Introduction
Endophthalmitis is an uncommon intraocular
inflammatory condition, but it may result in partial or complete
vision loss. It may occur as a complication of intraocular surgery
or as a result of non-surgical trauma or systemic infection
(1). Bacterial endophthalmitis is
an infection of the intraocular cavities that usually occurs
following the introduction of microbial organisms into the eye
(2). During a bacterial
infection, irreversible damage to the photoreceptor cells of the
retina frequently occurs (3).
Among the bacterial pathogens, Pseudomonas aeruginosa
(PA), a Gram-negative rod rod-shaped organism, is frequently
a cause of nosocomial infections (4). PA can cause ocular
infections, such as keratitis and endophthalmitis (5). Lipopolysaccharides (LPS), components
of Gram-negative bacteria, lead to the activation of immune cells,
resulting in the release of pro-inflammatory mediators (6). Retinal pigment epithelial cells (RPE
cells) secrete pro-inflammatory mediators within the eye when
stimulated with infectious agents, such as Gram-negative bacteria
(7). The retinal pigment
epithelium (RPE) is a monolayer of pigmented cells originating from
the neural ectoderm. The RPE lies between the photoreceptor cell
layer of the neural retina and Bruch's membrane and choroid and
contains blood vessels that nourish the retina (8). The major function of the RPE is the
phagocytosis of photoreceptor segments, converting light impulses
into vision and it is involved in the pathology of a variety of
retinal diseases, such as age-related macular degeneration (AMD)
and diabetic retinopathy (9). The
RPE cells play an important role in the ocular immune system.
Inflammatory mediators play a critical role in
orchestrating cellular infiltration to the site of infection.
Increased cellular infiltration is followed by severe retinal
damage. Interleukin (IL)-6 is a pleiotropic cytokine with a wide
spectrum of biological activities in a broad variety of ocular
inflammatory diseases and wound healing processes (10–12). IL-6 is an important mediator in a
variety of diseases, including inflammatory, autoimmune and
malignant diseases. The local production of IL-6 by resident cells
and infiltrating inflammatory cells has been detected during a
variety of inflammatory ocular conditions (13,14). IL-6 is involved in the recruitment
of polymorphonuclear leukocytes (PMNs) to the site of inflammation
by upregulating the expression of intercellular adhesion molecule-1
(ICAM-1), a key molecule involved in the migration of neutrophils
(15–17). ICAM-1 is important for the
transendothelial migration of leukocytes through blood vessels and
the retention of leukocytes in the inflamed tissue by means of firm
attachment to the extracellular matrix (18). Monocyte chemotactic protein-1
(MCP-1), a member of the C-C subfamily of chemokines, may elicit
its inflammatory effect, leading to leukocyte accumulation and
activation in areas of injury and inflammation under pathological
and physiological conditions (19–21). IL-8, a member of the C-X-C
chemokines, is an important mediator of various acute and chronic
infectious conditions that attract and activate neutrophils and
eosinophils (22).
Damage to the delicate tissues of the retina occurs
due to oxidative stress (23).
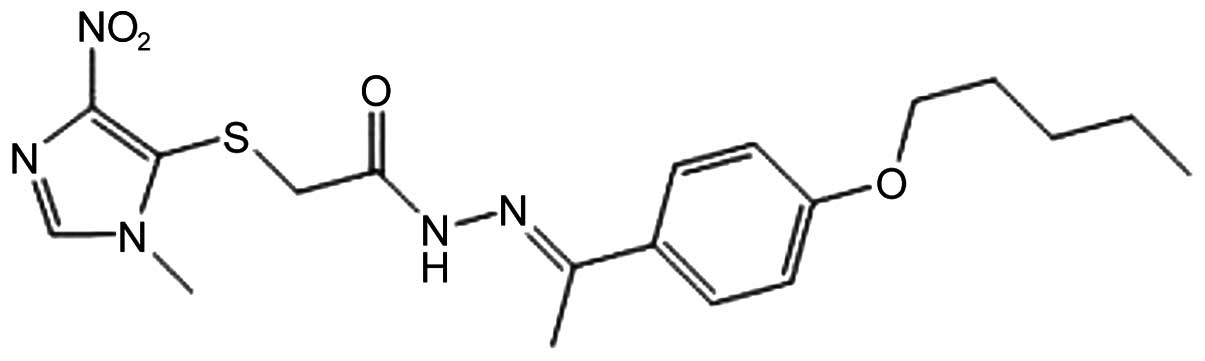
YCG063 (Fig. 1), a novel
anti-angiogenic small molecule, inhibits reactive oxygen species
(ROS) production (24); however,
its anti-inflammatory activity in response to LPS has not
previously been identified. In the present study, we examined
cultured human RPE cells for the production of IL-6, IL-8, ICAM-1
and MCP and the signaling pathways involved in response to
stimulation by PA-LPS. Additionally, we evaluated the
effects of YCG063 on these inflammatory responses.
Materials and methods
Reagents
PA-LPS was purchased from Sigma Chemical Co.
(St. Louis, MO, USA). YCG063 was obtained from Millipore
(Billerica, MA, USA). LY294002 (an inhibitor of AKT) was purchased
from Calbiochem (La Jolla, CA, USA). Antibodies against p65 (Cat.
no. 14-6731) and Toll-like receptor (TLR)2 (Cat. no. 16-9024-83;
clone T2.5) were obtained from eBioscience (San Diego, CA, USA).
