Introduction
Aging is generally associated with progressive
changes in body composition and a decline in muscle strength,
muscle mass (also known as sarcopenia) and aerobic capacity,
leading to a reduction in mobility and an impaired quality of life
in elderly subjects (1,2). The reduction of force-generating
capacity with increasing age is a characteristic of muscle aging.
At the age of 80, the muscle-force generating capacity is, on
average, approximately 60% lower than that at the age of 20–30, and
continues to decrease with age (3). Since aging is a continuous, complex
process, a multitude of factors are involved in the decline in
muscle force-generating capacity. The reduction in muscle mass
contributes to a large proportion of the loss in muscle
force-generating capacity with age. A previous study examining the
anatomical cross-sectional area (ACSA) indicated that an average
decrease in muslce mass of the human quadriceps femoris, elbow
flexors and extensors was <35% in aged males (68–75 years old)
compared to young males (24–31 years old). Muscle fiber atrophy and
muscle fiber cross-sectional area (CSA) also contributed to the
age-related loss of muscle mass (4). A comparison of muscle fiber CSA
between young and elderly subjects demonstrated that aging is
accompanied by a 20–50% atrophy of type II muscle fibers, while
type I muscle fiber size is not altered (5).
In addition to muscle atrophy, a decline in muscle
regenerative capacity is associated with aging (6). It has been reported that new fiber
formation could compensate for the loss of muscle mass in aged
rodents; however, the regenerative capacity of muscles decreases
progressively with age (6). The
number and regenerative ability of progenitor cells decline during
aging due to age-related changes in endocrine factors that alter
their myogenic potential (7).
Based on the above-mentioned impact of age-related skeletal muscle
changes on the quality of life of elderly subjects, a deeper
understanding of muscle aging and the development of adequate
strategies to maintain muscle function are urgently required.
Eriobotrya japonica (E. japonica), also known
as loquat, is an evergreen fruit tree and is widely cultivated in
China, Japan and Korea. The leaves, seeds and fruit of loquat are
widely used as teas, food and folk medicines. The leaves of E.
japonica (also known as Folium Eriobotryae) have been widely
used in Korea, China and Japan, as a traditional medicine due to
their beneficial effects on fever and chronic diseases, including
headaches, lower back pain, dysmenorrhea, asthma, phlegm, chronic
bronchitis and gastroenteric diseases (8). The dried leaf of the loquat is the
part most commonly used in the treatment of diabetes mellitus
(9). Various active compounds,
such as triterpenes, flavonoids and tannins have been identified in
the loquat leaf, and some of them have been reported to possess
anti-metastatic, anti-hyperglycaemic and immunomodulatory
properties (10–12). For example, triterpenes from the
loquat leaf have been shown to exert anti-inflammatory properties
(13–15) and have anti-inflammatory and
anti-tumor properties (16).
Recently, the anti-osteoporotic effects of the loquat leaves have
been reported. In a previous study, the dietary supplementation
with loquat leaf extract (LE) significantly prevented bone mineral
density loss in ovariectomized mice and suppressed the
RANKL-induced osteoclast differentiation of RAW 264.7 cells
(17).
Among the triterpenoids, ursolic acid (UA) has been
identified as a pharmacologically active constituent of the loquat
leaf (18). Recently, Kunkel
et al (19) reported that
UA enhanced skeletal muscle insulin/insulin-like growth factor-I
signaling and inhibited atrophy-associated skeletal muscle mRNA
expression, thus suppressing muscle atrophy and stimulating muscle
hypertrophy in mice. They also demonstrated that UA increased
skeletal muscle mass, fast- and slow-twitch muscle fiber size, grip
strength and exercise capacity (20). UA has also been shown to promote
the differentiation of murine myoblasts (21), although the effects of LE on the
muscles of aged animals and myoblast differentiation in
vitro have not been evaluated. The aim of the present study was
to investigate the effects of LE on the mass and function of muscle
in aged rats. We also examined the hypothesis that LE promotes
myogenic differentiation by modulating myogenesis-related gene
products.
Materials and methods
Materials
All chemical reagents were obtained from
Sigma-Aldrich (St. Louis, MO, USA) onless otherwise stated.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
was obtained from AMRESCO (Solon, OH, USA). Antibodies against
myosin heavy chain (MyHC; sc-20641), MyoD (sc-760), myogenin
(sc-576), phosphorylated (p-)Akt (Ser473; sc-7985-R), Akt1/2/3
(sc-8312), anti-rabbit IgG-horseradish peroxidase (HRP)-conjugated
antibody (sc-2054), and anti-mouse IgG-HRP-conjugated antibody
(sc-2031) were obtained from Santa Cruz Biotechnology, Inc.,
(Dallas, TX, USA). Antibodies against eukaryotic translation
initiation factor 4E binding protein 1 (4E-BP1; no. 9452), p-4E-BP1
(Thr37/46; no. 2855), mammalian target of rapamycin (mTOR; no.
2983), p-mTOR (Ser2488; no. 5536), and p-p70 S6 kinase (Thr389; no.
9234) were purchased from Cell Signaling Technology, Inc.,
(Danvers, MA, USA). β-actin antibody was purchased from
Sigma-Aldrich, and polyclonal antibody against p70 S6 kinase
(bs-6370R) was obtained from Bioss, Inc. (Woburn, MA, USA).
Dulbecco's modified Eagle's medium (DMEM) was purchased from
WelGENE, Inc. (Daegu, Korea), and horse serum (HS) was from
Invitrogen Life Technologies (Grand Island, NY, USA). Fetal bovine
serum (FBS) and penicillin/streptomycin were purchased from GE
Healthcare Life Sciences (Logan, UT, USA). A creatine kinase (CK)
enzymatic assay kit (MaxDiscovery® Creatine Kinase
Enzymatic Assay kit) was purchased from Bioo Scientific Corp.
(Austin, TX, USA). Polyvinylidene difluoride (PVDF) membranes were
obtained from Merck Millipore (Billerica, MA, USA).
Preparation of LE
An ethanol (EtOH) extract of loquat leaf (hereafter
termed LE) was prepared as previously described in the study by
Jung et al (22) with some
modifications, and was provided by the Marine Bio-industry
Development Center (Busan, Korea). The yield of LE based on the
dried weight of the loquat leaf was 4.68%. The final concentration
of UA in the LE was 104.14 mg/g. LE was dissolved in EtOH as a 5
mg/ml stock solution. The stock solution was stored at −20°C and
diluted with medium to the desired concentration prior to use.
Animal experiments
Male Sprague-Dawley rats (aged 5 and 18–19 months)
were obtained from Samtako (Osan, Korea). The rats were maintained
under controlled environmental conditions (23±1°C, 50±10% relative
humidity, 12 h/12 h light/dark cycle) with ad libitum access
to water and a basal diet (FORMULA M07; FEEDLAB, Guri, Korea).
After an acclimation period (1 week), the rats were randomly
divided into 4 study groups (4–6 animals per group) as follows: i)
young rats not administered LE (Y-Con group); ii) young rats
administered LE (Y-LE group); iii) aged rats not administered LE
(O-Con group); and iv) aged rats administered LE (O-LE group).
Animals in the LE-treated groups were administered
LE mixed in their food (based on food intake, daily dose of LE=50
mg/kg of body weight). During the experiments, forelimb grip
strength was determined using a grip strength meter equipped with a
T-shaped pull bar (Columbus Instruments, Columbus, OH, USA). After
the scheduled experiment, the rats were sacrificed by decapitation
and soleus and gastrocnemius muscles were quickly removed. Muscle
weights were measured immediately, and muscles were divided for
biological analysis and histological examination. The animal
protocol used in the present study was reviewed and approved by the
Pusan National University Institutional Animal Care and Use
Committee (PNU-IACUC; Approval no. PNU 2013-0461) with respect to
ethical issues and scientific care.
Histological analysis
Medial portions of soleus muscles were fixed in 10%
formalin solution for 24 h, routinely embedded in paraffin blocks,
transversely sectioned (3-µm-thick sections) and stained
with hematoxylin and eosin (H&E).
