Introduction
The treatment of head and neck cancer remains
challenging. Due to the high rate of locally advanced and
metastatic tumors, intensified adjuvant treatment regimes,
including radiotherapy and chemotherapy, are performed. However,
local recurrence is a common occurrence, and the cumulative 5-year
overall survival is approximately 50% (1). In addition to TNM staging and
histological grading, molecular markers may aid in the
identification of high-risk patients. Melanoma-associated
antigens-A (MAGE-A) proteins belong to the very large family of
cancer/testis antigens (CTA) (2).
With the exception of the placenta, testis and fetal keratinocytes,
MAGE-A expression is restricted to malignant tissues. In general,
MAGE-A expression has been reported in malignant melanoma (3), breast cancer (4), lung cancer (5), urothelial carcinoma (6), colorectal carcinoma (7), hepatocellular carcinoma (8) and head and neck cancer (9). Typically, the different subgroups
are co-expressed, but there is no unique expression pattern for
specific tumor entities (10).
Remarkably, MAGE-A expression has been found in oral carcinoma
in situ and leukoplakia with dysplasia, but has not been
detected in oral ulcers, oral lichen planus and leukoplakia without
dysplasia (11). Recently, Han
et al reported that a high MAGE-A9 expression is a poor
prognostic marker in laryngeal squamous cell carcinoma (12). Of note, in their study, MAGE-A9
expression did not correlate with the TNM status, but a highly
significant correlation was observed with grading and overall
survival, indicating that the MAGE-A9 expression status may thus be
another way of identifying high-risk patients, apart from TNM. Our
group previously demonstrated a correlation between the treatment
efficacy of several drugs and the expression of certain MAGE-A
subgroups (13,14). This correlation may be mediated,
at least in part, by MAGE-induced p53-inhibition (15) and/or MAGE-associated expression of
taxol resistance-associated gene-3 (TRAG-3) (16). Additionally, MAGE-A3 expression
has been shown to contribute to an increased tumor size and the
size of metastatic foci in an animal xenograft model of thyroid
cancer (17). As antigens, MAGE
proteins can be recognized by T-cells and thus lead to immune
activation. This property makes them interesting players in terms
of tumor vaccination. For example, in non-small cell lung cancer
(NSCLC) patients, MAGE-A3 vaccine has been shown to contribute to
the reduction of recurrence rates compared with the control group
(18).
This study was designed to evaluate the prognostic
value of all known MAGE-A antigens in terms of response to
chemotherapy. As the evaluation of the distinct functional role of
all MAGE-A antigens is almost unmanageable, the total and clustered
MAGE-A expression in the context of chemoresistance was
examined.
Materials and methods
Cell lines
We used cell lines that were originally established
(Table I) at the Cancer Institute
of the University of Pittsburgh (19) and used in our previous studies
(13,14,20). As previously described, the cells
were cultured in a humidified atmosphere of 5% CO2/95%
air at 37°C and fed 2 to 3 times/week (14,20).
 | Table IName, origin and TNM status of the 5
cell lines used in this study. |
Table I
Name, origin and TNM status of the 5
cell lines used in this study.
| Name | Origin | TNM
classification |
|---|
| PCI-1 | Laryngeal carcinoma
of the glottis of a male patient | pT2N0M0G2 |
| PCI-9 | Primary carcinoma at
the base of the tongue of a male patient | pT4N3M0G2 |
| PCI-13 | Male patient
diagnosed with oral squamous cell carcinoma of the retromolar
triangle | pT4pN1M0G3 |
| PCI-52 | Primary carcinoma of
the aryepiglottic fold of a male patient | pT2N0M0G2 |
| PCI-68 | Primary tongue
carcinoma of a male patient | pT4N0M0G1 |
Drugs
For the antineoplastic treatment of the cell lines,
we selected agents that are widely used in different protocols for
induction or for neoadjuvant or adjuvant chemotherapy in head and
neck cancer. Cisplatin was purchased from Teva (Radebeul, Germany)
and 5-fluorouracil was purchased from Medac (Hamburg, Germany).
Paclitaxel, docetaxel, cetuximab and panitumumab were provided by
the pharmacy of the University Hospital of Würzburg, Germany.
Measurement of cytotoxicity by real-time
cell analysis (RTCA)
For the determination of cytotoxicity, we used an
impedance-based, non-colorimetric and interference-free RTCA system
(xCELLigence RTCA SP System; Roche, Mannheim, Germany). Measurement
intervals were 30 min. This method is established and has been
previously described for cytotoxicity measurement in head and neck
cancer cell lines (21). We
seeded 10,000 cells/well of each cell line. Following overnight
incubation, cisplatin (25–400 μM), 5-fluo-rouracil (0.75–12
mM), docetaxel (1.56–25 nM), paclitaxel (1.56–25 nM), cetuximab
(0.01–100 μg/ml) and panitumumab (0.01–100 μg/ml),
all dissolved in 20 μl Dulbecco’s modified Eagle’s medium
(Life Technologies GmbH/ThermoFisher Scientific, Darmstadt,
Germany), were added to the cultures, which were incubated for an
additional 40 h. All of the experiments were performed in
triplicate, and further evaluations used mean values.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
For RNA isolation, the cells were detached from the
culture plate and dissolved in TRIzol reagent (Life Technologies
GmbH/ThermoFisher Scientific). After repeated centrifugations (at
12,000 x g), the RNA pellet was resolved in 20 μl of fully
desalinated water. Subsequently, the RNA concentration was
determined using a NanoDrop 2000 system (Thermo Fisher Scientific,
Waltham, MA, USA). The absorption was measured at a wavelength of
260 nm and was normalized to the absorption of the fully
desalinated water control at the same wavelength. All specimens
were diluted to a concentration of 0.2 μg RNA/ml.