Antibodies against phosphorylated (p)-extracellular
signal-regulated protein kinase (ERK)1/2 (Cat. no. 9106), AKT (Cat.
no. 9272), p-AKT (Cat. no. 4058) and p-p38 MAPK (Cat. no. 9211)
were purchased from Cell Signaling Technology (Beverly, MA, USA).
Antibodies against ERK1/2 (Cat. no. sc-94) and p38 MAPK (Cat. no.
sc-535) were purchased from Santa Cruz Biotechnology, Inc. (Santa
Cruz, CA, USA). 2′,7′-Dichlorofluorescein diacetate (DCFDA) was
purchased from Invitrogen (Carlsbad, CA, USA). MitoSOX and MitoPY1
were purchased from Tocris Bioscience (Bristol, UK). BAY 11-7082
and parthenolide [nuclear factor-κB (NF-κB) inhibitors] were
purchased from Santa Cruz Biotechnology, Inc. Nitrocellulose
membranes and an enhanced chemiluminescence (ECL) kit were obtained
from Amersham Pharmacia Biotech (Uppsala, Sweden). Phycoerythrin
(PE)-conjugated anti-human TLR2 (clone TL2.1) and TLR4 (clone
HTA125) monoclonal antibodies or isotope controls were obtained
from BD Biosciences (San Jose, CA, USA).
ARPE-19 cell culture
The ARPE-19 cells were obtained from the American
Type Culture Collection (ATCC, Manassas, VA, USA) and cultured in
Dulbecco's modified Eagle's medium (DMEM)/F12 medium supplemented
with 10% fetal bovine serum plus a 100 IU/ml penicillin and 100
µg/ml streptomycin mixture (Gibco/BRL, Gaithersburg, MD,
USA) in a humidified atmosphere (5% CO2) at 37°C. The
ARPE-19 cells were trypsinized, seeded in 10-cm diameter dishes,
and incubated overnight until attachment.
Cell viability assay
The viability of the ARPE-19 cells was determined
using the cell counting kit-8 (CCK-8) according to the
manufacturer's instructions (Dojindo Laboratories, Kumamoto,
Japan). Briefly, the cells were seeded in triplicate at a density
of 1×104 cells/well into 96-well culture plates and
allowed to attach overnight. The cells were stimulated with the
indicated concentrations of PA-LPS (0.5, 1, 5, 10, 20 and 30
µg/ml) for 24 h. The medium was then replaced with 100
µl of DMEM/F12 medium containing 5, 10 or 50 µM
YCG063. The plates were incubated for 24 h, and 10 µl of
CCK-8 reagent was added to each well. Following incubation for a
further 2 h at 37°C, the plates were read at 450 nm using a
microplate reader (Model EL800; Bio-Tek Instruments, Winooski, VT,
USA).
Enzyme-linked immunosorbent assay
(ELISA)
The levels of cytokines in the cell culture medium
were assessed by ELISA. The cells were treated with various
concentrations of YCG063, BAY 11-7082 and parthenolide for 2 h
prior to PA-LPS stimulation. Following incubation for 24 h,
the culture supernatants were collected, and the levels of
inflammatory mediators were measured. ELISA kits purchased from
BioLegend (San Diego, CA, USA) were used to measure the levels of
IL-6, IL-8 and MCP-1, and a kit obtained from R&D Systems
(Minneapolis, MN, USA) was used to measure the ICAM-1 levels. The
absorbance at 450 nm was measured using a microplate reader (Model
EL800; Bio-Tek Instruments).
Western blot analysis
The ARPE-19 cells were pre-treated with YCG063 and
LY294002 for 2 h prior to stimulation with PA-LPS. The ARPE-19
cells were washed 3 times with phosphate-buffered saline (PBS) and
lysed with lysis buffer (Mammalian Cell-PE LB, G-Biosciences, St.
Louis, MO, USA). Equal amounts of protein were separated on 10%
sodium dodecyl sulfate (SDS)-polyacrylamide minigels and
transferred onto nitrocellulose transfer membranes. Follownig
incubation with the appropriate primary antibody (ERK, p-ERK, p38,
p-38, AKT, p-AKT and p65), the membranes were incubated for 1 h at
room temperature with a secondary antibody conjugated to
horseradish peroxidase [goat anti-rabbit IgG (Cat. no. 31460;
Pierce, Rockford, IL, USA), goat anti-mouse IgG (Cat. no. sc-2031;
Santa Cruz Biotechnology, Inc.)]. Following 3 washes in
Tris-buffered saline Tween-20 (TBST), the immunoreactive bands were
visualized using the ECL detection system (Pierce).
