Introduction
Ankylosing spondylitis (AS) is a chronic
inflammatory disorder that primarily affects the spine and
sacroiliac joint. It is a type of spondyloarthritis (SpA) and has a
particularly strong genetic association with human leukocyte
antigen (HLA)-B27 (1).
The simultaneous destruction and excessive formation
of bone are the most distinctive features of AS (2–4),
and these processes lead to the formation of syndesmophytes
combined with systemic bone loss (5–8).
There has been marked progress in the treatment of AS, due to the
development of tumor necrosis factor-α (TNF-α) blocking agents, and
anti-TNF-α therapy has become the standard of care for patients
with AS over the past decade. However, to the best of our
knowledge, the pathophysiological mechanisms responsible for the
effects of TNF-α on new bone formation and osteoporosis in AS have
not been have not yet been fully elucidated.
Peptidyl arginine deiminase, type IV (PADI4) is an
enzyme that catalyzes the conversion of arginine residues to
citrulline residues. It is predominantly expressed in granulocytes
and monocytes (9) and plays an
important role in inflammation and the immune response (10–13). Emerging evidence has indicated
that the expression of PADI4 is associated with the development of
rheumatoid arthritis (RA), osteoarthritis (OA) and AS (11,14,15). However, little is known about the
precise role of PADI4 in the pathogenic process in
vitro.
In the present study, we examined the expression of
PADI4 in the synovial tissue of patients with AS and in normal
controls. We then carried out an in vitro experiment to
investigate the potential effects of PADI4 on human mesenchymal
stem cell (hMSC) proliferation and osteogenic differentiation under
normal and pathological conditions. This study indicates that there
is a novel mechanism underlying anti-TNF-α therapy for patients
with AS.
Materials and methods
Sample collection
A total of 18 patients diagnosed with AS and 11
healthy control subjects with traumatic fractures were enrolled in
the present study. The patients and controls provided informed
written consent prior to enrollment in this study, and the study
protocol was approved by the Ethics Committee of Xinyu Hospital of
Nanchang University, Xinyu, China. Synovial tissue samples were
collected during hip replacement surgery from the patients with AS
and the healthy controls with traumatic fractures. The synovial
tissue samples were immediately stored at −80°C following
dissection from the connective tissue.
Cell culture and transfection
Bone marrow-derived hMSCs (registration no.
PCS-500-012; ATCC, Manassas, VA, USA) were cultured in mesenchymal
stem cell basal medium (ATCC) supplemented with 10% fetal bovine
serum (FBS), 15 ng/ml insulin-like growth factor (IGF)-1, 125 pg/ml
fibroblast growth factor-basic (FGF-b) and 2.4 mM
L-Alanyl-L-Glutamine. The cells were grown at 37°C with 5%
CO2 in a humidified atmosphere. TNF-α was purchased from
PeproTech (Rocky Hill, NJ, USA) and diluted in 1% bovine serum
albumin (BSA; Sigma-Aldrich, St. Louis, MO, USA). Cells in the
vehicle group were treated with 1% BSA only. Cell transfection with
PADI4 siRNA or scrambled control siRNA was performed using
Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) following the
manufacturer’s instructions. Briefly, 2 µg siRNA were
diluted with Opti-MEM I (Gibco-BRL, Grand Island, NY, USA) and
incubated with Lipofectamine 2000 at 37°C. The lipid-DNA complexes
were then added to each well, and the cells were incubated at 37°C
for 4 h.
Reverse transcription-quantitative
(real-time) PCR (RT-qPCR)
Total RNA was extracted using TRIzol reagent
(Invitrogen). A total of 6 µg of each RNA sample was used
for reverse transcription using the RevertAid First Strand cDNA
Synthesis kit (Fermentas, Vilnius, Lithuania). Quantitative PCR
(qPCR) was performed on a Corbett Rotor-Gene 6000 system (Corbett
Research, Westburg, Leusden, The Netherlands) using the PerfeCTa
qPCR FastMix (Quanta Biosciences, Inc., Gaithersburg, MD, USA)
following the manufacturer’s instructions. The primer sequences
were as follows: PADI4 forward, 5′-tttgggaacctggaagtgag-3′ and
reverse, 5′-ggcacaaagctcaggaactc-3′; and β-actin forward,
5′-cattaaggagaagctgtgct-3′ and reverse, 5′-gttgaaggtagtttcgt
gga-3′. β-actin was used as a housekeeping gene. The Ct value was
calculated using the ΔΔCt method.