In order to remove the DNA contaminants, the RNA was
heated up to 42°C for 2 min and then chilled on ice. This step was
performed in the presence of a gDNA Wipeout Buffer (Qiagen, Venlo,
The Netherlands) (Table II).
Reverse transcription was carried out by incubation at 42°C for 15
min using a Reverse Transcription kit from QuantiTect (Qiagen). The
reverse transcriptase was denatured by heating the sample to 95°C
for an additional 3 min (Table
III). Polymerase chain reaction was conducted using a
QuantiTect SYBR-Green PCR kit 200 (Qiagen) and a C1000 Thermal
Cycler (Bio-Rad, Hercules, CA, USA). The protocol and the primers
(Qiagen) differ from previous publications by our group and are
described in Tables IVTable VVI. Quantification of expression was
calculated relative to β-actin.
 | Table IIComposition of the gDNA Wipeout Buffer
with RNA. |
Table II
Composition of the gDNA Wipeout Buffer
with RNA.
| gDNA Wipeout Buffer,
7X | 2 μl |
| Template RNA | 5 μl |
| RNase-free water | 7 μl |
 | Table IIIComposition of the reverse
transcription reaction with purified RNA. |
Table III
Composition of the reverse
transcription reaction with purified RNA.
| Quantiscript
reverse transcriptase | 1 μl |
| Quantiscript RT
buffer, 5X | 4 μl |
| RT Primer mix | 1 μl |
| Purified RNA | 14 μl |
 | Table IVPolymerase chain reaction
protocol. |
Table IV
Polymerase chain reaction
protocol.
| 1× | Polymerase
activation | 15 min | 95°C |
| 40× | Denaturation | 15 sec | 94°C |
| Annealing | 30 sec | 50–60°C |
| Elongation | 30 sec | 72°C |
 | Table VComposition of the polymerase chain
reaction mixture. |
Table V
Composition of the polymerase chain
reaction mixture.
| SYBR Green Master
Mix | 12.5 μl |
| QuantiTect Primer
assay | 2.75 μl |
| cDNA (concentration
of 10 ng/μl) | 2.5 μl |
| Water | 7.25 μl |
 | Table VIMAGE-A primers. |
Table VI
MAGE-A primers.
| Hs_ACTB_2_SG | #QT01680476 |
| Hs_MAGEA1_2_SG | #QT01669430 |
| Hs_MAGEA2_2_SG | #QT01668688 |
|
Hs_MAGEA2B_1_SG | #QT01033529 |
| Hs_MAGEA3_1_SG | #QT00064799 |
| Hs_MAGEA4_1_SG | #QT00008862 |
| Hs_MAGEA5_3_SG | #QT01849750 |
| Hs_MAGEA6_1_SG | #QT00059129 |
| Hs_MAGEA8_1_SG | #QT00094668 |
| Hs_MAGEA9_1_SG | #QT00230874 |
|
Hs_MAGEA10_1_SG | #QT00005376 |
|
Hs_MAGEA11_1_SG | #QT01004094 |
|
Hs_MAGEA12_1_SG | #QT00033873 |
| Hs_SPP1_1_SG | #QT01008798 |
Statistical analysis
For statistical analysis, we used MEDAS software
(version 2014; Grund, Margetshöchheim, Germany). Global treatment
efficacy is represented as the mean number of viable cells at
different drug concentrations and incubation times for all cell
lines used. We analyzed the influence of the concentration of each
drug at a fixed incubation time with the cell lines as repeats with
Friedman 2-way ANOVA by ranks. To evaluate significant differences
among MAGE-A subgroup expression, we also used the Friedman test.
To simplify expression analysis, factor analysis was performed.
Afterwards, the factors were correlated with treatment efficacy by
Spearman’s rank correlation. Spearman’s Rho is the correlation
coefficient and expresses the relationship between the two
investigated parameters. A Rho value of 1 represents a perfect
correlation between both investigated parameters, whereas a Rho
value of -1 means a perfect inverse correlation of both parameters.
Rho ≥0.7 is considered a high (positive) correlation, whereas Rho
values ≤−0.7 are considered a high inverse correlation. In our
analysis, we correlated the total and the clustered MAGE-A
expression with the results of the RTCA experiments. A high number
of viable cells is represented by a high RTCA cell index and
indicates a low efficacy of the drug used in the experiment. The
significance level was set at p≤0.05.
Results
Treatment efficacy
A brief overview on the efficacy of cisplatin
incubation is provided in Fig.
1A. Of note, the viable fraction after 5 h of incubation is
higher than the control (25/50/100/200 μM cisplatin). The
same effect can be observed after 10 and 20 h of incubation for
concentrations of 25/50/100 μM and 25/50 μM,
respectively. After 40 h of incubation, the viable fraction of all
concentrations was <100%. The effect of cisplatin on the number
of viable cells was significantly associated with the incubation
time. Based on all incubation intervals, the concentration of
cisplatin was not significantly associated with the viable fraction
(Table VII).
