Introduction
Embryonic stem cells (ESCs) have the ability to
differentiate into functional neurons and glia through a mechanism
akin to in vivo development (1–6).
However, several issues, such as ethical concerns and the immune
rejection of allograft transplants, impede the use of ESCs.
A major advance in stem cell biology has been the
direct reprogramming of somatic cells through the induction of
transcription factor expression to produce induced pluripotent stem
cells (iPSCs) (7,8). This technique has attracted
considerable attention worldwide due to its applications in human
disease modeling, drug screening and translational medicine
(7,8). iPSCs derived from adult somatic
cells can differentiate into various cell types, including cells of
the three embryonic germ layers. This finding facilitates in
vitro studies on neuronal disorders by enabling the generation
of neural lineage cells, such as oligodendrocytes, astrocytes and
neurons. However, iPSC-based applications are limited by safety
concerns and their complexity, as the generation of iPSCs typically
involves the integration of exogenous transcription factor-encoding
genes (8).
Spermatogonial stem cells (SSCs) are adult male germ
cells that develop after birth and serve as a reservoir of cells
that can differentiate into spermatozoa throughout the lifetime of
an organism (9). Several studies
have demonstrated that SSCs can acquire pluripotency under
appropriate culture conditions (10–16). Such multipotent germline stem
cells (mGSCs) express markers of pluripotency in culture and
produce teratomas when transplanted into immunodeficient mice.
These cells can differentiate into cell types derived from all
three embryonic germ layers (10–16). We recently reported that mGSCs
derived from adult mouse testis are pluripotent and can
differentiate similarly to ESCs and iPSCs (17). These data demonstrate that the
male GSC lineage can be altered in vitro and that unipotent
cells can become pluripotent without the addition of exogenous
transcription factors. mGSCs can be used without ethical and
immunological concerns in personalized cell-based therapies for
patients. In addition, Glaser et al demonstrated that mGSCs
develop into functional neurons with active functional networks and
engage in synchronized oscillatory activity (18).
Fluorescence-activated cell sorting (FACS) is used
to sort cellular populations based on fluorescent labeling
(19,20). A neuronal lineage marker profile
is required, similar to the lineage specification profile of
cluster of differentiation (CD) antigens used for hematopoiesis
(21,22). For translational stem cell
research, we hypothesized that a neuronal precursor cell separation
methodology based on a neuronal lineage marker profile would enable
the efficient transplantation of viable neural stem cell
populations.
In the present study, we generated neural precursor
cells expressing CD24, a neural precursor marker, from pluripotent
stem cell (PSC) lines and demonstrated that these cells effectively
differentiated along the neural lineage in vitro. Using
mGSCs, we also investigated the effects of paracrine molecules on
CD24 expression during neural lineage differentiation.
Materials and methods
All procedures were performed according to the
guidelines for the ethical treatment of animals and were approved
by the Institutional Animal Care and Use Committee of Chung-Ang
University, Seoul, Korea.
Cell culture and differentiation
Unless otherwise stated, all reagents were purchased
from Sigma-Aldrich (St. Louis, MO, USA). The mGSCs were cultured
according to the method described in our previous study [Kim et
al (17)]. Testicular cells
were collected from the testes of adult (6-week-old) POU class 5
homeobox 1 (Pou5f1)-green fluorescent protein (GFP)
transgenic mice [B6; CBA-Tg (Pou5f1-EGFP) 2Mnn/J;
Jackson Laboratory, Bar Harbor, ME, USA] using a two-step enzymatic
digestion procedure. Following enzymatic digestion,
1×107 testicular cells were plated onto 100-mm culture
dishes coated with 0.1% (w/v) gelatin and cultured in GSC culture
medium. After 7 days of continuous culture, GSC clumps were
collected by gentle pipetting. The harvested GSCs were cultured on
a monolayer of mitomycin C-inactivated mouse embryonic fibroblasts
(MEFs) in a 24-well culture dish and passaged once every 7 days at
a dilution of 1:2 to 1:3. The expression of Pou5f1-GFP was
monitored, and morphologically atypical transitional colonies were
mechanically selected. To convert the GSCs into mGSCs, the colonies
were replated onto MEFs in standard ESC medium [Dulbecco's modified
Eagle's medium (DMEM; Invitrogen, Grand Island, NY, USA)
supplemented with 15% fetal bovine serum (FBS; HyClone/Thermo
Scientific, Logan, UT, USA), 1% minimal essential medium
non-essential amino acids (Invitrogen), 2 mM L-glutamine
(Invitrogen), penicillin/streptomycin (penicillin, 50 units/ml;
streptomycin, 50 μg/ml; Invitrogen), 50 μM
β-mercaptoethanol and 103 U/ml leukemia inhibitory factor (Thermo
Scientific)]. Neural stem cells (NSCs)-iPSCs were cultured
according to the method described in the study by Do et al
(46). Briefly, embryos were extracted from parthenogenetic pregnant
female mice at 10.5 days post-coitum and brain tissue was collected
from the embryos (OG2+/−) for the generation of NSCs. To
induce the expression of Pou5f1, SRY (sex determining region Y)-box
2 (Sox2), Krüppel-like factor 4 (KLf4) and c-Myc, the NSCs were
transduced with retrovirus-containing supernatants for 24 h. The
cells were replated onto MEF feeders in ESC medium. The
Pou5f1-GFP+ iPSCs were sorted with FACS and
subcultured onto MEF feeders. Prior to differentiation, the PSCs
were plated onto gelatin-coated dishes with ESC medium for 40 min
to remove the feeder cells. For differentiation, the PSCs were
cultivated as embryoid bodies (EBs) using different media. The
cells were transferred to 24-well ultra-low attachment plates
(Corning, Midland, MI, USA) and cultured at 4×104
cells/ml/well in standard ESC medium, NeuroCult (NeuroCult basal
medium with 1X NeuroCult NSC proliferation supplements; StemCell
Technology, Vancouver, BC, Canada), or N2/B27 medium [DMEM-F12
(Invitrogen) supplemented with 1% B27 (Invitrogen), 0.5% N2
(Invitrogen), 100 μM β-mercaptoethanol and 2 mM
L-glutamine]. The cells were seeded in the presence of either
growth factors or inhibitors, as indicated.