Protein extraction and western blot
analysis
Total protein was extracted using lysis buffer (150
mM Tris pH 7.4, 150 mM NaCl, 1% Triton X-100, 0.2% SDS and 1 mM
PMSF). After 30 min on ice, the lysates were centrifuged for 1 min
at 4°C, and the supernatant was then collected. The protein content
was assessed using the bicinchoninic acid assay (BCA) method with
reagents from Pierce Biotechnology, Inc. (Rockford, IL, USA).
Protein (40 µg) was separated on a 12% SDS polyacrylamide
gel and transferred onto polyvinylidene difluoride membranes
(Millipore, Billerica, MA, USA). Following blocking with 5% non-fat
milk, the membranes were incubated with anti-PADI4 rabbit
polyclonal antibody (Cat. no. sc-98991, 1:500 dilution; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA), anti-bone morphogenetic
protein 2 (BMP-2) mouse monoclonal antibody (Cat. no. ab6285, 1:400
dilution), anti runt-related transcription factor 2 (Runx2) rabbit
polyclonal antibody (Cat. no. ab102711, 1:400 dilution),
anti-Osterix mouse monoclonal antibody (Cat. no. ab57335, 1:800
dilution) (all from Abcam, Cambridge, MA, USA) and anti β-actin
mouse monoclonal antibody (Cat. no. BM0627, 1:1,000 dilution;
Boster, Wuhan, China) at 37°C for 2 h. The membranes were washed 3
times with TBST and incubated with rabbit-anti mouse IgG (Cat. no.
sc-358913, 1:2,000 dilution) or mouse-anti rabbit IgG (Cat. no.
sc-2357, 1:2,000 dilution) horseradish peroxidase (HRP)-conjugated
secondary antibody (both from Santa Cruz Biotechnology) at 37°C for
1 h. The signals were detected using an ECL detection kit (Pierce
Biotechnology, Inc.). The intensity of the protein bands was
quantified using Image J software (National Institutes of Health,
Bethesda, MD, USA). The relative protein levels were normalized
against β-actin.
Cell proliferation assay
The cells were seeded onto 96-well plates and
incubated with 10 ng/ml TNF-α for 12, 24, 48 and 72 h. Cell
proliferation was examined by MTT assay. MTT reagent (20 µl;
Beyotime Institute of Biotechnology, Shanghai, China) was added to
the cell cultures and incubated at 37°C for 4 h. Subsequently, 150
µl of dimethyl sulfoxide (DMSO) were added and mixed gently for 10
min. The absorbance at 570 nm was measured using a microplate
reader (Ascent 354; Thermo Labsystems, Waltham, MA, USA).
Measurement of alkaline phosphatase (ALP)
activity
ALP activity in the hMSCs was measured using an
Alkaline Phosphatase Activity Colorimetric assay kit (BioVision
Inc., Milpitas, CA, USA) according to the manufacturer’s
instructions. Briefly, the cells were homogenized and centrifuged
at 13,000 x g for 3 min. The samples and the p-nitrophenyl
phosphate (pNPP) standard were added to the 96-well plates, and the
ALP enzyme solution was then added to each well followed by
incubation at room temperature for 60 min. The reactions were
terminated by the addition of Stop Solution. The optical density at
405 nm was measured using a microplate reader (Ascent 354; Thermo
Labsystems). The amount of purine nucleoside phosphorylase (pNP)
generated by the ALP sample was calculated by applying sample
readings to the standard curve.
Statistical analyses
Statistical analyses of the data were performed
using SPSS 16.0 statistical software (SPSS, Inc., Chicago, IL,
USA). The student’ t-test was used to assess the statistical
differences between 2 groups. A P-value <0.05 was considered to
indicate a statistically significant difference.
