levels. This was confirmed by the decreased serum ALT and AST
levels observed in the IPC group compared with the I/R group. In
agreement with our findings, remote ischemic preconditioning (RIP)
and N-acetylcysteine with RIP, as well as other ischemic
preconditioning methods have been shown to exert protective effects
against reperfusion injury in rats (26–28). Of note, in our study, IPC reversed
the I/R-induced increase in MDA activity, as well as the
I/R-induced decrease in SOD activity in the liver tissue, as
mentioned above. These data indicate that IPC modulates the liver
oxidant-antioxidant system during I/R injury in rats as has been
reported for other ischemic preconditioning methods (28–30).
Liver I/R leads to severe injury and even liver
failure, and IPC is a simple method for ameliorating liver I/R
injury. The mechanisms underlying the protective effects of IPC
against I/R injury remain controversial. Previous studies (31,32) have demonstrated that IPC
significantly reduces inflammatory cell infiltration so as to
improve hepatic microcirculation through the activation of
transcription regulators, such as nuclear factor-κB (nf-κb) and hypoxia-inducible factor-α
(HIF-α). Consequently, the transcription of inducible nitric oxide
synthase (iNOS) is regulated and NO synthesis is increased, which
results in the amelioration of hepatic microcirculation and
decreased oxygen free radical damage. Moreover, the expression of
tumor necrosis factor-α (TNF-α) and interleukin (IL)-1β has been
shown to be downregulated after liver I/R; this significantly
reduces the inflammatory response and cell apoptosis (33). In this study, we demonstrated that
IPC activated the GSK3β/β-catenin signaling pathway.
GSK-3 is a widely expressed and multifunctional
serine/threonine protein kinase, and includes the GSK-3α and GSK-3β
subtypes. GSK-3β is constitutively active in its dephosphorylated
form and has a pleiotropic function in the regulation of cell
activation, differentiation and survival. It has previously
(34) been suggested that GSK-3β
regulates the expression of cAMP-response element binding protein
(CREB), heat shock factor 1 (HSF1) and heat shock protein 70
(HSP70), as well as that of caspase and Bax, leading to cell
apoptosis. Several signaling pathways participate in the regulation
of GSK-3β activity, including the PI3KPKB/Akt and Wnt pathways. The
phosphorylation of GSK-3β leads to the release, stability and
accumulation of β-catenin in the cytoplasm, followed by its
translocation into the nucleus and combination with TCF/LEF, which
activates or inhibits target genes, such as cMyc, cyclin D1,
fibrin, VEGF, Bcl-2 and survivin (13,14,18–20,33). Tong et al (36) reported that IPC leads to the
phosphorylation and inactivation of GSK-3β, exerting marked
cardioprotective effects. The stability and accumulation of
β-catenin in the cytoplasm is the core event, while its
translocation to the nucleus translates into the activation of the
Wnt pathway. Kaga et al (37) found that IPC accelerates
angiogenesis and anti-apoptosis by upregulating the expression of
VEGF, Bcl-2 and survivin through GSK-3β/β-catenin signaling,
significantly ameliorating myocardial I/R injury in rats.
Therefore, in this study, we aimed to verify whether the same
mechanism of GSK-3β/β-catenin signaling contributes to the
protective effects of IPC against liver I/R injury.
Of note, p-GSK-3β was barely detectable in the
sham-operated group; however, its expression increased following
I/R and IPC, while the total GSK-3β levels were maintained at
relatively high levels. These findings further confirmed the liver
injury in the I/R and IPC groups. Importantly, it has been
demonstrated that the inhibition of GSK-3β ameliorates hepatic I/R
injury through the GSK-3β/β-catenin signaling pathway (38) and an IL-10-mediated immune
regulatory mechanism (39). In
this study, β-catenin significantly accumulated in the cytosol and
nucleus along with the increased VEGF expression in the IPC group
in comparison with the I/R group. These data suggest that IPC
activates the GSK-3β/β-catenin signaling pathway, alleviating liver
I/R injury.
The majority of neuronal and cardiac studies have
suggested that the protective effects of GSK-3β inhibition occur
through anti-apoptosis. Koh et al (40) used a transient middle cerebral
artery occlusion model and verified that GSK-3β inhibition
protected neuronal tissue from occlusion-induced damage through
anti-apoptosis. Using adeno-shRNA, Thirunavukkarasu et al
(41) found that the knockdown of
β-catenin abolished the IPC-mediated cardioprotective effects by
downregulating the target genes, Bcl-2 and survivin, in the
ischemic rat myocardium. Kaga et al (37) clarified that SB216763 increased
the accumulation of β-catenin in both the cytosol and nucleus,
which activated GSK-3β/β-catenin and further enhanced
anti-apoptotic signaling through the induction of Bcl-2 and
survivin expression in the rat IPC myocardium.