Preparation of nuclear extracts and
electrophoretic mobility shift assay (EMSA)
Nuclear extracts were prepared using the NE-PER
nuclear extraction reagent (Pierce). As a probe for the gel
retardation assay, an oligonucleotide containing the immunoglobulin
κ-chain binding site (κB, 5′-GATCTCAGA GGGGACTTTCCGAGAGA-3′) was
synthesized. A non-radioactive method in which the 3′ end of the
probe was labeled with biotin was used in these experiments
(Pierce). The binding reactions contained 5 µg of nuclear
extract protein, buffer (10 mM Tris, pH 7.5, 50 mM KCl, 5 mM
MgCl2, 1 mM dithiothreitol, 0.05% Nonidet P-40 and 2.5%
glycerol), 50 ng of poly(dI-dC) and 20 fM of the biotin-labeled
DNA. The reactions were incubated for 20 min at room temperature in
a final volume of 20 µl. The competition reactions were
conducted by adding a 100-fold excess of unlabeled p65 NF-κB to the
reaction mixture. The mixture was then separated by electrophoresis
on a 5% polyacrylamide gel in 0.5X Tris-borate buffer and
transferred onto nylon membranes. The biotin-labeled DNA was
detected using a LightShift chemiluminescent EMSA kit (Pierce).
Measurement of ROS levels
The ARPE-19 cells (1×104 cells/well) were
seeded in 96-well plates in a humidified atmosphere containing 5%
CO2 at 37°C for 16 h. Following 16 h of incubation, the
cells were treated with 10 µg/ml PA-LPS and further
incubated for 5 h. For the intracellular ROS assay, the cells were
incubated with 10 µM DCFDA for 30 min. The cells were then
fixed with an equal volume of 4% formaldehyde and measured
immediately. The intracellular ROS levels were measured using a
fluoresence microplate reader (SpetraMax M2; Molecular Devices,
Sunnyvale, CA, USA) at an excitation wavelength of 492 nm and an
emission wavelength of 515 nm. For the mitochondrial ROS assay, the
cells (1 ml, 1×105 cells/ml) were incubated with 5
µM MitoSOX and MitoPY1 for 30 min. Subsequently, the cells
were washed with PBS and examined immediately. The levels of
mitochondrial ROS were measured using FACSort and CellQuest Pro
Software (BD Biosciences).
Flow cytometric analysis
The cells were stained with PE-conjugated anti-human
TLR2 monoclonal antibody (mAb; clone TL2.1) and PE-conjugated
anti-human TLR4 mAb (clone HTA125) and analyzed using FACSort and
Cell QuestPro software (BD Biosciences).
Statistical analysis
Data values represent the means ± standard deviation
(SD). To analyze the data produced from the experiments with 2
independent variables, one-way analysis of variance (ANOVA) was
performed using GraphPad Prism software (GraphPad Software, La
Jolla, CA, USA). A value of p<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of PA-LPS and YCG063 on the
viability of ARPE-19 cells
Initially, we examined the viability of the ARPE-19
cells stimulated with PA-LPS (0.5, 1, 5, 10, 20 and 30
µg/ml) for 24 h and of those treated with YCG063 (DMSO, 5,
10 and 50 µM) by CCK-8 assay. No significant cytotoxicity
was observed following stimulation with PA-LPS at the lower
doses; however, cell viability significnatly decreased following
stimulation with PA-LPS at 30 µg/ml (Fig. 2A). There was no significant
cytotoxicity observed to the ARPE-19 cells following treatment with
YCG063 at doses up to 50 µM (Fig. 2B). Based on these results, a
concentration range of PA-LPS of 10 µg/ml and of
YCG063 of 5 to 50 µM was selected for use in the subsequent
experiments.
Effects of YCG063 on the expression of
IL-6, IL-8, MCP-1, and ICAM-1 in PA-LPS-stimulated ARPE-19
cells
The levels of IL-6, IL-8, MCP-1 and ICAM-1
considerably increased following stimulation of the ARPE-19 cells
with PA-LPS (Fig. 3). To
evaluate the effects of YCG063 on the protein expression of IL-6,
IL-8, MCP-1 and ICAM-1 in the ARPE-19 cells, we treated the cells
with YCG063 (5, 10 and 50 µM) prior to stimulation with
PA-LPS (10 µg/ml). Treatment with YCG063 suppressed
the PA-LPS-induced production of the IL-6, IL-8, MCP-1 and
ICAM-1 proteins (Fig. 3).
Expression of TLR2 in PA-LPS-stimulated
ARPE-19 cells
To determine whether TLRs are involved in the
PA-LPS-stimulated inflammatory responses in ARPE-19 cells,
the cells were treated with LPS (10 µg/ml) for 12 h. The
cells were then stained with PE-conjugated TLR2 and TLR4, and their
expression levels were measured by flow cytometry (Fig. 4). The unstimulated cells did not
express TLR2, whereas upon stimulation with LPS, the protein
expression of TLR2 was upregulated (Fig. 4). However, TLR4 expression was not
found to be upregulated during PA-LPS stimulation (Fig. 4). Therefore, as indicatd by the
results of flow cytometry, only TLR2 is involved in the
PA-LPS-induced inflammatory responses of ARPE-19 cells.