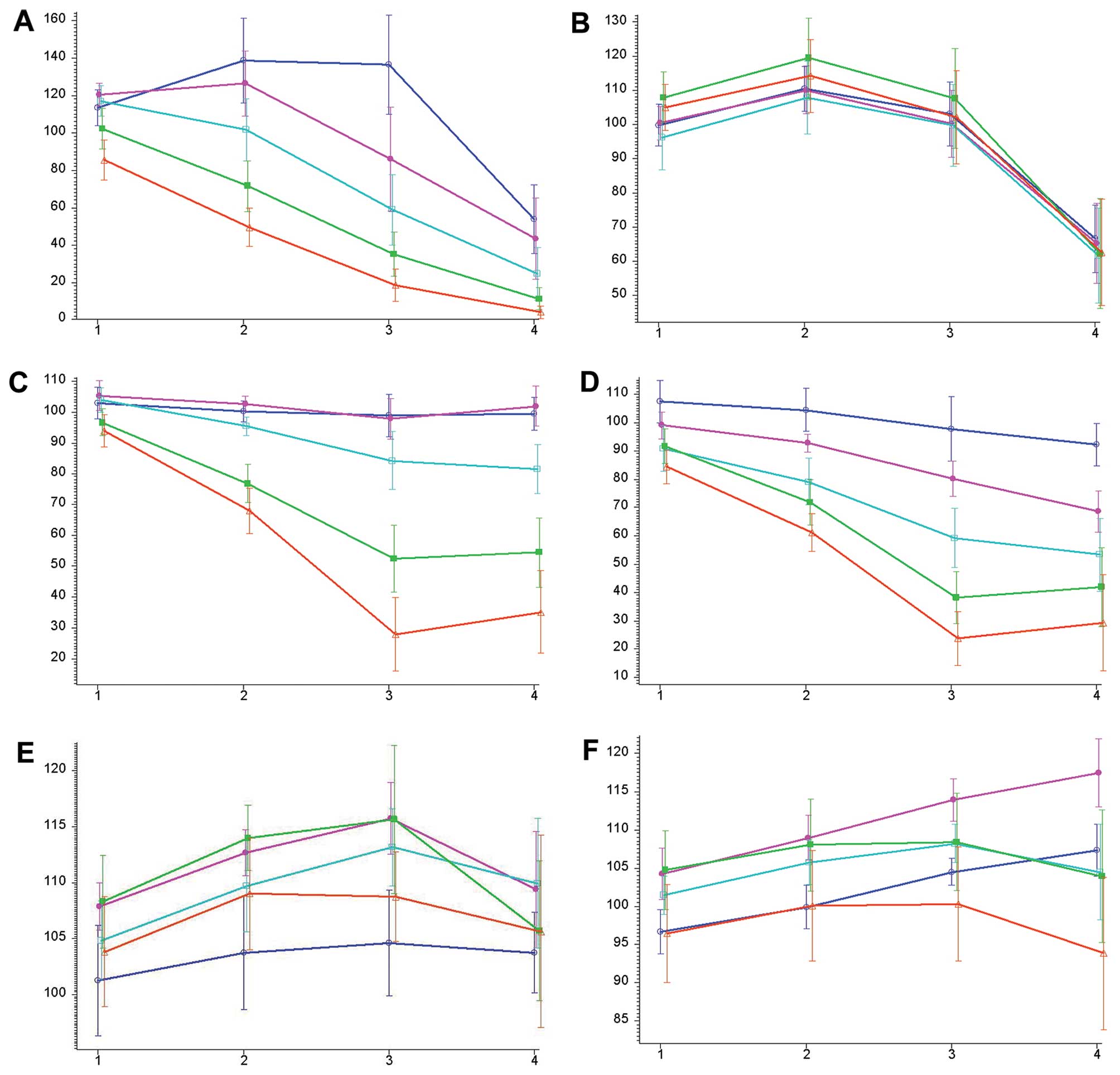 | Figure 1The y-axis represents the viable
fraction compared with the control (100). The x-axis shows
different time intervals (1, 5 h; 2, 10 h; 3, 20 h; 4, 40 h). The
bars indicate the standard deviation of the mean. (A) Cumulative
treatment efficacy of cisplatin. The concentrations are as follows:
blue, 25 μM; pink, 50 μM; turquoise, 100 μM;
green, 200 μM; red, 400 μM. (B) Cumulative treatment
efficacy of 5-fluorouracil. The concentrations are as follows:
blue, 750 μM; pink, 1.5 mM; turquoise, 3 mM; green, 6 mM;
red, 12 mM. (C) Cumulative treatment efficacy of paclitaxel. The
concentrations are as follows: blue, 1.56 nM; pink, 3.12 nM;
turquoise, 6.25 nM; green, 12.5 nM; red, 25 nM. (D) Cumulative
treatment efficacy of docetaxel. The concentrations are as follows:
blue, 1.56 nM; pink, 3.12 nM; turquoise, 6.25 nM; green, 12.5 nM;
red, 25 nM. (E) Cumulative treatment efficacy of cetuximab. The
concentrations are as follows: blue, 0.01 μg/ml; pink, 0.1
μg/ml, turquoise, 1 μg/ml; green, 10 μg/ml;
red, 100 μg/ml. (F) Cumulative treatment efficacy of
panitumumab. The concentrations are as follows: blue, 0.01
μg/ml; pink, 0.1 μg/ml; turquoise, 1 μg/ml;
green, 10 μg/ml; red, 100 μg/ml. |
 | Table VIIAssociation between incubation time
or concentration and the treatment efficacy for all drugs
tested. |
Table VII
Association between incubation time
or concentration and the treatment efficacy for all drugs
tested.
| Drug | p-value (incubation
time) | p-value
(concentration) |
|---|
| Cisplatin |
<0.000005a | 0.19 |
| 5-Fluorouracil | 0.00000a | 0.92 |
| Paclitaxel |
<0.000005a | 0.11 |
| Docetaxel |
<0.000005a | 0.098 |
| Cetuximab | 0.00005a | 0.72 |
| Panitumumab | 0.00005a | 0.51 |
Fig. 1B provides
an overview of the effects of 5-fluorouracil treatment on the cell
lines. After an initial increase in the number of viable cells, the
viable fraction at all concentrations after 40 h of incubation is
clearly below that of the controls. The effect of 5-fluorouracil on
the viable fraction is signifi-cantly associated with the
incubation time. Like cisplatin, the concentration of
5-fluorouracil is not significantly associated with the viable
fraction (Table VII).