Results
PADI4 expression is increased in the
synovial tissues of patients with AS
The expression levels of PADI4 in the synovial
tissues from 18 patients with AS and 11 controls were measured by
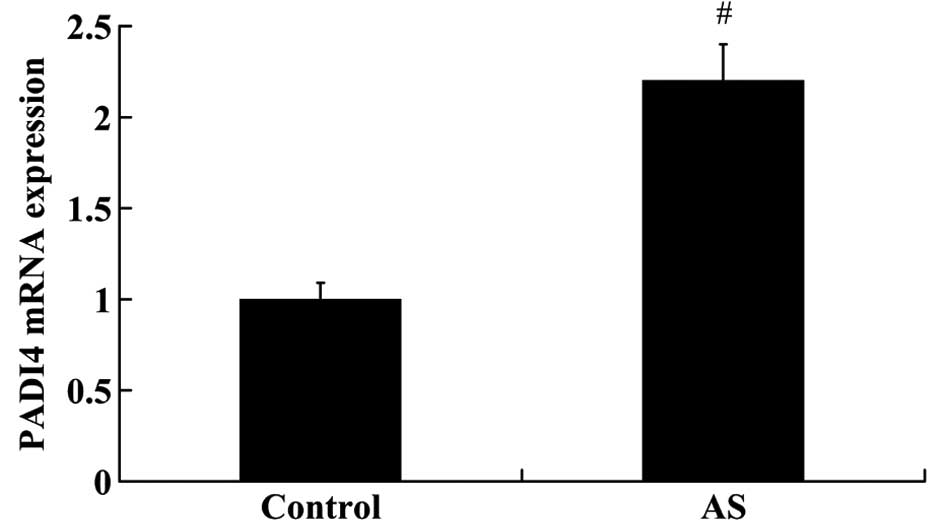
RT-qPCR and western blot analysis. As shown in Fig. 1, the relative mRNA expression of
PADI4 was significantly higher in the patients with AS compared
with the controls (fold change >2.0, P<0.01). The results
from western blot analysis revealed that PADI4 protein expression
was also increased in the patients with AS compared with the
controls (0.23±0.04 vs. 0.13±0.02, P<0.05; Fig. 2).
TNF-α promotes PADI4 expression in
hMSCs
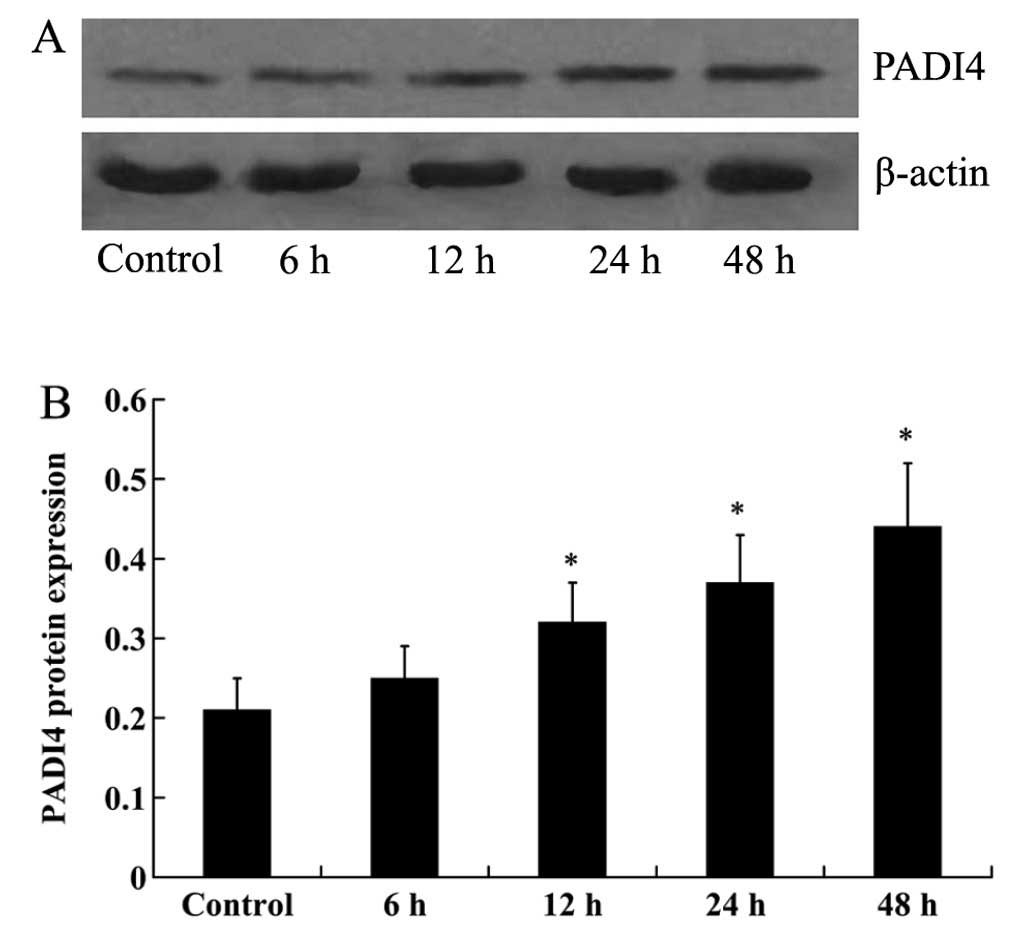
In order to investigate the effect of TNF-α o n
PADI4 expression, the hMSCs were cultured with 10 ng/ml TNF-α for
6, 12, 24 and 48 h. Western blot analysis was then performed to
