Introduction
A progressive loss of muscle mass and strength,
known as sarcopenia, represents an important risk factor for
disability and mortality. The loss of skeletal muscle mass has a
profound effect on the daily life of patients, particularly on
physical activity. The resulting decrease in physical activity
induces further skeletal muscle atrophy, leading to a vicious cycle
of atrophic processes (1,2). The main factors that cause muscle
atrophy are denervation, musculoskeletal injury, joint
immobilization, ligament and joint injury, joint inflammation,
prolonged bed rest, glucocorticoid (GLU) treatment, sepsis, cancer
and aging (3,4). Atrophy begins with a reduction in
muscle tension, which is reflected in both a decrease in synthesis
and an increase in protein degradation (5). Four systems of proteolytic
degradation are involved in muscle atrophy: the lysosomal protease
system (cathepsin), calpain calcium-dependent signaling, caspase
signaling and the ubiquitin-proteasome system (5,6).
Oxidative stress has also been well-established as an important
inducer of muscle atrophy in both disuse and muscle catabolic
cachexia (7).
Various animal models of skeletal muscle atrophy
have been used in research, including unloading (8), immobilization (9), starvation (10), denervation (11) and the administration of GLU
(12). Among these, the
administration of high concentrations of dexamethasone (a
representative GLU) causes catabolic changes in skeletal muscle,
mainly due to the stimulation of muscle proteolysis. This
GLU-induced protein degradation is mainly mediated by the
activation of the ubiquitin-proteasome and lysosomal pathways
(13,14). In particular, the muscle-specific
E3-ligases, atrogin-1 and muscle RING-finger protein-1 (MuRF1), and
the lysosomal enzyme, cathepsin L, are highly stimulated by GLUs
(15,16). The upregulation of myostatin, a
member of the transforming growth factor (TGF)-β family, is also an
important negative regulator of skeletal muscle mass that is
involved in GLU-induced catabolic muscle atrophy (17). These findings suggest that
GLU-induced skeletal muscle atrophy may serve as a useful and rapid
animal model for screening agents that can prevent abnormal
catabolic muscle atrophy (18,19).
The dried fruit of Schizandra chinensis
Baillon (S. chinensis), Fructus Schisandrae (FS), is
a well-known traditional herb used for pharmacological purposes in
Asian countries (e.g., Korea, China and Japan) and in Russia to
increase physical working capacity and for its stress-protective
effects against aseptic inflammation and heavy metal intoxication.
It also has beneficial effects on the central nervous, sympathetic
nervous, endocrine, immune, respiratory, cardiovascular and
gastrointestinal systems. It inhibits the development of
experimental atherosclerosis, controls blood sugar and acid-base
balance, and regulates uterus myotonic activity (20,21). In addition, recent studies have
suggested that FS exerts favorable effects on diabetes and related
complications (22–25) due to its smooth muscle relaxant
effects (26,27). However, the effectiveness of FS
administration in the prevention of GLU-induced muscle atrophy
remains unclear.
The aim of the present study was to investigate the
effects of the administration of FS ethanol extracts on
dexamethasone-induced skeletal muscle atrophy in vivo. In
addition, we evaluated the molecular mechanisms involved in
dexamethasone-induced muscle atrophy and the inhibitory effects of
FS on these molecular events, in an aim to determine whether the
administration of FS has therapeutic value as a treatment for
GLU-induced muscle atrophy.
Materials and methods
Test materials
The fruits of S. chinensis were collected
from an area around the city of Mungyeong (Gyeongsangbuk-do, Korea)
and washed 3 times with tap water before being stored at −20°C. The
frozen samples were lyophilized and homogenized using a grinder
prior to extraction. The materials were extracted with 20% ethanol
(FS) at room temperature for 24 h. The extract solution was
filtered and concentrated using a rotary vacuum evaporator (Buchi
Rotavapor R-144, Büchi Labortechnik AG, Flawil, Switzerland).
Oxymetholone
[17β-hydroxy-2-(hydroxymethylene)-17-methyl-5α-androstane-3-one;
Celltrion Pharm Inc., Jincheon, Korea], which is an orally active
17α-alkylated anabolic-androgenic steroid, was used as the
reference drug. Oxymetholone was dissolved at 5 mg/ml in distilled
water and FS was dissolved at 50 mg/ml in distilled water, and
dexamethasone (Sigma-Aldrich Chemical Co., St. Louis, MO, USA) was
dissolved at 50 mg/ml in distilled water. They were stored in at
4°C and diluted with medium to the desired concentration prior to
use.
Animals and experimental design
Adult SPF/VAF outbred CrljOri:CD1 (ICR) mice
(OrientBio Inc., Seungnam, Korea) were used in the experiments in
the present study. The animals were maintained under controlled
environmental conditions under a 12 h/12 h light/dark cycle and
were allowed ad libitum access to water and a standard
laboratory diet. Six groups of 8 mice in each group [i) the intact
vehicle control, ii) the dexamethasone control group, iii) the
oxymetholone-treated group and the FS-treated groups: iv) FS 125
mg/kg-treated group, v) FS 250 mg/kg-treated group and SF 500 mg/kg
treated group] were created in which the mice were selected based
on body weight (35.76±1.32 g; range, 33.40–38.50 g) and calf
thickness (3.15±0.14 mm; range, 2.84–3.42 mm) after 8 days of
acclimatization. Three different concentrations of FS (125, 250 and
500 mg/kg body mass) were orally administered, once a day, for 24
days; treatment with FS was initiated 2 weeks before dexamethasone
treatment, and 50 mg/kg of oxymetholone were also orally
administered in the same time period as FS administration. In this
study, muscle atrophy was induced by a subcutaneous injection of
dexamethasone (1 mg/kg), once a day for 10 days according to a
previously established method (14). An equal volume of distilled water
was orally administered to the mice in the intact vehicle control
and dexamethasone control groups, instead of FS or oxymetholone,
and saline was subcutaneously injected into the mice in the intact
vehicle control gorup instead of dexamethasone. The dosage of
oxymetholone was selected as 50 mg/kg based on a previous efficacy
test in mice (28). This
experiment was conducted according to the international regulations
of the usage and welfare of laboratory animals, and approved by the
Institutional Animal Care and Use Committee of Daegu Haany
University (Gyeongsan, Korea) (Approval no. DHU2014-003).
Measurement of body weight, calf
thickness and gastrocnemius muscle thickness
The body weights of all the mice and the thickness
(mm/mouse) of the left hind calf were measured at 1 day before, and
on days 0, 1, 7, 14, 19, 23 and 24 of the test material
administration using an automatic electronic balance machine
(Precisa Instruments, Dietikon, Switzerland) and an electronic
digital caliper (Mytutoyo, Tokyo, Japan), respectively. The
gastrocnemius muscle thickness of the left hind limb was also
measured using the same method following the exposure of the muscle
at sacrifice to reduce the differences from surrounding tissues,
and the changes in calf thickness during the 14 days prior to the
administration of the test material, during the 10 days of
administration, and for the total 24 days of administration were
additionally calculated to reduce individual differences. Mice were
sacrificed by exsanguination under anesthesia with a 25 mg/kg
intraperitoneal injection of zoletil (Zoletil 50®;
Virbac, Nice, France).
Measurement of gastrocnemius muscle
weight
After measuring gastrocnemius muscle thickness at
sacrifice, the gastrocnemius muscle masses were carefully separated
from the tibia and fibula bones. The weights of individual
gastrocnemius muscle masses were measured at the g levels (absolute
wet weight) using an automatic electronic balance machine, and the
relative weight (% of body weight) was also calculated to reduce
the differences from individual body weights, using body weight at
sacrifice and absolute weight, as follows: relative muscle weight
(% of body weight) = [(absolute organ weight/body weight at
sacrifice) ×100].
Measurement of calf muscle strength
One hour after the final (24th) administration of
the vehicle, oxymetholone or FS (10 days after the initial
dexamethasone treatment), the calf muscle strength of individual
mice was measured as tensile strength (N) using a computerized
testing machine (SV-H1000, Japan Instrumentation System Co., Tokyo,
Japan). Briefly, the animals were restrained in the machine using
two separated 1-0 silk suture ties on the left ankle and chest, and
the peak tensile loads were recorded as calf muscle strength, at
knee angles of 0° (10–20 mm distances).
Serum biochemistry
Sera for blood biochemistry were obtained on the day
of necropsy by centrifuging the blood samples in a separation tube
at 3,000 rpm for 10 min. Serum creatine and creatine kinase (CK)
levels were measured using an autoanalyzer (Hemagen Analyst,
Hemagen Diagnostics, Columbia, MD, USA) and lactate dehydrogenase
(LDH) levels were measured on an automated serum biochemistry
analyzer (SP-4410, Spotochem, Tokyo, Japan).