The characteristics of paclitaxel treatment are
shown in Fig. 1C. Cells incubated
with 1.56 and 3.12 nM of paclitaxel grew faster than the controls
after 5, 10, 20 and 40 h. Higher concentrations of paclitaxel
(6.25, 12.5 and 25 nM) lead to a smaller viable fraction than the
control cells after 10, 20 and 40 h. The effect of paclitaxel on
the viable fraction is significantly associated with the incubation
time. The concentration of paclitaxel is not significantly
associated with the viable fraction (Table VII).
The effects of docetaxel treatment are comparable to
those of paclitaxel treatment and are shown in Fig. 1D. With the exception of 1.56 nM of
docetaxel after 5 and 10 h, all other concentrations and time
intervals showed a smaller viable fraction than the controls. The
effect of docetaxel on the viable fraction is significantly
associated with the incubation time. The concentration of docetaxel
is not significantly associated with the viable fraction (Table VII).
The effects of cetuximab treatment are shown in
Fig. 1E. Cetuximab treatment at
all concentrations and time intervals led to a higher fraction of
viable cells compared with the controls. This effect is
time-dependent but not concentration-dependent (Table VII).
An overview on panitumumab treatment efficacy is
provided in Fig. 1F. Panitumumab
has no relevant cytostatic effect. However, as with the other
drugs, there was a significant association between treatment
efficacy and time but not concentration (Table VII).
MAGE-A expression
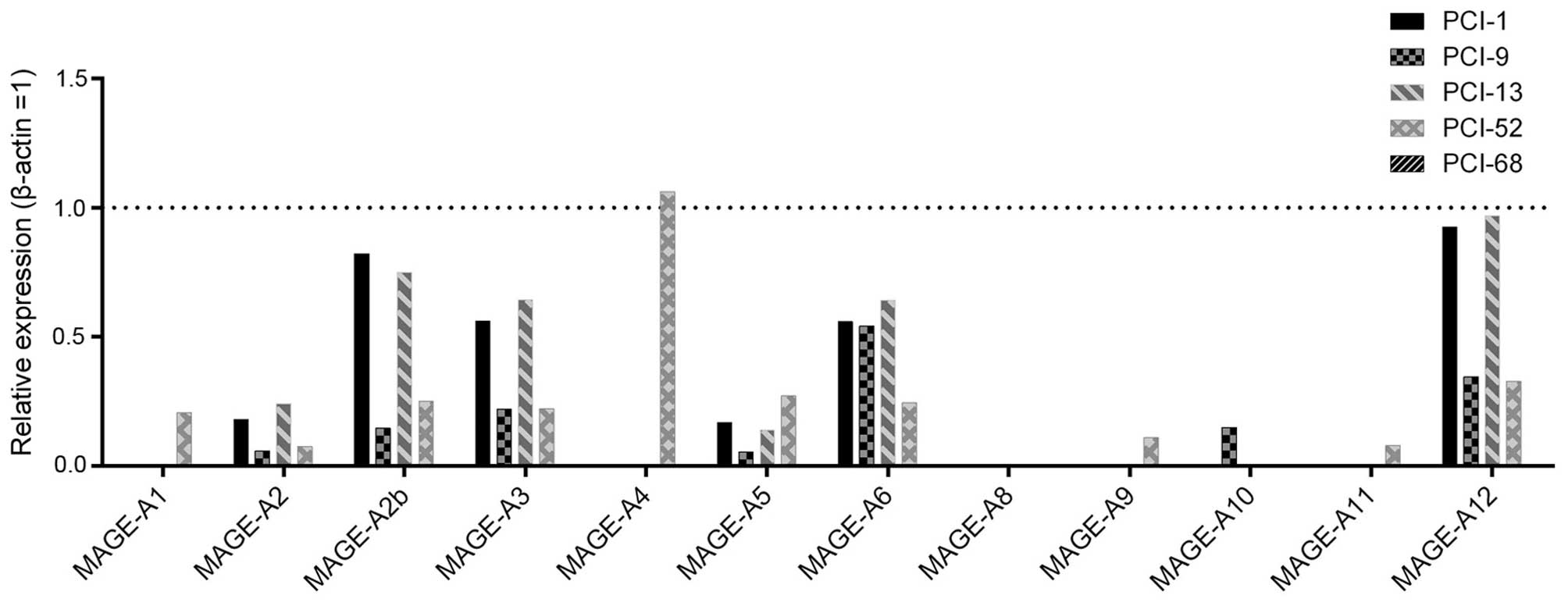
MAGE-A expression analysis showed distinct
differences among the cell lines (Fig. 2). Of note, one cell line (PCI-68)
showed only a marginal expression of MAGE-A antigens, whereas
another cell line (PCI-52) showed a distinct expression of almost
all MAGE-A subgroups (10/12). The expression pattern of the other
cell lines ranged between that of these cell lines. MAGE-A4 showed
the highest expression level of all subgroups, and MAGE-A8 could
not be detected. PCI-52 had the highest levels of MAGE-A1 (0.206),
-A4 (1.062), -A5 (0.271), -A9 (0.108) and -A11 (0.079). PCI-13
showed the strongest expression of MAGE-A2 (0.242), -A3 (0.643),
-A6 (0.641) and A12 (0.969). PCI-1 showed the highest levels of
MAGE-A2b (0.822), and PCI-9 had the strongest expression of
MAGE-A10 (0.149). With the exception of MAGE-A6, -A8, -A9 and -A10,
the expression levels differed significantly among the cell lines
(Table VIII).