measure the expression levels of PADI4 in the hMSCs. At 6 h, no
significant difference in PADI4 expression was detected. However,
the increased expression of PADI4 in the hMSCs was detected at 12,
24 and 48 h (Fig. 3), with the
maximal response being observed at 48 h. The hMSCs were then
cultured with 0.01, 0.1, 1 and 10 ng/ml TNF-α for 48 h. As shown in
Fig. 4, PADI4 protein expression
was increased in a dose-dependent manner by TNF-α, with the maximal
response being observed following treatment with TNF-α at 10
ng/ml.
Silencing of PADI4 attenuates the
TNF-α-induced proliferation of hMSCs
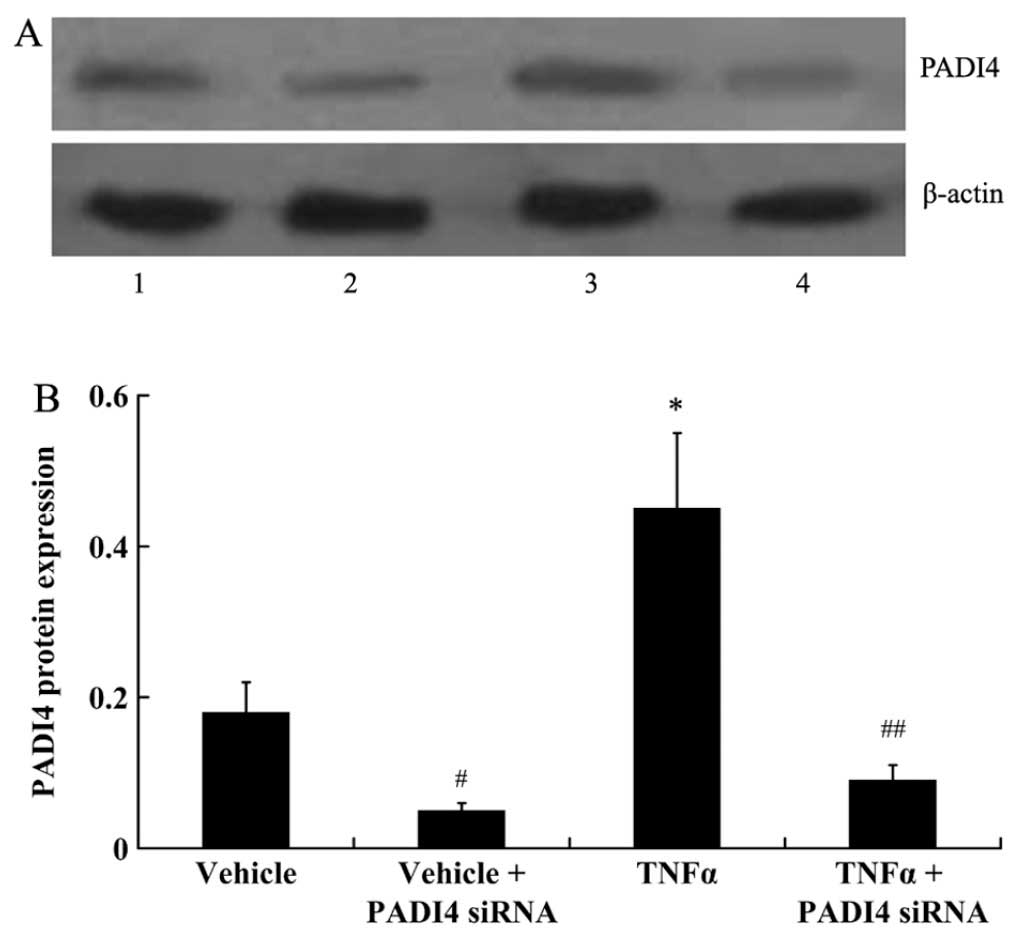
In order to silence PADI4 expression in the hMSCs,
the cells were transfected with siRNA against PADI4. As shown by
the results of western blot analysis, PADI4 protein expression was
significantly decreased in the hMSCs transfected with PADI4 siRNA
in the presence or absence of TNF-α (P<0.01; Fig. 5).
