Introduction
Acetaminophen (APAP) is commonly used as an
analgesic and antipyretic agent (1–3),
and is considered safe at therapeutic doses (4). It is readily available, and high
doses of APAP may be provided to patients over a short time-period.
However, APAP is the most common drug to cause clinical
hepatotoxicity and nephrotoxicity in several countries (5–7). A
number of studies have demonstrated that high-dose APAP (10–15 g)
causes serious damage to liver and renal cells (8,9).
High-dose APAP can increase the levels of reactive oxygen species
(ROS), thus increasing cellular oxidative stress and causing liver
and renal injury (10–12). Therefore, several studies have
examined the ability of antioxidants to target high-dose
APAP-induced liver and renal damage through the reduction of
cellular ROS levels and oxidative stress (13–16). At present, N-acetylcysteine (NAC),
an antioxidant, has been used to treat APAP-induced hepatotoxicity
and nephrotoxicity in emergency cases (17–19).
In order to improve the understanding of the
mechanisms underlying APAP-induced toxicity, several animal and
cell models have been developed for hepatotoxic and nephrotoxic
investigations. In general, high-dose APAP (>5 mM) is used to
induce cell death in renal and liver cell models (20–26), and high-dose APAP (300–2,500
mg/kg) is used to induce liver and kidney damage in animal models
(27–31). These studies have observed that
APAP can stimulate apoptotic or necrotic death pathway activation
in different cell models (24,31,32). In addition, several cellular
effects and signals are stimulated in high-dose APAP-treated cells,
including increased levels of ROS and oxidative stress, decreased
levels of glutathione, induction of the mitogen-activated protein
kinase (MAPK) signaling pathway and activation of caspase cascades
(21,25,26,31,33–36).
High-dose APAP-induced clinical intoxication is
predominantly found in liver and renal cells; therefore, the
majority of previous studies have focussed on the mechanisms
underlying high-dose APAP-triggered liver and renal injury
(17,37,38). Furthermore, certain studies have
indicated that APAP can exhibit antitumor activities in certain
tumor types, including breast cancer, liver cancer and
neuroblastoma (26,39–43). These studies also demonstrated
that APAP-induced cell death is linked to nuclear factor-κB, the
B-cell lymphoma 2 family or glycogen synthase kinase-3 in different
tumor cells.
At present, with the exception of liver, renal and
tumor cells, almost no cellular effects have been reported in other
human cells following APAP therapy (10,12,39–43). Therefore, whether APAP causes
toxic cellular effects in other human cells remains to be
elucidated. APAP can freely cross the placenta (44,45); thus, high-dose APAP can cause
cellular damage in maternal as well as fetal liver cells. In
addition, several previous studies have suggested that stem cells
are critical during fetal development (46–48). However, whether APAP can induce
toxic cellular effects in stem cells during fetal development
remains to be elucidated. APAP-induced cellular effects in human
stem cells have not been reported previously, therefore, the aim of
the present study was to investigate the cellular responses of
APAP-treated human stem cells.
Based on the above-mentioned studies, the aim of our
study was to determine the cytotoxic effects of APAP on human
mesenchymal stem cells (hMSCs). Furthermore, the ROS levels
(H2O2 and O2−) and the
role of caspase death pathways and MAPK signaling pathways were
also determined in the APAP-treated hMSCs.
Materials and methods
Chemicals
Caspase-3, caspase-8, caspase-9, cleaved caspase-3,
cleaved caspase-8 and cleaved caspase-9 monoclonal antibodies were
purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA).
Extracellular-signal-regulated kinase (ERK), p38, JNK,
phosphorylated (p)-p38, p-ERK and p-JNK monoclonal antibodies were
purchased from BD Transduction Laboratories (San Diego, CA, USA).
Secondary mouse anti-human antibody was purchased from GE
Healthcare (Piscataway, NJ, USA). Tubulin monoclonal antibody,
luminol, lucigenin, vitamin C and Hoechst 33342 were purchased from
Sigma-Aldrich (St. Louis, MO, USA). The
3-(4,5-dimethylthiazol-2-yl)-2,5-di-phenyltetrazolium bromide (MTT)
kits were purchased from Bio Basic, Inc. (Markham, ON, Canada).
Fetal bovine serum, Dulbecco’s modified Eagle’s medium (DMEM),
DMEM-low glucose (DMEM-LG), non-essential amino acid L-glutamine
and penicillin/streptomycin were obtained from GE Healthcare Life
Sciences (Logan, UT, USA).
Cells and cell cultures
The NRK-52E rat renal tubular cells were obtained
from Bioresource Collection and Research Center (Hsinchu, Taiwan).
The hMSCs (Bioresources Collection and Research Center, Hsin Chu,
Taiwan) were cultured in DMEM-LG supplemented with 10% fetal bovine
serum, 2 mM L-glutamine, 100 IU/ml penicillin/streptomycin and 0.1
mM non-essential amino acids. The NRK-52E cells were cultured in
DMEM supplemented with 10% fetal bovine serum, 2 mM L-glutamine,
100 IU/ml penicillin/streptomycin, and 0.1 mM non-essential amino
acids. The two cell lines were maintained in a humidified 37°C
incubator containing 5% carbon dioxide.
Cell survival rate assay
The survival rates of the NRK-52E and hMSCs were
determined using MTT assay kits, as described in a previous study
(26). Briefly, 1,500 cells were
cultured in each well of 96-well plates at 37°C. After 24 h, the
cells were divided into control and experimental groups and the
cell survival rates were examined for 4 days. Each day, 100
μl MTT (0.005 g/ml in PBS) were added to each well,
according to the manufacturer’s instructions. After 3 h incubation
at 37°C, the absorbance (570 nm) was measured under a multi-well
enzyme-linked immunosorbent assay reader (SpectraMax Paradigm
Multi-Mode Microplate Reader; Molecular Devices, Sunnyvale, CA,
USA). The cell survival rate was determined using the following
formula: A570 experimental group / A570
control group × 100%.