Analysis of CSA
To analyze the muscle fiber CSA, images of the
soleus muscle were captured using a microscope (Axiovert 100;
Zeiss, Göettingen, Germany). The CSA of each muscle fiber in each
field was measured using ImageJ software (version 1.49m, National
Institutes of Health, Bethesda, MD, USA). The CSA was examined in 3
rats from each group.
CK activity assay
The cytosolic fraction of the muscle homogenate was
used for the CK activity assay. Briefly, the tissues were
homogenized in homogenate buffer containing 50 mM HEPES (pH 7.4),
10 mM KCl, 2 mM MgCl2, 1 mM DTT, 0.1 mM EDTA, 0.1 mM
phenylmethylsulfonyl fluoride (PMSF), 20 mM β-glycerophosphate, 20
mM sodium fluoride, 2 mM sodium orthovanadate, 1 mM pepstatin, 2 mM
leupeptin and 5 mM aprotinin, and the homogenates obtained were
placed on ice for 15 min. Nonidet P-40 (NP-40; 10%, 125 µl/1
ml homogenate) solution was then added, mixed for 15 sec, and the
mixture was then centrifuged at 14,000 x g for 2 min. The
supernatants obtained were used as cytosolic fractions.
For the CK assay using the C2C12 cells, the cells
were collected, washed with Dulbecco's phosphate-buffered saline
(DPBS) and then lysed with lysis buffer [40 mM Tris (pH. 8.0), 120
mM NaCl, 0.5% NP-40, 100 µg/ml PMSF and complete protease
inhibitor] and stored at −70°C until use. CK activity was
determined using a CK enzymatic assay kit (Bioo Scientific Corp.),
according to the manufacturer's instructions. Briefly, 250
µl of CK reagent were added to 5 µl of lysate or
homogenate in a microplate. CK activity was immediately measured 2
times at 5-min time intervals, at 340 nm, using a multi-well reader
(GENios; Tecan Austria GmbH, Grödig, Austria). The assay was
performed in duplicate. The average 5-min increase in absorbance
was multiplied by 2,186 (conversion factor) to obtain the CK
activity (IU/l).
Cell culture
C2C12 murine myoblasts were purchased from the
American Type Culture Collection (ATCC; Manassas, VA, USA). For all
experiments, the C2C12 cells were cultured from 4 to 9 passages to
70–80% confluence in growth medium (GM) containing DMEM
supplemented with 10% FBS, 100 U/ml penicillin and 100 µg/ml
streptomycin. The cells were maintained in humidified 95% air and
5% CO2 at 37°C.
For differentiation, the cells were plated at an
approximate initial density of 1×105 cells/well in
6-well culture plates and grown in GM. When the cells reached
80–90% confluence, the GM was removed and the cells were washed
with DPBS and fed differentiation medium (DM) containing DMEM
supplemented with 2% HS to induce differentiation. To examine the
effects of LE on myogenic differentiation, LE was added to the DM.
The medium was changed every other day until day 6, and the LE was
replaced with each medium change.
Cell viability assay
Cell viability was evaluated by measuring the
mitochondrial-dependent conversion of the yellow tetrazolium salt,
MTT, to purple formazan crystals by metabolically active cells.
Briefly, the C2C12 cells were seeded and induced to differentiate
as described above. At the end of the differentiation period, the
cells were incubated with 0.5 mg/ml MTT at 37°C for 2 h.
Subsequently, the MTT was removed and the formazan crystals were
dissolved in dimethyl sulfoxide. The absorbance was measured at 540
nm using a multi-well reader (Thermo Fisher Scientific, Vantaa,
Finland).
Western blot analysis
Following treatment, the cells were harvested and
washed with cold DPBS. The cells were lysed in lysis buffer.
Following centrifugation, the supernatant was collected and the
protein concentration was determined using protein assay reagents
(Bio-Rad, Hercules, CA, USA). Equal amounts of protein were boiled
for 5 min in 2X Laemmli sample buffer (Bio-Rad). The protein
samples were then separated by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis on 6–15% acrylamide gels
and transferred onto PVDF membranes. The membranes were blocked
with 5% non-fat dry milk in Tris-buffered saline with Tween-20
buffer (TBST; 20 mM Tris, 100 mM NaCl, pH 7.5 and 0.1% Tween-20)
for 1 h, incubated with various primary antibodies at 4°C
overnight, washed 3 times with TBST buffer and then incubated with
HRP-conjugated secondary antibodies (Santa Cruz Biotechnology,
Inc.) at room temperature for 1 h. Antigen-antibody complexes were
detected using an enhanced chemiluminescence (ECL) detection system
(GE Healthcare Life Sciences, Piscataway, NJ, USA). Densitometric
analysis (for optical density) was performed using Fluorchem SP
AlphaEase® FC (version 6.0.0) software (Alpha Innotech,
San Leandro, CA, USA), normalized to actin or other control
proteins, and expressed as a fold change compared with the
untreated controls.
Statistical analysis
Data are expressed as the means ± SEM, and analyzed
using GraphPad Prism software (version 5.02, GraphPad Software,
Inc., La Jolla, CA, USA). Treatments were compared by one-way ANOVA
followed by Tukey's post hoc test for pairwise comparisons.
P-values <0.05 were considered to indicate statistically
significant differences.
Results
Effects of LE supplementation on body
weight in aged rats
Changes in body weight were measured every 5 days,
and food and water intake were measured every 3 days over the
entire experimental period. No adverse effects were observed in
behavior, cleanliness and in the appearance of hair and eyes. No
significant differences in food and water intake were observed
between the beginning and the end of the treatment period for each
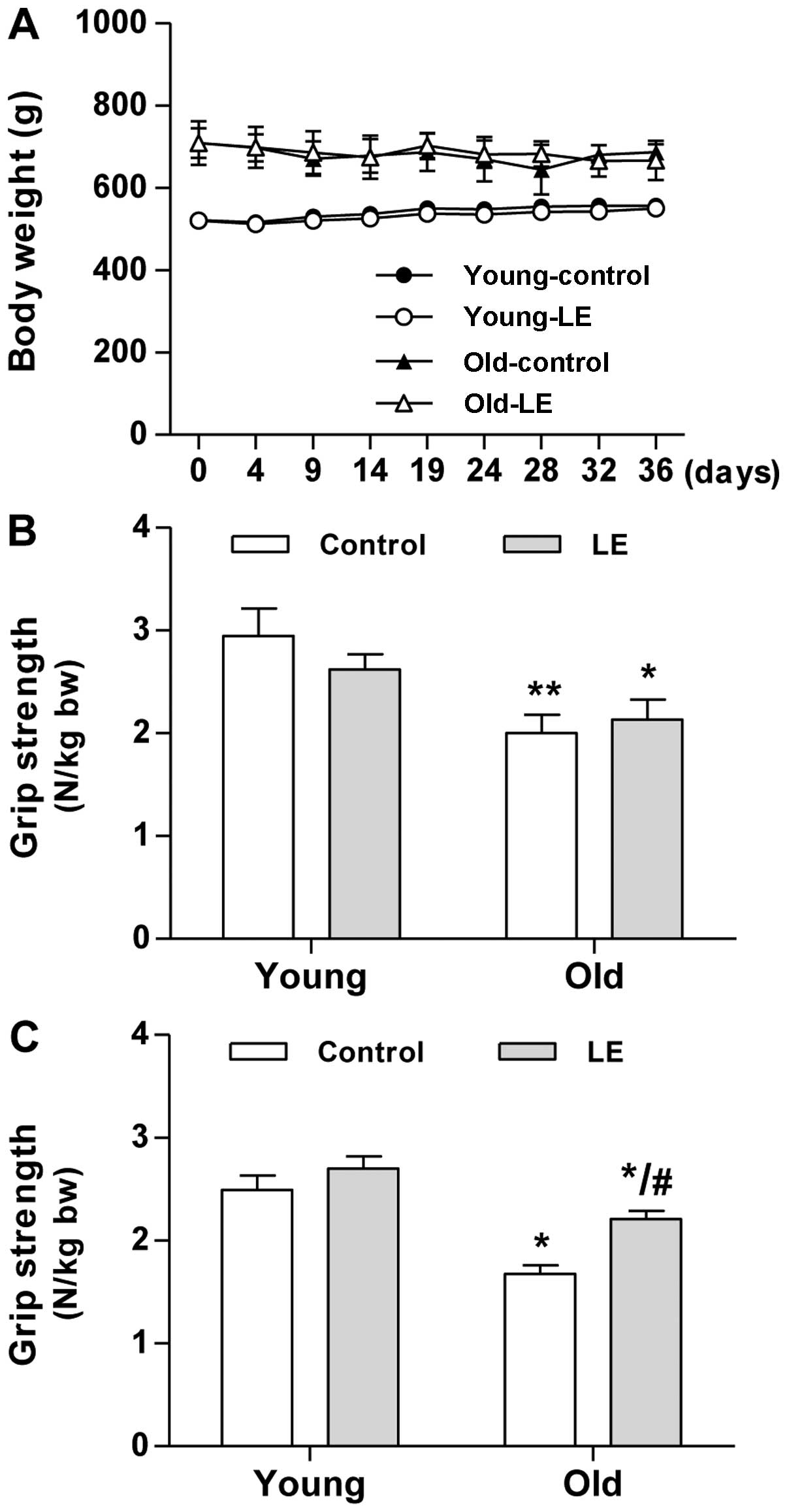
experimental group (data not shown). As shown in Fig. 1A, the young-aged groups, either
the control or the group fed LE, continuously gained body weight
during the experimenatl period, whereas the old-aged groups showed
insignificant changes in body weight.