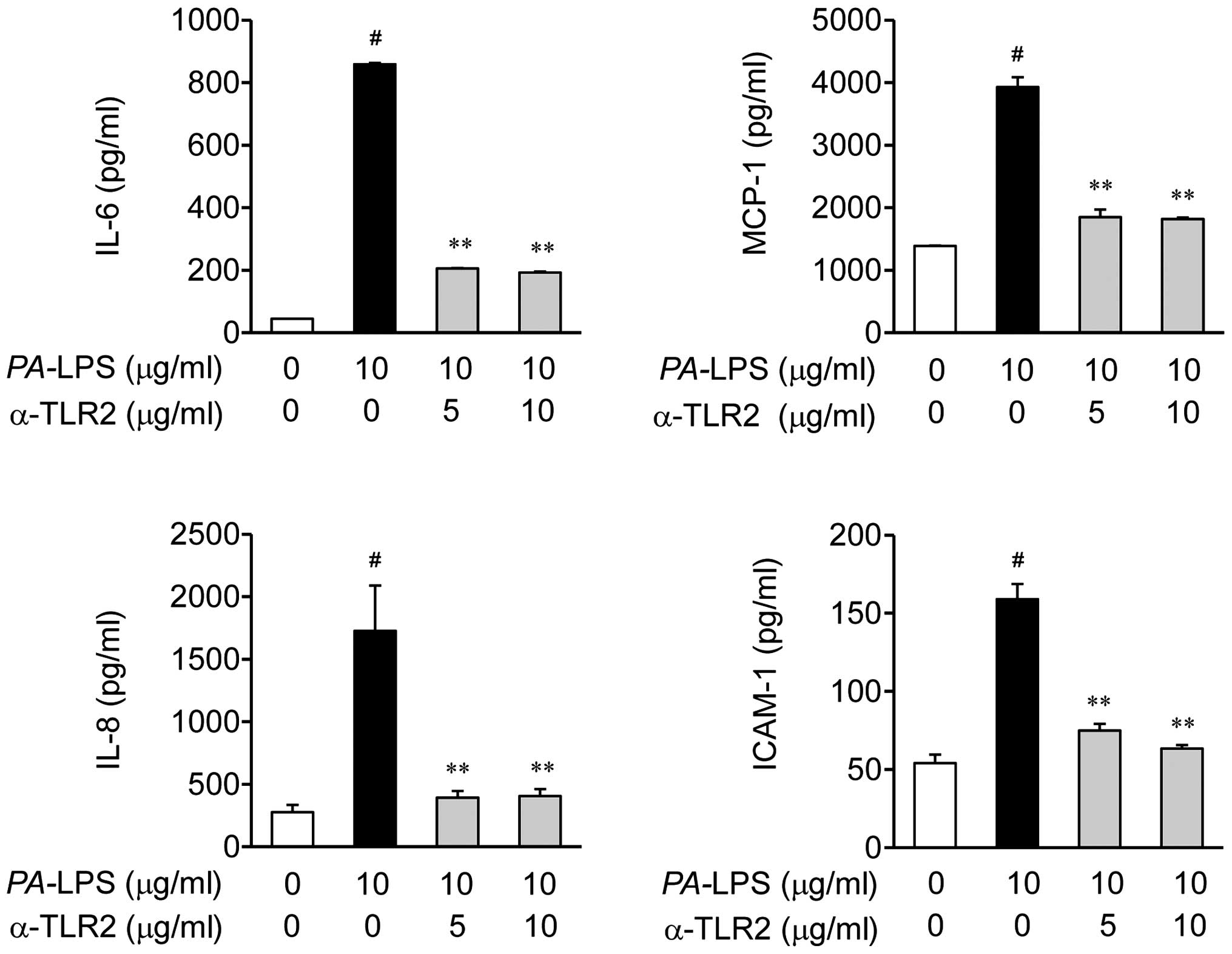
Effects of TLR2 antibody on the
expression of IL-6, IL-8, MCP-1 and ICAM-1 in PA-LPS-stimulated
ARPE-19 cells
Based on the results shown in Fig. 4, we investigated whether the TLR2
antibody can suppress PA-LPS-induced inflammation (Fig. 5). As expected, the TLR2 blocking
antibody (T2.5) inhibited the LPS-induced expression of
inflammatory mediators, including the expression of IL-6, IL-8,
MCP-1 and ICAM-1 in the ARPE-19 cells, as assessed by ELISA
(Fig. 5).