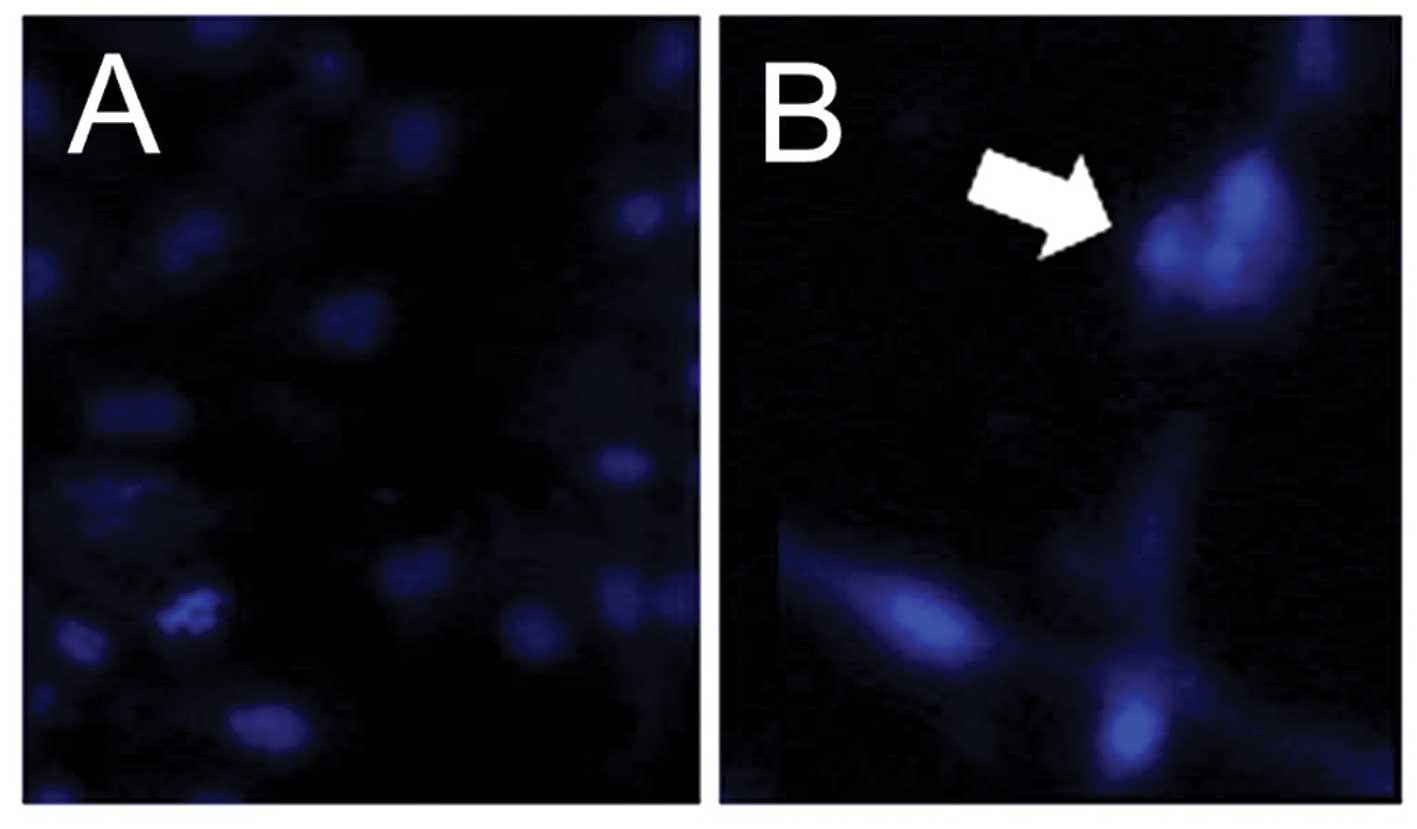
Observation of nuclear condensation
The examine the presence of apoptotic cells
exhibiting nuclear condensation, a Hoechst 33342 staining method
was used (26,49). The cells (approximately
104) in the control group and experimental group were
treated with 10 μg/ml Hoechst 33342 for 5 min. Nuclear
condensation was observed under an Olympus BX61 fluorescent
microscope (excitation, 352 nm; emission, 450 nm; Olympus
Corporation, Tokyo, Japan).
Sodium dodecyl sulfate (SDS)
electrophoresis and western blot analysis
SDS electrophoresis and western blot analysis were
performed, according to previous described methods (50,51). Briefly, the cells (approximately
107) were treated with radioimmunoprecipitation assay
lysis buffer (50 mM Tris-HCl, 120 mM NaCl, 1 mM EDTA, 1% NP-40, pH
7.5) and centrifuged (16,000 × g) for 10 min at 4°C. The protein
was collected from the supernatant layer and the concentration was
determined using a BSA Protein Assay Reagent kit (Pierce, Rockford,
IL, USA) with a DU-530 spectrophotometer (OD562 nm; Beckman
Coulter, Inc., Brea, CA, USA). Equal quantities of protein (60
μg) were separated on a SDS-polyacrylamide gel (13.3%) using
GHE320 Mini-STD Vertical Gel Electrophoresis Tank and transferred
onto a polyvinylidene difluoride membrane (Millipore, Billerica,
MA, USA). The membranes were blocked with 5% milk for 2 h at 25°C
and then washed with phosphate-buffered saline (PBS). The membranes
were incubated with 5% milk containing the primary antibodies
(1:500) for 2 h at 25°C. The membranes were then washed with PBS
buffer and treated with secondary antibodies (1:2,000) for 1 h at
25°C. Finally, the proteins were detected using 400 μl
Western Lightning Chemiluminescence Reagent Plus (PerkinElmer,
Inc., Waltham, MA, USA).
Determination of oxygen
(O2−) and H2O2
levels
The levels of O2− and
H2O2 were examined using a
lucigenin-amplified chemiluminescence technique, as previously
described (52,53). Briefly, to determine the levels of
H2O2, 200 μl of the sample (containing
8,000 cells) was treated with 0.2 mmol/l luminol solution (100
μl), followed by examination using a chemiluminescence
analyzing system (CLA-FS1; Tohoku Electronic Industrial Co., Ltd.,
Sendai, Miyagi, Japan). Similarly, to determine the levels of
O2−, 200 μl of the sample (containing
8,000 cells) was treated with 0.1 mmol/l of the lucigenin solution
(500 μl), followed by examination using the CLA-FS1
system.
Statistical analysis
Data were calculated from four independent
triplicate experiments and are presented as the means ± standard
deviation. Statistical differences between 2 groups were analyzed
using the Student’s t-test. A P-value <0.05 was considered to
indicate a statistically significant difference.
Results
APAP decreases the survival of kidney
tubular epithelial cells and hMSCs
Previous studies have demonstrated that high-dose
APAP (> 5 mM) can decrease the cell survival rate of liver and
kidney cells (20–26). Similar to these studies, the
present study revealed that high-dose APAP (7.94 mM) reduced cell
survival in the NRK-52E kidney tubular epithelial cells (Fig. 1A). Until now, the cytotoxic
effects of APAP treatment in human stem cells have not been
investigated. The present study is the first, to be best of our
knowledge, to demonstrate that high-dose APAP reduced the survival
rate of hMSCs (Fig. 1B). The
results following low-dose APAP treatment (0.794 mM) revealed no
significant cytotoxic effects in the NRK-52E cells or the hMSCs
(Fig. 1). The survival rates
following high-dose APAP therapy between the NRK-52E cells and
hMSCs were also compared. The survival rate on day 3 was ~60% in
the APAP-treated NRK-52E cells (Fig.
1A) and ~30% in the APAP-treated hMSCs (Fig. 1B). Therefore, high-dose APAP
exerted a more marked cytotoxic effect in the hMSCs, compared with
the NRK-52E cells. These findings indicated that APAP induced more
damage in the stem cells than in the kidney cells.
High-dose APAP induces apoptosis and
activates the caspase-9/-3 cascade in hMSCs
The present study subsequently examined whether the
apoptotic death pathway is involved in hMSC death following
high-dose APAP treatment. Upon examination of the nuclear
morphology, nuclear condensation, an apoptotic feature (26,54), was identified in the APAP-treated
hMSCs (Fig. 2). Thus, the results
demonstrated that high-dose APAP induced apoptosis in the hMSCs.