LE supplementation increases muscle
strength in aged rats
We then examined the effects of LE supplementation
on age-associated muscle function in rats. To monitor and quantify
muscle function, we conducted a forelimb grip strength test using a
grip strength meter. Grip strength was measured in each rat once
every 5 days. As expected, the old-aged groups showed a
significantly lower grip strength than the young-aged groups at the
start of the experimental period (day 0, Fig. 1B). Within the age-matched
experimental groups, no significant differences in muscle strength
were observed on day 0 of the experimental period (Fig. 1B). After 35 days of LE
supplementation, the mean grip strength of the old-aged control
group (O-Con) decreased by 33% compared with that of the young-aged
control group (Y-Con, Fig. 1C).
Following LE supplementation, only an 18% decrease in grip strength
was observed in the old-aged group supplemented with LE (O-LE
group) compared with the Y-Con group. LE supplementation resulted
in a significantly increased grip strength in the O-LE group
compared with the O-Con group. The grip strength of the rats in the
Y-LE group tended to slightly increase, although this change was
not significant compared to the rats in the Y-Con group (Fig. 1C). These results suggest that LE
supplementation has beneficial effects on muscle strength.
LE supplementation increases muscle mass
and muscle CK activity in aged rats
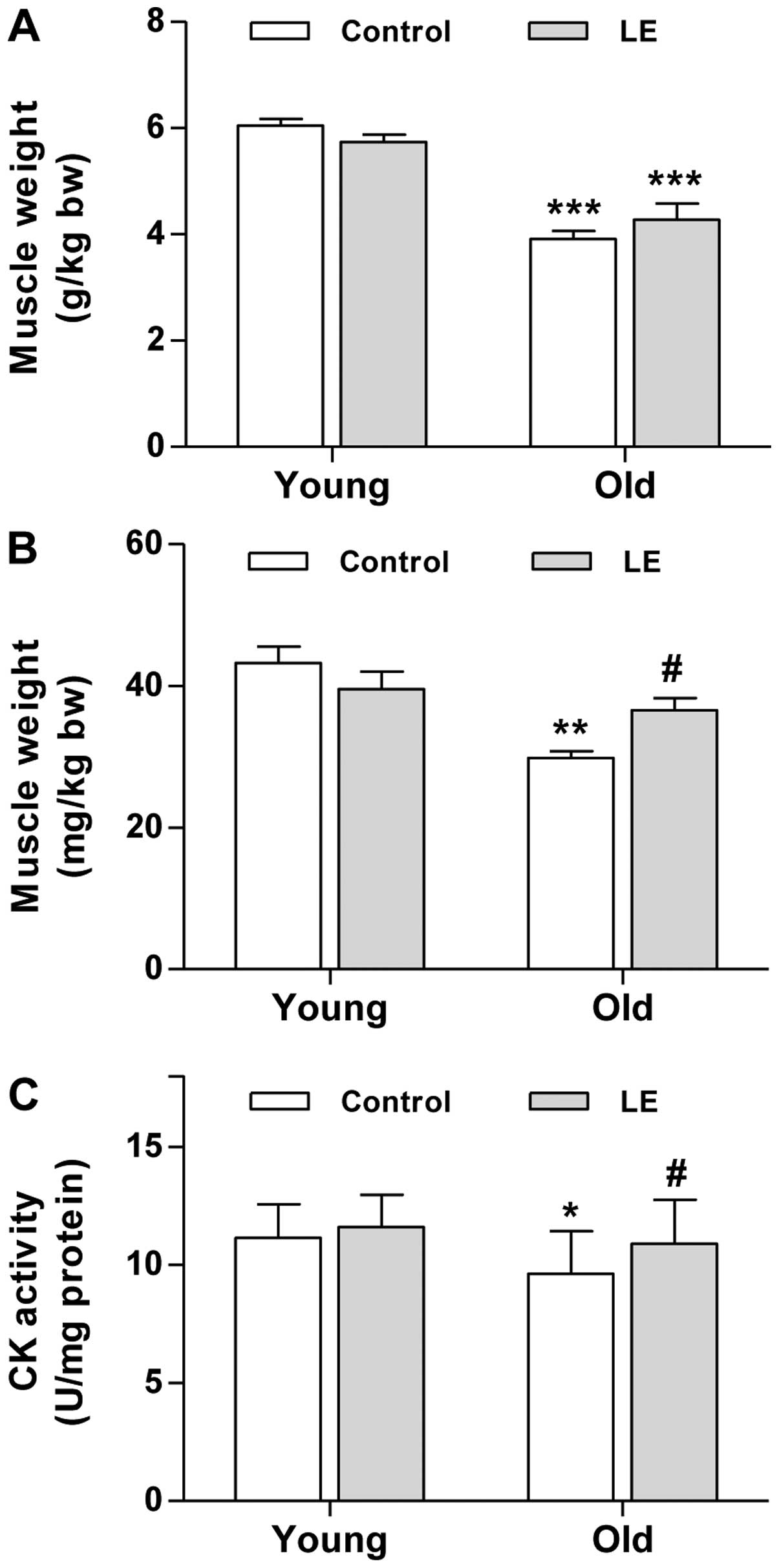
Grip strength decreased in the aged rats in
comparison with the young ones, and we then wished to determine
whether this decline in grip strength is associated with muscle
mass. To determine muscle mass, the weights of the soleus and
gastrocnemius muscles were measured immediately after biopsy. Aging
resulted in a significant decrease in muscle mass in both the
gastrocnemius (Fig. 2A) and
soleus muscle (Fig. 2B).
Moreover, gastrocnemius muscle mass in the O-LE group tended to
increase; however, this trend was not significant compared with the
O-Con group (Fig. 2A).
As regards the soleus muscle, the supplementation of
LE significantly increased muscle mass in the rats in the O-LE
group compared with their age-matched counterparts (Fig. 2B). However, LE intake did not
significantly affect the mass of either muscle in the young-aged
group (Fig. 2A and B). As the
decrease in muscle-specific CK activity may be a major contributor
to the loss of muscle function associated with aging (23), we assessed the effects of LE
supplementation on CK activity in muscles from young and aged rats.