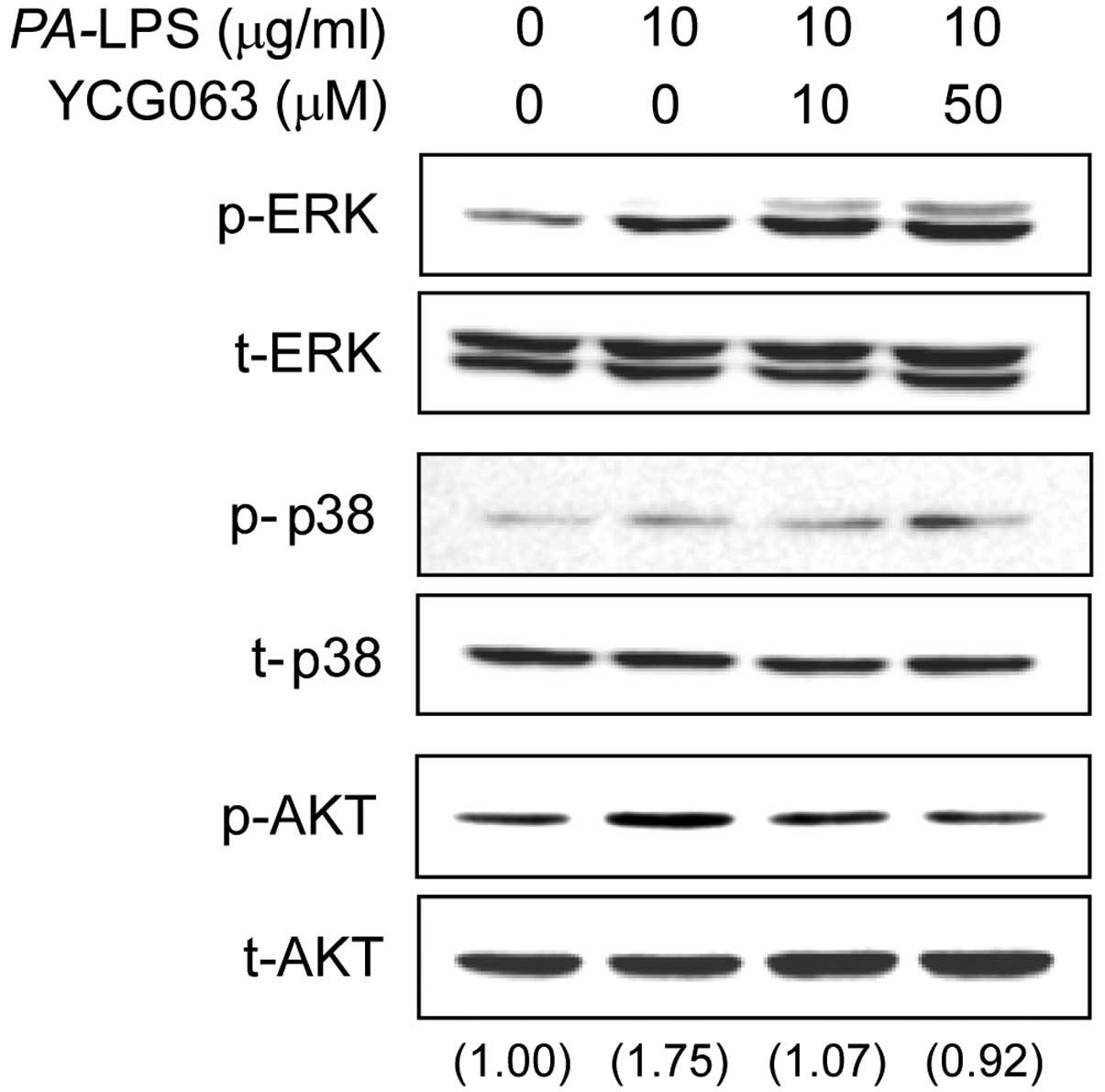
Effects of YCG063 on the activation of
the MAPK and AKT signaling pathways in LPS-stimulated ARPE-19
cells
To elucidate the mechanisms underlying the effects
of YCG063 on the expression of inflammatory mediators, we examined
the activation of MAPKs and AKT by western blot analysis.
Stimulation of the ARPE-19 cells with PA-LPS resulted in an
increase in the levels of phosphorylated (p-)AKT and p-ERK, but not
in those of p-p38. Pre-treatment with YCG063 (10 or 50 µM)
for 2 h attenuated the phosphorylation of AKT induced by a 30-min
incubation with 10 µg/ml LPS, but it did not affect the
phosphorylation of ERK (Fig.
6).
Effects of YCG063 on the activation of
NF-κB in PA-LPS-stimulated ARPE-19 cells
The production of pro-inflammatory cytokines,
chemokines and adhesion molecules is regulated by the transcription
factor, NF-κB, upon PA-LPS stimulation. Therefore, to
investigate the mechanism through which YCG063 affects the
expression of IL-6, IL-8, MCP-1 and ICAM-1, we examined the effects
of YCG063 on NF-κB activation. We found that YCG063 inhibited the
LPS-induced expression of NF-κB p65 in the nuclei (Fig. 7A). We then investigated the
effects of YCG063 on the DNA-binding activity of NF-κB by EMSA
(Fig. 7B). Stimulation with
PA-LPS caused a significant increase in the DNA-binding
activity of NF-κB, whereas pre-treatment with YCG063 markedly
reduced the PA-LPS-induced DNA-binding activity of NF-κB
(Fig. 7B). Moreover, the
upregulation in the expression of NF-κB p65 was significantly
inhibited by treatment with LY294002 (an inhibitor of AKT; Fig. 7C).
Effects of NF-κB inhibitors (BAY 11-7082
and parthenolide) on the expression of IL-6, IL-8, MCP-1 and ICAM-1
in PA-LPS-stimulated ARPE-19 cells
Based on the results shown in Fig. 7, we investigated whether BAY
11-7082 and parthenolide can suppress the LPS-induced expression of
inflammatory mediators (Fig. 8).
BAY 11-7082 and parthenolide inhibited the expression of IL-6,
IL-8, MCP-1 and ICAM-1 in the LPS-stimulated ARPE-19 cells
(Fig. 8).
Generation of ROS in PA-LPS-stimulated
ARPE-19 cells
Since the production of inflammatory mediators in
LPS-induced inflammation is associated with the generation of ROS
(25), we investigated whether
PA-LPS induces ROS generation. As shown by DCFDA staining,
the intracellular levels of ROS did not increase following
stimulation of the cells with 10 µg/ml PA-LPS
compared the untreated cells (basal levels; Fig. 9A). Additionally, according to the
results of flow cytometry, the levels of two types of mitochondrial
ROS (superoxide and hydrogen peroxide) did not increase following
stimulation with 10 µg/ml PA-LPS compared to the
untreated cells (basal level; Fig.