Antioxidant defense systems
After measuring the muscle weight, the gastrocnemius
muscles were separated for the assessment of malondialdehyde (MDA),
reactive oxygen species (ROS) and glutathione (GSH) contents, as
well as catalase (CAT) and superoxide dismutase (SOD) enzyme
activity in individual muscle. The separated gastrocnemius muscles
were weighed and homogenized in ice-cold 0.01 M Tris-HCl (pH 7.4),
and then centrifuged, at 12,000 g for 15 min, as previously
described by Del Rio et al (29). The concentrations of muscle lipid
peroxides (nmoles MDA/g tissue) were determined by estimating MDA
levels using the thiobarbituric acid test at an absorbance of 525
nm with a UV/Vis spectrometer (Optizen POP Mecasys, Daejeon,
Korea), as previoulsy described (30). Total protein levels were measured
using a previously described method (31) using bovine serum albumin
(Invitrogen, Carlsbad, CA, USA) as a standard. Skeletal muscles
were homogenized and the ROS levels were analyzed using
2′,7′-dichlorofluorescein diacetate fluorescent dye as a probe and
fluorescence density measurement at 490/520 nm according to the
manufacturer’s instructions (ROS assay kit; Abcam, Cambridge, MN,
USA). The measured optical density values [relative fluorescence
unit (RFU)] were corrected by the protein concentrations of the
samples and were expressed as RFU/μg protein, as previoulsy
described (32). The prepared
homogenates were mixed with 0.1 ml of 25% trichloroacetic acid
(Merck, San Francisco, CA, USA), and then centrifuged at 4,200 rpm
for 40 min at 4°C. GSH contents (mg/g tissue) were measured at
absorbance of 412 nm using 2-nitrobenzoic acid (Sigma-Aldrich
Chemical Co.), as previously described (33). The decomposition of
H2O2 in the presence of CAT was followed by
measuring the absorbance at 240 nm, as previously described
(34). CAT activity was defined
as the amount of enzyme required to decompose 1 nM of
H2O2 per minute, at 25°C and pH 7.8. The
results were expressed as U/mg protein. Measurements of SOD
activity were made according the method described in the study by
Sun et al (35). The
estimation of SOD activity was based on the generation of
superoxide radicals produced by xanthine and xanthine oxidase,
which react with nitrotetrazolium blue to form a formazan dye. SOD
activity (U/mg protein) was then measured at 560 nm as the degree
of inhibition of this reaction. One unit of SOD enzymatic activity
is equal to the amount of enzyme that diminishes the initial
absorbance of nitroblue tetrazolium by 50% during 1 min.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RNA was extracted using TRIzol reagent (Invitrogen),
according to the manufacturer’s instructions. The RNA
concentrations and quality were determined using a CFX96™ Real-Time
PCR detection system (Bio-Rad, Hercules, CA, USA). Contaminated DNA
was removed by treating the samples with recombinant DNase I
(DNA-free; Ambion, Austin, TX, USA). RNA was reverse transcribed
using the reagent High-Capacity cDNA Reverse Transcription kit
(Applied Biosystems, Foster City, CA, USA) according to the
manufacturer’s instructions. The internal control was 18S ribosomal
RNA. The sequences of the PCR oligonucleotide primers are listed in
Table I.
 | Table IOligonucleotide primers used for
RT-qPCR in this study. |
Table I
Oligonucleotide primers used for
RT-qPCR in this study.
| Target | | Primer sequence
(5′→3′) | Size (bp) | Gene ID |
|---|
| Atrogin-1 | Forward |
CAGCTTCGTGAGCGACCTC | 244 | 67731 |
| Reverse |
GGCAGTCGAGAAGTCCAGTC | | |
| MuRF 1 | Forward |
GACAGTCGCATTTCAAAGCA | 194 | 433766 |
| Reverse |
GCCTAGCACTGACCTGGAAG | | |
| PI3K p85α | Forward |
GCCAGTGGTCATTTGTGTTG | 236 | 18708 |
| Reverse |
ACACAACCAGGGAAGTCCAG | | |
| Akt1 | Forward |
ATGAACGACGTAGCCATTGTG | 116 | 11651 |
| Reverse |
TTGTAGCCAATAAAGGTGCCAT | | |
| Adenosine A1R | Forward |
TGTTCCCAGGGCCTTTCAC | 155 | 11539 |
| Reverse |
TAATGGACTGAGACTAGCTTGACTGGTA | | |
| TRPV4 | Forward |
CAGGACCTCTGGAAGAGTGC | 165 | 63873 |
| Reverse |
AAGAGCTAGCCTGGACACCA | | |
| Myostatin | Forward |
CCTCCACTCCGGGAACTGA | 185 | 17700 |
| Reverse |
AAGAGCCATCACTGCTGTCATC | | |
| SIRT1 | Forward |
TTCACATTGCATGTGTGTGG | 175 | 93759 |
| Reverse |
TGAGGCCCAGTGCTCTAACT | | |
| 18s Ribosomal
RNA | Forward |
AGCCTGAGAAACGGCTACC | 252 | 19791 |
| Reverse |
TCCCAAGATCCAACTACGAG | | |
Histopathological anlaysis
The samples of gastrocnemius muscle were separated
and fixed in 10% neutral-buffered formalin, embedded in paraffin,
sectioned (3–4 μm) and then stained with hematoxylin and
eosin (H&E) for general histopathological anlaysis, as
previously described (36) or
with Sirius red for detecting collagen fibers, as previously
described (37). The
histopathological profiles of each sample were then determined by
light microscopy observation (Nikkon, Japan). More detailed changes
in the gastrocnemius muscle samples were obtained by calculating
the mean muscle fiber diameters (μm/fiber) and the regions
occupied by collagen fibers (%/mm2 of muscle bundles) in
the muscle bundles for general histomorphometrical analysis using
an automated image analyzer (iSolution FL version 9.1, IMT
i-solution Inc., Quebec, Canada), according to previously described
methods (17,36) with minor modifications.
Immunohistochemistry
The sections were deparaffinized and pre-treated for
citrate buffer antigen (epitope) retrieval, as previously described
(38). Briefly, a staining dish
containing 10 mM citrate buffer (pH 6.0) was heated in a water bath
to a temperature of 95–100°C. The slides were immersed in the
staining dish, loosely covered, incubated for 20 min and then left
to cool for 20 min at room temperature. Following epitope
retrieval, the sections were then immunostained using avidin-biotin
complex (ABC) methods for nitrotyrosine, 4-hydroxynonenal (4-HNE),
inducible nitric oxide synthase (iNOS), and myostatin (Table II), according to a previously
described method (39). The cells
or muscle fibers occupying >20% of immunoreactivities, the
density, of each antiserum for nitrotyrosine, 4-HNE, iNOS,
cyclooxygenase-2 (COX-2), tumor necrosis factor (TNF)-α and
myostatin as compared with the intact muscles, were regarded as
positive, and the mean number of nitrotyrosine, 4-HNE, iNOS and
myostatin-immunoreactive fibers or COX-2 and TNF-α- immunoreactive
cells dispersed in the mm2 of muscle bundles was counted
using an automated image analysis process as per previously
established methods (40,41) with minor modifications. A
histopathologist blinded to the group distribution performed the
analysis.
 | Table IIPrimary antisera and detection kits
used in this study. |
Table II
Primary antisera and detection kits
used in this study.
| Antisera or
detection kits | Code | Source | Dilution |
|---|
| Primary
antiseraa |
|
Anti-4-Hydroxynonenal polyclonal
antibody | Ab46545 | Abcam, Cambridge,
UK | 1:100 |
| Anti-Nitrotyrosine
polyclonal antibody | 06-284 | Millipore
Corporation, Billerica, CA, USA | 1:200 |
| Anti-nitric oxide
synthase2 (N-20) polyclonal antibody | sc-651 | Santa Cruz
Biotechnology, Burlingame, CA, USA | 1:100 |
|
Anti-GDF8/Myostatin antibody | Ab71808 | Abcam, Cambridge,
UK | 1:50 |
| Detection kits |
| Vectastain Elite
ABC kit | PK-6200 | Vector Laboratories
Inc., Burlingame, CA, USA | 1:50 |
| Peroxidase
substrate kit | SK-4100 | Vector Laboratories
Inc., Burlingame, CA, USA | 1:50 |
Statistical analyses
Multiple comparison tests were conducted for the
different groups. Variance homogeneity was examined using the
Levene test (42). If the Levene
test indicated no significant deviations from variance homogeneity,
the obtained data were analyzed by one-way ANOVA followed by a
least-significant differences multi-comparison (LSD) test to
determine which pairs of group comparisons were significantly
different. When significant deviations from variance homogeneity
were observed with the Levene test, a non-parametric comparison
test (Kruskal-Wallis H test) was conducted. When a significant
difference was observed in the Kruskal-Wallis H test, the
Mann-Whitney U (MW) test was conducted to determine which specific
pairs of the group comparison were significantly different.
Statistical analyses were conducted using SPSS software for Windows
(Release 14K, SPSS Inc., USA), as previously described (43).
Results
The administration of FS mitigates the
dexamethasone-induced loss of body weight and calf thickness
As shown in Fig.
1, the mice in the dexamethasone control group presented with
progressive body weight loss throughout the study as compared with
the intact vehicle control group. However, this decrease in body
weight was significantly inhibited by treatment with oxymetholone
and all 3 concentrations of FS at the final (24th) administration
and at sacrifice. As was expected, treatment with dexamethasone
caused a significant decrease in calf thickness compared with the
intact vehicle controls from day 19 after the administration of the
first test substance, the day of the 5th dexamethasone treatment
until sacrifice (Fig. 2). This
decrease in calf thickness was also significantly inhibited in a
dose-dependent manner by treatment with FS compared with the
dexamethasone controls. In this study, the oxymetholone-treated
mice showed a significant increase in calf thickness from the 14th
treatment compared to the mice in the intact vehicle control and
the dexamethasone control groups.