As shown in Fig. 2C, CK activity
in the young rats tended to increase slightly, although this change
was not statistically significant. By contrast, aging resulted in a
significant decrease in muscle CK activity (Fig. 2C). Moreover, LE intake
significantly increased CK activity in the O-LE group compared with
the O-Con group. Taken together, these findings suggest that LE
supplementation improves muscle function in aged rats.
LE supplementation affects age-related
muscle damage and muscle fiber CSA
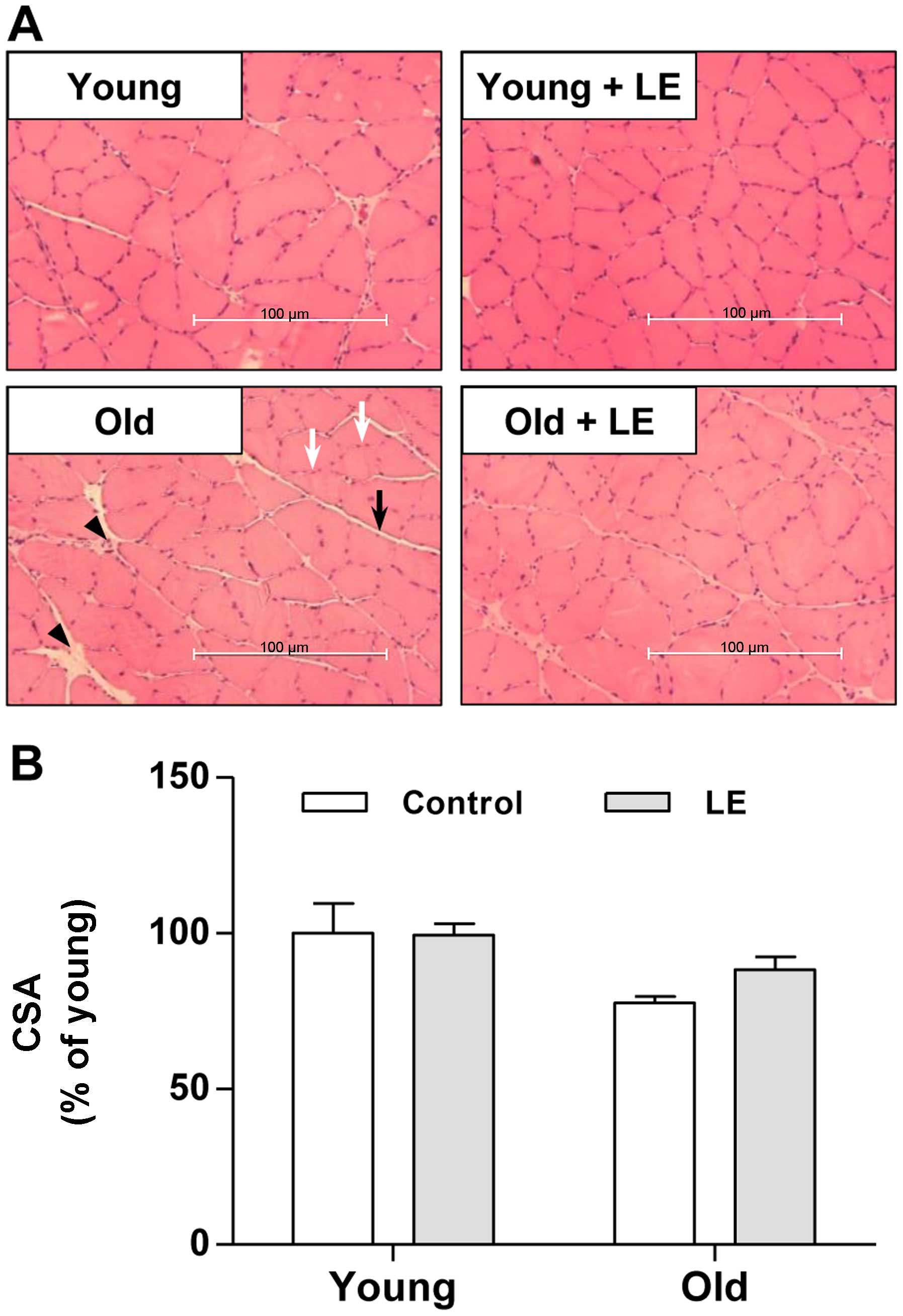
In order to investigate the effects of LE on
age-associated muscle weakness, we performed histological analysis
on the medial sections of soleus muscles using H&E staining. As
shown in Fig. 3A, muscle fibers
in the Y-Con group were in intimate contact in muscle bundles. LE
supplementation to young rats also produced similar histological
results as those observed in the rats in the Y-Con group. In the
aged rats, the amount of connective tissue increased compared with
that in young rats (Fig. 3A,
arrowhead). Thus, connective tissue surrounding the muscle fibers
(endomysium) and fiber bundles (perimysium) was observed in the
soleus muscle of the O-Con group compared with the young groups;
however, these histological changes were attenuated by LE
supplementation (Fig. 3A). We
also examined the effects of aging and LE intake on muscle CSA. The
CSA in the Y-LE group did not differ from that in the Y-Con group
(Fig. 3B). Moreover, the CSA in
the aged rats (O-Con group) was lower than that in the Y-Con group,
although the difference was not statistically significant (Fig. 3B). In the aged rats, LE
supplementation increased the CSA slightly, although not
significantly (Fig. 3B).
LE promotes the myogenic differentiation
of C2C12 cells
To investigate the effects of LE on muscle
regeneration, we examined whether LE affects the myogenic
differentiation of C2C12 myoblasts. Since it normally takes up to 6
days for C2C12 myoblasts to fully differentiate into myotubes, we
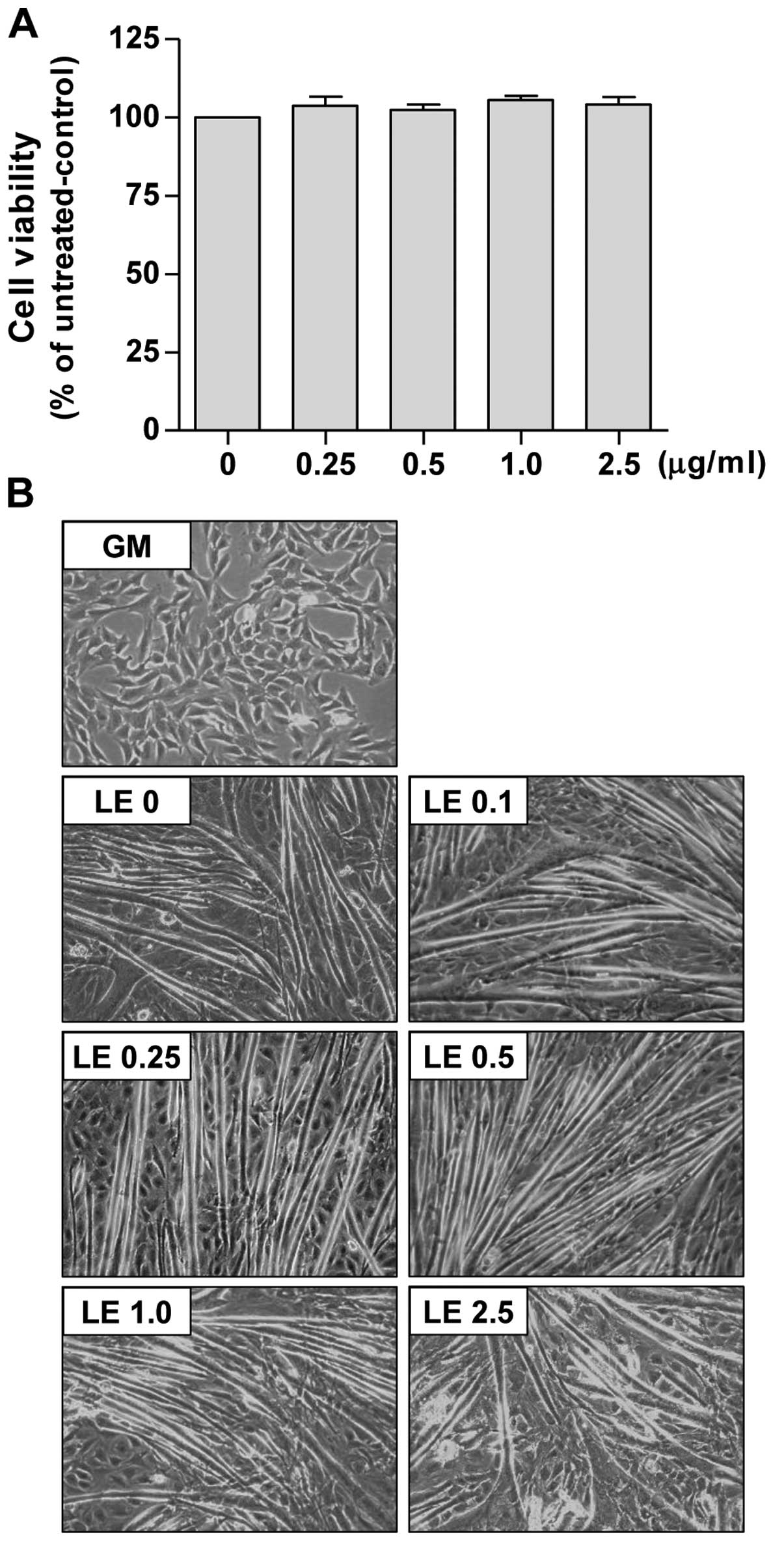
first assessed the effects of LE on C2C12 cell viability by MTT
assay under identical conditions of differentiation. As shown in
Fig. 4A, LE (0.25–2.5
µg/ml) did not induce the significant cell death of
myoblasts.