Studies assessing different types of liver damage
have also suggested that the protective effects of GSK-3β
inhibition occur through anti-apoptosis (42–44). In this sutdy, we demonstrate that
IPC leads to the phosphorylation and inactivation of GSK-3β, thus
function as a GSK-3β inhibitor. Of note, the results from western
blot analysis and RT-qPCR revealed higher levels of the
anti-apoptotic factors, such as Bcl-2 and survivin in the IPC group
compared with the I/R group, suggesting decreased apoptosis in the
former group. The anti-apoptotic effects of GSK-3β inactivation in
our model of liver I/R injury were consistent with those reported
for other models (43,44).
In conclusion, in this study, to the best of our
knowledge, we present the first evidence that the inactivation of
GSK-3β by IPC in liver I/R induces β-catenin signaling and
subsequently upregulates anti-apoptotic factors, such as Bcl-2 and
survivin, leading to a significant amelioration of liver I/R
injury. As reported in a previous study (45), liver I/R induces hepatocyte and
non-parenchymal cell death through necrosis and apoptosis, as well
as proliferation. However, it remains unclear whether the
IPC-induced inactivation of GSK-3β also relieves liver
proliferation and necrosis; further studies are required to clarify
this. Furthermore, further studies are required to clarify whether
inhibitors of GSK-3β and β-catenin affect other downstream target
genes, such as HIF-α and nf-κb, which may enhance our
understanding of these events. Overall, our data demonstrate partly
how IPC ameliorates liver I/R injury and enhances anti-apoptosis
through GSK-3β/β-catenin signaling.
Acknowledgments
This study was supported by Dr Xiaomei Xu, Dr Yan
Hu, Dr Xiaohan Zhai and Dr Musen Lin, who were provided guidance on
the analysis of the data.
References
|
1
|
Burroughs AK, Sabin CA, Rolles K, et al:
3-month and 12-month mortality after first liver transplant in
adults in Europe: predictive models for outcome. Lancet.
367:225–232. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li Q and Li JD: Progress in liver
transplantation ischemic preconditioning. Chin J Curr Adv Gen Surg.
13:470–473. 2010.In Chinese.
|
|
3
|
Murry CE, Jennings RB and Reimer KA:
Preconditioning with ischemia: a delay of lethal cell injury in
ischemic myocardium. Circulation. 74:1124–1136. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Peralta C, Closa D, Xaus C, Gelpi E,
Rosello-Catafau J and Hotter G: Hepatic preconditioning in rats is
defined by a balance of adenosine and xanthine. Hepatology.
28:768–773. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Glazier SS, O’Rourke DM, Graham DI and
Welsh FA: Induction of ischemic tolerance following brief focal
ischemia in rat brain. J Cereb Blood Flow Metab. 14:545–553. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Du ZY, Hicks M, Winlaw D, Spratt P and
MacDonald P: Ischemic preconditioning enhances donor lung
preservation in the rat. J Heart Lung Transplant. 15:1258–1267.
1996.PubMed/NCBI
|
|
7
|
Hotter G, Closa D, Prados M,
Fernandez-Cruz L, Prats N, Gelpi E and Rosello-Catafau J:
Intestinal preconditioning is mediated by a transient increase in
nitric oxide. Biochem Biophys Res Commun. 222:27–32. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ferdinandy P, Schulz R and Baxter GF:
Interaction of cardiovascular risk factors with myocardial
ischemia/reperfusion injury, preconditioning, and postconditioning.
Pharmacol Rev. 59:418–458. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Halestrap AP, Clarke SJ and Khaliulin I:
The role of mitochondria in protection of the heart by
preconditioning. Biochim Biophys Acta. 1767:1007–1031. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hausenloy DJ, Tsang A and Yellon DM: The
reperfusion injury salvage kinase pathway: a common target for both
ischemic preconditioning and postconditioning. Trends Cardiovasc
Med. 15:69–75. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Alchera E, Dal Ponte C, Imarisio C, Albano
E and Carini R: Molecular mechanisms of liver preconditioning.