Effects of the administration of FS on
the dexamethasone-induced loss of gastrocnemius muscle thickness
and weight
To investigate muscle loss following treatment,
gastrocnemius muscle thickness and weight were measured immediately
after biopsy. As shown in Fig. 3,
a significant decrease in gastrocnemius muscle thickness after the
exposure of the muscle (through skin removal) was observed in the
mice in the dexamethasone control group compared with the mice in
the intact vehicle control group. A significant increase in
gastrocnemius muscle thickness was observed in the mice
administered oxymetholone and all 3 different concentrations of FS
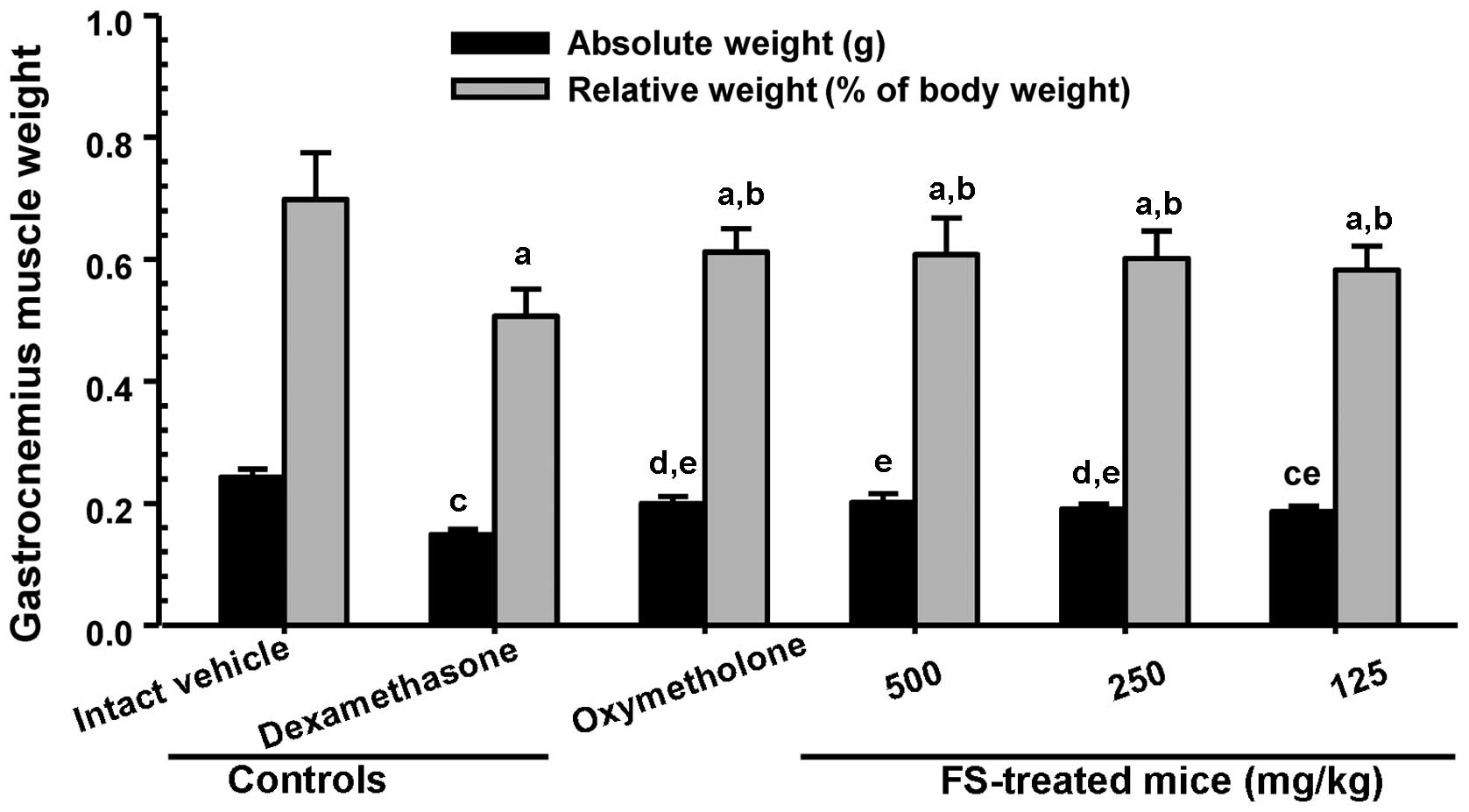
compared with the dexamethasone controls. A significant decrease in
absolute wet-weight and the relative weight of the gastrocnemius
muscle were also observed in the mice in the dexamethasone control
group compared with the mice in the intact vehicle control group
(Fig. 4). The adminsitration of
FS effectively prevented the dexamethasone-induced decrease in
muscle weight compared with the dexamethasone controls (Fig. 4).
The administration of FS inhibits the
dexamethasone-induced decrease in muscle strength
Since reduced absolute muscle strength may reflect
the loss of muscle mass (1,2),
we analyzed the effects of the administration of FS on calf muscle
strength. As was expected, treatment with dexamethasone resulted in
a significant decrease in the tensile strength of the calf muscles
when compared with the intact vehicle control mice (Fig. 5). However, a significant increase
in calf muscle strength was observed in the mice administered
oxymetholone and 500 and 250 mg/kg FS compared with the
dexamethasone controls. In addition, the mice treated with 125
mg/kg FS also showed an increase in calf muscle strength compared
with the dexamethasone controls, although this difference was not
statistically significant (when compared with the dexamethasone
controls).
Effects of the administration of FS on
serum biochemistry
Since the appearance of CK associated with the
decrease in LDH levels in blood serum is considered a surrogate
marker of muscle damage (3,4),
we measured the levels of serum creatine, CK and LDH. A significant
increase in serum creatine and CK levels, and a decrease in serum
LDH levels were observed in the mice in the dexamethasone control
group compared with the mice in the intact vehicle control group;
however, the administration of oxymetholone and FS effectively
attenuated the dexamethasone-induced increase in creatine and CK
levels. Oxymetholone and FS also significantly increased the serum
LDH levels (Table III).
 | Table IIIChanges in serum biochemistry in the
mice with dexamethasone-induced muscle atrophy. |
Table III
Changes in serum biochemistry in the
mice with dexamethasone-induced muscle atrophy.
| Groups | Creatine serum
levels (mg/dl) | CK serum levels
(IU/l) | LDH serum levels
(IU/l) |
|---|
| Controls |
| Intact
vehicle | 0.34±0.05 | 79.75±19.91 | 566.63±135.65 |
| Dexamethasone | 0.76±0.11a |
258.50±50.32d |
144.25±50.47d |
| Reference |
| Oxymetholone | 0.45±0.11b,c |
146.88±30.17d,e |
330.38±84.23d,e |
| FS-treated
mice |
| 500 mg/kg | 0.46±0.12b,c |
148.75±37.44d,e |
319.25±108.50d,e |
| 250 mg/kg | 0.52±0.10b,c |
178.38±28.81d,e |
286.88±101.48d,e |
| 125 mg/kg | 0.60±0.12b,c |
202.13±18.15d,f |
230.75±41.82d,e |
Effects of the administration of FS on
gastrocnemius muscle antioxidant defense systems
Oxidative stress due to greater levels of ROS
production than those normally neutralized by intracellular
antioxidant defenses has recently gained much attention for its
possible involvement in muscle disuse atrophy (7). As shown in Table IV, a significant increase in
muscle lipid peroxidation (elevation of MDA levels) and ROS
contents were observed in the mice in the dexamethasone control
group compared with the mice in the intact vehicle control group.
These elevated levels of MDA and ROS were significantly decreased
by treatment with FS in a dose-dependent manner. In addition, the
elevated lipid peroxide and ROS levels in the oxymetholone-treated
mice were also significantly decreased compared with the
dexamethasone control mice. In addition, a significant decrease in
endogenous antioxidant (GSH) levels and antioxidative enzyme (SOD
and CAT) activity were detected in the dexamethasone controls
compared with the intact vehicle controls, and this decrease was
significantly inhibited by 24 days of continuous oral treatment
with oxymetholone or FS.
 | Table IVChanges in gastrocnemius muscle
antioxidant defense systems in mice with dexamethasone-induced
muscle atrophy. |
Table IV
Changes in gastrocnemius muscle
antioxidant defense systems in mice with dexamethasone-induced
muscle atrophy.
| Groups | Fundus antioxidant
defense systems
|
|---|
| MDA (nM/mg
protein) | ROS (RFU/μg
protein) | GSH (nM/mg
protein) | SOD (nM/mg
protein) | CAT (U/mg
protein) |
|---|
| Controls |
| Intact
vehicle | 1.72±0.57 | 20.17±10.07 | 0.66±0.11 | 27.74±6.76 | 6.32±1.30 |
| Dexamethasone | 8.12±1.12a | 61.22±10.95a | 0.19±0.06a | 11.64±1.46e | 2.06±0.38e |
| Reference |
| Oxymetholone | 4.59±0.61a,c | 35.76±11.06a,c | 0.36±0.07a,c | 19.39±3.19e,f | 3.40±0.49e,f |
| FS-treated
mice |
| 500 mg/kg | 4.21±1.23a,c | 34.01±11.69b,c | 0.37±0.09a,c | 20.93±2.18e,f | 3.50±0.77e,f |
| 250 mg/kg | 5.33±1.17a,c | 40.18±11.75a,c | 0.31±0.07a,c | 18.21±1.89e,f | 3.22±0.50e,f |
| 125 mg/kg | 6.35±0.82a,c | 44.82±8.53a,c | 0.28±0.05a,d | 16.54±2.54e,f | 2.74±0.28e,f |
Effects of the administration of FS on
mRNA expression levels in gastrocnemius muscle
To assess the mechanisms responsible for the
inhibitory effects of FS on dexamethasone-induced muscle atrophy,
we measured the levels of genes involved in muscle growth,
regeneration and the activation of protein synthesis. As presented
in Table V, a significant
increase in the expression of gastrocnemius muscle atrogin-1,
MuRF1, myostatin and sirtuin 1 (SIRT1) mRNA expression was observed
in the dexamethasone controls compared with the intact vehicle
controls. This elevated expression was significantly decreased by
treatment with FS in a dose-dependent manner. These expression
levels in the oxymetholone-treated mice also showed a significant
decrease compared with the mice in the dexamethasone control group.