 | Table VIIIDifferences in MAGE-A subgroup
expression among the cell lines analyzed by the Friedman test. |
Table VIII
Differences in MAGE-A subgroup
expression among the cell lines analyzed by the Friedman test.
| Subgroup | p-value |
|---|
| MAGE-A1 | 0.017a |
| MAGE-A2 | 0.024a |
| MAGE-A2b | 0.012a |
| MAGE-A3 | 0.032a |
| MAGE-A4 | 0.016a |
| MAGE-A5 | 0.019a |
| MAGE-A6 | 0.088 |
| MAGE-A8 | 0.22 |
| MAGE-A9 | 0.068 |
| MAGE-A10 | 0.080 |
| MAGE-A11 | 0.025a |
| MAGE-A12 | 0.036a |
Factor analysis
The factor analysis suggests the clustering of
MAGE-A expression into 4 groups (Table IX). Cluster 1 consists of
MAGE-A1, -A4, -A5, -A9 and -A11. Cluster 2 consists of MAGE-A2,
-A2b, -A3, -A6 and -A12. MAGE-A8 represents cluster 3, and MAGE-A10
represents cluster 4. Due to the low expression level of the MAGE-A
subgroups -A8 and -A10, clusters 3 and 4 were excluded from further
analyses. Due to the suggested clustering, 96.61% of the variance
was represented, and an excellent fitting was achieved.
 | Table IXResults of factor analysis. |
Table IX
Results of factor analysis.
| MAGE | Factor 1 | Factor 2 | Factor 3 | Factor 4 | Commonality |
|---|
| A1 | −0.108 |
0.990a | −0.055 | −0.041 | 0.997 |
| A2 |
0.967a | −0.048 | −0.029 | −0.071 | 0.943 |
| A2b |
0.885a | −0.133 | −0.265 | −0.081 | 0.877 |
| A3 |
0.986a | −0.047 | −0.026 | 0.002 | 0.975 |
| A4 | −0.098 |
0.989a | −0.056 | −0.043 | 0.992 |
| A5 | 0.548 |
0.791a | −0.095 | −0.033 | 0.936 |
| A6 |
0.776a | −0.077 | 0.570 | −0.162 | 0.960 |
| A8 | −0.120 | −0.093 | 0.081 |
0.984a | 0.998 |
| A9 | −0.126 |
0.970a | −0.074 | −0.056 | 0.965 |
| A10 | −0.143 | −0.173 |
0.955a | 0.121 | 0.977 |
| A11 | −0.117 |
0.989a | −0.025 | −0.039 | 0.995 |
| A12 |
0.987a | −0.068 | −0.011 | −0.042 | 0.980 |
| Variance | 4.65 | 4.57 | 1.34 | 1.03 | 11.593 |
| Variance (%) | 38.76 | 38.12 | 11.13 | 8.60 | 96.61 |
| Factor loading | 5 | 5 | 1 | 1 | 12 |
Correlation of MAGE-A expression and
treatment efficacy
In total, 120 experiments (6 drugs, 5
concentrations, 4 time intervals) represent the treatment efficacy
of the drugs in each cell line.
First, we analyzed the correlation of total MAGE-A
expression level and efficacy of all tested drugs at all
concentrations after all time periods. Of note, there was no
significant correlation between total MAGE-A expression and
treatment efficacy of all tested drugs.
In the next step, we correlated the clustered MAGE-A
expression (cluster 1 or 2) with the treatment efficacy of all
drugs. High correlations (Rho ≥0.7) between MAGE-A expression
(group 1 or 2) and lower treatment efficacy were observed in 38.3%
(46/120) of the experiments. The analysis revealed a significant
correlation between cluster 1 (-A1, -A4, -A5, -A9 and -A11) or
cluster 2 (-A2, -A2b, -A3, -A6 and -A12) expression and several
concentrations of cisplatin, 5-fluorouracil, docetaxel, paclitaxel,
cetuximab and panitumumab (Table
X). Overall, a significant correlation between clustered MAGE-A
expression and treatment efficacy was observed in 20.8% (25/120) of
the experiments.
 | Table XSignificant correlations between
group 1 or 2 MAGE-A expression and treatment efficacy. |
Table X
Significant correlations between
group 1 or 2 MAGE-A expression and treatment efficacy.
| Drug | Concentration | Time | Rho | p-value |
|---|
| Cisplatin | 100 μM | 5 h | 1.0000 | 0.00000b |
| 5-Fluorouracil | 750 μM | 5 h | 0.9000 | 0.037a |
| 5-Fluorouracil | 3 mM | 5 h | 0.9000 | 0.037a |
| 5-Fluorouracil | 3 mM | 20 h | 1.0000 | 0.00000b |
| 5-Fluorouracil | 12 mM | 20 h | 1.0000 | 0.00000b |
| 5-Fluorouracil | 12 mM | 40 h | 0.9000 | 0.037a |
| Paclitaxel | 6.25 nM | 20 h | 0.9000 | 0.037a |
| Paclitaxel | 12.5 nM | 5 h | 0.9000 | 0.037a |
| Paclitaxel | 25 nM | 20 h | 1.0000 | 0.00000b |
| Docetaxel | 1.56 nM | 5 h | 0.9000 | 0.037a |
| Docetaxel | 3.12 nM | 20 h | 1.0000 | 0.00000b |
| Docetaxel | 3.12 nM | 40 h | 0.9000 | 0.037a |
| Docetaxel | 6.25 nM | 5 h | 0.9000 | 0.037a |
| Docetaxel | 12.5 nM | 10 h | 1.0000 | 0.00000b |
| Cetuximab | 0.01
μg/ml | 5 h | 0.9000 | 0.037a |
| Cetuximab | 0.01
μg/ml | 20 h | 0.9000 | 0.037a |
| Cetuximab | 1 μg/ml | 5 h | 1.0000 | 0.00000b |
| Cetuximab | 10
μg/ml | 40 h | 1.0000 | 0.00000b |
| Cetuximab | 100
μg/ml | 5 h | 0.9000 | 0.037a |
| Cetuximab | 100
μg/ml | 20 h | 0.9000 | 0.037a |
| Cetuximab | 100
μg/ml | 40 h | 0.9000 | 0.037a |
| Panitumumab | 0.01
μg/ml | 40 h | 0.9000 | 0.037a |
| Panitumumab | 0.1
μg/ml | 5 h | 1.0000 | 0.00000b |
| Panitumumab | 1 μg/ml | 5 h | 0.9000 | 0.037a |
| Panitumumab | 1 μg/ml | 10 h | 0.9000 | 0.037a |
In all 25 cases, a significant correlation Rho was
between 0.9 and 1.0, indicating a positive correlation between
MAGE-A expression and the RTCA cell index. The RTCA cell index
represents the number of viable cells. A higher cell index
indicates a minor effective chemotherapeutic treatment. In summary,
a higher cluster 1 or 2 MAGE-A expression correlated with a minor
efficacy of the tested drugs.