Reverse-transcription-quantitative
PCR
Total RNA was isolated from the cells using a
PureLink RNA Mini kit (from Invitrogen) and reverse transcribed
using SuperScript III reverse transcriptase (Invitrogen), according
to the manufacturer's instructions. Quantitative PCR (qPCR) was
performed using the SYBR-Green PCR Mix (from Applied Biosystems,
Grand Island, NY, USA) and a 7500 Real-Time PCR system (Applied
Biosystems). All gene expression levels were determined by RT-qPCR
and normalized to the level of glyceraldehyde 3-phosphate
dehydrogenase (GAPDH). The primers used were as follows:
nestin forward, 5′-AGTTTGGTCGTGGGGAGATT-3′ and reverse,
5′-ACTTTGGGGAGGCAGGAG-3′; tyrosine hydroxylase (TH) forward,
5′-TTGAAGCCAAAATCCACCA-3′ and reverse, 5′-AGACACCCGACGCACAG-3′;
microtubule-associated protein (MAP)2 forward,
5′-GGGCACCTATTCAGATACCAAA-3′ and reverse,
5′-TCCTTCTCTTGTTCACCTTTCAG-3′; Mbp forward,
5′-GCTTCTTTAGCGGTGACAGG-3′ and reverse, 5′-TGTGTGAGTCCTTGCCAGAG-3′;
Tubb3 forward, 5′-GAATGACCTGGTGTCCGAGT-3′ and reverse,
5′-CCGATTCCTCGTCATCATCT-3′; and GAPDH forward,
5′-CCACTCACGGCAAATTCA-3′ and reverse,
5′-GACTCCACGACATACTCAGCAC-3′.
Flow cytometry and cell sorting
EBs generated from the differentiation experiments
with the PSCs were dissociated by incubating the cells with
trypsin-EDTA (Invitrogen). The cells were stained for specific
markers using the following antibodies: anti-mouse
CD24-allophycocyanin (APC; Cat. no. 17-0242) and IgG2a isotype
control-APC (Cat. no. 17-4210; eBioscience, San Diego, CA, USA).
The dissociated cells were suspended in Dulbecco′s
phosphate-buffered saline (PBS; Invitrogen) supplemented with 1%
FBS, 10 mM HEPES, 1 mM pyruvate, 50 U/ml penicillin (Invitrogen),
50 μg/ml streptomycin (Invitrogen) and 1 mg/ml glucose
(PBS-S). The cells were incubated with the appropriate antibodies
for 20 min on ice, washed twice with excess PBS-S and used for FACS
analysis. After the final wash, the cells were resuspended
(1×106 cells/ml) in PBS-S containing 1 μg/ml
propidium iodide (PI) and kept on ice in the dark until analysis.
Flow cytometric analyses and cell sorting were performed using a
dual-laser FACS Aria II (BD Biosciences, San Jose, CA, USA) at the
Center for Research Facilities, Chung-Ang University, Korea. The
isotype control was used to define the gate in all FACS analyses.
The sorted cells were centrifuged and plated onto 0.1%
gelatin-coated coverslips in N2/B27 medium containing 10 ng/ml
basic fibroblast growth factor (bFGF; BD Biosciences) and 5 nM
retinoic acid (RA).
To evaluate the effect of additional growth factors
on neural differentiation, RA (Cat. no. R2625; Sigma), bone
morphogenetic protein 4 (BMP4, Cat. no. 4050-BP; R&D Systems,
Minneapolis, MN, USA), Noggin (Cat. no. 719-NG; R&D Systems),
Sonic hedgehog (Shh, Cat. no. 461-SH-025; R&D Systems), glial
cell line-derived neurotrophic factor (GDNF, Cat. no. 212-GD;
R&D Systems), fibroblast growth factor 8 (FGF8, Cat. no.
110-25; Peprotech, Rocky Hill, NJ, USA), and bFGF (Cat. no. 354060;
BD Biosciences) were added during differentiation. Dimethyl
sulfoxide (DMSO, Cat. no. D2650; Sigma) was also used as a solvent
for RA.
Immunocytochemical staining
For immunocytochemical staining, the cells were
fixed with 4% paraformaldehyde for 30 min at room temperature,
permeabilized with 0.1% Triton X-100 (Sigma) for 15 min, and
incubated with 5% (w/v) bovine serum albumin (BSA; Roche, Basel,
Switzerland) at room temperature for 30 min. The cells were then
incubated with a primary antibody against MAP2 (Cat. no. sc-32791;
Santa Cruz Biotechnology, Dallas, TX, USA), TH (Cat. no. sc-14007;
Santa Cruz Biotechnology), or glial fibrillary acidic protein
(GFAP; Cat. no. sc-33673; Santa Cruz Biotechnology) diluted 1:200
in 5% BSA solution and incubated overnight at 4°C. Following 2
washes with PBS, the cells were incubated with the respective
secondary antibodies: Alexa Fluor 488 goat anti-mouse (Cat. no.
A11001; Invitrogen) for GFAP and Alexa Fluor 488 donkey anti-rabbit
(Cat. no. A21206; Invitrogen) for TH. The secondary antibodies were
diluted 1:200 in 5% BSA and incubated for 1 h at room temperature
in the dark. The cells were then washed twice with PBS and mounted
on glass slides using Vectashield containing
4′,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories,
Burlingame, CA, USA). The slides were viewed using a Nikon TE
2000-U fluorescence microscope (Nikon, Tokyo, Japan).
Statistical analysis
Statistical analysis was conducted using SPSS
version 18 software (SPSS Inc., Mechanicsburg, PA, USA).
Significant differences between mean values were assessed using
Tukey’s honestly significant difference test and an independent
t-test. Differences were considered statistically significant at
P<0.05.