We then assessed whether LE affects the
differentiation of myoblasts into myotubes. For this purpose, we
used DM containing 2% HS. The undifferentiated C2C12 cells
(Fig. 4B, panel GM) were flat,
fusiform or star-shaped. The myotubes began to appear 3–4 days
following the induction of differentiation (data not shown). After
6 days of incubation in DM, the C2C12 cells became differentiated
(Fig. 4B, panel LE 0). The
myotubes exhibited thick and fusiform structures, and they were
elongated in 3–4 directions. We found that treatment with LE
promoted the differentiation of the C2C12 myoblasts (Fig. 4B). When the LE-treated cells
(panels LE 0.1–2.5) were compared with the DM-treated cells (panel
LE 0) the myotubes from the LE-treated cells were more stretched
and longer in shape with syncytia and nuclei and were more abundant
in number than those in the DM-treated cells. Furthermore, thick
and Y-shaped (or spindly ring-shaped) myotubes were also
occasionally observed in the 2.5 µg/ml LE-treated cells.
LE enhances the expression of the
myogenic differentiation marker, MyHC, and increases CK
activity
To further confirm the effects of LE on myogenic
differentiation, we measured the expression of the differentiation
marker, MyHC, which is the major structural protein in myotubes
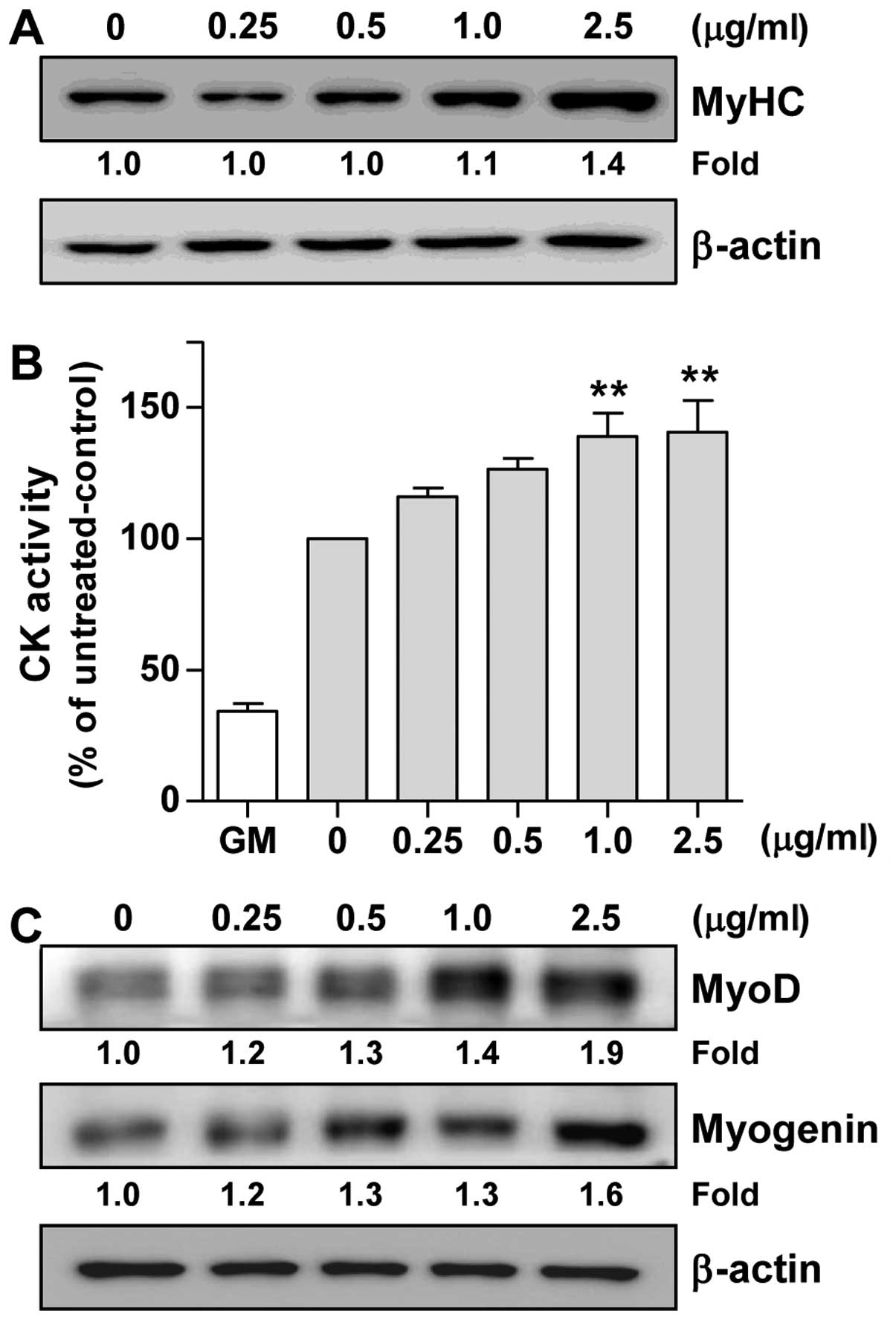
(24). As shown in Fig. 5A, the LE-treated C2C12 cells
exhibited an increased expression of MyHC in a
concentration-dependent manner compared with the untreated control
cells.
We then assessed CK activity, which is generally
accepted as an indicator of the differentiation state of muscle
cells (25). Since CK activity
gradually increased until reaching peak levels on day 6
post-differentiation (data not shown), we measured CK activity on
day 6 of differentiation. As shown in Fig. 5B, CK activity was induced 2.9-fold
by DM alone (0 mg/ml bar) in comparison with GM. In addition,
compared to treatment with DM alone (0 mg/ml bar), treatment with
LE significantly enhanced CK activity in a concentration-dependent
manner (Fig. 5B). Therefore,
these results suggest that LE enhances myoblast differentiation
into myotubes by increasing CK activity and upregulating MyHC
expression.
LE increases the expression of MyoD and
myogenin in C2C12 cells
To elucidate the mechanisms of myogenic
differentiation induced by LE, we examined the effects of LE on the
levels of myogenic regulatory factors (MRFs). Western blot analysis
(Fig. 5C) clearly indicated that
treatment with LE increased the expression of MyoD in C2C12 cells
in a concentration-dependent manner. In addition, it was evident
that LE induced the expression of myogenin (Fig. 5C). Overall, these results suggest
that LE promotes myogenic differentiation through the upregulation
of MyoD and myogenin.