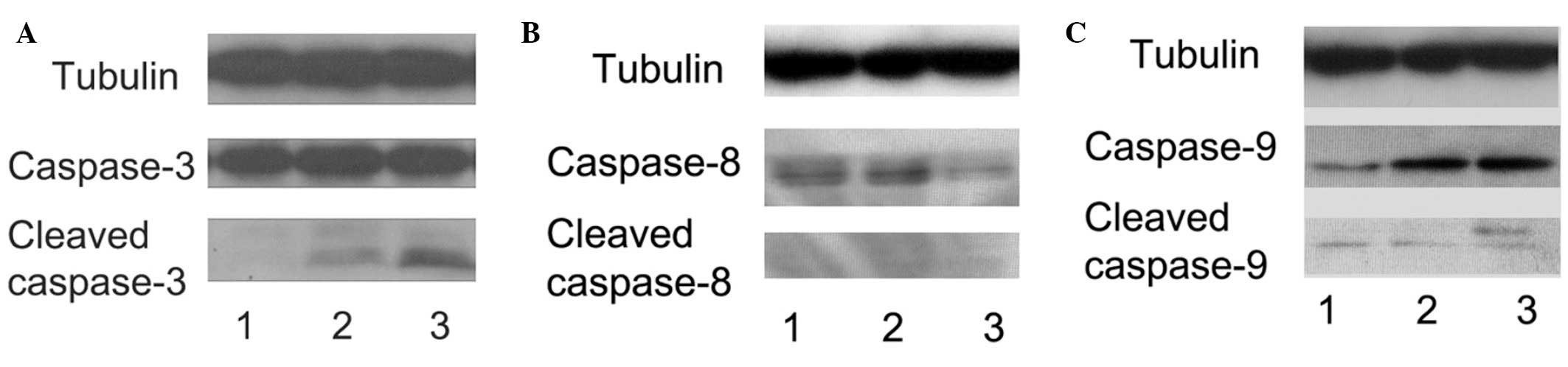
Caspase activation triggers apoptosis (49,55). Two major caspase signaling
pathways are associated with the apoptosis-caspase-9/-3 and
caspase-8/-3 cascades (26,56). Cleaved caspase-3, -8 and -9 were
observed following 3 days of APAP treatment using western blot
analysis. As shown in Fig. 3, the
levels of cleaved caspase-3 and -9 were increased in the high-dose
APAP-treated hMSCs (Fig. 3A, lane
3 and Fig. 3C, lane 3); however,
the levels of cleaved caspase-8 were unchanged (Fig. 3B). Therefore, these results
suggested that high-dose APAP stimulated apoptosis in the hMSCs via
the caspase-9/-3 signaling pathway.
APAP induces the phosphorylation of JNK
and p38 in hMSCs
APAP can induce liver injury via the MAPK signaling
pathways (57,58). In the present study, whether APAP
also activates the MAPK signaling pathways in hMSCs was examined.
JNK, p38 and ERK belong to the MAPK family (59,60). Therefore, the phosphorylation
levels of JNK, p38 and ERK were examined using western blot
analysis in the present study. As shown in Fig. 4, the levels of p- JNK and p-p38
were increased in the high-dose APAP-treated cells (Fig. 4B, lane 3), compared with the
control group (Fig. 4B, lane 1).
However, ERK phosphorylation was not observed in the APAP-treated
cells (Fig. 4B). These
experimental results suggested that APAP activated the JNK/p38 MAPK
signaling pathways, but not the ERK MAPK signaling pathway, in the
hMSCs.
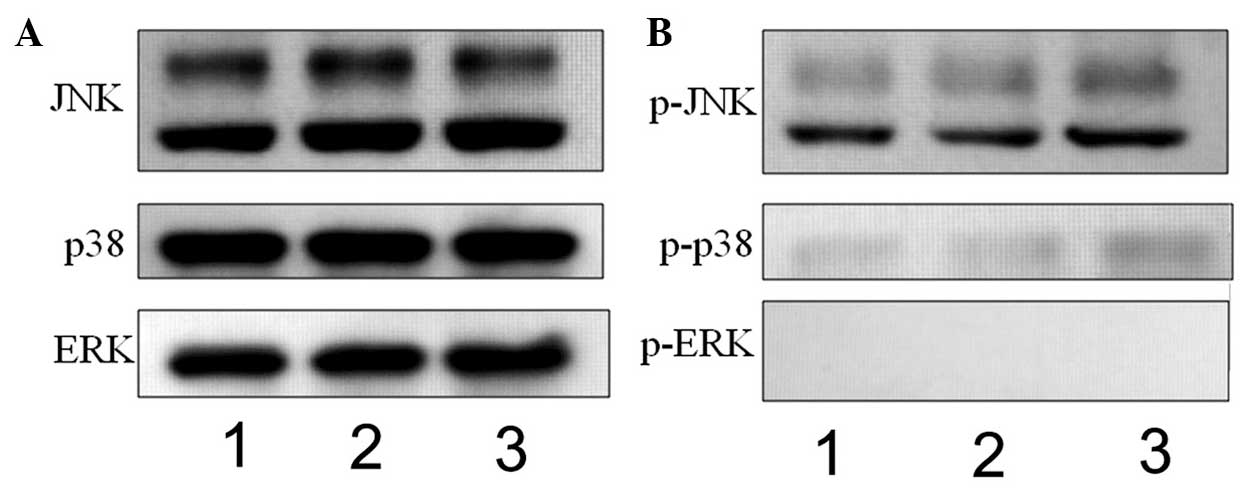 | Figure 4Western blot analysis to determine
the expression of mitogen-activated protein kinases, (JNK, p38 and
ERK) and their phosphorylation. (A) JNK, p38 and ERK, and (B)
p-JNK, p-p38 and p-ERK were observed at 30 min in the control (lane
1), 0.794 mM APAP-treated (lane 2) and 7.94 mM APAP-treated (lane
3) cells. The levels of p-JNK and p-p38 were increased in the 7.94
mM APAP-treated cells. JNK, c-Jun N-terminal kinase; ERK,
extracellular signal-regulated kinase; p-, phosphorylated; APAP,
acetaminophen. |
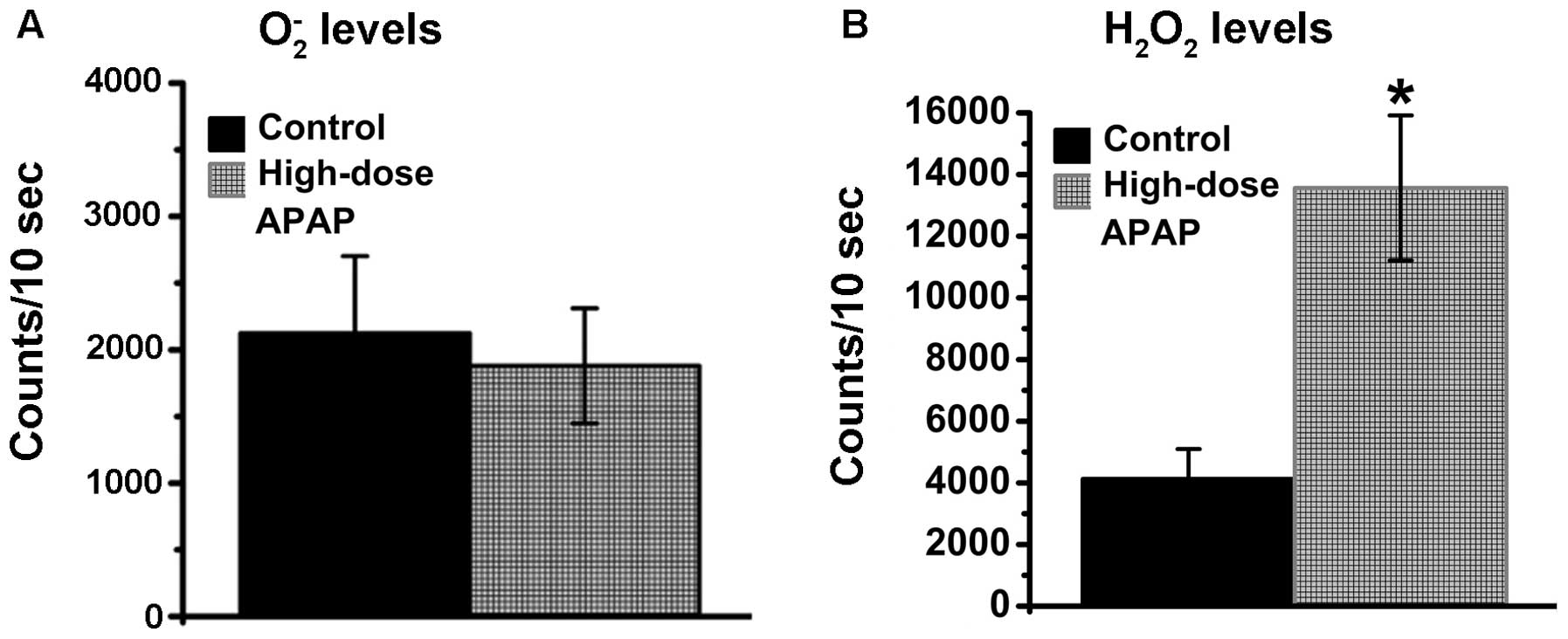
APAP stimulates increases in
H2O2 levels in hMSCs
Previous studies have demonstrated that APAP can
induce increases in ROS levels (61,62). In addition, a previous study
reported that augmentations in H2O2 levels
are found in APAP-treated kidney cells (26). O2− and
H2O2 belong to the ROS family and are
normally present in living cells, therefore, the levels of
O2− and H2O2 were
examined in the present study. As shown in Fig. 5, the O2−
levels remained constant (Fig.