A significant decrease in the mRNA expression of
phosphatidylinositol 3-kinase (PI3K), Akt1, adenosine A1 receptor
(A1R) and transient receptor potential cation cannel subfamily V
member 4 (TRPV4) was observed in the dexamethasone controls
compared with the intact vehicle controls. A significant increase
in these expression levels was observed with all 3 FS
concentrations used in a dose-dependent manner compared with the
dexamethasone controls.
 | Table VChanges in gastrocnemius muscle mRNA
expression in mice with dexamethasone-induced muscle atrophy. |
Table V
Changes in gastrocnemius muscle mRNA
expression in mice with dexamethasone-induced muscle atrophy.
| Target | Controls
| Reference
| FS-treated mice
(mg/kg)
|
|---|
| Intact vehicle | Dexamethasone | Oxymetholone | 500 | 250 | 125 |
|---|
| Atrogin-1 | 1.02±0.13 | 4.77±0.82e | 2.43±0.56e,f | 2.41±0.43e,f | 3.06±0.87e,f | 3.36±0.98e,g |
| MuRF 1 | 0.98±0.07 | 5.80±1.16e | 2.77±1.11e,f | 2.33±0.61e,f | 3.41±0.58e,f | 4.17±1.21e,g |
| PI3K p85α | 1.09±0.08 | 0.65±0.16a | 1.23±0.44c | 1.29±0.40c | 1.07±0.33c | 0.90±0.15 |
| Akt 1 | 0.95±0.12 | 0.52±0.10a | 0.83±0.11b,c | 0.87±0.08c | 0.78±0.16a,c | 0.73±0.12a,c |
| Adenosine A1R | 1.01±0.12 | 0.54±0.12a | 0.90±0.07b,c | 0.88±0.10b,c | 0.78±0.09a,c | 0.75±0.13a,c |
| TRPV4 | 0.99±0.12 | 0.41±0.12a | 0.71±0.15a,c | 0.74±0.11a,c | 0.64±0.15a,c | 0.57±0.12a,d |
| Myostatin | 1.09±0.14 | 6.97±0.72e | 3.22±1.36e,f | 2.96±1.12e,f | 3.89±0.99e,f | 4.91±1.58e,g |
| SIRT1 | 1.10±0.21 | 8.65±3.70e | 3.02±0.96e,f | 2.85±0.60e,f | 3.65±0.78e,f | 4.43±0.72e,f |
Histopathological and immunohistochemical
anlaysis of the effects of the administration of FS on
gastrocnemius muscle
In the histopathological analysis of muscle atrophy,
we observed that marked and classic catabolic muscle atrophic
changes, including diminishing muscle fibers, microvacuolation and
focal fibrosis in the muscle bundles were induced by treatment with
dexamethasone, as well as a significant decrease in the mean muscle
fiber diameters. An increase in the number of regions occupied by
collagen fibers in muscle bundles was detected in the mice in the
dexamethasone control group compared with the mice in the intact
vehicle control group (Table VI
and Fig. 6). These atrophic
changes were markedly decreased by treatment with FS in a
dose-dependent manner. The muscle atrophic changes were also
significantly inhibited in the oxymetholone-treated mice compared
with the dexamethasone control mice. Moreover, a marked and
significant increase in nitrotyrosine-, 4-HNE- and
iNOS-immunoreactive fibers was also observed in the mice in the
dexamethasone control group; however, FS normalized these
dexamethasone-related changes in a dose-dependent manner (Table VI). Oxymetholone also
significantly decreased the nitrotyrosine-, 4-HNE- and
iNOS-positive muscle fiber numbers compared with those obseved in
the mice in the dexamethasone control group. Furthermore, the
numbers of immunoreactive fibers stained for myostatin, a potent
negative regulator of muscle growth, were significantly increased
in the mice in the dexamethasone control group. However, FS
significantly normalized these changes and oxymetholone also
significantly decreased the numbers of myostatin-positive muscle
fibers compared with those observed in the mice in the
dexamethasone control group.
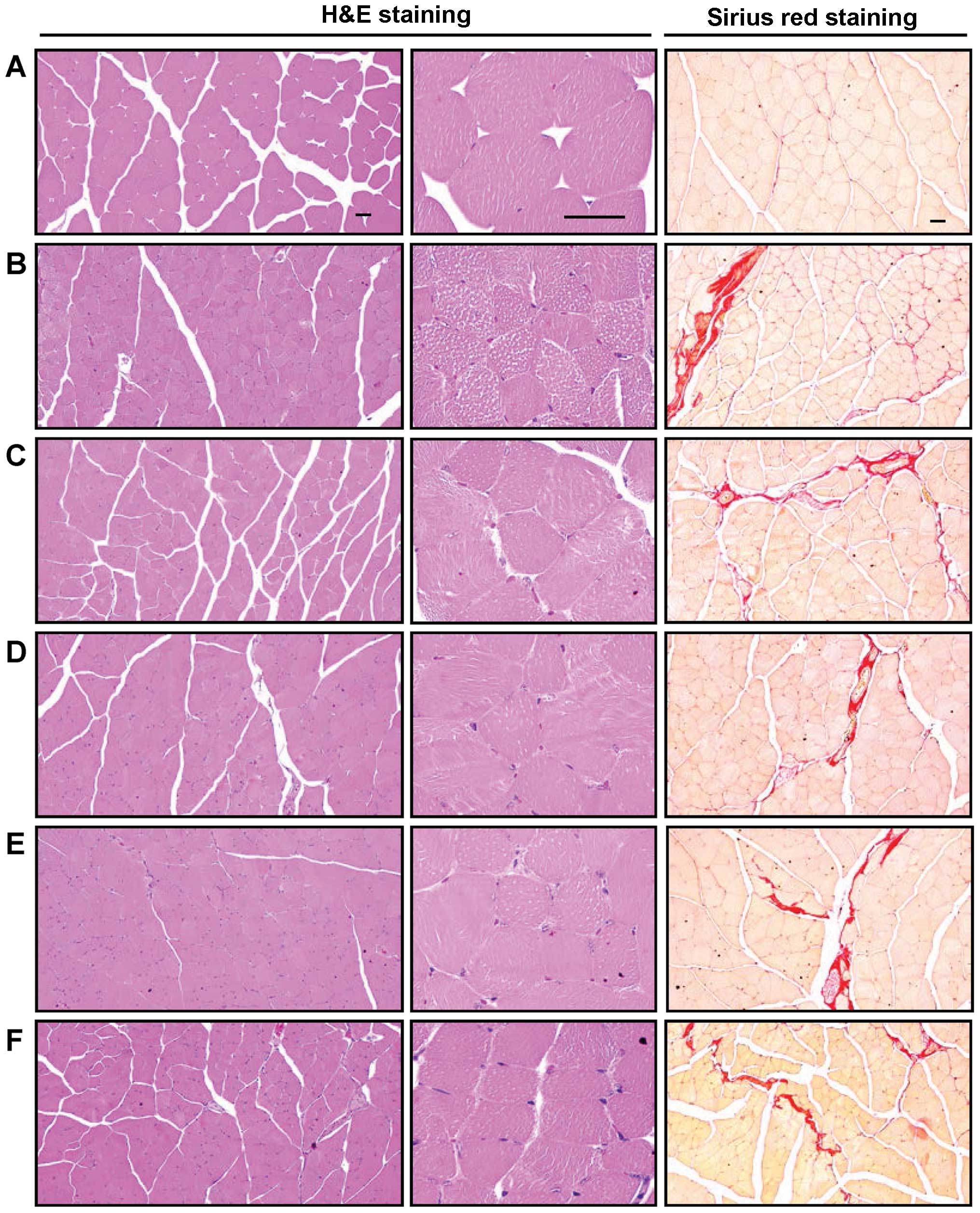 | Figure 6Representative gastrocnemius muscle
histological images, taken from mice in the intact vehicle control
or dexamethasone control groups. Samples from gastrocnemius muscle
were separated and fixed in 10% neutral buffered formalin, then
embedded in paraffin, sectioned and stained with H&E for
general histo-pathological analysis or with Sirius red for the
detection of collagen fibers, and the histopathological profiles of
each sample were determined by light microscopy. (A) Intact vehicle
control, (B) dexamethasone control, (C) 50 mg/kg
oxymetholone-treated mice, (D) 500 mg/kg FS orally-treated mice,
(E) 250 mg/kg FS mg/kg orally-treated mice, (F) 125 mg/kg FS
orally-treated mice. Scale bars, 40 μm. FS, Fructus
Schisandrae. |
 | Table VIHistopathological and
immunohistochemical anlaysis of the changes in gastrocnemius muscle
in mice with dexamethasone-induced muscle atrophy. |
Table VI
Histopathological and
immunohistochemical anlaysis of the changes in gastrocnemius muscle
in mice with dexamethasone-induced muscle atrophy.