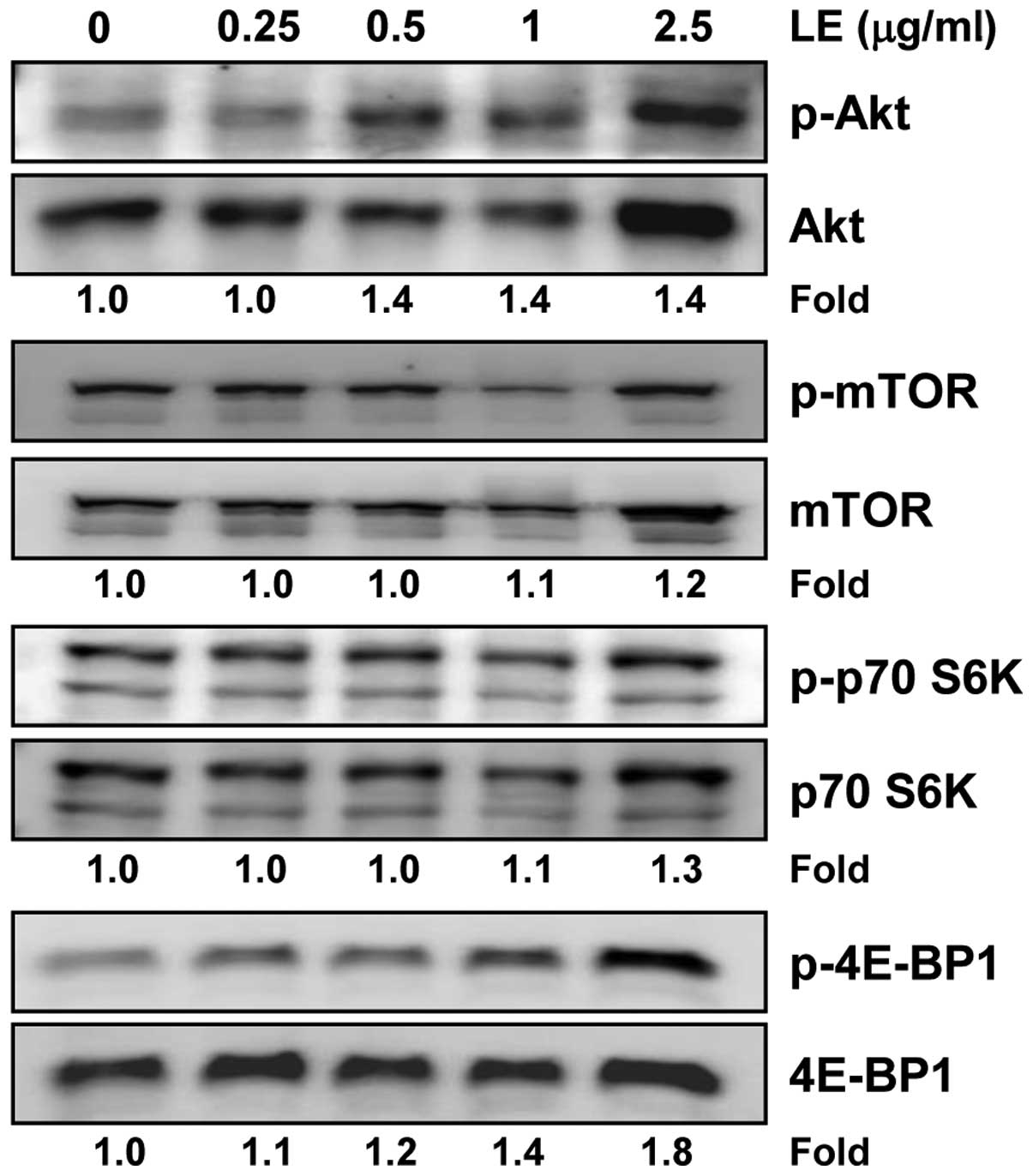
Treatment with LE activates the Akt/mTOR
signaling pathway during the myogenic differentiation of C2C12
cells
Phosphatidylinositol 3-kinase (PI3K)/Akt and its
downstream mTOR pathway have been shown to play an important role
in skeletal myogenesis (26,27). Thus, we examined whether LE can
modulate the activation of the PI3K/Akt/mTOR signaling pathway in
C2C12 cells. We found that LE induced the phosphorylation of Akt,
p70 S6K and 4E-BP1 in a concentration-dependent manner (Fig. 6). The effects of LE on 4E-BP1
activation, however, were clearly evident and occurred in a
concentration-dependent manner, whereas those on Akt and p70 S6K
were not so prominent. Of note, treatment with LE induced both the
phosphorylation and expression of Akt. By contrast, no significant
change in the expression of mTOR was observed in the LE-treated
C2C12 cells (Fig. 6). Taken
together, these results indicate that LE enhances myogenic
differentiation through the PI3K/Akt/mTOR signaling pathway;
however, the effects of LE on signaling molecules vary.
Discussion
As the proportion of older subjects to younger ones
is increasing worldwide, more attention is being paid toward
ʽhealthy aging' and ʽquality of life'. Although physical exercise
and an adequate diet, in terms of both calorie and protein intake,
contribute to the preservation of muscle function in older
subjects, these strategies are limited to healthy persons rather
than those who suffer from illness or inactivity (28). Therefore, in the present, we
investigated whether LE has the potential to prevent the
age-associated loss of muscle function. In myoblast culture and
aged rats, we found that LE abrogated the decline in skeletal
muscle function, including the loss of muscle mass. When examining
the mechanisms involved, we found that LE enhanced myogenic
differentiation through the upregulation of myogenic gene
expression. We also observed that LE activated the Akt/mTOR
pathway, which is a key cascade in skeletal muscle protein
synthesis.
To the best of our knowledge, this is the first
study to demonstrate that LE suppresses the age-associated loss of
skeletal muscle mass and muscle strength in rodents. Whether LE
affects the regenerative capacity of muscle fibers and the
expression of myogenic proteins has not been investigated
previously. However, LE is known to have therapeutic potential in
diabetes and inflammation, which are closely linked to age-related
muscle loss (9,10,14,29). Although it is well known that
certain nutritional interventions, such as essential amino acids,
milk-based proteins, creatine monohydrate, essential fatty acids
and vitamin D, in combination with resistance exercise, may further
enhance the beneficial effects on muscle mass and strength in aged
populations (30), a small number
of studies have reported the preventive effects of natural products
on skeletal muscle aging. In the present study, LE ameliorated the
decline in skeletal muscle function in aged rats, due to the
induction of myogenesis. LE stimulated myogenic proteins,
indicating a probable mechanism for the muscle regenerative
potential of LE. Other natural products, such as (-)-epicatechin
(Epi) and epigallocatechin-3-gallate (EGCG), have also been
reported to favorably modulate muscle cell differentiation in aged
animals (31,32). Similarly, LE has been shown to
increase the expression of myogenic genes, which is an effect that
is needed for myogenic differentiation, and the regenerative effect
of Epi and EGCG on aged-associated muscle function also requires
the activation of myogenic progenitor cells or myogenic genes
(31,32). In addition, an olive oil-derived
antioxidant mixture has also been reported to prevent the
aging-associated loss of skeletal muscle function, although through
a different mechanism (33).
We found that LE enhanced the expression of MyoD and
myogenin in C2C12 myoblasts. These observations are important as
MyoD and myogenin are known as MRFs which play master roles in the
process of generating muscle (also known as myogenesis). Myogenesis
can occur during both embryonic development and post-natal life
with many similarities in molecular mechanisms (34). In adult skeletal muscle, as in all
renewing organs, myogenesis depends on a mechanism that compensates
for the turnover of terminally differentiated cells to maintain
tissue homeostasis. Therefore, myogenesis in adult skeletal muscle
depends on the activation of satellite cells that have the
potential to differentiate into new fibers (34). It is a multistep process that
involves withdrawal from the cell cycle, the activation of
muscle-specific genes and the fusion of differentiated myocytes in
multinucleated myotubes. This step is controlled by the MRFs, a
group of bHLH transcription factors composed of myogenic factor 5
(Myf5), MyoD, myogenic factor 6 (Myf6) and myogenin (35). In particular, MyoD and Myf5
function early in the commitment steps of myogenesis; myogenin and
Myf6 act at later stages by promoting myoblast fusion and the
differentiation of mature skeletal muscle fibers (34). Thes MRFs control the expression of
structural muscle-specific genes, such as MyHC. In addition, our
results demonstrated that LE is able to increase the expression
level of MyHC and thereby potentially enhance myogenic
differentiation.