By contrast, a high inverse correlation (Rho ≤−0.7)
between MAGE-A expression and treatment efficacy was not observed
(data not shown). An inverse correlation (Rho ≤0) was found in
19.2% of the cases (23/120). However, none of these correlations
were significant (data not shown).
Discussion
There is a general agreement that MAGE genes and
proteins are often overexpressed in cancer tissues and contribute
to the progression of malignancies (2,17,22). In addition, there is growing
evidence that MAGE expression is related to a lower efficacy of
systemic antitumor treatment and a poorer prognosis of cancer
patients. In particular, MAGE-A proteins appear to play a role as
regulator of transcription factors, such as p53 (15,23,24). Our group previously described
several correlations between MAGE-A expression patterns and
treatment efficacy in head and neck cancer (13,14,20). In terms of head and neck cancer,
simultaneous nuclear and cytoplasmic expression of MAGE-A proteins
is described as an independent marker for poor survival (9). Based on these findings and due to
the complexity of different roles of all MAGE-A subgroups, the aim
of this study was to investigate the role of total and clustered
MAGE-A expression in the context of chemotherapeutic treatment. To
the best of our knowledge, this study is the first to examine the
correlation of all known MAGE-A subgroups with the impact of
antineoplastic treatment.
As expected, the treatment efficacy showed a wide
range in the different cell lines. For all drugs, we noticed a
significant time-dependent effect. However, treatment with
cetuximab and panitumumab did not lead to relevant cytostatic
effects. This was previously described by our group (14).
Regarding the MAGE-A levels, our analysis yielded an
inhomogenous expression pattern in the different cell lines. One
cell line (PCI-52) expressed 10 out of 12 of the investigated
MAGE-A subgroups. By contrast, PCI-68 showed only a marginal
expression of the MAGE-A subgroups. In our previous studies, we
detected MAGE-A8 expression in this cell line panel (14). However, in this study, we did not
observe relevant MAGE-A8 expression. This may be explained by the
fact that another primer set was used in our previous study. The
MAGE-A2 gene is found on two different loci on the X chromosome
(MAGE-A2 and MAGE-A2b). Of note, in all cell lines expressing
MAGE-A2, we observed higher levels of MAGE-A2b than MAGE-A2. This
indicates that PCR-based quantitative MAGE-A expression analysis
should always include MAGE-A2 and MAGE-A2b. MAGE-A4 showed the
highest levels of all investigated subgroups. With the exception of
PCI-68, all of the cell lines expressed multiple MAGE-A subgroups.
This phenomenon has been reported in the literature and was
described in detail in the study by Ries et al in 2008
(25). Further statistical
analysis revealed significantly different expression levels for A1,
A2, A2b A3, A4, A5, A11 and A12, indicating that a diversified
panel of cell lines was used.
Since the MAGE-A proteins are considered to be
contributors to malignancy, the total amount of protein expression
may correlate with a poorer outcome of antineoplastic treatment. To
our surprise, total MAGE-A expression was not significantly
associated with any of the antineoplastic treatments used in our
experiments. This may be explained by complex interactions between
pro- and anti-apoptotic functions of different MAGE-A subgroups.
Remarkably, the cell line (PCI-52) that was least vulnerable to all
agents had the highest cumulative MAGE-A expression. Nevertheless,
this cell line was the only one expressing MAGE-A4. Of note, Bandić
et al described MAGE-A4 as a positive predictor of survival
in women with invasive ductal breast cancer (26). Furthermore, Sakurai et al
showed that MAGE-A4 interacts with Myc-interacting zinc finger
protein 1 (Miz1), thus leading to apoptosis in a panel of human
cancer cell lines (27). On the
other hand, PCI-52 is the only cell line expressing relevant
amounts of MAGE-A9 and -A11. A negative influence of MAGE-A9 on the
prognosis of breast cancer (28),
hepatocellular carcinoma (8),
renal cell carcinoma (29) and
head and neck cancer (12) has
been extensively described. MAGE-A11 expression also seems to
contribute to malignancy. Xia et al demonstrated that
MAGE-A11 distinctly enhances the proliferation of breast cancer
cells (30). A study by Lian
et al showed that MAGE-A11 expression is a poor prognostic
factor for overall survival in breast cancer (31). Taken together, these findings may
explain how different MAGE-A subgroups antagonize each other, thus
making it impossible to perform a cumulative analysis of all
subgroups.