Subsequently, MTT assay was used to examine the
proliferation of hMSCs in which PADI4 was silenced by siRNA. As
shown in Fig. 6, treatment with
10 ng/ml TNF-α resulted in a significant induction of hMSC
proliferation in comparison to the untreated cells. In the absence
of TNF-α, cell viability did not differ significantly between the
control and PADI4-silenced hMSCs. However, in the presence of
TNF-α, the PADI4-silenced hMSCs proliferated at a lower rate than
the control cells.
Silencing of PADI4 attenuates the
TNF-α-induced osteogenic differentiation of hMSCs
To investigate the osteogenic differentiation of
hMSCs, ALP activity and the expression of BMP-2, Runx2 and Osterix
was examined. TNF-α (10 ng/ml) was used to treat the hMSCs for 48
h. As shown in Fig. 7, ALP
activity increased significantly following treatment with TNF-α
compared with the control (not treated with TNF-α; P<0.05). The
silencing of PADI4 did not alter ALP activity in the untreated
cells. However, the silencing of PADI4 resulted in a significant
decrease in ALP activity in the TNF-α-treated hMSCs (P<0.05;
Fig. 7). The results from western
blot analysis indicated that TNF-α signifantly increased the
expression of BMP-2, Runx2 and Osterix in the hMSCs (P<0.05). In
the absence of TNF-α, the expression of BMP-2, Runx2 and Osterix
did not change significantly following transfection of the hMSCs
with PADI4 siRNA. However, in the presence of TNF-α, the expression
of BMP-2, Runx2 and Osterix decreased significantly following
transfection with PADI4 siRNA (P<0.05; Fig. 8).
Discussion
Using whole genome SNP scanning, PADI4 has been
identified as a risk factor for RA (16–18), and the elevated expression of
PADI4 has been detected in the synovial membrane and synovial fluid
of patients with RA (11,14). In the present study, we examined
the expression of PADI4 in patients with AS. Similar to the results
of the above-mentioned studies obtained from patients with RA, in
our study, the expression of PADI4 was found to be significantly
increased in the synovial tissues of patients with AS both at the
mRNA and protein level.
AS is characterized by massive bone loss and ectopic
bone formation (2–4). Bone remodeling is affected by
multiple factors, including cytokines, hormones and signaling
molecules (19–21). A number of cell types, such as
osteoblasts (OBs), osteoclasts (OCs) and osteocytes (OYs) have been
shown to be involved in this process (22,23). In general, the activation of OCs
is linked with bone loss and the development of erosion. By
contrast, the activation of OBs and the inhibition of OCs are
associated with new bone formation and ossification (24).
TNF-α is a highly potent pro-inflammatory molecule
in the immune system. It is well known that TNF-α plays an
important role in the regulation of bone homeostasis and is
involved in the pathogenisis of chronic immune and inflammatory
joint diseases (25,26). It has been established that TNF-α
acts as a stimulator of osteoclastogenesis (27–31) and an inhibitor of
osteoblastogenesis (32–36). These actions are dependent on the
TNF-α concentration, exposure time, and on the cell type (26,37,38). In addition, TNF-α possesses
osteogenic differentiation capabilities. Mesenchymal stem cells are
multipotent stromal cells and can differentiate into various cell
types, including those of connective tissue and bone (39). In the present study, we found that
TNF-α induced the proliferation of hMSCs, which is consistent with
the results of previous studies (26,40). BMP-2 and ALP are well-known
osteogenetic proteins, and RUNX2 and Osterix are two critical
regulators of osteogenic differentiation. In this study, we
examined the expression of proteins associated with osteogenic
differentiation. We found that TNF-α promoted the osteogenic
differentiation of hMSCs, as demonstrated by an increase in ALP
activity, and an increase in the expression of BMP-2, Runx2 and
Osterix.