Our results are also in agreement with those of
previous studies indicating that the stimulation of MRFs drives
myogenic differentiation. For instance, resveratrol, a polyphenol,
has been shown to promote myogenic differentiation by inducing the
expression of myogenin (36).
Recently, the natural products betaine, kazinol-P and
tetrahydropalmatine have also been shown to enhance myogenic
differentiation through upregulation of MRFs (37–39).
We further demonstrated that LE activated the
Akt/mTOR pathway. Akt, a seine/threonine kinase, affects several
other signaling pathways that positively or negatively regulate
growth, proliferation, survival and myogenic differentiation
(40). A protein that is likely
to have pleiotropic functions, mTOR, is best known for its role in
regulating translation initiation (41). Thus, 4E-BP1 and S6K1, two of the
most well characterized downstream effectors of the mTOR pathway,
have been known to regulate translation initiation (42,43). 4E-BP1 and S6K1 are regulated by
mTOR and the PI3K/Akt pathway in parallel (41). Notably, in this study, LE
treatment did not alter mTOR phosphorylation, while an increase in
the levels of phosphorylated Akt, p70 S6K and 4E-BP1 were observed
with LE treatment. Although mTOR is a direct substrate for Akt, and
Ser-2448 is identified as the Akt target site in mTOR,
kinase-dependent and kinase-independent functions of mTOR in
skeletal muscle myogenesis have been reported (26). Park and Chen (44) described that the kinase activity
of mTOR was not required for nascent myotube formation, but was
essential for myotube maturation. These findings may explain our
results regarding mTOR; however, further mechanistic studies on the
LE-induced activation of the Akt/mTOR pathway are required.
The data from the present study and our previous
study on the protective effects of LE against atrophy (45) present potentially important
observations with clinical implications for the population of
elderly persons who suffer from impaired mobility and fragility
fractures due to muscle wasting or muscle loss. Subsequent studies
should be conducted to determine whether, as shown by our
observations of aged rats, elderly humans will derive similar
benefits from consuming LE during a period of rehabilitation
following hospitalization or other inactivity. The daily
consumption of up to 294 mg of loquat leaf extract was used in a
randomized double-blinded clinical study with hyperlipidemia
volunteers apparently without negative side-effects (46). Assuming a 60 kg human, the 294
mg/day used in that study would be equivalent to 30.2 mg/kg of LE,
which is below the 50 mg/kg used in the present study. While the
analysis of ingredient composition is needed and the administration
period is different, such as 35 days vs. 3 months, their result
warrants further trial with LE (46). Even given the promising effects of
LE, an optimal clinical trial design would need to include a dose
establishment and a significant number of elderly subjects to
demonstrate the desired therapeutic effects of LE, i.e., increased
muscle mass, without undesirable side-effects.
Acknowledgments
This study was supported by the R&D program of
MOTIE/KIAT (N0000697; Establishment of Infrastructure for
Anti-aging Industry Support) and the R&D program of MOTIE/KEIT
(10040391; Development of Functional Food Materials and Device for
Prevention of Aging-associated Muscle Function Decrease). This
study was also supported by the National Research Foundation of
Korea (NRF) grant, funded by the Korean Government (MSIP, No.
2009-0083538). We thank the Aging Tissue Bank for providing
research information.
References
|
1
|
Wernette CM, White BD and Zizza CA:
Signaling proteins that influence energy intake may affect
unintentional weight loss in elderly persons. J Am Diet Assoc.
111:864–873. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Taekema DG, Gussekloo J, Maier AB,
Westendorp RG and de Craen AJ: Handgrip strength as a predictor of
functional, psychological and social health. A prospective
population-based study among the oldest old. Age Ageing.
39:331–337. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Doherty TJ: Invited review: aging and
sarcopenia. J Appl Physiol. 95:1717–1727. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lexell J, Taylor CC and Sjöström M: What
is the cause of the ageing atrophy? Total number, size and
proportion of different fiber types studied in whole vastus
lateralis muscle from 15- to 83-year-old men. J Neurol Sci.
84:275–294. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nilwik R, Snijders T, Leenders M, Groen
BB, van Kranenburg J, Verdijk LB and van Loon LJ: The decline in
skeletal muscle mass with aging is mainly attributed to a reduction
in type II muscle fiber size. Exp Gerontol. 48:492–498. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vinciguerra M, Musaro A and Rosenthal N:
Regulation of muscle atrophy in aging and disease. Adv Exp Med
Biol. 694:211–233. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shefer G, Rauner G, Yablonka-Reuveni Z and
Benayahu D: Reduced satellite cell numbers and myogenic capacity in
aging can be alleviated by endurance exercise. PLoS One.
5:e133072010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Uto T, Suangkaew N, Morinaga O, Kariyazono
H, Oiso S and Shoyama Y: Eriobotryae folium extract suppresses
LPS-induced iNOS and COX-2 expression by inhibition of NF-kappaB
and MAPK activation in murine macrophages. Am J Chin Med.
38:985–994. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Noreen W, Wadood A, Hidayat HK and Wahid
SA: Effect of Eriobotrya japonica on blood glucose levels of normal
and alloxan-diabetic rabbits. Planta Med. 54:196–199. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li WL, Wu JL, Ren BR, Chen J and Lu CG:
Pharmacological studies on anti-hyperglycemic effect of folium
eriobotryae. Am J Chin Med. 35:705–711. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cha DS, Shin TY, Eun JS, Kim DK and Jeon
H: Anti-metastatic properties of the leaves of Eriobotrya japonica.
Arch Pharm Res. 34:425–436. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Alshaker HA, Qinna NA, Qadan F, Bustami M
and Matalka KZ: Eriobotrya japonica hydrophilic extract modulates
cytokines in normal tissues, in the tumor of Meth-A-fibrosarcoma
bearing mice, and enhances their survival time. BMC Complement
Altern Med. 11:92011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang Y, Li J, Cao Q, Yu SC, Lv XW, Jin Y,
Zhang L, Zou YH and Ge JF: Anti-oxidative effect of triterpene
acids of Eriobotrya japonica (Thunb) Lindl. leaf in chronic
bronchitis rats. Life Sci. 78:2749–2757. 2006. View Article : Google Scholar
|
|
14
|
Ge JF, Wang TY, Zhao B, Lv XW, Jin Y, Peng
L, Yu SC and Li J: Anti-inflammatory effect of triterpenoic Aacids
of Eriobotrya japonica (Thunb.) Lindl. Leaf on rat model of chronic
bronchitis. Am J Chin Med. 37:309–321. 2009. View Article : Google Scholar
|
|
15
|
Yang Y, Huang Y, Huang C, Lv X, Liu L,
Wang Y and Li J: Antifibrosis effects of triterpene acids of
Eriobotrya japonica (Thunb.) Lindl. leaf in a rat model of
bleomycin-induced pulmonary fibrosis. J Pharm Pharmacol.
64:1751–1760. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Banno N, Akihisa T, Tokuda H, Yasukawa K,
Taguchi Y, Akazawa H, Ukiya M, Kimura Y, Suzuki T and Nishino H:
Anti-inflammatory and antitumor-promoting effects of the triterpene
acids from the leaves of Eriobotrya japonica. Biol Pharm Bull.