9B).
Discussion
Recurrent endogenous endophthalmitis due to
infection by PA has been previously reported (5). In the present study, we investigated
the anti-inflammatory effects of YCG063 (a well-known ROS
inhibitor) on the production of the inflammatory mediators, IL-6,
IL-8, MCP-1 and ICAM-1, induced by PA-LPS in ARPE-19
cells.
The infiltration of PMNs in the eye is an important
pathological process observed in intraocular infections, such as
bacterial endophthalmitis (26).
Inflammatory mediators induce increased PMN infiltration to the
infected eye and, subsequently, severe retinal damage. The
inflammatory mediators are expressed in response to Gram-negative
bacterial infections and are mediated by bacterially derived
products, such as LPS (27). In
this study, we first investigated the inhibitory effects of YCG063
on the PA-LPS-induced production of IL-6, IL-8, MCP-1 and
ICAM-1 in ARPE-19 cells. The levels of pro-inflammatory cytokines
and chemokines in RPE cells suggest a role of this cell type in the
clinical presentation of bacterial endophthalmitis. To the best of
our knowledge, although YCG063 has been investigated as an
anti-angiogenic small molecule (24), a possible role in ocular
inflammation has not been addressed. In this study, YCG063
significantly inhibited the LPS-induced expression of IL-6, IL-8,
MCP-1 and ICAM-1 at concentrations of YCG063 that were not
cytotoxic to the ARPE-19 cells (Fig.
3). Thus, the inhibition of the production of potent
inflammatory mediators by pre-treatment with YCG063 may be an
effective therapeutic approach with which to prevent retinal
damage.
We also investigated which TLR members are involved
in the inflammatory response induced by PA-LPS stimulation.
The TLR family members are associated with the innate immune
response and recognize specific pathogen-associated molecular
patterns (PAMPs) expressed by different groups of infectious
microbes (28). Ultimately, a
cascade of intracellular signaling reactions occur and this leads
to the activation of DNA binding proteins that, in turn, promote
the transcription of pro-inflammatory genes (29,30). They constitute as the first line
of defense against invading pathogens and play a significant role
in inflammation, immune cell regulation, survival and
proliferation. In this study, we found that TLR2 was upregulated in
the PA-LPS-stimulated ARPE-19 cells, whereas TLR4 was not
(Fig. 4). To confirm this
response, we investigated whether inflammatory mediators are
inhibited by blocking TLR2 with an antibody against TLR2. The TLR2
antibody significantly inhibited the production of IL-6, IL-8,
MCP-1 and ICAM-1 (Fig. 5). Our
findings demonstrated that PA-LPS predominantly enhanced
TLR2 expression in the ARPE-19 cells, leading to the increased
production of IL-6, IL-8, MCP-1 and ICAM-1. The blockade of TLR4
did not have an inhibitory effect on the PA-LPS-stimulated
ARPE-19 cells (data not shown).
Since YCG063 inhibited the expression of the
inflammatory mediators in the PA-LPS-stimulated ARPE-19
cells, we further explored the regulatory mechanisms of YCG063
involved in this process (Fig.
6). The MAPK and AKT pathways are important downstream pathways
of LPS/TLR signaling (31). To
elucidate the regulatory mechanisms of YCG063 involved in these
processes, we investigated whether the AKT and MAPK signaling
pathways are involved in regulating the inflammatory mediators. Our
findings demonstrated that ERK and AKT, but not p38 MAPK, were
involved in the inflammatory responses of the
PA-LPS-stimulated ARPE-19 cells. Therefore, the activation
of the ERK and AKT pathways plays an important role in the
PA-LPS-induced increase in the production of inflammatory
mediators in ARPE-19 cells. However, our results demonstrated that
YCG063 inhibited the PA-LPS-induced phosphorylation of AKT.
Therefore, we demonstrated the blockage of the
PA-LPS-induced phosphorylation of AKT by YCG063 in ARPE-19
cells. To further elucidate the regulatory mechanisms of YCG063, we
investigated whether the NF-κB signaling pathway is involved in
this process. NF-κB is known to be a pleiotropic regulator of
various genes involved in the production of a number of
pro-inflammatory cytokines and enzymes related to the inflammatory
process (32). Due to its key
role in several pathological conditions, NF-κB is a major drug
target in a variety of diseases (33). Our results revealed that the
LPS-induced activation of NF-κB p65 was completely abolished by
pretreatment of the cells with YCG063. Therefore, the inhibition of
the NF-κB signaling pathway in the ARPE-19 cells by YCG063 may
result in the downregulation of inflammatory responses. The
AKT-dependent activation of NF-κB has been described in a variety
of cell types stimulated with platelet-derived growth factor,
TNF-α, IL-1, as well as other agonists (34). We found that the inhibition of AKT
by LY-294002 blocked the translocation of NF-κB (Fig. 7C). Subsequently, we investigated
whether the NF-κB inhibitors (BAY 11-7082 and parthenolide) are
involved in regulating these inflammatory mediators. The NF-κB
inhibitors significantly suppressed the production of the IL-6,
IL-8, MCP-1 and ICAM-1 proteins (Fig.