It has been previously reported that TNF-α induces
PADI4 translocation to the nucleus and that this is followed by the
regulation of the expression of various genes (41). In the present study, we
investigated To the best of our knowledge, this is for the first
time that the effect of TNF-α on PADI4 expression has been studied.
In this study, the time- and dose-dependent induction of PADI4
protein expression by TNF-α was observed in the hMSCs. To further
elucidate the effects of PADI4 on hMSC proliferation and osteogenic
differentiation under normal and pathological conditions, the cells
were transfected with PADI4 siRNA in order to silence PADI4
expression. We observed that, under normal conditions, the
silencing of PADI4 did not have any effect on hMSC proliferation or
osteogenic differentiation. However, in the presence of TNF-α, hMSC
proliferation and osteogenic differentiation were induced; these
effects were attenuated by the silencing of PADI4. These results
indicate that both PADI4 and TNF-α are involved in the induction of
hMSC proliferation and osteogenic differentiation by TNF-α.
In conclusion, in this study, we demonstrated that
the expression of PADI4 differs between patients with AS and normal
subjects. TNF-α increased the protein expression of PADI4 in hMSCs
in a dose- and time-dependent manner. Our data indicate that PADI4
plays a role in hMSC proliferation and differentiation, which is
induced by TNF-α. This study identified a novel pathophysiological
mechanism responsible for new bone formation and osteoporosis in
patients with AS; thus, PADI4 may emerge as a novel therapeutic
target in AS.
References
|
1
|
McHugh K and Bowness P: The link between
HLA-B27 and SpA - new ideas on an old problem. Rheumatology
(Oxford). 51:1529–1539. 2012. View Article : Google Scholar
|
|
2
|
Zhang X, Aubin JE and Inman RD: Molecular
and cellular biology of new bone formation: insights into the
ankylosis of ankylosing spondylitis. Curr Opin Rheumatol.
15:387–393. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schett G: Bone formation versus bone
resorption in ankylosing spondylitis. Adv Exp Med Biol.
649:114–121. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Grisar J, Bernecker PM, Aringer M, Redlich
K, Sedlak M, Wolozcszuk W, Spitzauer S, Grampp S, Kainberger F,
Ebner W, et al: Ankylosing spondylitis, psoriatic arthritis, and
reactive arthritis show increased bone resorption, but differ with
regard to bone formation. J Rheumatol. 29:1430–1436.
2002.PubMed/NCBI
|
|
5
|
Miossec P: IL-17 and Th17 cells in human
inflammatory diseases. Microbes Infect. 11:625–630. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Koenders MI, Marijnissen RJ, Devesa I,
Lubberts E, Joosten LA, Roth J, van Lent PL, van de Loo FA and van
den Berg WB: Tumor necrosis factor-interleukin-17 interplay induces
S100A8, interleukin-1β, and matrix metalloproteinases, and drives
irreversible cartilage destruction in murine arthritis: rationale
for combination treatment during arthritis. Arthritis Rheum.
63:2329–2339. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kotake S, Udagawa N, Takahashi N,
Matsuzaki K, Itoh K, Ishiyama S, Saito S, Inoue K, Kamatani N,
Gillespie MT, et al: IL-17 in synovial fluids from patients with
rheumatoid arthritis is a potent stimulator of osteoclastogenesis.
J Clin Invest. 103:1345–1352. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lubberts E, Joosten LA, van de Loo FA,
Schwarzenberger P, Kolls J and van den Berg WB: Overexpression of
IL-17 in the knee joint of collagen type II immunized mice promotes
collagen arthritis and aggravates joint destruction. Inflamm Res.
51:102–104. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nakashima K, Hagiwara T, Ishigami A,
Nagata S, Asaga H, Kuramoto M, Senshu T and Yamada M: Molecular
characterization of peptidylarginine deiminase in HL-60 cells
induced by retinoic acid and 1alpha,25-dihydroxyvitamin D(3). J
Biol Chem. 274:27786–27792. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Asaga H, Nakashima K, Senshu T, Ishigami A
and Yamada M: Immunocytochemical localization of peptidylarginine
deiminase in human eosinophils and neutrophils. J Leukoc Biol.