Results
Effects of medium on the neural
differentiation of mGSCs
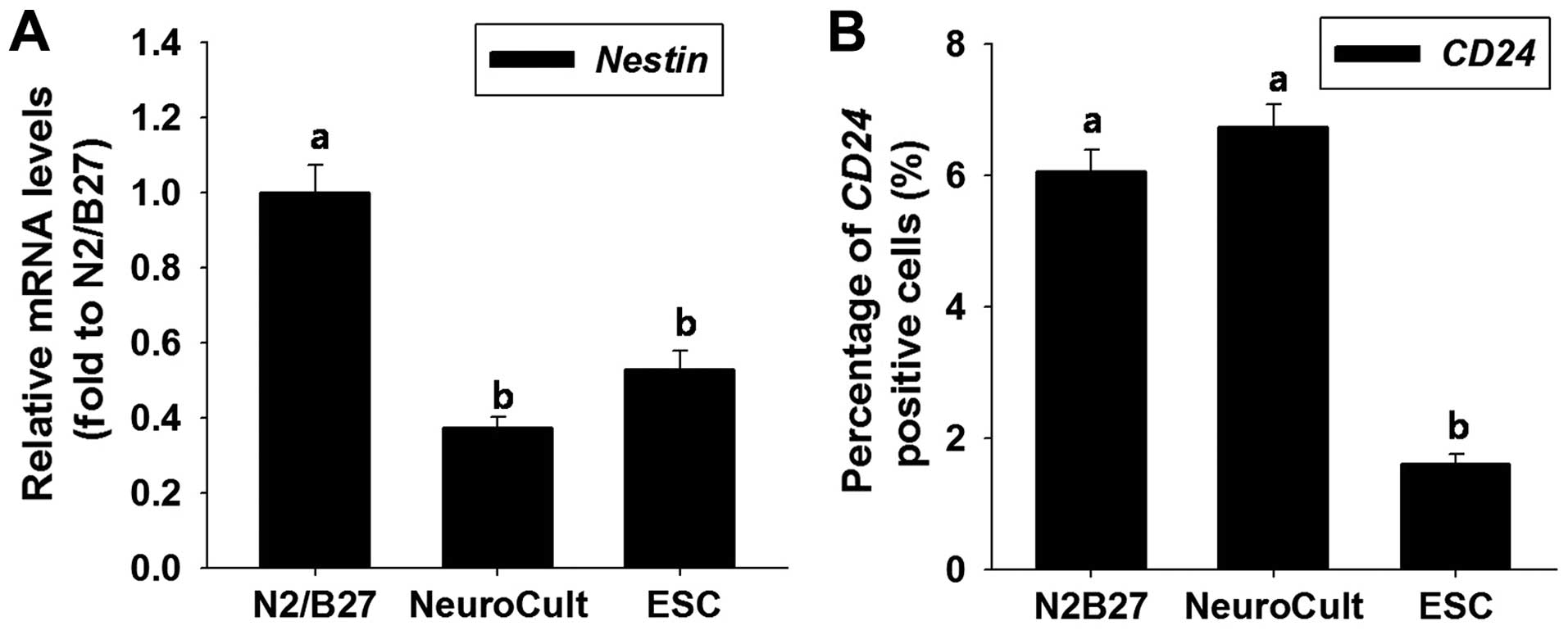
To assess the effects of the different media on the
differentiation of the mGSCs, EBs were generated and cultured for 2
days in N2/B27, NeuroCult, or standard ESC medium in the absence of
any factors, as previously described (17). Nestin is an intermediate
filament-related protein, which has to date been found only in
vertebrates. It is widely accepted as an NSC marker, both during
embryonic development and in the brain at later stages of
development (2,3). In this study, we examined the
temporal gene expression pattern of nestin, using RT-qPCR to
determine the level of nestin required to induce the
differentiation of the cells into the neural lineage. The RT-qPCR
data demonstrated a significant upregulation of nestin mRNA
expression during neural differentiation in N2/B27 medium (Fig. 1A).
EBs produced in the different media were harvested 3
days after they were formed. The cells were dissociated with
enzymatic digestion and analyzed by flow cytometry. CD24 was used
as a marker of neural precursor populations and was found to be
expressed in 6.1±0.3, 6.7±0.4 and 1.6±0.2% of the EBs following
culture in N2/B27, NeuroCult and standard ESC medium, respectively.
Thus, CD24 was expressed at low levels when the EBs were formed in
ESC medium (Fig. 1B). On the
basis of nestin and CD24 expression, subsequent experiments were
performed using N2/B27 medium for neural differentiation.
Evaluation of the neural differentiation
potential of sorted CD24+ cells
The functions of dopaminergic neurons and the
neurotransmitter, dopamine, have been extenstively investigated
[reviewed in (23)]. TH has been
widely used as a marker of dopaminergic neurons as it is the first
enzyme in the dopamine synthesis pathway, as well as the
rate-limiting enzyme in dopamine synthesis. Microtubule assembly
dynamics are regulated by proteins known as MAPs, including MAP1,
MAP2 and Tau, which are expressed primarily in neurons. Among the
neuronal MAPs, MAP2 expression is considered a marker of neural