8). Taken together, these results suggest that the activation
of AKT and NF-κB, but not that of MAPKs, is involved in the
inhibitory effects of YCG063 on the PA-LPS-induced
production of IL-6, IL-8, MCP-1 and ICAM-1.
In a previous study, ROS were generated with
PA-LPS in human adenocarcinoma alveolar basal epithelial
cells (A549 cells) (35). In
order to observe whether they contribute to the production of
inflammatory mediators, we investigated whether ROS are generated
in ARPE-19 cells by stimulation with PA-LPS (Fig. 9). The results revealed that the
levels of ROS were not enhanced in the PA-LPS-stimulated
ARPE-19 cells compared to the untreated cells. Thus, our findings
suggest that the production of pro-inflammatory cytokines and
chemokines triggered by PA-LPS is accomplished through
ROS-independent mechanisms.
In conclusion, in this study, we demonstrated that
PA-LPS induced IL-6, IL-8, MCP-1 and ICAM-1 expression in
the ARPE-19 cells. Pre-treatment with YCG063 inhibited the
expression of IL-6, IL-8, MCP-1 and ICAM-1 in the
PA-LPS-stimulated ARPE-19 cells. This inhibition by YCG063
may be the result of the suppression of the phosphorylation of AKT,
as well as the inhibition of the activation of NF-κB in modulating
TLR2-induced inflammation. We also demonstrated that ROS are not
involved in these inflammatory responses induced by PA-LPS.
Based on our results, the inhibitory effects of YCG063 on
inflammatory reactions may be considered as an effective
therapeutic strategy for the treatment of bacterial
endophthalmitis.
Acknowledgments
The present study was supported by a grant from the
Korea Healthcare Technology R&D Project, Ministry of Health and
Welfare and Family Affairs, Republic of Korea (HI12C0005).
Abbreviations:
|
PA-LPS
|
Pseudomonas aeruginosa lipopol
ysaccharide
|
|
TLR
|
Toll-like receptor
|
|
ROS
|
reactive oxygen species
|
|
RPE cells
|
retinal pigment epithelial cells
|
|
MCP-1
|
monocyte chemoattractant protein-1
|
|
ICAM-1
|
intracellular adhesion molecule-1
|
References
|
1
|
Bertino JS Jr: Impact of antibiotic
resistance in the management of ocular infections: The role of
current and future antibiotics. Clin Ophthalmol. 3:507–521. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Al-Amri MS: Endogenous endophthalmitis
secondary to pyogenic liver abscess. Int J Diabet Mellitus. 2:646.
2010. View Article : Google Scholar
|
|
3
|
Callegan MC, Engelbert M, Parke DW II,
Jett BD and Gilmore MS: Bacterial endophthalmitis: Epidemiology,
therapeutics, and bacterium-host interactions. Clin Microbiol Rev.
15:111–124. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Obritsch MD, Fish DN, MacLaren R and Jung
R: Nosocomial infections due to multidrug-resistant Pseudomonas
aeruginosa: Epidemiology and treatment options. Pharmacotherapy.
25:1353–1364. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Eifrig CW, Scott IU, Flynn HW Jr and
Miller D: Endophthalmitis caused by Pseudomonas aeruginosa.
Ophthalmology. 110:1714–1717. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ren Y, Xie Y, Jiang G, Fan J, Yeung J, Li
W, Tam PK and Savill J: Apoptotic cells protect mice against
lipopolysaccharide-induced shock. J Immunol. 180:4978–4985. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vallejo-Garcia JL, Asencio-Duran M,
Pastora-Salvador N, Vinciguerra P and Romano MR: Role of
inflammation in endo-phthalmitis. Mediators Inflamm.
2012:1960942012. View Article : Google Scholar
|
|
8
|
Campochiaro PA: Ocular neovascularization.
J Mol Med Berl. 91:311–321. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bonilha VL: Age and disease-related
structural changes in the retinal pigment epithelium. Clin
Ophthalmol. 2:413–424. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Arranz-Valsero I, Schulze U,
Contreras-Ruiz L, García-Posadas L, López-García A, Paulsen F and
Diebold Y: Involvement of corneal epithelial cells in the Th17
response in an in vitro bacterial inflammation model. Mol Vis.
19:85–99. 2013.PubMed/NCBI
|
|
11
|
Gallucci RM, Simeonova PP, Matheson JM,
Kommineni C, Guriel JL, Sugawara T and Luster MI: Impaired
cutaneous wound healing in interleukin-6-deficient and
immunosuppressed mice. FASEB J. 14:2525–2531. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gallucci RM, Sugawara T, Yucesoy B,
Berryann K, Simeonova PP, Matheson JM and Luster MI: Interleukin-6
treatment augments cutaneous wound healing in immunosup-pressed
mice. J Interferon Cytokine Res. 21:603–609. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Biswas PS, Banerjee K, Kinchington PR and
Rouse BT: Involvement of IL-6 in the paracrine production of VEGF
in ocular HSV-1 infection. Exp Eye Res. 82:46–54. 2006. View Article : Google Scholar
|
|
14
|
De Vos AF, Hoekzema R and Kijlstra A:
Cytokines and uveitis, a review. Curr Eye Res. 11:581–597. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fenton RR, Molesworth-Kenyon S, Oakes JE
and Lausch RN: Linkage of IL-6 with neutrophil chemoattractant
expression in virus-induced ocular inflammation. Invest Ophthalmol
Vis Sci. 43:737–743. 2002.PubMed/NCBI
|
|
16
|
Cole N, Bao S, Willcox M and Husband AJ:
Expression of interleukin-6 in the cornea in response to infection
with different strains of Pseudomonas aeruginosa. Infect Immun.