5A), whereas increases in H2O2 were found
in the APAP-treated hMSCs (Fig.
5B), whereas. Therefore, the APAP-induced augmentation of ROS
was associated with H2O2, but not
O2−, in the hMSCs.
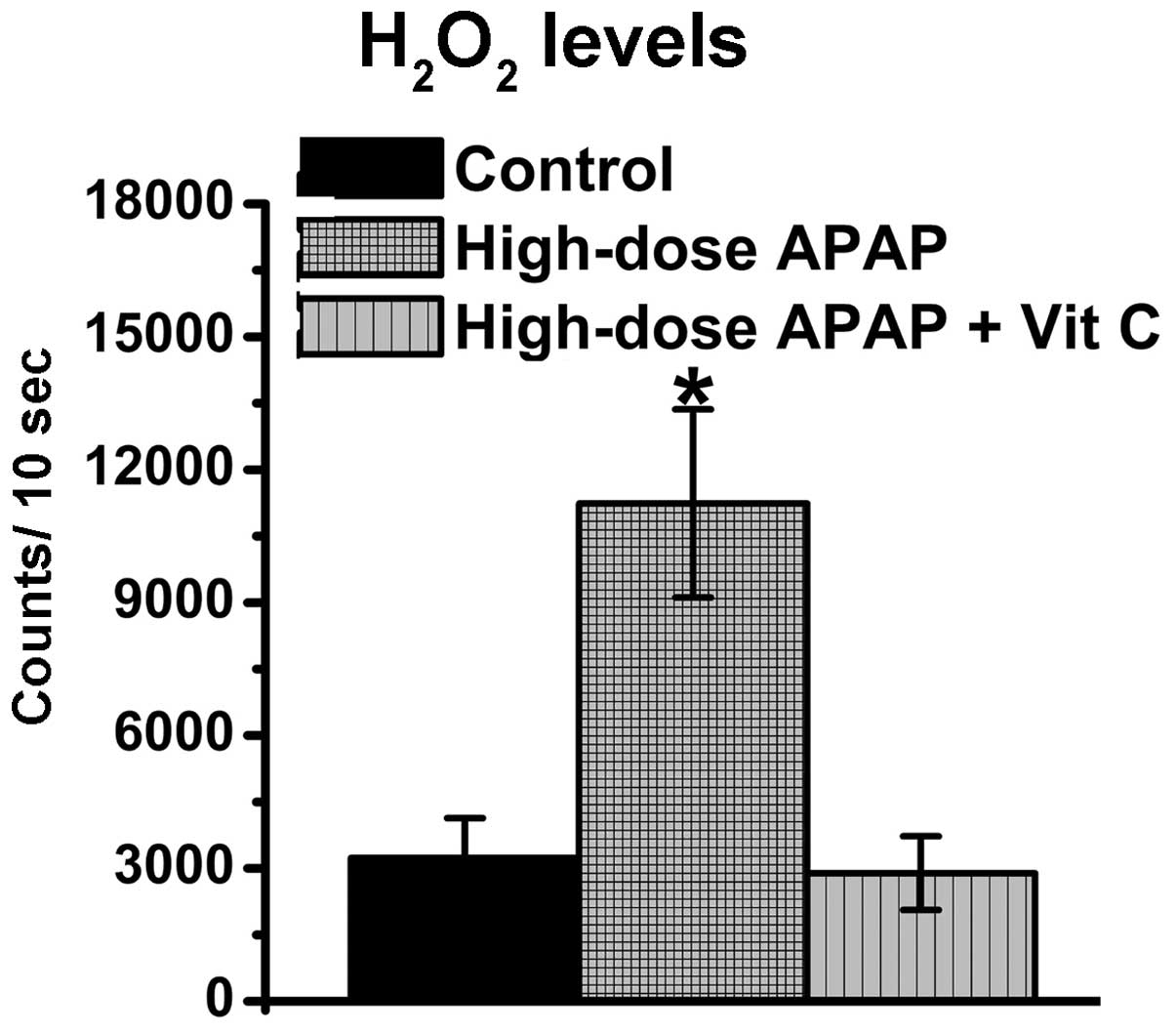
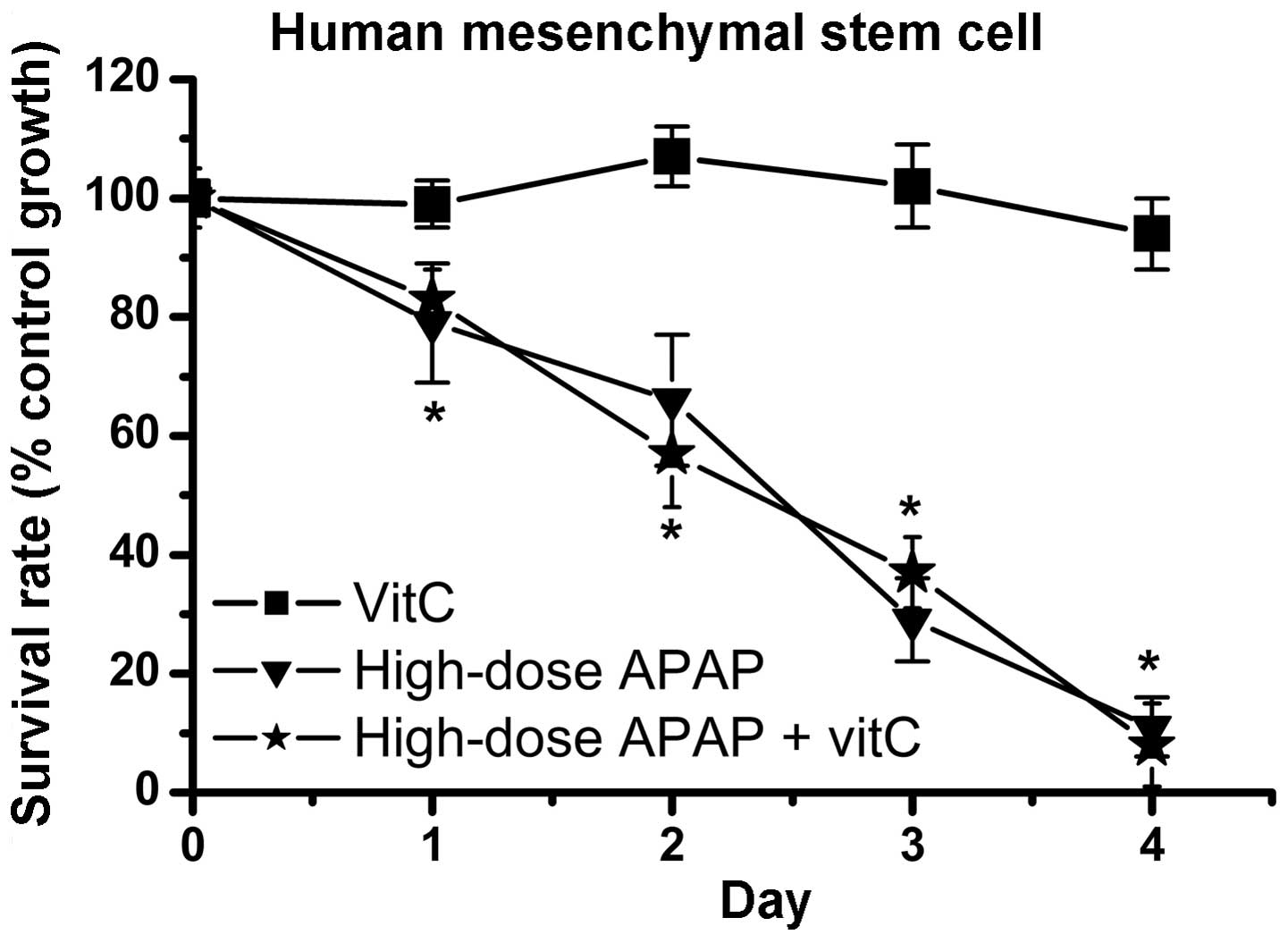
Vitamin C reduces APAP-induced increases
in H2O2 levels, but does not inhibit
APAP-induced cytotoxicity in hMSCs
APAP can stimulate elevations in ROS levels causing
cellular oxidative stress, which results in hepatotoxicity and
nephrotoxicity (16,63). Therefore, several antioxidant
drugs that prevent APAP-induced cellular damage have been
investigated (64–68). Vitamin C, an antioxidant, was used
to inhibit the cytotoxic effects of APAP in the hMSCs in the
present study. The resulting data revealed that vitamin C
effectively reduced the increases in H2O2
levels (Fig. 6). Therefore,
vitamin C had an antioxidative function in decreasing cellular
oxidative stress. Subsequently, whether vitamin C inhibited
APAP-induced cytotoxicity in the hMSCs was determined. As shown in
Fig. 7, cell survival rates were
markedly decreased in the high-dose APAP-treated group and
high-dose APAP + vitamin C-treated group, compared to the control
group. These findings indicated that inhibition of the increases in
H2O2 did not prevent APAP-induced
cytotoxicity in the hMSCs.
The present study was the first, to the best of our
knowledge, to demonstrate that high-dose APAP reduced the survival
rate of hMSCs, induced the JNK/p38 MAPK signaling pathways and
activated the caspase-9/-3 apoptotic death pathway. In addition,
the inhibition of increases in the levels of
H2O2 did not rescue the cell survival rate
following APAP treatment.
Discussion
APAP is regarded a safe medicine applied widely to
treat pain and fever in clinical cases (69–71). However, high-dose APAP can cause
clinical hepatotoxicity and nephrotoxicity (5–7).
Previous studies have demonstrated that APAP has antitumor effects
in various types of cancer, including liver cancer, breast cancer
and neuroblastoma (26,39,43). These studies indicated that APAP
can induce cytotoxicity in liver, renal and tumor cells. Therefore,
the majority of studies investigating APAP-induced cytotoxic
mechanisms have focused on renal, liver and tumor cells (26,39,43,58,62,72,73). The present study was the first, to
the best of our knowledge, to demonstrate that APAP also induces
cytotoxicity in hMSCs, suggesting APAP not only triggers clinical