differentiation (24,25). MAP2, found primarily in the
dendritic extensions of post-mitotic, terminally differentiated
neurons, plays a role in neurite outgrowth and dendrite induction
(26–28).
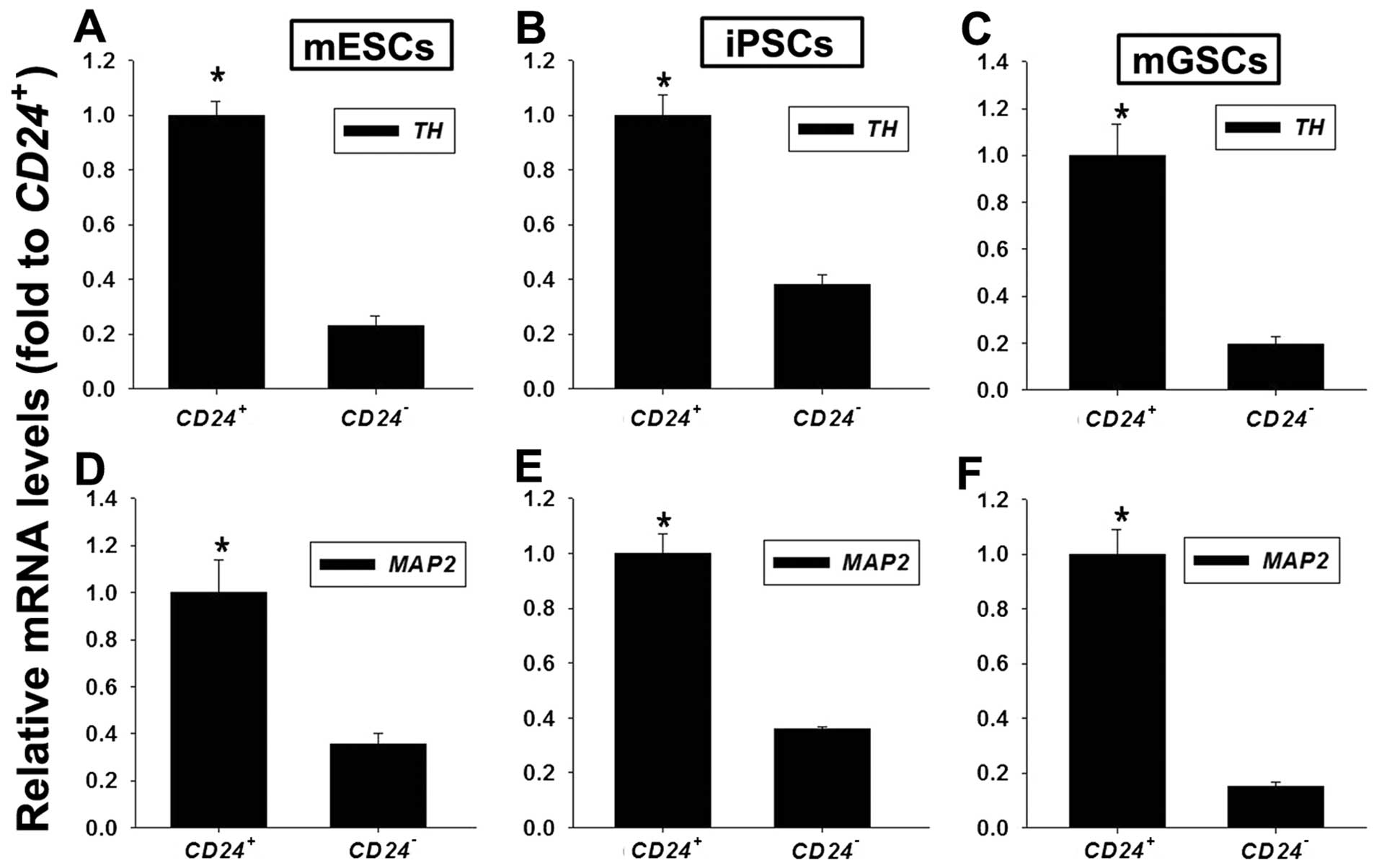
mESCs, iPSCs and mGSCs were cultured in the
indicated differentiation medium for 3 days to generate EBs, which
were then dissociated and sorted into CD24+ and
CD24− cells. We evaluated the differentiation potential
of these cells follwoing further differentiation in a monolayer
culture. On day 3 following differentiation, the cells were stained
with an APC-conjugated CD24 antibody, and the sorted cells were
plated onto 0.1% gelatin-coated 24-well plates in N2/B27 medium
containing 10 ng/ml of bFGF and 5 nM of RA. After 7 days, mRNA was
isolated from these cultures, and RT-qPCR was performed to assess
the TH and MAP2 expression levels. TH expression in the
CD24+ cells was upregulated when compared with its
expression in CD24− cells (4.3-, 2.6- and 5-fold higher
in CD24+ cells vs. CD24− cells derived from
mESCs, iPSCs and mGSCs, respectively) (Fig. 2A–C). Additionally, MAP2 expression
was higher in the CD24+ cells than in the
CD24− cells (2.9-, 2.8- and 6.7-fold higher in the
CD24+ cells vs. the CD24− cells derived from
mESCs, iPSCs and mGSCs, respectively) (Fig. 2D–F).
Effect of paracrine factors on
CD24+ neural precursor induction
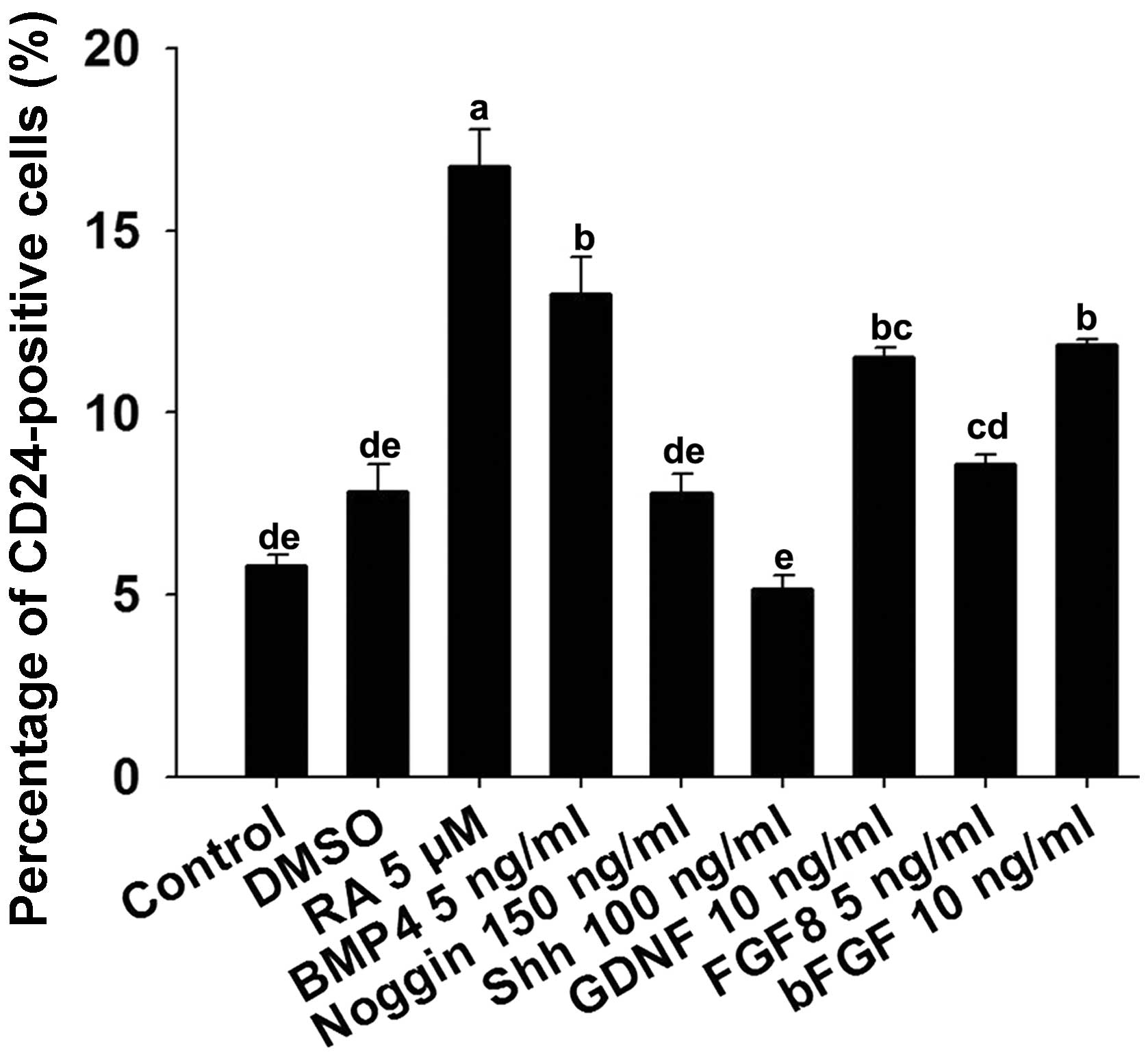
In order to determine whether paracrine factors
induce the specific differentiation of mGSCs into CD24-expressing
neural precursor cells, the mGSCs were treated with 100 ng/ml Shh,
5 μM RA, 150 ng/ml noggin, 5 ng/ml FGF8, 10 ng/ml bFGF, 10
ng/ml GDNF or 5 ng/ml BMP4. DMSO (1 μl/ml) in N2/B27 medium
was used as a solvent control. The FACS data demonstrated that the
percentage of cells expressing CD24 was higher in the RA-treated
group than in the other groups (control, 5.8±0.3%; DMSO, 7.8±0.7%;
RA, 16.8±1.0%; BMP4, 13.3±1.0%; noggin, 7.8±0.5%; Shh, 5.2±0.4%;
GDNF, 11.5±0.2%; FGF8, 8.6±0.3%; bFGF, 11.9±0.1%; mean ± SEM; n=3)
(Fig. 3).