| Index | Controls
| Reference
| FS-treated mice
(mg/kg)
|
|---|
| Intact vehicle | Dexamethasone | Oxymetholone | 500 | 250 | 125 |
|---|
| General
histomorphometry |
| Fiber diameter
(μm) | 50.16±5.62 | 24.01±5.13a | 39.24±2.79a,b | 39.84±3.80a,b | 35.75±5.55a,b | 30.16±2.81a,b |
| Collagen (%) | 4.46±1.54 | 30.54±3.83c | 18.15±1.92c,d | 18.87±3.26c,d | 20.84±2.44c,d | 25.58±2.92ce |
|
Immunohistomorphometry
(fibers/mm2) |
| Nitrotyrosine | 6.25±1.49 | 68.25±12.90c | 31.75±10.18c,d | 31.00±5.55c,d | 42.75±17.09c,d | 50.88±11.69ce |
| 4-HNE | 4.38±1.51 | 70.75±6.71a | 36.25±8.66a,b | 37.88±15.75a,b | 49.50±12.14a,b | 54.50±13.15a,b |
| iNOS | 7.38±2.77 | 46.50±5.29a | 21.00±5.10a,b | 29.50±4.17a,b | 32.75±4.86a,b | 36.75±7.65a,b |
| Myostatin | 2.00±1.31 | 52.25±11.16c | 25.38±10.50c,d | 27.38±7.73c,d | 34.88±7.26c,d | 39.75±6.27c,e |
Discussion
Catabolic muscle atrophy induced by GLU is
characterized by a reduction in protein content, the loss of
organelles, cytoplasm, fiber diameter, muscle strength and
resistance to fatigue (14,17). Millions of individuals take GLUs
as chronic therapy for the treatment of diseases, such as
rheumatoid arthritis, asthma, organ transplants and primary or
secondary adrenal insufficiency (44). Common side-effects of GLUs include
insomnia, nervousness, gastrointestinal upset, arthralgias,
immunosuppression, edema and myopathy (45). With over 50 years of use, GLUs are
one of the common medications known to cause myopathy, particularly
when used for prolonged periods ofm time at high concentrations
(46). The incidence of muscle
weakness and myopathy can be as high as 50% in persons receiving
long-term GLU therapy (47,48). The characteristics of myopathy
include muscle atrophy and weakness, insulin resistance, oxidative
stress and mitochondrial dysfunction. Steroid-induced myopathy is
proximal and symmetrical and may involve both the upper and lower
extremities. Steroid myopathy is more commonly associated with the
use of fluorinated steroids, such as dexamethasone, ;betamethasone
and triamcinolone, but can also be caused by non-fluorinated
steroids, such as prednisolone and hydrocortisone (49). In the present study, the
beneficial effects of FS on skeletal muscle were observed in the
mice with GLU (dexamethasone)-induced catabolic muscle atrophy.
All mice used in this study as intact vehicle
controls presented with a normal body weight throughout the
experimental period, which was within the normal range for
age-matched normal reference mice (50). No changes in body weight related
to treatment with the test materials were observed compared with
the mice in the intact vehicle control or dexamethasone control
groups, whereas a signifi-cant decrease in body weight was observed
in the mice in the dexamethasone control group compared with the
mice in the intact vehicle control beginning 5 days after the
initial dexamethasone treatment until sacrifice (Fig. 1). This decrease in body weight
induced by treatment with dexamethasone was considered to be due to
cachexia-related changes resulting from the potent catabolic
effects of dexamethasone itself (51,52), and the increase in body weight
detected with all 3 FS concentrations may be related at least in
part to the well-documented immuno modulatory effects of FS
(53,54). Generally, animals with enhanced
immune systems show relatively good growth patterns (55,56). In addition, oxymetholone, a
representative 17α-alkylated anabolic-androgenic steroid (28), may have inhibited the catabolic
cachexia-related decrease in body weight induced by GLU treatment
through its potent anabolic effects (57,58).
High concentrations of GLU can induce catabolic
muscle atrophy characterized by a reduction and degradation of the
protein content, a decrease in the fiber diameter, a reduction in
muscle strength and poor resistance to fatigue (17,44). Accordingly, in this study, a
decrease in calf thicknesses was noted from 5 days after the
initial dexamethasone treatment, and a decrease in calf muscle
strength, gastrocnemius muscle thickness and body weight at
sacrifice was also observed in the dexamethasone treated-mice as a
result of catabolic muscle atrophy (Figs. 2Figure 3Figure 4–5). Treatment with oral FS and 50 mg/kg
oxymetholone resulted in similar responses, thus providing direct
evidence that both FS and oxymetholone ameliorated the
dexamethasone-induced calf muscle atrophic changes. In this study,
treatment with 500 mg/kg FS showed similar favorable effects on
calf muscle preservation to those observed following treatment with
50 mg/kg oxymetholone.
Creatine is a nitrogenous organic acid that occurs
naturally in vertebrates and helps to supply energy to all cells in
the body, primarily muscle. Creatine synthesis occurs in the liver
and kidneys, but not in muscle, which has no creatine synthesis
capacity, and creatine is accumulated in muscle against a
concentration gradient through specific active transport from
plasma (59). An estimated 98% of
total-body creatine is found in skeletal muscle. The creatine
content in skeletal muscle is relatively constant (60). Creatine is metabolized to its
non-ionic cyclic derivative creatinine at a constant rate of over
1.7% per day (61) by a
non-enzymatic hydrolytic cyclization that is irreversible in
vivo (62). Creatinine
rapidly diffuses from muscle into the plasma and urine with no
re-uptake into muscle (59). It
is not significantly otherwise metabolized, and its excretion under
steady-state conditions therefore equals creatinine production and
is proportional to the total-body creatine pool size and skeletal
muscle mass (59,63). Plasma creatine levels can
therefore be used as a valuable serum biochemistry marker
indicating skeletal muscle damage, activity, or amounts (64,65). In the present study, a marked
increase in serum creatine levels was observed along with
GLU-related catabolic muscle atrophic changes, as previously
demonstrated (13); however,
treatment with FS significantly inhibited this increase in a
dose-dependent manner (Table
III). Treatment with FS at 500 mg/kg in particular, showed
inhibitory effects on serum creatine levels comparable to those
observed with 50 mg/kg oxymetholone, again suggesting that FS has
favorable effects on muscle preservation against muscle atrophy
induced by dexamethasone.
LDH is of medical significance as it is found
extensively in body tissue, such as blood cells and heart muscle,
and CK is an enzyme expressed by various tissues and cell types. CK
cata-lyzes the conversion of creatine and consumes adenosine. Since
these factors are released during tissue damage, they are serum
markers of common injury and disease, particularly muscle damage
(66,67). They are also markedly elevated in
animals with disuse muscle atrophy (68). In a previous study, muscle atrophy
induced by treatment with dexamethasone resulted in a marked
elevation in serum CK levels (69), but in another study, serum LDH
levels were generally decreased due to a reduction in physiological
activity, i.e., reduced contractions of skeletal muscle fibers
(70). A significant elevation in
serum CK levels indicating muscle damage and a decrease in serum
LDH levels suggesting a reduction in muscle activity were also
observed in the mice in the dexamethasone control group in the
present study. A similar concentration-dependent decrease in serum
CK levels and an increase in serum LDH levels were observed in the
FS- and oxymetholone-treated mice compared with the dexamethasone
controls, which provides indirect evidence that FS exerts favorable
and potent effects on muscle preservation (Table III).
Various toxic substances arising from lipid
peroxidation destroy surrounding tissue (71), and oxidative stress is also an
important inducer of muscle atrophy in both disuse and muscle
cachexia (7). GSH is a
representative endogenous antioxidant and prevents tissue damage by
keeping ROS at low levels and at specific cellular concentrations
and is recognized as a protective antioxidant factor in tissue
(72). SOD is one of the
antioxidant enzymes that contributes to enzymatic defense
mechanisms, and CAT is an enzyme that catalyzes the conversion of
H2O2 to H2O (73). The inhibition of the increase in
lipid peroxidation and ROS levels, together with an increase in the
GSH content and SOD and CAT activity in damaged muscle tissu is
also important in terms of protecting muscle against atrophic
changes (32,74). 4-HNE is an α,β-unsaturated
hydroxyalkenal produced by lipid peroxidation in cells, and has
been used as a valuable tissue lipid peroxidation marker. It is
considered as a possible causal agent of numerous diseases, such as
chronic inflammation, neurodegenerative diseases, adult respiratory
distress syndrome, atherogenesis, diabetes and different types of
cancer (75,76). Nitrotyrosine is a product of
tyrosine nitration mediated by reactive nitrogen species, such as
peroxynitrite anion and nitrogen dioxide. It is detected in large
amounts under pathological conditions, and is considered a marker
of iNOS-dependent, reactive nitrogen species-induced nitrative
stress (77,78). In the present study, FS protected
the gastrocnemius muscle against oxidative stress induced by
dexamethasone in a dose-dependent manner, particularly the increase
in lipid peroxidation and ROS formation, the decrease in the GSH
content and SOD and CAT activity, and the increase in nitrotyrosine
and 4-HNE in the muscle fibers (Tables IV and VI). Oxymetholone also showed potent
antioxidant effects against the dexamethasone-induced depletion of
antioxidant defense systems; this result is in accordance with
previously published studies on anabolic steroids (79,80).
Muscle mass and structure are determined by the
balance between protein degradation and synthesis (2). In the protein degradation pathway,
ATP-ubiquitin-dependent proteolysis is the process most responsible
for muscle wasting (81). Three
enzymes are involved in the polyubiquitination cascades in this
process: E1 (ubiquitin-activating), E2 (ubiquitin-conjugating) and
E3 (ubiquitin ligase). It has recently been established that
muscle-specific E3 ubiquitin ligases, such as atrogin-1 and MuRF1
play critical roles in muscle atrophy (2). Atrogin-1 contains an SCF complex
(Skp, Cull and Roc1) (82) and
directly interacts with calcineurin A and α-actinin-2 at the Z-disc
(83). MuRF1 is a member of the
RING finger-B-box-coiled-coil family (84) and interacts with titin at the M
band (85). Previous studies have
demonstrated that the expression levels of atrogin-1 and MuRF1 are
increased in atrophic skeletal muscles and that mice deficient in
either atrogin-1 or MuRF1 are resistant to muscle atrophy (3,82,86). In addition, a marked increase in
atrogin-1 and MuRF1 mRNA expression levels has been detected in
GLU-induced catabolic muscle atrophy (14). In the present study, a marked
elevation in the gastrocnemius muscle atrogin-1 and MuRF1 mRNA
expression levels was also observed in the dexamethasone controls
compared with the intact vehicle controls. This increase in mRNA
expression was inhibited by treatment with FS in a dose-dependent
manner, providing direct evidence that FS exerts potent protective
effects on muscle through the downregulation of atrogin-1 and
MuRF1, which are involved in muscle protein degradation (Table V).