hepatotoxicity and nephrotoxicity, but it is also harmful to stem
cells. Notably, as shown in Fig.
1, the present study demonstrated that APAP exerts a more
marked cytotoxic effect in hMSCs than in renal tubular cells. Stem
cells are important in fetal development, and stem cell dysfunction
may be harmful to fetus growth. In addition, previous studies have
demonstrated that APAP can cross the placenta (44,45). Therefore, the results of the
present study suggested the requirement for caution when treating
pregnant females with APAP for pain and fever.
The activation of apoptosis and necrosis have been
found in liver and renal cells following APAP treatment in
different animal and cell models (31,32). The majority of studies have
reported that APAP-induced apoptotic death in liver and renal cells
is associated with caspase-3 activation (74–76). There are two major caspase
cascades, caspase-9/-3 and caspase-8/-3 cascades (26,54,55). The caspase-9/-3 cascade is linked
to mitochondrial dysfunction and the caspase-8/-3 casecade is
associated with death receptor signal transduction. APAP-induced
liver and renal injury has been observed to trigger the
caspase-9/-3 pathway (11,77).
In addition, activation of the caspase-9/-3 cascade is also found
in APAP-treated hepatoma cells (26). In the present study, the data
revealed that APAP activated caspase-9 and -3 signaling in the
hMSCs but did not activate caspase-8 (Fig. 3). Taken together, these studies
indicated that mitochondrial damage is an important factor that
results in cell death in renal cells, liver cells, hepatoma cells
and stem cells following APAP treatment.
The MAPK signaling pathways undergo three major
phosphorylation reactions: ERK, JNK and p38 phosphorylation
(59,60). Previous studies have demonstrated
that APAP can induce acute liver injury via the JNK and ERK
phosphorylation signaling pathways (57,58). A previous study found that
APAP-induced liver damage not only activates JNK and ERK
phosphorylation, but also induces p38 phosphorylation in mouse
models (78), although the common
signaling pathways are the ERK and JNK phosphorylation pathways. In
the present study, JNK and p38 phosphorylation were observed in the
APAP-treated hMSCs, however, ERK phosphorylation was not observed
(Fig. 4). The observation of ERK
phosphorylation in the APAP-treated liver cells, but not in the
stem cells remains to be elucidated and requires investigation in
the future.