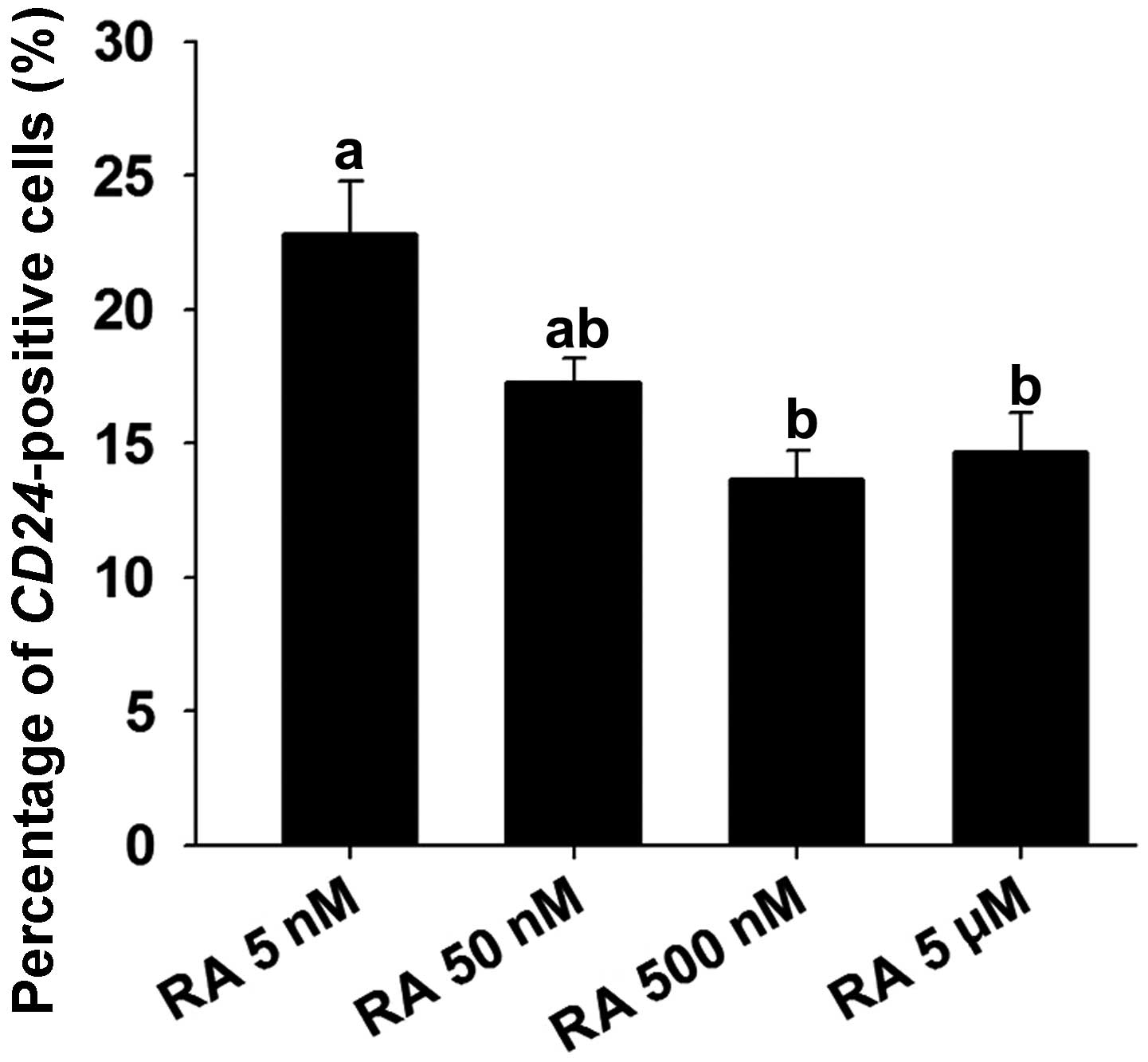
To evaluate the optimal concentration of RA required
for CD24 expression, 5, 50 and 500 nM, or 5 μM of RA were
added during neural differentiation. The percentage of cells
expressing CD24 was 22.8±1.9, 17.3±0.9, 13.7±1.1 and 14.7±1.5%,
respectively (mean ± SEM; n=3) (Fig.
4). Thus, CD24 expression was higher in the group treated with
5 nM RA than in the groups treated with 50 nM, 500 nM and 5
μM RA. The increasing concentration of RA inhibited CD24
expression in the cells.
On the basis of these results, we used N2/B27 medium
containing 5 nM RA as the basic condition for the neural
differentiation of mGSCs. We then examined the effect of additional
growth factors on CD24 expression. We added BMP4 (5 ng/ml), noggin
(150 ng/ml), Shh (100 ng/ml), GDNF (10 ng/ml), as well as FGF8 (5
ng/ml) and bFGF (10 ng/ml) to the N2/B27 medium containing 5 nM of
RA. The percentage of CD24+ cells was 17.9±2.1, 8.1±1.2,
17.1±0.7, 17.9±2.1, 18.4±1.6 and 19.1±0.3% in the groups of cells
treated with 5 nM RA alone or 5 nM RA with BMP4, noggin, Shh, GDNF
and FGF8/bFGF, respectively (mean ± SEM; n=3) (Fig. 5). There were no significant
differences observed between the groups, with the exception of the
BMP4-treated group, in which the expression of CD24 was lower than
that in the other groups (Fig.