70:46–51. 2001.PubMed/NCBI
|
|
11
|
Chang X, Yamada R, Suzuki A, Sawada T,
Yoshino S, Tokuhiro S and Yamamoto K: Localization of
peptidylarginine deiminase 4 (PADI4) and citrullinated protein in
synovial tissue of rheumatoid arthritis. Rheumatology (Oxford).
44:40–50. 2005. View Article : Google Scholar
|
|
12
|
György B, Tóth E, Tarcsa E, Falus A and
Buzás EI: Citrullination: a posttranslational modification in
health and disease. Int J Biochem Cell Biol. 38:1662–1677. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Anzilotti C, Pratesi F, Tommasi C and
Migliorini P: Peptidylarginine deiminase 4 and citrullination in
health and disease. Autoimmun Rev. 9:158–160. 2010. View Article : Google Scholar
|
|
14
|
Chang X, Zhao Y, Sun S, Zhang Y and Zhu Y:
The expression of PADI4 in synovium of rheumatoid arthritis.
Rheumatol Int. 29:1411–1416. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vossenaar ER, Nijenhuis S, Helsen MM, van
der Heijden A, Senshu T, van den Berg WB, van Venrooij WJ and
Joosten LA: Citrullination of synovial proteins in murine models of
rheumatoid arthritis. Arthritis Rheum. 48:2489–2500. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Suzuki A, Yamada R, Chang X, Tokuhiro S,
Sawada T, Suzuki M, Nagasaki M, Nakayama-Hamada M, Kawaida R, Ono
M, et al: Functional haplotypes of PADI4, encoding citrullinating
enzyme peptidylarginine deiminase 4, are associated with rheumatoid
arthritis. Nat Genet. 34:395–402. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Plenge RM, Padyukov L, Remmers EF, Purcell
S, Lee AT, Karlson EW, Wolfe F, Kastner DL, Alfredsson L, Altshuler
D, et al: Replication of putative candidate-gene associations with
rheumatoid arthritis in >4,000 samples from North America and
Sweden: association of susceptibility with PTPN22, CTLA4, and
PADI4. Am J Hum Genet. 77:1044–1060. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kang CP, Lee HS, Ju H, Cho H, Kang C and
Bae SC: A functional haplotype of the PADI4 gene associated with
increased rheumatoid arthritis susceptibility in Koreans. Arthritis
Rheum. 54:90–96. 2006. View Article : Google Scholar
|
|
19
|
Klein-Nulend J, Bacabac RG and Bakker AD:
Mechanical loading and how it affects bone cells: the role of the
osteocyte cytoskeleton in maintaining our skeleton. Eur Cell Mater.
24:278–291. 2012.PubMed/NCBI
|
|
20
|
Onal M, Xiong J, Chen X, Thostenson JD,
Almeida M, Manolagas SC and O’Brien CA: Receptor activator of
nuclear factor κB ligand (RANKL) protein expression by B
lymphocytes contributes to ovariectomy-induced bone loss. J Biol
Chem. 287:29851–29860. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vezeridis PS, Semeins CM, Chen Q and
Klein-Nulend J: Osteocytes subjected to pulsating fluid flow
regulate osteoblast proliferation and differentiation. Biochem
Biophys Res Commun. 348:1082–1088. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Walkley CR, Shea JM, Sims NA, Purton LE
and Orkin SH: Rb regulates interactions between hematopoietic stem
cells and their bone marrow microenvironment. Cell. 129:1081–1095.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schaffler MB and Kennedy OD: Osteocyte
signaling in bone. Curr Osteoporos Rep. 10:118–125. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Taylan A, Sari I, Akinci B, Bilge S,
Kozaci D, Akar S, Colak A, Yalcin H, Gunay N and Akkoc N:
Biomarkers and cytokines of bone turnover: extensive evaluation in
a cohort of patients with ankylosing spondylitis. BMC Musculoskelet
Disord. 13:1912012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Aggarwal BB: Signalling pathways of the
TNF superfamily: a double-edged sword. Nat Rev Immunol. 3:745–756.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Osta B, Benedetti G and Miossec P:
Classical and paradoxical effects of TNF-α on bone homeostasis.
Front Immunol. 5:482014. View Article : Google Scholar
|
|
27
|
Pacifici R: Estrogen, cytokines, and
pathogenesis of postmenopausal osteoporosis. J Bone Miner Res.