Previous studies have demonstrated that high-dose
APAP-induced hepatotoxicity and nephrotoxicity are associated with
increases in ROS levels (25,79–81). Severalantioxidants against
APAP-induced cytotoxicity have been investigated, including green
tea, honey, tofu and NAC (13,80,82–85). O2− and
H2O2 belong to the ROS family and are
produced by the electron transport chain. O2−
can be removed by superoxide dismutase, and
H2O2 can be removed by glutathione system
(19,26,85). NAC, a precursor for glutathione
synthesis, can effectively reduce H2O2 levels
and has been applied as a treatment method for APAP-induced
hepatotoxicity and nephrotoxicity in clinical cases (17–19). The levels of
O2− and H2O2 can be
determined in APAP-treated stem cells. The present study
demonstrated that APAP stimulated increases in the levels of
H2O2, but not O2−, in
human stem cells. This result is similar to a previous study, in
which only increases in H2O2 levels were
found in APAP-treated Hep3B cells (26). In addition, the present study
further demonstrated that vitamin C effectively reduced
APAP-induced elevations in H2O2, but does not
inhibit APAP-induced cytotoxicity, in human cells. This result
indicated that there are unknown cellular effects, in addition to
augmentations in the levels of ROS, resulting in APAP-induced
cytotoxicity in human stem cells. The present study demonstrated
that antioxidants agents prevented APAP-induced damage in liver and
renal cells, but not in stem cells.
In conclusion, this study was the first, to the best
of our knowledge, to demonstrate that APAP induced the p38/JNK MAPK
signaling pathway, activated the caspase-9/-3 cascade and decreased
survival rate in human stem cells. The present study also revealed
that APAP-induced cytotoxic effects were more marked in stem cells
than in renal cells, and antioxidants did not prevent stem cell
damage following APAP treatment.
Acknowledgments
This study was supported in part by the Ministry of
Science and Technology (MOST103 2320-B-039-052-MY3), the National
Health Research Institutes (NHRI-EX102-10245BI) and the Taipei Tzu
Chi Hospital (TCRD-TPE-102-26 and TCRD-TPE-103-48).
References
|
1
|
Cuzzolin L, Antonucci R and Fanos V:
Paracetamol (acetaminophen) efficacy and safety in the newborn.
Curr Drug Metab. 14:178–185. 2013.
|
|
2
|
Klotz U: Paracetamol (acetaminophen) - a
popular and widely used nonopioid analgesic. Drug Res. 62:355–359.
2012.
|
|
3
|
Section on Clinical Pharmacology
Therapeutics, Committee on Drugs; Sullivan JE and Farrar HC: Fever
and antipyretic use in children. Pediatrics. 127:580–587. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rumack BH: Acetaminophen misconceptions.
Hepatology. 40:10–15. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hawton K, Bergen H, Simkin S, et al:
Impact of different pack sizes of paracetamol in the United Kingdom
and Ireland on intentional overdoses: a comparative study. BMC
Public Health. 11:4602011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hawton K, Townsend E, Deeks J, et al:
Effects of legislation restricting pack sizes of paracetamol and
salicylate on self poisoning in the United Kingdom: before and
after study. BMJ. 322:1203–1207. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Daly FF, Fountain JS, Murray L, Graudins A
and Buckley NA; Panel of Australian and New Zealand clinical
toxicologists: Guidelines for the management of paracetamol
poisoning in Australia and New Zealand - explanation and
elaboration. A consensus statement from clinical toxicologists
consulting to the Australasian poisons information centres. Med J
Aust. 188:296–301. 2008.PubMed/NCBI
|
|
8
|
Young RJ: Dextropropoxyphene overdosage.
Pharmacological considerations and clinical management. Drugs.
26:70–79. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Simkin S, Hawton K, Kapur N and Gunnell D:
What can be done to reduce mortality from paracetamol overdoses? A
patient interview study. QJM. 105:41–51. 2012. View Article : Google Scholar
|
|
10
|
McGill MR, Li F, Sharpe MR, et al:
Circulating acylcarnitines as biomarkers of mitochondrial
dysfunction after acetaminophen overdose in mice and humans. Arch
Toxicol. 88:391–401. 2014. View Article : Google Scholar :
|
|
11
|
Ghosh J, Das J, Manna P and Sil PC:
Acetaminophen induced renal injury via oxidative stress and
TNF-alpha production: therapeutic potential of arjunolic acid.
Toxicology. 268:8–18. 2010. View Article : Google Scholar
|
|
12
|
Chandrasekaran VR, Wan CH, Liu LL, Hsu DZ
and Liu MY: Effect of sesame oil against acetaminophen-induced
acute oxidative hepatic damage in rats. Shock. 30:217–221.
2008.
|
|
13
|
Galal RM, Zaki HF, Seif El-Nasr MM and
Agha AM: Potential protective effect of honey against
paracetamol-induced hepatotoxicity. Arch Iran Med. 15:674–680.
2012.PubMed/NCBI
|
|
14
|
Abdul Hamid Z, Budin SB, Wen Jie N, Hamid
A, Husain K and Mohamed J: Nephroprotective effects of Zingiber
zerumbet Smith ethyl acetate extract against paracetamol-induced
nephrotoxicity and oxidative stress in rats. J Zhejiang Univ Sci B.
13:176–185. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kheradpezhouh E, Panjehshahin MR, Miri R,
et al: Curcumin protects rats against acetaminophen-induced
hepatorenal damages and shows synergistic activity with N-acetyl
cysteine. Eur J Pharmacol. 628:274–281. 2010. View Article : Google Scholar
|
|
16
|
Anoush M, Eghbal MA, Fathiazad F, Hamzeiy
H and Kouzehkonani NS: The protective effects of garlic extract
against acetaminophen-induced oxidative stress and glutathione
depletion. Pak J Biol Sci. 12:765–771. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mehrpour O, Shadnia S and Sanaei-Zadeh H:
Late extensive intravenous administration of N-acetylcysteine can
reverse hepatic failure in acetaminophen overdose. Hum Exp Toxicol.
30:51–54. 2011. View Article : Google Scholar
|
|
18
|
Blackford MG, Felter T, Gothard MD and
Reed MD: Assessment of the clinical use of intravenous and oral
N-acetylcysteine in the treatment of acute acetaminophen poisoning
in children: a retrospective review. Clin Ther. 33:1322–1330. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tsai CL, Chang WT, Weng TI, Fang CC and
Walson PD: A patient-tailored N-acetylcysteine protocol for acute
acetaminophen intoxication. Clin Ther. 27:336–341. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Amaral SS, Oliveira AG, Marques PE, et al:
Altered responsiveness to extracellular ATP enhances acetaminophen
hepatotoxicity. Cell Commun Signal. 11:102013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Badmann A, Langsch S, Keogh A, Brunner T,
Kaufmann T and Corazza N: TRAIL enhances paracetamol-induced liver
sinusoidal endothelial cell death in a Bim- and Bid-dependent
manner. Cell Death Dis. 3:e4472012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Badmann A, Keough A, Kaufmann T, Bouillet
P, Brunner T and Corazza N: Role of TRAIL and the pro-apoptotic
Bcl-2 homolog Bim in acetaminophen-induced liver damage. Cell Death
Dis. 2:e1712011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
McGill MR, Yan HM, Ramachandran A, Murray
GJ, Rollins DE and Jaeschke H: HepaRG cells: a human model to study
mechanisms of acetaminophen hepatotoxicity. Hepatology. 53:974–982.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao X, Cong X, Zheng L, Xu L, Yin L and
Peng J: Dioscin, a natural steroid saponin, shows remarkable
protective effect against acetaminophen-induced liver damage in
vitro and in vivo. Toxicol Lett. 214:69–80. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mobasher MA, Gonzalez-Rodriguez A,
Santamaria B, et al: Protein tyrosine phosphatase 1B modulates
GSK3β/Nrf2 and IGFIR signaling pathways in acetaminophen-induced
hepatotoxicity. Cell Death Dis. 4:e6262013. View Article : Google Scholar
|
|
26
|
Yu YL, Yiang GT, Chou PL, et al: Dual role
of acetaminophen in promoting hepatoma cell apoptosis and kidney
fibroblast proliferation. Mol Med Rep. 9:2077–2084. 2014.PubMed/NCBI
|
|
27
|
Gopi KS, Reddy AG, Jyothi K and Kumar BA:
Acetaminophen-induced hepato- and nephrotoxicity and amelioration
by silymarin and Terminalia chebula in rats. Toxicol Int. 17:64–66.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Abdel-Zaher AO, Abdel-Hady RH, Mahmoud MM
and Farrag MM: The potential protective role of alpha-lipoic acid
against acetaminophen-induced hepatic and renal damage. Toxicology.