28:1995–1999. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tan H, Furuta S, Nagata T, Ohnuki K,
Akasaka T, Shirouchi B, Sato M, Kondo R and Shimizu K: Inhibitory
effects of the leaves of loquat (Eriobotrya japonica) on bone
mineral density loss in ovariectomized mice and osteoclast
differentiation. J Agric Food Chem. 62:836–841. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
De Tommasi N, De Simone F, Pizza C,
Mahmood N, Moore PS, Conti C, Orsi N and Stein ML: Constituents of
Eriobotrya japonica. A study of their antiviral properties. J Nat
Prod. 55:1067–1073. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kunkel SD, Suneja M, Ebert SM, Bongers KS,
Fox DK, Malmberg SE, Alipour F, Shields RK and Adams CM: mRNA
expression signatures of human skeletal muscle atrophy identify a
natural compound that increases muscle mass. Cell Metab.
13:627–638. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kunkel SD, Elmore CJ, Bongers KS, Ebert
SM, Fox DK, Dyle MC, Bullard SA and Adams CM: Ursolic acid
increases skeletal muscle and brown fat and decreases diet-induced
obesity, glucose intolerance and fatty liver disease. PLoS One.
7:e393322012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim M, Sung B, Kang YJ, Kim DH, Lee Y,
Hwang SY, Yoon JH, Yoo MA, Kim CM, Chung HY and Kim ND: The
combination of ursolic acid and leucine potentiates the
differentiation of C2C12 murine myoblasts through the mTOR
signaling pathway. Int J Mol Med. 35:755–762. 2015.
|
|
22
|
Jung HA, Park JC, Chung HY, Kim J and Choi
JS: Antioxidant flavonoids and chlorogenic acid from the leaves of
Eriobotrya japonica. Arch Pharm Res. 22:213–218. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nuss JE, Amaning JK, Bailey CE, DeFord JH,
Dimayuga VL, Rabek JP and Papaconstantinou J: Oxidative
modification and aggregation of creatine kinase from aged mouse
skeletal muscle. Aging (Albany NY). 1:557–572. 2009.
|
|
24
|
Novitch BG, Mulligan GJ, Jacks T and
Lassar AB: Skeletal muscle cells lacking the retinoblastoma protein
display defects in muscle gene expression and accumulate in S and
G2 phases of the cell cycle. J Cell Biol. 135:441–456. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dufresne MJ, MacLeod J, Rogers J and
Sanwal BD: Serine auxotrophy of myoblasts in primary and secondary
culture. Biochem Biophys Res Commun. 70:1085–1090. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ge Y and Chen J: Mammalian target of
rapamycin (mTOR) signaling network in skeletal myogenesis. J Biol
Chem. 287:43928–43935. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ge Y, Wu AL, Warnes C, Liu J, Zhang C,
Kawasome H, Terada N, Boppart MD, Schoenherr CJ and Chen J: mTOR
regulates skeletal muscle regeneration in vivo through
kinase-dependent and kinase-independent mechanisms. Am J Physiol
Cell Physiol. 297:C1434–C1444. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sarti S, Ruggiero E, Coin A, Toffanello
ED, Perissinotto E, Miotto F, Pintore G, Inelmen EM, Manzato E and
Sergi G: Dietary intake and physical performance in healthy elderly
women: a 3-year follow-up. Exp Gerontol. 48:250–254. 2013.
View Article : Google Scholar
|
|
29
|
Cha DS, Eun JS and Jeon H:
Anti-inflammatory and anti-nociceptive properties of the leaves of
Eriobotrya japonica. J Ethnopharmacol. 134:305–312. 2011.
View Article : Google Scholar
|
|
30
|
Candow DG, Forbes SC, Little JP, Cornish
SM, Pinkoski C and Chilibeck PD: Effect of nutritional
interventions and resistance exercise on aging muscle mass and
strength. Biogerontology. 13:345–358. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gutierrez-Salmean G, Ciaraldi TP, Nogueira
L, Barboza J, Taub PR, Hogan MC, Henry RR, Meaney E, Villarreal F,
Ceballos G and Ramirez-Sanchez I: Effects of (-)-epicatechin on
molecular modulators of skeletal muscle growth and differentiation.
J Nutr Biochem. 25:91–94. 2014. View Article : Google Scholar
|
|
32
|
Alway SE, Bennett BT, Wilson JC, Edens NK
and Pereira SL: Epigallocatechin-3-gallate improves plantaris
muscle recovery after disuse in aged rats. Exp Gerontol. 50:82–94.
2014. View Article : Google Scholar :
|
|
33
|
Pierno S, Tricarico D, Liantonio A, Mele
A, Digennaro C, Rolland JF, Bianco G, Villanova L, Merendino A,
Camerino GM, et al: An olive oil-derived antioxidant mixture
ameliorates the age-related decline of skeletal muscle function.
Age (Dordr). 36:73–88. 2014. View Article : Google Scholar :
|
|
34
|
Bentzinger CF, Wang YX and Rudnicki MA:
Building muscle: molecular regulation of myogenesis. Cold Spring
Harb Perspect Biol. 4:a0083422012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Parker MH, Seale P and Rudnicki MA:
Looking back to the embryo: Defining transcriptional networks in
adult myogenesis. Nat Rev Genet. 4:497–507. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kaminski J, Lançon A, Aires V, Limagne E,
Tili E, Michaille JJ and Latruffe N: Resveratrol initiates
differentiation of mouse skeletal muscle-derived C2C12 myoblasts.
Biochem Pharmacol. 84:1251–1259. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Senesi P, Luzi L, Montesano A, Mazzocchi N
and Terruzzi I: Betaine supplement enhances skeletal muscle
differentiation in murine myoblasts via IGF-1 signaling activation.
J Transl Med. 11:1742013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hwang J, Lee SJ, Yoo M, Go GY, Lee Y, Kim
YK, Seo DW, Kang JS, Ryu JH and Bae GU: Kazinol-P from Broussonetia
kazinoki enhances skeletal muscle differentiation via p38MAPK and
MyoD. Biochem Biophys Res Commun. 456:471–475. 2015. View Article : Google Scholar
|
|
39
|
Lee SJ, Yoo M, Go GY, Hwang J, Lee HG, Kim
YK, Seo DW, Baek NI, Ryu JH, Kang JS and Bae GU:
Tetrahydropalmatine promotes myoblast differentiation through
activation of p38MAPK and MyoD. Biochem Biophys Res Commun.
455:147–152. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wilson EM and Rotwein P: Selective control
of skeletal muscle differentiation by Akt1. J Biol Chem.
282:5106–5110. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gingras AC, Raught B and Sonenberg N:
Regulation of translation initiation by FRAP/mTOR. Genes Dev.
15:807–826. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gingras AC, Raught B and Sonenberg N: eIF4
initiation factors: Effectors of mRNA recruitment to ribosomes and
regulators of translation. Annu Rev Biochem. 68:913–963. 1999.
View Article : Google Scholar
|
|
43
|
Magnuson B, Ekim B and Fingar DC:
Regulation and function of ribosomal protein S6 kinase (S6K) within
mTOR signalling networks. Biochem J. 441:1–21. 2012. View Article : Google Scholar
|
|
44
|
Park IH and Chen J: Mammalian target of
rapamycin (mTOR) signaling is required for a late-stage fusion
process during skeletal myotube maturation. J Biol Chem.
280:32009–32017. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Noh KK, Chung KW, Sung B, Kim MJ, Park CH,
Yoon C, Choi JS, Kim MK, Kim CM, Kim ND and Chung HY: Loquat
(Eriobotrya japonica) extract prevents dexamethasone-induced muscle
atrophy by inhibiting the muscle degradation pathway in
Sprague-Dawley rats. Mol Med Rep. 12:3607–3614. 2015.PubMed/NCBI
|
|
46
|
Said O, Saad B, Fulder S, Amin R, Kassis E
and Khalil K: Hypolipidemic activity of extracts from Eriobotrya
japonica and Olea europaea, traditionally used in the Greco-Arab
medicine in maintaining healthy fat levels in the blood. Open
Complement Med J. 1:84–91. 2009.
|