11:1043–1051. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kudo O, Fujikawa Y, Itonaga I, Sabokbar A,
Torisu T and Athanasou NA: Proinflammatory cytokine
(TNFalpha/IL-1alpha) induction of human osteoclast formation. J
Pathol. 198:220–227. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mucci JM, Scian R, De Francesco PN, García
FS, Ceci R, Fossati CA, Delpino MV and Rozenfeld PA: Induction of
osteoclastogenesis in an in vitro model of Gaucher disease is
mediated by T cells via TNF-α. Gene. 509:51–59. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Matsubara R, Kukita T, Ichigi Y, Takigawa
I, Qu PF, Funakubo N, Miyamoto H, Nonaka K and Kukita A:
Characterization and identification of subpopulations of
mononuclear preosteoclasts induced by TNF-α in combination with
TGF-β in rats. PLoS One. 7:e479302012. View Article : Google Scholar
|
|
31
|
Kagiya T and Nakamura S: Expression
profiling of microRNAs in RAW264.7 cells treated with a combination
of tumor necrosis factor alpha and RANKL during osteoclast
differentiation. J Periodontal Res. 48:373–385. 2013. View Article : Google Scholar
|
|
32
|
Abbas S, Zhang YH, Clohisy JC and Abu-Amer
Y: Tumor necrosis factor-alpha inhibits pre-osteoblast
differentiation through its type-1 receptor. Cytokine. 22:33–41.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gilbert L, He X, Farmer P, Boden S,
Kozlowski M, Rubin J and Nanes MS: Inhibition of osteoblast
differentiation by tumor necrosis factor-alpha. Endocrinology.
141:3956–3964. 2000.PubMed/NCBI
|
|
34
|
Gilbert LC, Rubin J and Nanes MS: The p55
TNF receptor mediates TNF inhibition of osteoblast differentiation
independently of apoptosis. Am J Physiol Endocrinol Metab.
288:E1011–E1018. 2005. View Article : Google Scholar
|
|
35
|
Mukai T, Otsuka F, Otani H, Yamashita M,
Takasugi K, Inagaki K, Yamamura M and Makino H: TNF-alpha inhibits
BMP-induced osteoblast differentiation through activating SAPK/JNK
signaling. Biochem Biophys Res Commun. 356:1004–1010. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lee HL, Yi T, Baek K, Kwon A, Hwang HR,
Qadir AS, Park HJ, Woo KM, Ryoo HM, Kim GS and Baek JH: Tumor
necrosis factor-α enhances the transcription of Smad ubiquitination
regulatory factor 1 in an activating protein-1- and Runx2-dependent
manner. J Cell Physiol. 228:1076–1086. 2013. View Article : Google Scholar
|
|
37
|
Hess K, Ushmorov A, Fiedler J, Brenner RE
and Wirth T: TNFalpha promotes osteogenic differentiation of human
mesenchymal stem cells by triggering the NF-kappaB signaling
pathway. Bone. 45:367–376. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Feng X1, Feng G, Xing J, Shen B, Li L, Tan
W, Xu Y, Liu S, Liu H, Jiang J, et al: TNF-α triggers osteogenic
differentiation of human dental pulp stem cells via the NF-κB
signalling pathway. Cell Biol Int. 37:1267–1275. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jaiswal N, Haynesworth SE, Caplan AI and
Bruder SP: Osteogenic differentiation of purified, culture-expanded
human mesenchymal stem cells in vitro. J Cell Biochem. 64:295–312.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Böcker W, Docheva D, Prall WC, Egea V,
Pappou E, Rossmann O, Popov C, Mutschler W, Ries C and Schieker M:
IKK-2 is required for TNF-alpha-induced invasion and proliferation
of human mesenchymal stem cells. J Mol Med (Berl). 86:1183–1192.
2008. View Article : Google Scholar
|
|
41
|
Mastronardi FG, Wood DD, Mei J, Raijmakers
R, Tseveleki V, Dosch HM, Probert L, Casaccia-Bonnefil P and
Moscarello MA: Increased citrullination of histone H3 in multiple
sclerosis brain and animal models of demyelination: a role for
tumor necrosis factor-induced peptidylarginine deiminase 4
translocation. J Neurosci. 26:11387–11396. 2006. View Article : Google Scholar : PubMed/NCBI
|