5).
Effect of paracrine factors on neural
lineage-specific marker expression
Class III β-tubulin (Tubb3), a microtubule protein,
is selectively expressed in neural cells (29). Tubb3 antibodies and RT-qPCR-based
gene expression studies have been used to identify cells of the
neural lineage and to quantify neuronal cells during stem cell
differentiation (29). Myelin
basic protein (MBP) is an important factor in the nerve myelination
process within the central nervous system (29) and is used as a marker of
oligodendrocytes (30). In this
study, to evaluate the effects of additional growth factors on
mature cells of the neural lineage, BMP4 (5 ng/ml), noggin (150
ng/ml), Shh (100 ng/ml), GDNF (10 ng/ml) or FGF8 (5 ng/ml) were
added to the N2/B27 medium containing 5 nM RA and the EBs were
allowed to differentiate for 5 days. To determine the expression of
neural Tubb3 and Mbp, the EBs were harvested at 3, 4
and 5 days following differentiation, and RT-qPCR experiments were
performed. We found that, 4 days after EB formation, Tubb3
expression was higher in the cells treated with noggin or FGF8 than
in the cells treated with the other factors (Fig. 6A). Additionally, 4 days after
neural induction, the expression of Mbp was higher in the
cells treated with noggin or FGF8 than in the cells treated with RA
alone or with Shh (Fig. 6B).
These results indicate that noggin and FGF8 induce the
differentiation of mGSCs into mature neural cells and that the
expression of the neural markers, Tubb3 and Mbp, is
maximal on day 4.
CD24+ cell populations induced with RA (5
nM), FGF8 (5 ng/ml) and Shh (100 ng/ml) were isolated through cell
sorting on day 3 of differentiation. The cells were re-aggregated
on V-shaped, ultra-low attachment 96-well plates at a density of
5×103 cells/well and incubated for 2 days. The
re-aggregated EBs were transferred to a 96-well plate as a
monolayer. After 5 days in a monolayer culture, the expression of
TH and GFAP was analyzed. As shown in Fig. 7, CD24+ progenitor
cell-derived neural cells induced with a combination of paracrine
factors expressed the mature neuron marker, TH, and the astrocyte
marker, GFAP.
Discussion
In the present study, we report that neuronal cells
can be efficiently generated from a mouse mGSC line by combining a
FACS-based system for the isolation of CD24-expressing neural
precursors with paracrine factor treatment. Using this system, we
generated PSC lines, assessed the expression of the neuron-specific
genes, TH and MAP2, and demonstrated that
CD24+ cells have greater potential than CD24−
cells to differentiate into cells of the neural lineage.
The expression of nestin, a NSC marker, was
upregulated when serum-free N2/B27 medium was used for
differentiation (Fig. 1A). As
serum contains several factors that may influence the
differentiation process, a number of researchers have investigated
the in vitro differentiation of ESCs into neurons or neural
precursors using serum-free media (31–37). In addition, the serum-based
protocols used to induce cell differentiation have been modified,
with the goal of obtaining cells with specific neural phenotypes
more efficiently (38–40). Several of these studies have used
proprietary medium or serum, wherein it is difficult to identify
the specific factors responsible for efficient ESC differentiation
(41).
For translational approaches, it is best to purify
transplanted cells, such as neural precursor cells, that have the
capacity to differentiate into functional neurons. To this end, a
transplantable cell candidate that can be efficiently purified
needs to be identified. CD24 is a cell surface glycoprotein with
numerous carbohydrate structures and a small protein core that
attaches to the membrane through a glycosylphosphatidylinositol
tail (42,43). In the adult central nervous
system, CD24 expression is restricted to immature neurons in two
regions of the brain that exhibit ongoing neurogenesis, the
subventricular zone of the lateral ventricle pathway and the
dentate gyrus of the hippocampal formation. CD24 is also strongly
expressed in ciliated ependymal cells (44). In the present study, FACS-based
sorting of CD24-expressing cells revealed that the CD24 surface
antigen defined subsets of cells differentiating along the neural
precursor lineage. To investigate whether CD24+ cells
undergo neurogenesis efficiently in vitro, we sorted
CD24+ and CD24− cells derived from mESCs,
iPSCs and mGSCs on day 3 of differentiation and plated them for an
additional 7 days in a monolayer culture. In the CD24+
cells derived from ESCs, iPSCs and mGSCs, the TH and MAP2 mRNA
levels were upregulated to a similar extent relative to levels in
CD24− cells (Fig.
2).
To induce the efficient differentiation of PSCs
along a specific lineage, signaling pathways must be controlled by
means of paracrine factors that regulate lineage-specific
differentiation during embryonic development. Several factors that
can promote the differentiation of PSCs into cells in the neural
lineage have been identified (45–49). In the present study, the highest
enrichment of the neuronal population was obtained with N2/B27
medium, regardless of exposure to RA, which is commonly used in
vitro to induce the differentiation of stem cell populations
into cells of the neural lineage, including adult NSCs. Retinoids,
which include vitamin A (retinol) and its subtypes, are critical
contributors to the development of organs in the vertebrate central
nervous system, particularly the spinal cord (50,51).
The analysis of Tubb3 and Mbp
expression indicated that the medium containing noggin and FGF8
promoted the induction of neural cell differentiation in mGSCs.
Noggin, a signaling protein, plays an important role in promoting
somite patterning in the developing embryo (52). Released from the notochord, noggin
regulates BMP during development and blocks BMP4 signaling
(53), which results in neural
differentiation patterning in the developing embryo. Additionally,
in Xenopus, BMP4 inhibition induces the neural
differentiation of the ectoderm (54). In the present study, treatment
with BMP4 (5 ng/ml) decreased CD24 expression and downregulated
neural-related gene expression.
In conclusion, the findings of this study
demonstrate that CD24 expression, enhanced by a combination of RA,
noggin and FGF8 under serum-free conditions, promotes the
differentiation of cells into neural cell precursors. Using a
simple cell-sorting method, we obtained neural precursor cells from
differentiated mGSCs with the potential to differentiate into
mature neurons and astrocytes in vitro. The use of mGSCs
avoids the ethical quandaries surrounding embryo destruction.