World J Gastroenterol. 16:6058–6067. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jellestad L, Fink T, Pradarutti S, Kubulus
D, Wolf B, Bauer I, Thiemermann C and Rensing H: Inhibition of
glycogen synthase kinase (GSK)-3-β improves liver microcirculation
and hepatocellular function after hemorrhagic shock. Eur J
Pharmacol. 724:175–184. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ding VW, Chen RH and McCormick F:
Differential regulation of glycogen synthase kinase 3beta by
insulin and Wnt signaling. J Biol Chem. 275:32475–32481. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kusano S and Raab-Traub N: I-mfa domain
proteins interact with Axin and affect its regulation of the Wnt
and c-Jun N-terminal kinase signaling pathways. Mol Cell Biol.
22:6393–6405. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hart MJ, de los Santos R, Albert IN,
Rubinfeld B and Polakis P: Downregulation of beta-catenin by human
Axin and its association with the APC tumor suppressor,
beta-catenin and GSK3 beta. Curr Biol. 8:573–581. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aberle H, Bauer A, Stappert J, Kispert A
and Kemler R: beta-catenin is a target for the ubiquitin-proteasome
pathway. EMBO J. 16:3797–3804. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rubinfeld B, Albert I, Porfiri E, Fiol C,
Munemitsu S and Polakis P: Binding of GSK3beta to the
APC-beta-catenin complex and regulation of complex assembly.
Science. 272:1023–1026. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bienz M: TCF: transcriptional activator or
repressor? Curr Opin Cell Biol. 10:366–372. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Easwaran V, Lee SH, Inge L, et al:
beta-Catenin regulates vascular endothelial growth factor
expression in colon cancer. Cancer Res. 63:3145–3153.
2003.PubMed/NCBI
|
|
20
|
Behrens J, von Kries JP, Kuhl M, Bruhn L,
Wedlich D, Grosschedl R and Birchmeier W: Functional interaction of
beta-catenin with the transcription factor LEF-1. Nature.
382:638–642. 1996. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kuroda S, Tashiro H, Igarashi Y, et al:
Rho inhibitor prevents ischemia-reperfusion injury in rat steatotic
liver. J Hepatol. 56:146–152. 2012. View Article : Google Scholar
|
|
22
|
Nakayama H, Yamamoto Y, Kume M, et al:
Pharmacologic stimulation of adenosine A2 receptor supplants
ischemic preconditioning in providing ischemic tolerance in rat
livers. Surgery. 126:945–954. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Uysal AI, Ocmen E, Akan M, Ozkardesler S,
Ergur BU, Guneli E, Kume T, Koca U and Unal Togrul B: The effects
of remote ischemic preconditioning and N-acetylcysteine with remote
ischemic preconditioning in rat hepatic ischemia reperfusion injury
model. Biomed Res Int. 2014:8927042014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qi BN, Yi JH, Tang GH, Miao JL and Guo JF:
The experimental study of n-hexane on lipid peroxidation and DNA
damage of hepatic cell in rats. J Xi’an Jiaotong Univ Med Sci.
28:145–148. 2007.In Chinese.
|
|
25
|
Jia YX and Chen ZW: The effects of
Cu2+, Cd2+ on superoxide activities in
carassius auratus. Acta Hydrobiol Sin. 27:323–325. 2003.In
Chinese.
|
|
26
|
Jiang Y, Tang JJ, Wu BQ, Yuan B and Qu Z:
The protective effects of different-time-ischemic preconditioning
on the reperfusion injury in fatty livers in rats. PLoS One.
8:e580862013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jin LM, Jin SF, Liu YX, Zhou L, Xie HY,
Yan S, Xu X and Zheng SS: Ischemic preconditioning enhances
hepatocyte proliferation in the early phase after ischemia under
hemi-hepatectomy in rats. Hepatobiliary Pancreat Dis Int.
11:521–526. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yuan GJ, Ma JC, Gong ZJ, Sun XM, Zheng SH
and Li X: Modulation of liver oxidant-antioxidant system by
ischemic preconditioning during ischemia/reperfusion injury in
rats. World J Gastroenterol. 11:1825–1828. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yong J, Bo Y, Bao-qiang W, Jian-jun T and
Zhen Q: The optimal time window of ischemic preconditioning (IPC)
on the reperfusion injury in moderate to severe hepatocirrhosis in
rats. Ann Clin Lab Sci. 43:64–69. 2013.PubMed/NCBI
|
|
30
|
Camargo CA Jr, Madden JF, Gao W, Selvan RS
and Clavien PA: Interleukin-6 protects liver against warm
ischemia/reperfusion injury and promotes hepatocyte proliferation
in the rodent. Hepatology. 26:1513–1520. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Serafin A, Rosello-Catafau J, Prats N,
Xaus C, Gelpi E and Peralta C: Ischemic preconditioning increases
the tolerance of Fatty liver to hepatic ischemia-reperfusion injury
in the rat. Am J Pathol. 161:587–601. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Peralta C, Bulbena O, Xaus C, Prats N,
Cutrin JC, Poli G, Gelpi E and Rosello-Catafau J: Ischemic
preconditioning: a defense mechanism against the reactive oxygen
species generated after hepatic ischemia reperfusion.