The insulin-like growth factor 1 (IGF-1)/PI3K/Akt
signaling pathway is known to play a pivotal role in activating
protein synthesis (2). PI3K,
which is activated by insulin or IGF, in turn activates Akt, a
serine/threonine kinase, which is located downstream of PI3K,
phosphorylates glycogen synthase kinase-3β and mammalian target of
rapamycin (mTOR), thereby inducing hypertrophy (6). As shown in Table V, a marked downregulation in the
Akt1 and PI3K mRNA expression levels was observed in the mice in
the dexamethasone control group, indicating catabolic muscle
atrophic changes. However, FS upregulated the Akt1 and PI3K mRNA
expression levels in a dose-dependent manner compared with the
dexamethasone controls, providing direct evidence that FS promotes
muscle protein synthesis and prevents dexamethasone-induced
catabolic muscle atrophy, similar to the effects of
oxymetholone.
Adenosine is known to modulate various physiological
functions of the cardiovascular system and of most tissues,
including skeletal muscle (87,88). The involvement of adenosine is
proposed in both the regulation of blood flow to skeletal muscle
(89) and in the synergistic
effect of contraction and insulin-stimulated glucose uptake in
skeletal muscle (90). The
majority of the physiological effects of adenosine are believed to
be mediated through specific adenosine receptors (91). Among these, adenosine A1R has
shown cytoprotective effects on skeletal muscle (92). TRPV4, a member of the TRP channel
superfamily (93,94), is a Ca2+-permeable
non-selective cation channel that appears to play a mechanosensory
or osmosensory role in several musculoskeletal tissue and prevents
muscle atrophy or bone loss (94,95). In this study, dexamethasone
significantly decreased the adenosine A1R and TRPV4 mRNA expression
levels in gastrocnemius muscle due to proteolysis related to
catabolic muscle atrophy. However, FS upregulated the mRNA
expression levels of adenosine A1R and TRPV4 (involved in muscle
growth) in a dose-dependent manner when compared with the
dexamethasone controls (Table V),
again providing direct evidence that FS increased muscle growth and
resistance to dexamethasone-induced catabolic muscle atrophy to a
similar extent as oxymetholone.
Myostatin, a secreted growth differentiation factor,
is a member of the TGF-β protein family that inhibits muscle
differentiation and growth in the process known as myogenesis.
Myostatin is produced primarily in skeletal muscle cells,
circulates in the blood and acts on muscle tissue by binding a
cell-bound receptor called the activin type II receptor (96,97). Myostatin is a potent negative
regulator of muscle growth (14,81). The sirtuin family of proteins
possesses NAD+-dependent deacetylase activity and/or ADP
ribosyltransferase activity. The 7 mammalian sirtuins, SIRT1-7, are
localized differentially within cells and have a variety of
functions. SIRT1 is the most extensively studied member of the
family and regulates diverse biological processes ranging from cell
proliferation, differentiation, apoptosis and metabolism (98). It controls the transcription of
the peroxisome proliferator-activated receptor-γ co-activator 1 α
in skeletal muscle (99) and
induces cachexia by inhibiting muscle regeneration (100). In catabolic muscle atrophy, the
mRNA expression levels of myostatin and SIRT1 have been detected
along with a decrease in muscle mass (14,99), and this was also observed
following treatment with dexamethasone in the present study. This
increase in myostatin mRNA expression was inhibited by FS in a
dose-dependent manner, and FS also inhibited the increase in the
numbers of myostatin-immunoreactive fibers observed in
immunohistochemical analysis in a dose-dependent manner, again
providing direct evidence that FS exerts sufficiently potent muscle
protective effects through the downregulation of myostatin and
SIRT1 (Table V).
Catabolic muscle atrophic changes induced by GLUs
include characteristic histopathological changes involving
diminishing muscle fiber diameters, microvacuolation, collagen
deposition and fibrosis, along with protein degradation (13,17), which were also observed in the
present study. The histopatho-logical inhibition of muscle atrophic
changes by treatment with FS or oxymetholone, demonstrated in this
study, is considered valuable evidence that these substances can
preserve denervation-related muscle atrophy (Fig. 6 and Table VI).
In conclusion, the results from the present study
support a favorable ameliorating effect of FS on muscle atrophy
induced by dexamethasone, by exerting anti-inflammatory and
antioxidant effects related to muscle fiber protection that may be
due to an increase in protein synthesis and a decrease in protein
degradation. These effects of FS may help improve various muscle
atrophies with various etiologies. The effects of treatment with
500 mg/kg FF were comparable to those obseved with treatment with
50 mg/kg oxymetholone, a 17α-alkylated anabolic-androgenic steroid,
which has been used for the treatment of various muscle
disorders.
Acknowledgments
This study was supported by the R&D program of
MOTIE/KEIT (10040391, Development of Functional Food Materials and
Device for Prevention of Aging-associated Muscle Function
Decrease).
References
|
1
|
Metter EJ, Talbot LA, Schrager M and
Conwit R: Skeletal muscle strength as a predictor of all-cause
mortality in healthy men. J Gerontol A Biol Sci Med Sci.
57:B359–B365. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Glass DJ: Signaling pathways perturbing
muscle mass. Curr Opin Clin Nutr Metab Care. 13:225–229. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bodine SC, Latres E, Baumhueter S, Lai VK,
Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K,
et al: Identification of ubiquitin ligases required for skeletal
muscle atrophy. Science. 294:1704–1708. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ramírez C, Russo TL, Sandoval MC, Dentillo
AA, Couto MA, Durigan JL and Salvini TF: Joint inflammation alters
gene and protein expression and leads to atrophy in the tibialis
anterior muscle in rats. Am J Phys Med Rehabil. 90:930–939.
2011.PubMed/NCBI
|
|
5
|
Jackman RW and Kandarian SC: The molecular
basis of skeletal muscle atrophy. Am J Physiol Cell Physiol.
287:C834–C843. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sandri M: Signaling in muscle atrophy and
hypertrophy. Physiology (Bethesda). 23:160–170. 2008. View Article : Google Scholar
|
|
7
|
Powers SK, Kavazis AN and McClung JM:
Oxidative stress and disuse muscle atrophy. J Appl Physiol 1985.
102:2389–2397. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hofer T, Marzetti E, Xu J, Seo AY, Gulec
S, Knutson MD, Leeuwenburgh C and Dupont-Versteegden EE: Increased
iron content and RNA oxidative damage in skeletal muscle with aging
and disuse atrophy. Exp Gerontol. 43:563–570. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Booth FW: Physiologic and biochemical
effects of immobilization on muscle. Clin Orthop Relat Res.
219:15–20. 1987.PubMed/NCBI
|
|
10
|
Thomas DR: Loss of skeletal muscle mass in
aging: Examining the relationship of starvation, sarcopenia and
cachexia. Clin Nutr. 26:389–399. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Léger B, Senese R, Al-Khodairy AW, Dériaz
O, Gobelet C, Giacobino JP and Russell AP: Atrogin-1, MuRF1, and
FoXO, as well as phosphorylated GSK-3beta and 4E-BP1 are reduced in
skeletal muscle of chronic spinal cord-injured patients. Muscle
Nerve. 40:69–78. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kawano F, Tanihata J, Sato S, Nomura S,
Shiraishi A, Tachiyashiki K and Imaizumi K: Effects of
dexamethasone on the expression of beta(1)-, beta (2)- and beta
(3)-adrenoceptor mRNAs in skeletal and left ventricle muscles in
rats. J Physiol Sci. 59:383–390. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kanda F, Takatani K, Okuda S, Matsushita T
and Chihara K: Preventive effects of insulinlike growth factor-I on
steroid-induced muscle atrophy. Muscle Nerve. 22:213–217. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gilson H, Schakman O, Combaret L, Lause P,
Grobet L, Attaix D, Ketelslegers JM and Thissen JP: Myostatin gene
deletion prevents glucocorticoid-induced muscle atrophy.
Endocrinology. 148:452–460. 2007. View Article : Google Scholar
|
|
15
|
Auclair D, Garrel DR, Chaouki Zerouala A
and Ferland LH: Activation of the ubiquitin pathway in rat skeletal
muscle by catabolic doses of glucocorticoids. Am J Physiol.
272:C1007–C1016. 1997.PubMed/NCBI
|
|
16
|
Komamura K, Shirotani-Ikejima H, Tatsumi
R, Tsujita-Kuroda Y, Kitakaze M, Miyatake K, Sunagawa K and Miyata
T: Differential gene expression in the rat skeletal and heart
muscle in glucocorticoid-induced myopathy: Analysis by microarray.
Cardiovasc Drugs Ther. 17:303–310. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qin J, Du R, Yang YQ, Zhang HQ, Li Q, Liu
L, Guan H, Hou J and An XR: Dexamethasone-induced skeletal muscle
atrophy was associated with upregulation of myostatin promoter
activity. Res Vet Sci. 94:84–89. 2013. View Article : Google Scholar
|
|
18
|
Benveniste O, Jacobson L, Farrugia ME,
Clover L and Vincent A: MuSK antibody positive myasthenia gravis
plasma modifies MURF-1 expression in C2C12 cultures and mouse
muscle in vivo. J Neuroimmunol. 170:41–48. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang Z, Nakagawa K, Sarkar A, Maruyama J,
Iwasa H, Bao Y, Ishigami-Yuasa M, Ito S, Kagechika H, Hata S, et
al: Screening with a novel cell-based assay for TAZ activators
identifies a compound that enhances myogenesis in C2C12 cells and
facilitates muscle repair in a muscle injury model. Mol Cell Biol.