The finding that total MAGE-A expression showed no
significant correlation with treatment efficacy led us to perform a
cluster analysis of the MAGE-A subgroups. Factor analysis suggested
clustering MAGE-A expression into two clusters. The first cluster
consists of A1, A4, A5, A9 and A11, whereas A2, A2b, A3, A6, A12
represent the second cluster. MAGE-A8 and -A10 were excluded due to
low expression levels. Of note, a high correlation was observed
between clustered expression and minor treatment efficacy in 38.3%
of the experiments (46/120). Remarkably, this finding was
significant in 54.3% (25/46) of the cases. This result is quite
unexpected as MAGE-A4, -A9 and -A11 were clustered in one group but
may have opposite functional roles in terms of apoptosis
regulation. Factor analysis revealed a contrast between PCI-13 and
PCI-52. These two cell lines showed the highest expression levels
of specific MAGE-A subgroups and also had the lowest response to
the antineoplastic drugs. Hence, these findings indicate that
MAGE-A expression, in general, is related to a poorer efficacy of
antineoplastic treatment. Additional analysis is required to
clarify why particular MAGE-A subgroups are clustered together and
others are not.
Even if the majority (7/25) of significant
correlations between high MAGE-A expression and a lower impact of
antineoplastic treatment was found in patients treated with
cetuximab, we were unable to draw any specific conclusions for
clinical use. This notion is supported by the fact that cetuximab
and panitumumab treatment, in general, showed no relevant effect,
and our statistical findings regarding the EGFR antibodies should
be considered an artifact. In clear contrast, high correlations
(Rho ≤−0.7) between strong MAGE-A cluster expression and a better
treatment efficacy were not observed in any of the cases (0/120).
Taken together, these results provide further evidence that the
co-expression of MAGE-A subgroups contributes to the minor efficacy
of antineoplastic treatment.
Since this was a pilot study, there are no direct
clinical implications for antineoplastic treatment at this time.
Further analysis using tissue samples and an in vitro
investigation of the possible interactions among several subgroups
are warranted to clarify the role of the MAGE-A expression
landscape as a predictor for prognosis in different types of
cancer. However, cluster analysis rather than individual subgroup
analysis may provide more rapid results and may be less expensive
in the future.
Acknowledgments
Language editing support was provided by the
American Journal Experts (AJE).
References
|
1
|
Gupta S, Kong W, Peng Y, Miao Q and
Mackillop WJ: Temporal trends in the incidence and survival of
cancers of the upper aerodigestive tract in Ontario and the United
States. Int J Cancer. 125:2159–2165. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Simpson AJ, Caballero OL, Jungbluth A,
Chen YT and Old LJ: Cancer/testis antigens, gametogenesis and
cancer. Nat Rev Cancer. 5:615–625. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
van der Bruggen P, Traversari C, Chomez P,
Lurquin C, De Plaen E, Van den Eynde B, Knuth A and Boon T: A gene
encoding an antigen recognized by cytolytic T lymphocytes on a
human melanoma. Science. 254:1643–1647. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Abd-Elsalam EA and Ismaeil NA:
Melanoma-associated antigen genes: A new trend to predict the
prognosis of breast cancer patients. Med Oncol. 31:2852014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li G, Song P and Zhang B: Expression and
significance of MAGE genes in human lung cancer. Zhongguo Fei Ai Za
Zhi. 16:308–313. 2013.In Chinese. PubMed/NCBI
|
|
6
|
Dyrskjøt L, Zieger K, Kissow Lildal T,
Reinert T, Gruselle O, Coche T, Borre M and Ørntoft TF: Expression
of MAGE-A3, NY-ESO-1, LAGE-1 and PRAME in urothelial carcinoma. Br
J Cancer. 107:116–122. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang QM, He SJ, Shen N, Luo B, Fan R, Fu
J, Luo GR, Zhou SF, Xiao SW and Xie XX: Overexpression of MAGE-D4
in colorectal cancer is a potentially prognostic biomarker and
immunotherapy target. Int J Clin Exp Pathol. 7:3918–3927.
2014.PubMed/NCBI
|
|
8
|
Gu X, Fu M, Ge Z, Zhan F, Ding Y, Ni H,
Zhang W, Zhu Y, Tang X, Xiong L, et al: High expression of MAGE-A9
correlates with unfavorable survival in hepatocellular carcinoma.
Sci Rep. 4:66252014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Laban S, Atanackovic D, Luetkens T, Knecht
R, Busch CJ, Freytag M, Spagnoli G, Ritter G, Hoffmann TK, Knuth A,
et al: Simultaneous cytoplasmic and nuclear protein expression of
melanoma antigen-A family and NY-ESO-1 cancer-testis antigens
represents an independent marker for poor survival in head and neck
cancer. Int J Cancer. 135:1142–1152. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sahin U, Türeci O, Chen YT, Seitz G,
Villena-Heinsen C, Old LJ and Pfreundschuh M: Expression of
multiple cancer/testis (CT) antigens in breast cancer and melanoma:
Basis for polyvalent CT vaccine strategies. Int J Cancer.
78:387–389. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Krauss E, Rauthe S, Gattenlöhner S,
Reuther T, Kochel M, Kriegebaum U, Kübler AC and Müller-Richter UD:
MAGE-A antigens in lesions of the oral mucosa. Clin Oral Investig.
15:315–320. 2011. View Article : Google Scholar
|
|
12
|
Han L, Jiang B, Wu H, Zhang S and Lu X:
Expression and prognostic value of MAGE-A9 in laryngeal squamous
cell carcinoma. Int J Clin Exp Pathol. 7:6734–6742. 2014.PubMed/NCBI
|
|
13
|
Hartmann S, Kriegebaum U, Küchler N,
Brands RC, Linz C, Kübler AC and Müller-Richter UD: Correlation of
MAGE-A tumor antigens and the efficacy of various chemotherapeutic
agents in head and neck carcinoma cells. Clin Oral Investig.