243:261–270. 2008. View Article : Google Scholar
|
|
29
|
Cermik H, Taslipinar MY, Aydin I, et al:
The relationship between N-acetylcysteine, hyperbaric oxygen, and
inflammation in a rat model of acetaminophen-induced
nephrotoxicity. Inflammation. 36:1145–1152. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ucar F, Taslipinar MY, Alp BF, et al: The
effects of N-acetylcysteine and ozone therapy on oxidative stress
and inflammation in acetaminophen-induced nephrotoxicity model. Ren
Fail. 35:640–647. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liang YL, Zhang ZH, Liu XJ, et al:
Melatonin protects against apoptosis-inducing factor
(AIF)-dependent cell death during acetaminophen-induced acute liver
failure. PLoS One. 7:e519112012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ramachandran A, McGill MR, Xie Y, Ni HM,
Ding WX and Jaeschke H: Receptor interacting protein kinase 3 is a
critical early mediator of acetaminophen-induced hepatocyte
necrosis in mice. Hepatology. 58:2099–2108. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ahmad ST, Arjumand W, Nafees S, et al:
Hesperidin alleviates acetaminophen induced toxicity in Wistar rats
by abrogation of oxidative stress, apoptosis and inflammation.
Toxicol Lett. 208:149–161. 2012. View Article : Google Scholar
|
|
34
|
Inkielewicz-Stepniak I and Knap N: Effect
of exposure to fluoride and acetaminophen on oxidative/nitrosative
status of liver and kidney in male and female rats. Pharmacol Rep.
64:902–911. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Slitt AM, Dominick PK, Roberts JC and
Cohen SD: Effect of ribose cysteine pretreatment on hepatic and
renal acetaminophen metabolite formation and glutathione depletion.
Basic Clin Pharmacol Toxicol. 96:487–494. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yousef MI, Omar SA, El-Guendi MI and
Abdelmegid LA: Potential protective effects of quercetin and
curcumin on paracetamol-induced histological changes, oxidative
stress, impaired liver and kidney functions and haematotoxicity in
rat. Food Chem Toxicol. 48:3246–3261. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
De-Giorgio F, Lodise M, Chiarotti M,
d’Aloja E, Carbone A and Valerio L: Possible fatal acetaminophen
intoxication with atypical clinical presentation. J Forensic Sci.
58:1397–1400. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Brusilow SW and Cooper AJ: Encephalopathy
in acute liver failure resulting from acetaminophen intoxication:
new observations with potential therapy. Crit Care Med.
39:2550–2553. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Posadas I, Santos P and Cena V:
Acetaminophen induces human neuroblastoma cell death through NFKB
activation. PLoS One. 7:e501602012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Posadas I, Vellecco V, Santos P,
Prieto-Lloret J and Cena V: Acetaminophen potentiates
staurosporine-induced death in a human neuroblastoma cell line. Br
J Pharmacol. 150:577–585. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jaeschke H: Comments on ‘glycogen synthase
kinase-3 mediates acetaminophen-induced apoptosis in human hepatoma
cells’. J Pharmacol Exp Ther. 314:1401–1402; author reply
1403–1404. 2005. View Article : Google Scholar
|
|
42
|
Macanas-Pirard P, Yaacob NS, Lee PC,
Holder JC, Hinton RH and Kass GE: Glycogen synthase kinase-3
mediates acetaminophen-induced apoptosis in human hepatoma cells. J
Pharmacol Exp Ther. 313:780–789. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bilir A, Guneri AD and Altinoz MA:
Acetaminophen and DMSO modulate growth and gemcitabine cytotoxicity
in FM3A breast cancer cells in vitro. Neoplasma. 51:460–464.
2004.
|
|
44
|
Thiele K, Kessler T, Arck P, Erhardt A and
Tiegs G: Acetaminophen and pregnancy: short- and long-term
consequences for mother and child. J Reprod Immunol. 97:128–139.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wilkes JM, Clark LE and Herrera JL:
Acetaminophen overdose in pregnancy. South MedJ. 98:1118–1122.
2005. View Article : Google Scholar
|
|
46
|
Tsunekawa Y, Kikkawa T and Osumi N:
Asymmetric inheritance of Cyclin D2 maintains proliferative neural
stem/progenitor cells: A critical event in brain development and
evolution. Dev Growth Differ. 56:349–357. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Di Bernardo J, Maiden MM, Jiang G,
Hershenson MB and Kunisaki SM: Paracrine regulation of fetal lung
morphogenesis using human placenta-derived mesenchymal stromal
cells. J Surg Res. 190:255–263. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Dambrot C, Buermans HP, Varga E, et al:
Strategies for rapidly mapping proviral integration sites and
assessing cardiogenic potential of nascent human induced
pluripotent stem cell clones. Exp Cell Res. 327:297–306. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yiang GT, Chen YH, Chou PL, Chang WJ, Wei
CW and Yu YL: The NS3 protease and helicase domains of Japanese
encephalitis virus trigger cell death via caspase-dependent and
-independent pathways. Mol Med Rep. 7:826–830. 2013.PubMed/NCBI
|
|
50
|
Yu YL, Su KJ, Chen CJ, et al: Synergistic
anti-tumor activity of isochaihulactone and paclitaxel on human
lung cancer cells. J Cell Physiol. 227:213–222. 2012. View Article : Google Scholar
|
|
51
|
Wei CW, Hu CC, Tang CH, Lee MC and Wang
JJ: Induction of differentiation rescues HL-60 cells from Rana
catesbeiana ribonuclease-induced cell death. FEBS Lett.
531:421–426. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chen KH, Li PC, Lin WH, Chien CT and Low
BH: Depression by a green tea extract of alcohol-induced oxidative
stress and lipogenesis in rat liver. Biosci Biotechnol Biochem.
75:1668–1676. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lin BR, Yu CJ, Chen WC, et al: Green tea
extract supplement reduces D-galactosamine-induced acute liver
injury by inhibition of apoptotic and proinflammatory signaling. J
Biomed Sci. 16:352009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yiang GT, Yu YL, Chou PL, et al: The
cytotoxic protein can induce autophagocytosis in addition to
apoptosis in MCF-7 human breast cancer cells. In Vivo. 26:403–409.