34:1607–1621. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Panossian A and Wikman G: Pharmacology of
Schisandra chinensis Bail.: An overview of Russian research and
uses in medicine. J Ethnopharmacol. 118:183–212. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lu Y and Chen DF: Analysis of Schisandra
chinensis and Schisandra sphenanthera. J Chromatogr A.
1216:1980–1990. 2009. View Article : Google Scholar
|
|
22
|
Park S, Hong SM, Ahn IS, Kim YJ and Lee
JB: Huang-Lian-Jie-Du-Tang supplemented with Schisandra chinensis
Baill. and Polygonatum odoratum Druce improved glucose tolerance by
potentiating insulinotropic actions in islets in 90%
pancreatectomized diabetic rats. Biosci Biotechnol Biochem.
73:2384–2392. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang J, Shi LL and Zheng YN:
Dibenzocyclooctadiene lignans from Fructus Schisandrae Chinensis
improve glucose uptake in vitro. Nat Prod Commun. 5:231–234.
2010.PubMed/NCBI
|
|
24
|
Kwon DY, Kim S, Yang HJ and Park S: The
lignan-rich fractions of Fructus Schisandrae improve insulin
sensitivity via the PPAR-γ pathways in in vitro and in vivo
studies. J Ethnopharmacol. 135:455–462. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jang HI, Do GM, Lee HM, Ok HM, Shin JH and
Kwon O: Schisandra chinensis Baillon regulates the gene expression
of phase II antioxidant/detoxifying enzymes in hepatic damage
induced rats. Nutr Res Pract. 8:272–277. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang JM, Ip PS, Che CT and Yeung JH:
Relaxant effects of Schisandra chinensis and its major lignans on
agonists-induced contraction in guinea pig ileum. Phytomedicine.
18:1153–1160. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Young Park J, Wook Yun J, Whan Choi Y, Ung
Bae J, Won Seo K, Jin Lee S, Youn Park S, Whan Hong K and Kim CD:
Antihypertensive effect of gomisin A from Schisandra chinensis on
angiotensin II-induced hypertension via preservation of nitric
oxide bioavailability. Hypertens Res. 35:928–934. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pavlatos AM, Fultz O, Monberg MJ, Vootkur
A and Pharmd: Review of oxymetholone: A 17alpha-alkylated
anabolic-androgenic steroid. Clin Ther. 23:789–801; discussion 771.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Del Rio D, Stewart AJ and Pellegrini N: A
review of recent studies on malondialdehyde as toxic molecule and
biological marker of oxidative stress. Nutr Metab Cardiovasc Dis.
15:316–328. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jamall IS and Smith JC: Effects of cadmium
on glutathione peroxidase, superoxide dismutase, and lipid
peroxidation in the rat heart: A possible mechanism of cadmium
cardiotoxicity. Toxicol Appl Pharmacol. 80:33–42. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lowry OH, Rosebrough NJ, Farr AL and
Randall RJ: Protein measurement with the Folin phenol reagent. J
Biol Chem. 193:265–275. 1951.PubMed/NCBI
|
|
32
|
He HJ, Wang GY, Gao Y, Ling WH, Yu ZW and
Jin TR: Curcumin attenuates Nrf2 signaling defect, oxidative stress
in muscle and glucose intolerance in high fat diet-fed mice. World
J Diabetes. 3:94–104. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sedlak J and Lindsay RH: Estimation of
total, protein-bound, and nonprotein sulfhydryl groups in tissue
with Ellman’s reagent. Anal Biochem. 25:192–205. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Aebi H: Catalase. Methods in enzymatic
analysis. Bergmeyer HU: Academic Press; New York: pp. 673–686.
1974, View Article : Google Scholar
|
|
35
|
Sun Y, Oberley LW and Li Y: A simple
method for clinical assay of superoxide dismutase. Clin Chem.
34:497–500. 1988.PubMed/NCBI
|
|
36
|
Ogawa T, Nikawa T, Furochi H, Kosyoji M,
Hirasaka K, Suzue N, Sairyo K, Nakano S, Yamaoka T, Itakura M, et
al: Osteoactivin upregulates expression of MMP-3 and MMP-9 in
fibroblasts infiltrated into denervated skeletal muscle in mice. Am
J Physiol Cell Physiol. 289:C697–C707. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tipoe GL, Leung TM, Liong EC, Lau TY, Fung
ML and Nanji AA: Epigallocatechin-3-gallate (EGCG) reduces liver
inflammation, oxidative stress and fibrosis in carbon tetrachloride
(CCl4)-induced liver injury in mice. Toxicology.
273:45–52. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shi SR, Chaiwun B, Young L, Cote RJ and
Taylor CR: Antigen retrieval technique utilizing citrate buffer or
urea solution for immunohistochemical demonstration of androgen
receptor in formalin-fixed paraffin sections. J Histochem Cytochem.
41:1599–1604. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ki SH, Yang JH, Ku SK, Kim SC, Kim YW and
Cho IJ: Red ginseng extract protects against carbon
tetrachloride-induced liver fibrosis. J Ginseng Res. 37:45–53.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lee HS, Choi SH and Ku SK: Regional
distribution and relative frequency of gastrointestinal endocrine
cells in the ddN mice: An immunohistochemical study. Anat Histol
Embryol. 39:521–528. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Song MY, Ku SK, Kim HJ and Han JS: Low
molecular weight fucoidan ameliorating the chronic
cisplatin-induced delayed gastrointestinal motility in rats. Food
Chem Toxicol. 50:4468–4478. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Levene A: Pathological factors influencing
excision of tumours in the head and neck. Part I. Clin Otolaryngol
Allied Sci. 6:145–151. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ludbrook J: Update: Microcomputer
statistics packages. A personal view. Clin Exp Pharmacol Physiol.
24:294–296. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Dirks-Naylor AJ and Griffiths CL:
Glucocorticoid-induced apoptosis and cellular mechanisms of
myopathy. J Steroid Biochem Mol Biol. 117:1–7. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Seale JP and Compton MR: Side-effects of
corticosteroid agents. Med J Aust. 144:139–142. 1986.PubMed/NCBI
|
|
46
|
Zoorob RJ and Cender D: A different look
at corticosteroids. Am Fam Physician. 58:443–450. 1998.PubMed/NCBI
|
|
47
|
Bowyer SL, LaMothe MP and Hollister JR:
Steroid myopathy: Incidence and detection in a population with
asthma. J Allergy Clin Immunol. 76:234–242. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Covar RA, Leung DY, McCormick D, Steelman
J, Zeitler P and Spahn JD: Risk factors associated with
glucocorticoid-induced adverse effects in children with severe
asthma. J Allergy Clin Immunol. 106:651–659. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Owczarek J, Jasińska M and
Orszulak-Michalak D: Drug-induced myopathies. An overview of the
possible mechanisms. Pharmacol Rep. 57:23–34. 2005.PubMed/NCBI
|
|
50
|
Fox JG, Cohen BJ and Loew FM: Laboratory
Animal Medicine. Academic Press Inc; Orlando, FL: 1984
|
|
51
|
Gupta AK and Chow M: Prednicarbate
(Dermatop): Profile of a corticosteroid. J Cutan Med Surg.
8:244–247. 2004. View Article : Google Scholar
|
|
52
|
Cho YH, Chung IK, Cheon WH, Lee HS and Ku
SK: Effect of DHU001, a polyherbal formula on formalin-induced paw
chronic inflammation of mice. Toxicol Res (Camb). 27:95–102. 2011.
View Article : Google Scholar
|
|
53
|
Yip AY, Loo WT and Chow LW: Fructus
Schisandrae (Wuweizi) containing compound in modulating human
lymphatic system - a Phase I minimization clinical trial. Biomed
Pharmacother. 61:588–590. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Tang SH, He RR, Huang T, Wang CZ, Cao YF,
Zhang Y and Kurihara H: The protective effect of Schisandra lignans
on stress-evoked hepatic metastases of P815 tumor cells in
restraint mice. J Ethnopharmacol. 134:141–146. 2011. View Article : Google Scholar
|
|
55
|
Duarte CG, dos Santos GL, Azzolini AE and
de Assis Pandochi AI: The effect of the antithyroid drug
propylthiouracil on the alternative pathway of complement in rats.
Int J Immunopharmacol. 22:25–33. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lee HS, Yang KJ, Shin HD, Park BR, Son CW,
Jang HJ, et al: Single oral dose toxicity studies of Polycan,
β-glucan originated from Aureobasidium in mice. J Toxicol Pub
Health. 21:361–365. 2005.
|
|
57
|
Hengge UR, Baumann M, Maleba R, Brockmeyer
NH and Goos M: Oxymetholone promotes weight gain in patients with
advanced human immunodeficiency virus (HIV-1) infection. Br J Nutr.
75:129–138. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Hengge UR, Stocks K, Wiehler H, Faulkner
S, Esser S, Lorenz C, Jentzen W, Hengge D, Goos M, Dudley RE and
Ringham G: Double-blind, randomized, placebo-controlled phase III
trial of oxymetholone for the treatment of HIV wasting. AIDS.
17:699–710. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wyss M and Kaddurah-Daouk R: Creatine and
creatinine metabolism. Physiol Rev. 80:1107–1213. 2000.PubMed/NCBI
|
|
60
|
Balsom PD, Söderlund K and Ekblom B:
Creatine in humans with special reference to creatine
supplementation. Sports Med. 18:268–280. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Fitch CD, Lucy DD, Bornhofen JH and
Dalrymple GV: Creatine metabolism in skeletal muscle. II. creatine
kinetics in man. Neurology. 18:32–42. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Bloch K and Schoenheimer R: Studies in
protein metabolism. XI. The metabolic relation of creatine and
creatinine studies with isotopic nitrogen. J Biol Chem.