18:189–197. 2014. View Article : Google Scholar
|
|
14
|
Hartmann S, Kriegebaum U, Küchler N,
Lessner G, Brands RC, Linz C, Schneider T, Kübler AC and
Müller-Richter UD: Efficacy of cetuximab and panitumumab in oral
squamous cell carcinoma cell lines: Prognostic value of MAGE-A
subgroups for treatment success. J Craniomaxillofac Surg.
41:623–629. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Monte M, Simonatto M, Peche LY, Bublik DR,
Gobessi S, Pierotti MA, Rodolfo M and Schneider C: MAGE-A tumor
antigens target p53 transactivation function through histone
deacetylase recruitment and confer resistance to chemotherapeutic
agents. Proc Natl Acad Sci USA. 103:11160–11165. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Duan Z, Duan Y, Lamendola DE, Yusuf RZ,
Naeem R, Penson RT and Seiden MV: Overexpression of MAGE/GAGE genes
in paclitaxel/doxorubicin-resistant human cancer cell lines. Clin
Cancer Res. 9:2778–2785. 2003.PubMed/NCBI
|
|
17
|
Liu W, Cheng S, Asa SL and Ezzat S: The
melanoma-associated antigen A3 mediates fibronectin-controlled
cancer progression and metastasis. Cancer Res. 68:8104–8112. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vansteenkiste J, Zielinski M, Linder A, et
al: Adjuvant therapy in stage IB/II non-small cell lung cancer
(NSCLC): Final results of a multi-center, double-blind, randomized,
placebocontrolled phase II study evaluating the MAGE-A3 cancer
immunotherapeutic. EJC Suppl. 5:3612007. View Article : Google Scholar
|
|
19
|
Heo DS, Snyderman C, Gollin SM, Pan S,
Walker E, Deka R, Barnes EL, Johnson JT, Herberman RB and Whiteside
TL: Biology, cytogenetics, and sensitivity to immunological
effector cells of new head and neck squamous cell carcinoma lines.
Cancer Res. 49:5167–5175. 1989.PubMed/NCBI
|
|
20
|
Hartmann S, Seher A, Brands RC, Linz C,
Lessner G, Böhm H, Kübler AC and Müller-Richter UD: Influence of
epidermal growth factor receptor expression on the cetuximab and
pani-tumumab response rates of head and neck carcinoma cells. J
Craniomaxillofac Surg. 42:1322–1328. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wheeler SE, Shi H, Lin F, Dasari S,
Bednash J, Thorne S, Watkins S, Joshi R and Thomas SM: Enhancement
of head and neck squamous cell carcinoma proliferation, invasion,
and metastasis by tumor-associated fibroblasts in preclinical
models. Head Neck. 36:385–392. 2014. View Article : Google Scholar :
|
|
22
|
Yang B, O’Herrin SM, Wu J, Reagan-Shaw S,
Ma Y, Bhat KM, Gravekamp C, Setaluri V, Peters N, Hoffmann FM, et
al: MAGE-A, mMAGE-b, and MAGE-C proteins form complexes with KAP1
and suppress p53-dependent apoptosis in MAGE-positive cell lines.
Cancer Res. 67:9954–9962. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Feng Y, Gao J and Yang M: When MAGE meets
RING: Insights into biological functions of MAGE proteins. Protein
Cell. 2:7–12. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Marcar L, Maclaine NJ, Hupp TR and Meek
DW: MAGE-A cancer/testis antigens inhibit p53 function by blocking
its interaction with chromatin. Cancer Res. 70:10362–10370. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ries J, Vairaktaris E, Mollaoglu N,
Wiltfang J, Neukam FW and Nkenke E: Expression of
melanoma-associated antigens in oral squamous cell carcinoma. J
Oral Pathol Med. 37:88–93. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bandić D, Juretić A, Sarcević B, Separović
V, Kujundzić-Tiljak M, Hudolin T, Spagnoli GC, Cović D and Samija
M: Expression and possible prognostic role of MAGE-A4, NY-ESO-1,
and HER-2 antigens in women with relapsing invasive ductal breast
cancer: Retrospective immunohistochemical study. Croat Med J.
47:32–41. 2006.
|
|
27
|
Sakurai T, Itoh K, Higashitsuji H, Nagao
T, Nonoguchi K, Chiba T and Fujita J: A cleaved form of MAGE-A4
binds to Miz-1 and induces apoptosis in human cells. J Biol Chem.
279:15505–15514. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu X, Tang X, Lu M, Tang Q, Zhang H, Zhu
H, Xu N, Zhang D, Xiong L, Mao Y, et al: Overexpression of MAGE-A9
predicts unfavorable outcome in breast cancer. Exp Mol Pathol.
97:579–584. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hatiboglu G, Pritsch M, Macher-Goeppinger
S, Zöller M, Huber J, Haferkamp A, Pahernik S, Wagener N and
Hohenfellner M: Prognostic value of melanoma-associated antigen A9
in renal cell carcinoma. Scand J Urol. 47:311–322. 2013. View Article : Google Scholar
|
|
30
|
Xia LP, Xu M, Chen Y and Shao WW:
Expression of MAGE-A11 in breast cancer tissues and its effects on
the proliferation of breast cancer cells. Mol Med Rep. 7:254–258.
2013.
|
|
31
|
Lian Y, Sang M, Ding C, Zhou X, Fan X, Xu
Y, Lü W and Shan B: Expressions of MAGE-A10 and MAGE-A11 in breast
cancers and their prognostic significance: A retrospective clinical
study. J Cancer Res Clin Oncol. 138:519–527. 2012. View Article : Google Scholar
|