Transplantation. 73:1203–1211. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Peralta C, Fernandez L, Panes J, Prats N,
Sans M, Pique JM, Gelpi E and Rosello-Catafau J: Preconditioning
protects against systemic disorders associated with hepatic
ischemia-reperfusion through blockade of tumor necrosis
factor-induced P-selectin up-regulation in the rat. Hepatology.
33:100–113. 2001. View Article : Google Scholar
|
|
34
|
Chung H, Seo S, Moon M and Park S:
Phosphatidylinositol-3-kinase/Akt/glycogen synthase kinase-3 beta
and ERK1/2 pathways mediate protective effects of acylated and
unacylated ghrelin against oxygen-glucose deprivation-induced
apoptosis in primary rat cortical neuronal cells. J Endocrinol.
198:511–521. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Miller JR, Hocking AM, Brown JD and Moon
RT: Mechanism and function of signal transduction by the
Wnt/beta-catenin and Wnt/Ca2+ pathways. Oncogene.
18:7860–7872. 1999. View Article : Google Scholar
|
|
36
|
Tong H, Imahashi K, Steenbergen C and
Murphy E: Phosphorylation of glycogen synthase kinase-3bet a during
preconditioning through a phosphatidylinositol-3-kinase-dependent
pathway is cardioprotective. Circ Res. 90:377–379. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kaga S, Zhan L, Altaf E and Maulik N:
Glycogen synthase kinase-3beta/beta-catenin promotes angiogenic and
anti-apoptotic signaling through the induction of VEGF, Bcl-2 and
survivin expression in rat ischemic preconditioned myocardium. J
Mol Cell Cardiol. 40:138–147. 2006. View Article : Google Scholar
|
|
38
|
Xia YX, Lu L, Wu ZS, Pu LY, Sun BC and
Wang XH: Inhibition of GSK-3beta ameliorates hepatic
ischemia-reperfusion injury through GSK-3beta/beta-catenin
signaling pathway in mice. Hepatobiliary Pancreat Dis Int.
11:278–284. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ren F, Duan Z, Cheng Q, et al: Inhibition
of glycogen synthase kinase 3 beta ameliorates liver ischemia
reperfusion injury by way of an interleukin-10-mediated immune
regulatory mechanism. Hepatology. 54:687–696. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Koh PO, Won CK and Cho JH: Estradiol
prevents the injury-induced decrease of Akt/glycogen synthase
kinase 3beta phosphorylation. Neurosci Lett. 404:303–308. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Thirunavukkarasu M, Han Z, Zhan L,
Penumathsa SV, Menon VP and Maulik N: Adeno-sh-beta-catenin
abolishes ischemic preconditioning-mediated cardioprotection by
downregulation of its target genes VEGF, Bcl-2, and survivin in
ischemic rat myocardium. Antioxid Redox Signal. 10:1475–1484. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Monga SP, Monga HK, Tan X, Mule K,
Pediaditakis P and Michalopoulos GK: Beta-catenin antisense studies
in embryonic liver cultures: role in proliferation, apoptosis, and
lineage specification. Gastroenterology. 124:202–216. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ibrahim SH, Akazawa Y, Cazanave SC, et al:
Glycogen synthase kinase-3 (GSK-3) inhibition attenuates hepatocyte
lipoapoptosis. J Hepatol. 54:765–772. 2011. View Article : Google Scholar :
|
|
44
|
Johnston A, Ponzetti K, Anwer MS and
Webster CR: cAMP-guanine exchange factor protection from bile
acid-induced hepatocyte apoptosis involves glycogen synthase kinase
regulation of c-Jun NH2-terminal kinase. Am J Physiol
Gastrointest Liver Physiol. 301:G385–G400. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gujral JS, Bucci TJ, Farhood A and
Jaeschke H: Mechanism of cell death during warm hepatic
ischemia-reperfusion in rats: apoptosis or necrosis? Hepatology.
33:397–405. 2001. View Article : Google Scholar : PubMed/NCBI
|