Spandidos Publications style
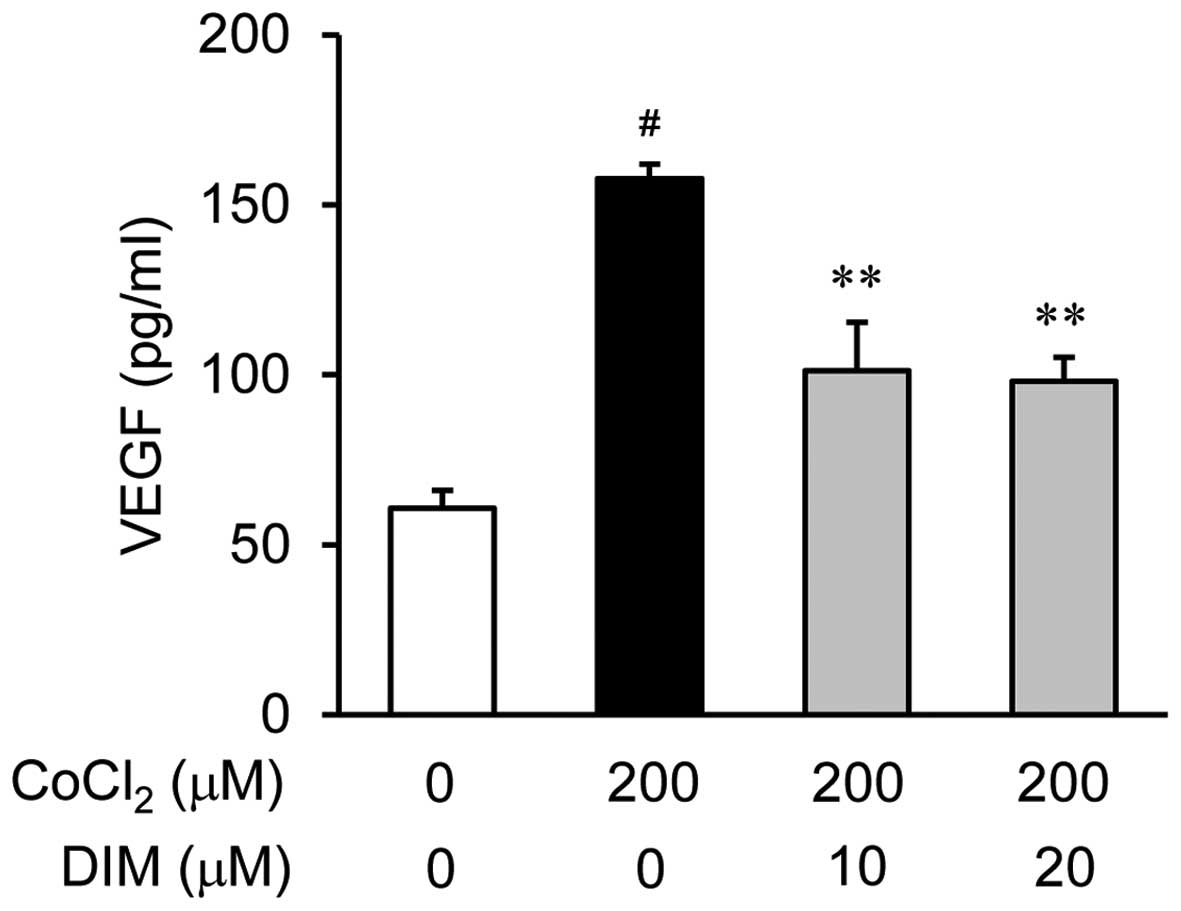
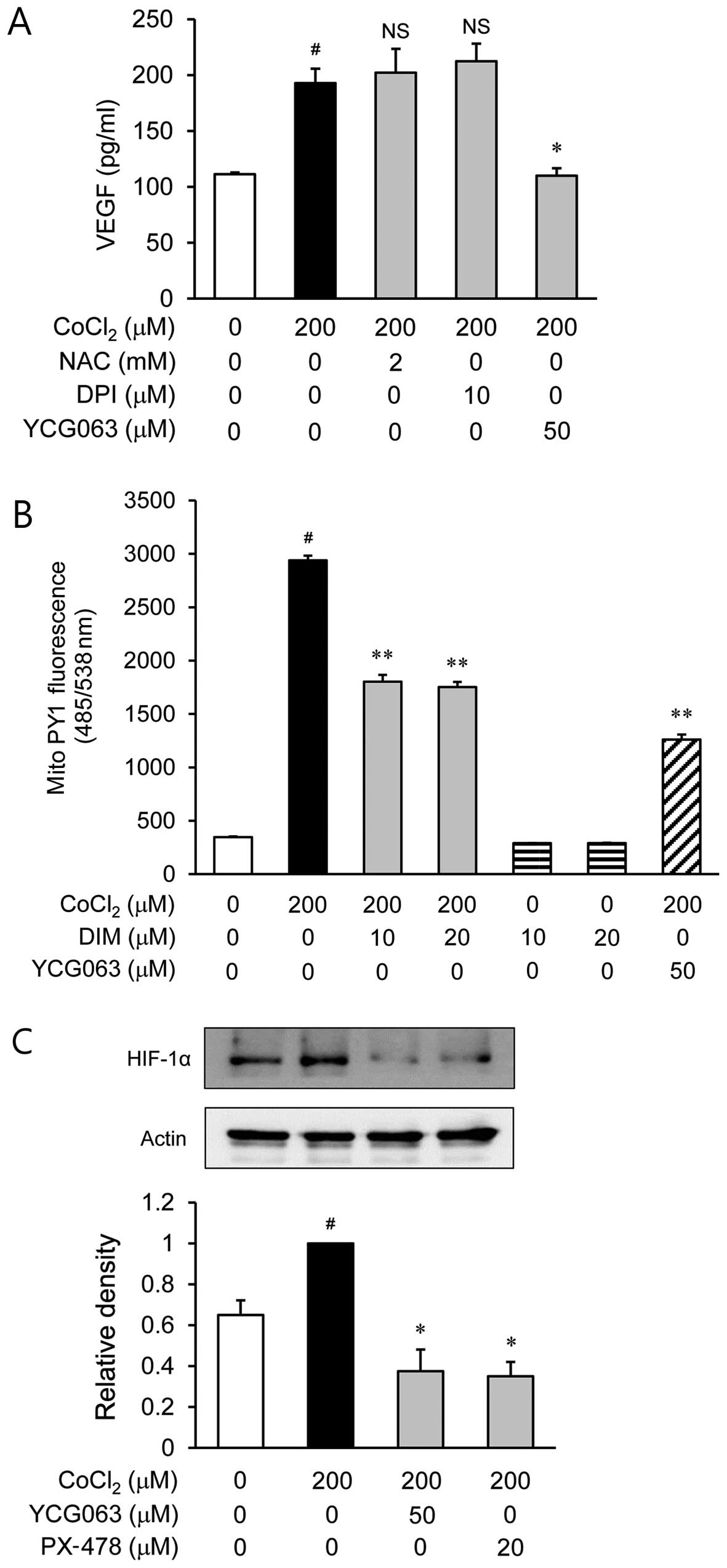
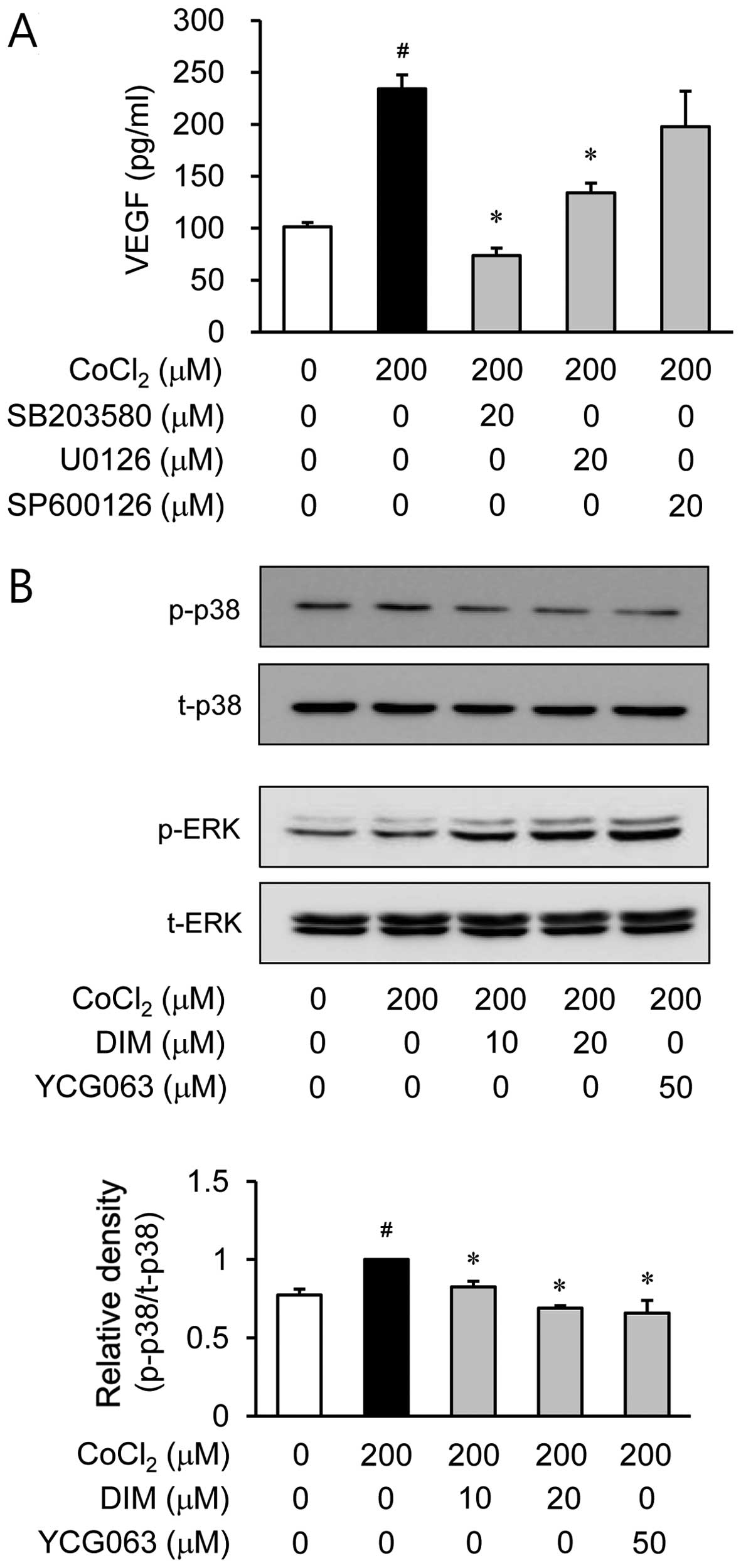
Park H, Lee D, Yim M, Choi YH, Park S, Seo S, Choi JS, Jang WH, Yea SS, Park WS, Park WS, et al: 3,3'-Diindolylmethane inhibits VEGF expression through the HIF-1α and NF-κB pathways in human retinal pigment epithelial cells under chemical hypoxic conditions. Int J Mol Med 36: 301-308, 2015.
APA