Furthermore, with the use of mGSCs, the opportunity for
auto-transplantation would circumvent immunological problems. The
neural differentiation potential of CD24+ cells derived
from ESCs, iPSCs and mGSCs was similar. A CD24-based neural
precursor marker system using mGSCs derived from the adult testis
may be a preferred strategy for future therapeutic applications. In
addition, it has the advantage of providing cells that are
genetically matched to their donor without the introduction of
reprogramming transcription factors.
Acknowledgments
The present study was supported by the
Next-Generation BioGreen 21 Program (no. PJ011347) and the
Excellent Student Scholarship 2015 from Chung-Ang University,
Republic of Korea.
References
|
1
|
Hu BY, Du ZW, Li XJ, Ayala M and Zhang SC:
Human oligodendrocytes from embryonic stem cells: Conserved SHH
signaling networks and divergent FGF effects. Development.
136:1443–1452. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li XJ, Du ZW, Zarnowska ED, Pankratz M,
Hansen LO, Pearce RA and Zhang SC: Specification of motoneurons
from human embryonic stem cells. Nat Biotechnol. 23:215–221. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Perrier AL, Tabar V, Barberi T, Rubio ME,
Bruses J, Topf N, Harrison NL and Studer L: Derivation of midbrain
dopamine neurons from human embryonic stem cells. Proc Natl Acad
Sci USA. 101:12543–12548. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Roy NS, Cleren C, Singh SK, Yang L, Beal
MF and Goldman SA: Functional engraftment of human ES cell-derived
dopaminergic neurons enriched by coculture with
telomerase-immortalized midbrain astrocytes. Nat Med. 12:1259–1268.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Watanabe K, Ueno M, Kamiya D, Nishiyama A,
Matsumura M, Wataya T, Takahashi JB, Nishikawa S, Nishikawa S,
Muguruma K and Sasai Y: A ROCK inhibitor permits survival of
dissociated human embryonic stem cells. Nat Biotechnol. 25:681–686.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang L, Soonpaa MH, Adler ED, Roepke TK,
Kattman SJ, Kennedy M, Henckaerts E, Bonham K, Abbott GW, Linden
RM, et al: Human cardiovascular progenitor cells develop from a
KDR+ embryonic-stem-cell-derived population. Nature.
453:524–528. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nishikawa S, Goldstein RA and Nierras CR:
The promise of human induced pluripotent stem cells for research
and therapy. Nat Rev Mol Cell Biol. 9:725–729. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Takahashi K and Yamanaka S: Induction of
pluripotent stem cells from mouse embryonic and adult fibroblast
cultures by defined factors. Cell. 126:663–676. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Spradling A, Drummond-Barbosa D and Kai T:
Stem cells find their niche. Nature. 414:98–104. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Conrad S, Renninger M, Hennenlotter J,
Wiesner T, Just L, Bonin M, Aicher W, Bühring HJ, Mattheus U, Mack
A, et al: Generation of pluripotent stem cells from adult human
testis. Nature. 456:344–349. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guan K, Nayernia K, Maier LS, Wagner S,
Dressel R, Lee JH, Nolte J, Wolf F, Li M, Engel W and Hasenfuss G:
Pluripotency of spermatogonial stem cells from adult mouse testis.
Nature. 440:1199–1203. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Izadyar F, Pau F, Marh J, Slepko N, Wang
T, Gonzalez R, Ramos T, Howerton K, Sayre C and Silva F: Generation
of multipotent cell lines from a distinct population of male germ
line stem cells. Reproduction. 135:771–784. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kanatsu-Shinohara M, Inoue K, Lee J,
Yoshimoto M, Ogonuki N, Miki H, Baba S, Kato T, Kazuki Y, Toyokuni
S, et al: Generation of pluripotent stem cells from neonatal mouse
testis. Cell. 119:1001–1012. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kanatsu-Shinohara M, Lee J, Inoue K,
Ogonuki N, Miki H, Toyokuni S, Ikawa M, Nakamura T, Ogura A and
Shinohara T: Pluripotency of a single spermatogonial stem cell in
mice. Biol Reprod. 78:681–687. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ko K, Tapia N, Wu G, Kim JB, Bravo MJ,
Sasse P, Glaser T, Ruau D, Han DW, Greber B, et al: Induction of
pluripotency in adult unipotent germline stem cells. Cell Stem
Cell. 5:87–96. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Seandel M, James D, Shmelkov SV,
Falciatori I, Kim J, Chaval S, Scherr DS, Zhang F, Torres R, Gale
NW, et al: Generation of functional multipotent adult stem cells
from GPR125+ germline progenitors. Nature. 449:346–350.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim BJ, Lee YA, Kim YH, Kim KJ, Jung MS,
Ha SJ, Kang HG, Kim BG, Do JT, Yang HS and Ryu BY: Establishment of
adult mouse testis-derived multipotent germ line stem cells and
comparison of lineage-specific differentiation potential. Tissue
Eng Regen Med. 11:121–130. 2014. View Article : Google Scholar
|
|
18
|
Glaser T, Opitz T, Kischlat T, Konang R,
Sasse P, Fleischmann BK, Engel W, Nayernia K and Brüstle O: Adult
germ line stem cells as a source of functional neurons and glia.
Stem Cells. 26:2434–2443. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Baumgarth N and Roederer M: A practical
approach to multi-color flow cytometry for immunophenotyping. J
Immunol Methods. 243:77–97. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kantor AB and Roederer M: FACS analysis of
leukocytes. Handbook of Experimental Immunology. Herzenberg LA,
Weir DM and Blackwell C: Blackwell Science; Boston: pp. 43–49.
1996
|
|
21
|
Morrison SJ, Uchida N and Weissman IL: The
biology of hematopoietic stem cells. Annu Rev Cell Dev Biol.
11:35–71. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zola H: Medical applications of leukocyte
surface molecules-the CD molecules. Mol Med. 12:312–316. 2006.
|
|
23
|
Iversen SD and Iversen LL: Dopamine: 50
years in perspective. Trends Neurosci. 30:188–193. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fraichard A, Chassande O, Bilbaut G, Dehay
C, Savatier P and Samarut J: In vitro differentiation of embryonic
stem cells into glial cells and functional neurons. J Cell Sci.
108:3181–3188. 1995.PubMed/NCBI
|
|
25
|
Megiorni F, Mora B, Indovina P and
Mazzilli MC: Expression of neuronal markers during NTera2/cloneD1
differentiation by cell aggregation method. Neurosci Lett.