2012.PubMed/NCBI
|
|
55
|
Yiang GT, Yu YL, Hu SC, Chen MH, Wang JJ
and Wei CW: PKC and MEK pathways inhibit caspase-9/-3-mediated
cytotoxicity in differentiated cells. FEBS Lett. 582:881–885. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Tang CH, Hu CC, Wei CW and Wang JJ:
Synergism of Rana catesbeiana ribonuclease and IFN-gamma triggers
distinct death machineries in different human cancer cells. FEBS
Lett. 579:265–270. 2005. View Article : Google Scholar
|
|
57
|
Wang AY, Lian LH, Jiang YZ, Wu YL and Nan
JX: Gentiana manshurica Kitagawa prevents acetaminophen-induced
acute hepatic injury in mice via inhibiting JNK/ERK MAPK pathway.
World J Gastroenterol. 16:384–391. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Win S, Than TA, Han D, Petrovic LM and
Kaplowitz N: c-Jun N-terminal kinase (JNK)-dependent acute liver
injury from acetaminophen or tumor necrosis factor (TNF) requires
mitochondrial Sab protein expression in mice. J Biol Chem.
286:35071–35078. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Chen JY, Zhang L, Zhang H, Su L and Qin
LP: Triggering of p38 MAPK and JNK signaling is important for
oleanolic acid-induced apoptosis via the mitochondrial death
pathway in hypertrophic scar fibroblasts. Phytother Res.
28:1468–1478. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Liu X, Ye F, Xiong H, et al: IL-1β
upregulates IL-8 production in human Müller cells through
activation of the p38 MAPK and ERK1/2 signaling pathways.
Inflammation. 37:1486–1495. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Levanon D, Manov I and Iancu TC:
Qualitative and quantitative analysis of the effects of
acetaminophen and N-acetylcysteine on the surface morphology of
Hep3B hepatoma cells in vitro. Ultrastruct Pathol. 28:3–14.
2004.PubMed/NCBI
|
|
62
|
Manov I, Hirsh M and Iancu TC:
Acetaminophen hepatotoxicity and mechanisms of its protection by
N-acetylcysteine: a study of Hep3B cells. Exp Toxicol Pathol.
53:489–500. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Kumari A and Kakkar P: Lupeol protects
against acetaminophen-induced oxidative stress and cell death in
rat primary hepatocytes. Food Chem Toxicol. 50:1781–1789. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Okokon JE, Nwafor PA, Charles U, Dar A and
Choudhary MI: Antioxidative burst and hepatoprotective effects of
ethanol root extract of Hippocratea africana against
paracetamol-induced liver injury. Pharm Biol. 51:872–880. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Lee NH, Seo CS, Lee HY, et al:
Hepatoprotective and antioxidative activities of Cornus officinalis
against acetaminophen-induced hepatotoxicity in mice. Evid Based
Complement Alternat Med. 2012:8049242012. View Article : Google Scholar
|
|
66
|
Olaleye MT, Akinmoladun AC, Ogunboye AA
and Akindahunsi AA: Antioxidant activity and hepatoprotective
property of leaf extracts of Boerhaavia diffusa Linn against
acetaminophen-induced liver damage in rats. Food Chem Toxicol.
48:2200–2205. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Nkosi CZ, Opoku AR and Terblanche SE: In
vitro antioxidative activity of pumpkin seed (Cucurbita pepo)
protein isolate and its in vivo effect on alanine transaminase and
aspartate transaminase in acetaminophen-induced liver injury in low
protein fed rats. Phytother Res. 20:780–783. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Bhattacharjee R and Sil PC: The protein
fraction of Phyllanthus niruri plays a protective role against
acetaminophen induced hepatic disorder via its antioxidant
properties. Phytother Res. 20:595–601. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Needleman SM: Safety of rapid intravenous
of infusion acetaminophen. Proc (Bayl Univ Med Cent). 26:235–238.
2013.
|
|
70
|
Engstrom Ruud L, Wilhelms DB, Eskilsson A,
et al: Acetaminophen reduces lipopolysaccharide-induced fever by
inhibiting cyclooxygenase-2. Neuropharmacology. 71:124–129. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Zuppa AF, Hammer GB, Barrett JS, et al:
Safety and population pharmacokinetic analysis of intravenous
acetaminophen in neonates, infants, children, and adolescents with
pain or fever. J Pediatr Pharmacol Ther. 16:246–261. 2011.
|
|
72
|
Boulares AH, Zoltoski AJ, Stoica BA,
Cuvillier O and Smulson ME: Acetaminophen induces a
caspase-dependent and Bcl-XL sensitive apoptosis in human hepatoma
cells and lymphocytes. Pharmacol Toxicol. 90:38–50. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Ghanizadeh A: Acetaminophen may mediate
oxidative stress and neurotoxicity in autism. Med Hypotheses.
78:3512012. View Article : Google Scholar
|
|
74
|
Wang C, Blough ER, Arvapalli R, et al:
Metabolic syndrome-induced tubulointerstitial injury: role of
oxidative stress and preventive effects of acetaminophen. Free
Radic Biol Med. 65:1417–1426. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Fouad AA, Al-Mulhim AS, Jresat I and Gomaa
W: Therapeutic role of telmisartan against acetaminophen
hepatotoxicity in mice. Eur J Pharmacol. 693:64–71. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Choi J, Park KH, Kim SZ, Shin JH and Jang
SI: The ameliorative effects of L-2-oxothiazolidine-4-carboxylate
on acetaminophen-induced hepatotoxicity in mice. Molecules.
18:3467–3478. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Kumari A and Kakkar P: Lupeol prevents
acetaminophen-induced in vivo hepatotoxicity by altering the
Bax/Bcl-2 and oxidative stress-mediated mitochondrial signaling
cascade. Life Sci. 90:561–570. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Ji L, Jiang P, Lu B, Sheng Y, Wang X and
Wang Z: Chlorogenic acid, a dietary polyphenol, protects
acetaminophen-induced liver injury and its mechanism. J Nutr
Biochem. 24:1911–1919. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Nafisi S, Heidari R, Ghaffarzadeh M, et
al: Cytoprotective effects of silafibrate, a newly-synthesised
siliconated derivative of clofibrate, against acetaminophen-induced
toxicity in isolated rat hepatocytes. Arh Hig Rada Toksikol.
65:169–178. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Colle D, Arantes LP, Gubert P, et al:
Antioxidant properties of Taraxacum officinaleleaf extract are
involved in the protective effect against hepatoxicity induced by
acetaminophen in mice. J Med Food. 15:549–556. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Zhao YL, Zhou GD, Yang HB, et al: Rhein
protects against acetaminophen-induced hepatic and renal toxicity.
Food Chem Toxicol. 49:1705–1710. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Lu Y, Sun J, Petrova K, et al:
Metabolomics evaluation of the effects of green tea extract on
acetaminophen-induced hepatotoxicity in mice. Food Chem Toxicol.
62:707–721. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Yakubu N, Oboh G and Olalekan AA:
Antioxidant and hepatoprotective properties of tofu (curdle
soymilk) against acetaminophen-induced liver damage in rats.
Biotechnol Res Int. 2013:2301422013. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Prescott LF, Park J, Ballantyne A,
Adriaenssens P and Proudfoot AT: Treatment of paracetamol
(acetaminophen) poisoning with N-acetylcysteine. Lancet. 2:432–434.
1977. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Allameh A, Vansoun EY and Zarghi A: Role
of glutathione conjugation in protection of weanling rat liver
against acetaminophen-induced hepatotoxicity. Mech Ageing Dev.
95:71–79. 1997. View Article : Google Scholar : PubMed/NCBI
|