Park, H., Lee, D., Yim, M., Choi, Y.H., Park, S., Seo, S. ... Choi, I. (2015). 3,3'-Diindolylmethane inhibits VEGF expression through the HIF-1α and NF-κB pathways in human retinal pigment epithelial cells under chemical hypoxic conditions. International Journal of Molecular Medicine, 36, 301-308. https://doi.org/10.3892/ijmm.2015.2202
MLA
Park, H., Lee, D., Yim, M., Choi, Y. H., Park, S., Seo, S., Choi, J. S., Jang, W. H., Yea, S. S., Park, W. S., Lee, C., Jung, W., Choi, I."3,3'-Diindolylmethane inhibits VEGF expression through the HIF-1α and NF-κB pathways in human retinal pigment epithelial cells under chemical hypoxic conditions". International Journal of Molecular Medicine 36.1 (2015): 301-308.
Chicago
Park, H., Lee, D., Yim, M., Choi, Y. H., Park, S., Seo, S., Choi, J. S., Jang, W. H., Yea, S. S., Park, W. S., Lee, C., Jung, W., Choi, I."3,3'-Diindolylmethane inhibits VEGF expression through the HIF-1α and NF-κB pathways in human retinal pigment epithelial cells under chemical hypoxic conditions". International Journal of Molecular Medicine 36, no. 1 (2015): 301-308. https://doi.org/10.3892/ijmm.2015.2202