373:105–109. 2005. View Article : Google Scholar
|
|
26
|
Caceres A, Mautino J and Kosik KS:
Suppression of MAP2 in cultured cerebellar macroneurons inhibits
minor neurite formation. Neuron. 9:607–618. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dehmelt L, Smart FM, Ozer RS and Halpain
S: The role of microtubule-associated protein 2c in the
reorganization of microtubules and lamellipodia during neurite
initiation. J Neurosci. 23:9479–9490. 2003.PubMed/NCBI
|
|
28
|
Harada A, Teng J, Takei Y, Oguchi K and
Hirokawa N: MAP2 is required for dendrite elongation, PKA anchoring
in dendrites, and proper PKA signal transduction. J Cell Biol.
158:541–549. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tesar PJ, Chenoweth JG, Brook FA, Davies
TJ, Evans EP, Mack DL, Gardner RL and McKay RD: New cell lines from
mouse epiblast share defining features with human embryonic stem
cells. Nature. 448:196–199. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang SC: Defining glial cells during CNS
development. Nat Rev Neurosci. 2:840–843. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bouhon IA, Kato H, Chandran S and Allen
ND: Neural differentiation of mouse embryonic stem cells in
chemically defined medium. Brain Res Bull. 68:62–75. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Finley MF, Devata S and Huettner JE: BMP-4
inhibits neural differentiation of murine embryonic stem cells. J
Neurobiol. 40:271–287. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Okabe S, Forsberg-Nilsson K, Spiro AC,
Segal M and McKay RD: Development of neuronal precursor cells and
functional postmitotic neurons from embryonic stem cells in vitro.
Mech Dev. 59:89–102. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tropepe V, Hitoshi S, Sirard C, Mak TW,
Rossant J and van der Kooy D: Direct neural fate specification from
embryonic stem cells: A primitive mammalian neural stem cell stage
acquired through a default mechanism. Neuron. 30:65–78. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Watanabe K, Kamiya D, Nishiyama A,
Katayama T, Nozaki S, Kawasaki H, Watanabe Y, Mizuseki K and Sasai
Y: Directed differentiation of telencephalic precursors from
embryonic stem cells. Nat Neurosci. 8:288–296. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wiles MV and Johansson BM: Embryonic stem
cell development in a chemically defined medium. Exp Cell Res.
247:241–248. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ying QL, Stavridis M, Griffiths D, Li M
and Smith A: Conversion of embryonic stem cells into
neuroectodermal precursors in adherent monoculture. Nat Biotechnol.
21:183–186. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kawasaki H, Mizuseki K, Nishikawa S,
Kaneko S, Kuwana Y, Nakanishi S, Nishikawa SI and Sasai Y:
Induction of midbrain dopaminergic neurons from ES cells by stromal
cell-derived inducing activity. Neuron. 28:31–40. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lee SH, Lumelsky N, Studer L, Auerbach JM
and McKay RD: Efficient generation of midbrain and hindbrain
neurons from mouse embryonic stem cells. Nat Biotechnol.
18:675–679. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wichterle H, Lieberam I, Porter JA and
Jessell TM: Directed differentiation of embryonic stem cells into
motor neurons. Cell. 110:385–397. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cai C and Grabel L: Directing the
differentiation of embryonic stem cells to neural stem cells. Dev
Dyn. 236:3255–3266. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kay R, Rosten PM and Humphries RK: CD24, a
signal transducer modulating B cell activation responses, is a very
short peptide with a glycosyl phosphatidylinositol membrane anchor.
J Immunol. 147:1412–1416. 1991.PubMed/NCBI
|
|
43
|
Williams LA, McLellan AD, Summers KL, Sorg
RV, Fearnley DB and Hart DN: Identification of a novel dendritic
cell surface antigen defined by carbohydrate specific CD24 antibody
cross-reactivity. Immunology. 89:120–125. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Calaora V, Chazal G, Nielsen PJ, Rougon G
and Moreau H: mCD24 expression in the developing mouse brain and in
zones of secondary neurogenesis in the adult. Neuroscience.
73:581–594. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Axell MZ, Zlateva S and Curtis M: A method
for rapid derivation and propagation of neural progenitors from
human embryonic stem cells. J Neurosci Methods. 184:275–284. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Do JT, Joo JY, Han DW, Araúzo-Bravo MJ,
Kim MJ, Greber B, Zaehres H, Sobek-Klocke I, Chung HM and Schöler
HR: Generation of parthenogenetic induced pluripotent stem cells
from parthenogenetic neural stem cells. Stem Cells. 27:2962–2968.
2009.PubMed/NCBI
|
|
47
|
Gerrard L, Rodgers L and Cui W:
Differentiation of human embryonic stem cells to neural lineages in
adherent culture by blocking bone morphogenetic protein signaling.
Stem Cells. 23:1234–1241. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hicks AU, Lappalainen RS, Narkilahti S,
Suuronen R, Corbett D, Sivenius J, Hovatta O and Jolkkonen J:
Transplantation of human embryonic stem cell-derived neural
precursor cells and enriched environment after cortical stroke in
rats: Cell survival and functional recovery. Eur J Neurosci.
29:562–574. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Koch P, Opitz T, Steinbeck JA, Ladewig J
and Brüstle O: A rosette-type, self-renewing human ES cell-derived
neural stem cell with potential for in vitro instruction and
synaptic integration. Proc Natl Acad Sci USA. 106:3225–3230. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Maden M: Retinoid signalling in the
development of the central nervous system. Nat Rev Neurosci.
3:843–853. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
51
|
Pierani A, Brenner-Morton S, Chiang C and
Jessell TM: A sonic hedgehog-independent, retinoid-activated
pathway of neurogenesis in the ventral spinal cord. Cell.
97:903–915. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hirsinger E, Duprez D, Jouve C, Malapert
P, Cooke J and Pourquié O: Noggin acts downstream of Wnt and Sonic
Hedgehog to antagonize BMP4 in avian somite patterning.
Development. 124:4605–4614. 1997.PubMed/NCBI
|
|
53
|
Marcelino J, Sciortino CM, Romero MF,
Ulatowski LM, Ballock RT, Economides AN, Eimon PM, Harland RM and
Warman ML: Human disease-causing NOG missense mutations: Effects on
noggin secretion, dimer formation, and bone morphogenetic protein
binding. Proc Natl Acad Sci USA. 98:11353–11358. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Sasai Y, Lu B, Steinbeisser H and De
Robertis EM: Regulation of neural induction by the Chd and Bmp-4
antagonistic patterning signals in Xenopus. Nature. 376:333–336.
1995. View Article : Google Scholar : PubMed/NCBI
|