67:2497–2502. 1999.PubMed/NCBI
|
|
17
|
Youker K, Smith CW, Anderson DC, Miller D,
Michael LH, Rossen RD and Entman ML: Neutrophil adherence to
isolated adult cardiac myocytes. Induction by cardiac lymph
collected during ischemia and reperfusion. J Clin Invest.
89:602–609. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu Y, Shaw SK, Ma S, Yang L, Luscinskas
FW and Parkos CA: Regulation of leukocyte transmigration: Cell
surface interactions and signaling events. J Immunol. 172:7–13.
2004. View Article : Google Scholar
|
|
19
|
Tacke F and Randolph GJ: Migratory fate
and differentiation of blood monocyte subsets. Immunobiology.
211:609–618. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Van Riper G, Siciliano S, Fischer PA,
Meurer R, Springer MS and Rosen H: Characterization and species
distribution of high affinity GTP-coupled receptors for human
rantes and monocyte chemoattractant protein 1. J Exp Med.
177:851–856. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Carr MW, Roth SJ, Luther E, Rose SS and
Springer TA: Monocyte chemoattractant protein 1 acts as a
T-lymphocyte chemoattractant. Proc Natl Acad Sci USA. 91:3652–3656.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kawashima A, Suzuki T, Nishihara F,
Kobayashi T, Takaku Y, Nakagome K, Soma T, Hagiwara K, Kanazawa M
and Nagata M: Effect of formoterol on eosinophil trans-basement
membrane migration induced by interleukin-8-stimulated neutrophils.
Int Arch Allergy Immunol. 161(Suppl 2): 10–15. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen YJ, Huang YS, Chen JT, Chen YH, Tai
MC, Chen CL and Liang CM: Protective effects of glucosamine on
oxidative-stress and ischemia/reperfusion-induced retinal injury.
Invest Ophthalmol Vis Sci. 56:1506–1516. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim KH, Park JY, Jung HJ and Kwon HJ:
Identification and biological activities of a new antiangiogenic
small molecule that suppresses mitochondrial reactive oxygen
species. Biochem Biophys Res Commun. 404:541–545. 2011. View Article : Google Scholar
|
|
25
|
Meng Z, Yan C, Deng Q, Gao DF and Niu XL:
Curcumin inhibits LPS-induced inflammation in rat vascular smooth
muscle cells in vitro via ROS-relative TLR4-MAPK/NF-κB pathways.
Acta Pharmacol Sin. 34:901–911. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Novosad BD, Astley RA and Callegan MC:
Role of Toll-like receptor (TLR) 2 in experimental Bacillus cereus
endo-phthalmitis. PLoS One. 6:e286192011. View Article : Google Scholar
|
|
27
|
Schwarz JM and Bilbo SD: LPS elicits a
much larger and broader inflammatory response than Escherichia coli
infection within the hippocampus of neonatal rats. Neurosci Lett.
497:110–115. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Aderem A and Ulevitch RJ: Toll-like
receptors in the induction of the innate immune response. Nature.
406:782–787. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Akira S and Takeda K: Toll-like receptor
signalling. Nat Rev Immunol. 4:499–511. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vogel SN, Fitzgerald KA and Fenton MJ:
TLRs: Differential adapter utilization by toll-like receptors
mediates TLR-specific patterns of gene expression. Mol Interv.
3:466–477. 2003. View Article : Google Scholar
|
|
31
|
McGuire VA, Gray A, Monk CE, Santos SG,
Lee K, Aubareda A, Crowe J, Ronkina N, Schwermann J, Batty IH, et
al: Cross talk between the Akt and p38α pathways in macrophages
downstream of Toll-like receptor signaling. Mol Cell Biol.
33:4152–4165. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jung WK, Lee DY, Park C, et al: Cilostazol
is anti-inflammatory in BV2 microglial cells by inactivating
nuclear factor-kappaB and inhibiting mitogen-activated protein
kinases. Br J Pharmacol. 159:1274–1285. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dąbek J, Kułach A and Gąsior Z: Nuclear
factor kappa-light-chain-enhancer of activated B cells (NF-κB): a
new potential therapeutic target in atherosclerosis? Pharmacol Rep.
62:778–783. 2010. View Article : Google Scholar
|
|
34
|
Chen BC, Wu WT, Ho FM and Lin WW:
Inhibition of interleukin-1beta-induced NF-kappa B activation by
calcium/calmodulin-dependent protein kinase kinase occurs through
Akt activation associated with interleukin-1 receptor-associated
kinase phosphorylation and uncoupling of MyD88. J Biol Chem.
277:24169–24179. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li W, Yan F, Zhou H, Lin X, Wu Y, Chen C,
Zhou N, Chen Z, Li JD and Shen H: P. aeruginosa
lipopolysaccharide-induced MUC5AC and CLCA3 expression is partly
through Duox1 in vitro and in vivo. PLoS One. 8:e639452013.
View Article : Google Scholar : PubMed/NCBI
|