131:111–119. 1939.
|
|
63
|
Heymsfield SB, Arteaga C, McManus C, Smith
J and Moffitt S: Measurement of muscle mass in humans: Validity of
the 24-hour urinary creatinine method. Am J Clin Nutr. 37:478–494.
1983.PubMed/NCBI
|
|
64
|
Sala A, Tarnopolsky M, Webber C, Norman G
and Barr R: Serum creatinine: A surrogate measurement of lean body
mass in children with acute lymphoblastic leukemia. Pediatr Blood
Cancer. 45:16–19. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Stimpson SA, Turner SM, Clifton LG, Poole
JC, Mohammed HA, Shearer TW, Waitt GM, Hagerty LL, Remlinger KS,
Hellerstein MK and Evans WJ: Total-body creatine pool size and
skeletal muscle mass determination by creatine-(methyl-D3) dilution
in rats. J Appl Physiol (1985). 112:1940–1948. 2012. View Article : Google Scholar
|
|
66
|
Zhang Y, Huang JJ, Wang ZQ, Wang N and Wu
ZY: Value of muscle enzyme measurement in evaluating different
neuromuscular diseases. Clin Chim Acta. 413:520–524. 2012.
View Article : Google Scholar
|
|
67
|
Choi M, Park H, Cho S and Lee M: Vitamin
D3 supplementation modulates inflammatory responses from the muscle
damage induced by high-intensity exercise in SD rats. Cytokine.
63:27–35. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Cohen I, Bogin E, Chechick A and Rzetelny
V: Biochemical alterations secondary to disuse atrophy in the rat’s
serum and limb tissues. Arch Orthop Trauma Surg. 119:410–417. 1999.
View Article : Google Scholar
|
|
69
|
Orzechowski A, Ostaszewski P, Wilczak J,
Jank M, Bałasińska B, Wareski P and Fuller J Jr: Rats with a
glucocorticoid-induced catabolic state show symptoms of oxidative
stress and spleen atrophy: The effects of age and recovery. J Vet
Med A Physiol Pathol Clin Med. 49:256–263. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Pellegrino MA, D’Antona G, Bortolotto S,
Boschi F, Pastoris O, Bottinelli R, Polla B and Reggiani C:
Clenbuterol antagonizes glucocorticoid-induced atrophy and fibre
type transformation in mice. Exp Physiol. 89:89–100. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Comporti M: Lipid peroxidation and
cellular damage in toxic liver injury. Lab Invest. 53:599–623.
1985.PubMed/NCBI
|
|
72
|
Odabasoglu F, Cakir A, Suleyman H, Aslan
A, Bayir Y, Halici M and Kazaz C: Gastroprotective and antioxidant
effects of usnic acid on indomethacin-induced gastric ulcer in
rats. J Ethnopharmacol. 103:59–65. 2006. View Article : Google Scholar
|
|
73
|
Cheeseman KH and Slater TF: An
introduction to free radical biochemistry. Br Med Bull. 49:481–493.
1993.PubMed/NCBI
|
|
74
|
Süleyman H, Cadirci E, Albayrak A, Polat
B, Halici Z, Koc F, Hacimuftuoglu A and Bayir Y: Comparative study
on the gastroprotective potential of some antidepressants in
indomethacin-induced ulcer in rats. Chem Biol Interact.
180:318–324. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Zarkovic N: 4-hydroxynonenal as a
bioactive marker of pathophysiological processes. Mol Aspects Med.
24:281–291. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Smathers RL, Galligan JJ, Stewart BJ and
Petersen DR: Overview of lipid peroxidation products and hepatic
protein modification in alcoholic liver disease. Chem Biol
Interact. 192:107–112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Pacher P, Beckman JS and Liaudet L: Nitric
oxide and peroxynitrite in health and disease. Physiol Rev.
87:315–424. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Chen JH, Tipoe GL, Liong EC, So HS, Leung
KM, Tom WM, Fung PC and Nanji AA: Green tea polyphenols prevent
toxin-induced hepatotoxicity in mice by down-regulating inducible
nitric oxide-derived prooxidants. Am J Clin Nutr. 80:742–751.
2004.PubMed/NCBI
|
|
79
|
Delgado J, Saborido A and Megías A:
Prolonged treatment with the anabolic-androgenic steroid stanozolol
increases antioxidant defences in rat skeletal muscle. J Physiol
Biochem. 66:63–71. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Yoo YE and Ko CP: Dihydrotestosterone
ameliorates degeneration in muscle, axons and motoneurons and
improves motor function in amyotrophic lateral sclerosis model
mice. PLoS One. 7:e372582012. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Onda A, Jiao Q, Nagano Y, Akimoto T,
Miyamoto T, Minamisawa S and Fukubayashi T: Acupuncture ameliorated
skeletal muscle atrophy induced by hindlimb suspension in mice.
Biochem Biophys Res Commun. 410:434–439. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Gomes MD, Lecker SH, Jagoe RT, Navon A and
Goldberg AL: Atrogin-1, a muscle-specific F-box protein highly
expressed during muscle atrophy. Proc Natl Acad Sci USA.
98:14440–14445. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Li HH, Kedar V, Zhang C, McDonough H, Arya
R, Wang DZ and Patterson C: Atrogin-1/muscle atrophy F-box inhibits
calcineurin-dependent cardiac hypertrophy by participating in an
SCF ubiquitin ligase complex. J Clin Invest. 114:1058–1071. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Centner T, Yano J, Kimura E, McElhinny AS,
Pelin K, Witt CC, Bang ML, Trombitas K, Granzier H, Gregorio CC, et
al: Identification of muscle specific ring finger proteins as
potential regulators of the titin kinase domain. J Mol Biol.
306:717–726. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
McElhinny AS, Kakinuma K, Sorimachi H,
Labeit S and Gregorio CC: Muscle-specific RING finger-1 interacts
with titin to regulate sarcomeric M-line and thick filament
structure and may have nuclear functions via its interaction with
glucocor-ticoid modulatory element binding protein-1. J Cell Biol.
157:125–136. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Cohen S, Brault JJ, Gygi SP, Glass DJ,
Valenzuela DM, Gartner C, Latres E and Goldberg AL: During muscle
atrophy, thick, but not thin, filament components are degraded by
MuRF1-dependent ubiquitylation. J Cell Biol. 185:1083–1095. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Berne RM, Knabb RM, Ely SW and Rubio R:
Adenosine in the local regulation of blood flow: A brief overview.
Fed Proc. 42:3136–3142. 1983.PubMed/NCBI
|
|
88
|
Segal SS and Kurjiaka DT: Coordination of
blood flow control in the resistance vasculature of skeletal
muscle. Med Sci Sports Exerc. 27:1158–1164. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Dobson JG Jr, Rubio R and Berne RM: Role
of adenine nucleotides, adenosine, and inorganic phosphate in the
regulation of skeletal muscle blood flow. Circ Res. 29:375–384.
1971. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Vergauwen L, Hespel P and Richter EA:
Adenosine receptors mediate synergistic stimulation of glucose
uptake and transport by insulin and by contractions in rat skeletal
muscle. J Clin Invest. 93:974–981. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Lynge J and Hellsten Y: Distribution of
adenosine A1, A2A and A2B receptors in human skeletal muscle. Acta
Physiol Scand. 169:283–290. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Zheng J, Wang R, Zambraski E, Wu D,
Jacobson KA and Liang BT: Protective roles of adenosine A1, A2A,
and A3 receptors in skeletal muscle ischemia and reperfusion
injury. Am J Physiol Heart Circ Physiol. 293:H3685–H3691. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Caterina MJ, Schumacher MA, Tominaga M,
Rosen TA, Levine JD and Julius D: The capsaicin receptor: A
heat-activated ion channel in the pain pathway. Nature.
389:816–824. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
94
|
Guilak F, Leddy HA and Liedtke W:
Transient receptor potential vanilloid 4: The sixth sense of the
musculoskeletal system? Ann N Y Acad Sci. 1192:404–409. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Mizoguchi F, Mizuno A, Hayata T, Nakashima
K, Heller S, Ushida T, Sokabe M, Miyasaka N, Suzuki M, Ezura Y, et
al: Transient receptor potential vanilloid 4 deficiency suppresses
unloading-induced bone loss. J Cell Physiol. 216:47–53. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Carnac G, Ricaud S, Vernus B and Bonnieu
A: Myostatin: Biology and clinical relevance. Mini Rev Med Chem.
6:765–770. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Gonzalez-Cadavid NF, Taylor WE, Yarasheski
K, Sinha-Hikim I, Ma K, Ezzat S, Shen R, Lalani R, Asa S, Mamita M,
et al: Organization of the human myostatin gene and expression in
healthy men and HIV-infected men with muscle wasting. Proc Natl
Acad Sci USA. 95:14938–14943. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Haigis MC and Guarente LP: Mammalian
sirtuins - emerging roles in physiology, aging, and calorie
restriction. Genes Dev. 20:2913–2921. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Amat R, Planavila A, Chen SL, Iglesias R,
Giralt M and Villarroya F: SIRT1 controls the transcription of the
peroxisome proliferator-activated receptor-gamma
Co-activator-1alpha (PGC-1alpha) gene in skeletal muscle through
the PGC-1alpha autoregulatory loop and interaction with MyoD. J
Biol Chem. 284:21872–21880. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Toledo M, Busquets S, Ametller E,
López-Soriano FJ and Argilés JM: Sirtuin 1 in skeletal muscle of
cachectic tumour-bearing rats: A role in impaired regeneration? J
Cachexia Sarcopenia Muscle. 2:57–62. 2011. View Article : Google Scholar : PubMed/NCBI
|