Introduction
Increased rates of population aging are causing the
problems associated with aging to attract increasing attention. By
the year 2050, the number of individuals over the age of 80 will
triple globally (1). More than
70% of individuals over 65 years of age suffer from at least two
chronic diseases, such as heart disease, stroke, cancer, arthritis
and diabetes (2). Studies of food
or medicine as a means of extending longevity have been taking
place since ancient times.
The underlying mechanisms of aging remain unknown,
despite tremendous progress being made in this area. There is
growing evidence to suggest that oxidative stress increases with
age (3). The endogenous enzymes,
superoxide dismutase (SOD) and catalase (CAT), are involved in the
human antioxidant defense network of human (4). Lipofuscin (LF) accumulation, which
is deemed to be a hallmark of aging (5), has been shown to be related to the
loss of protein homeostasis (6)
and the rate of accumulation has been linked to age-dependent
mortality (7).
Recently, great interest has arisen in the
possibility that antioxidants, particularly naturally occurring
antioxidants from edible materials, may reduce the risk of aging
(8).
Cordyceps sinensis, one of the most valued
edible, medical entomopathogenic fungi, used in traditional Chinese
medicine for thousands of years, is commonly used as a tonic for
promoting vitality and longevity, as well as a herbal medicine for
treating various intractable diseases (9,10).
It has been demonstrated to possess multiple pharmacological
properties, such as antioxidant (11), immunomodulatory (12) and anti-tumor (13) properties. Furthermore,
Cordyceps sinensis extract has been reported to improve
learning and memory function in a mouse model of
D-galactose-induced aging (14).
The aqueous polysaccharides of Cordyceps taii have been
shown to possess antioxidant activity in a mouse model of
D-galactose-induced aging (15).
However, to the best of our knowlewdge, the anti-aging effects of
Cordyceps sinensis in vivo, under physiological conditions,
have not been confirmed to date.
Cordyceps sinensis oral liquid (CSOL) was
studied and manufactured by the Naval Medical Research Institute
(Shanghai, China) (16), and has
been used as an immunomodulator, an adjunctive therapy during
chemoradiation treatment and to ameliorate chronic bronchitis more
than twenty years (17). In a
previous study of ours, we demonstrated that CSOL inhibited damage
induced by oxygen and glucose deprivation in vitro
(unpublished data) and thus, CSOL has potential for use as an agent
to ameliorate stroke-induced brain damage.
The aim of the present study was to assess the
anti-aging/life-prolonging effects of CSOL in Drosophila
melanogaster (D. melanogaster, fruit fly) under
conditions of physiological (normal) and pathological (premature)
aging. In addition, the mechanisms involving the anti-oxidative
stress pathway were investigated.
Materials and methods
CSOL
CSOL (lot no. 20130906; the Naval Medical Research
Institute), consists of wild Cordyceps sinensis extract
(CSE) and Cordyceps sinensis mycelial fermentation broth
(CSMFB). CSE and CSMFB were mixed in a ratio of 6:4 (v/v). The CSOL
concentration was expressed as 100 mg of wild Cordyceps
sinensis per 10 ml of CSOL (100 mg/10 ml). Adenosine (no less
than 1.0 mg/100 ml) was used as a marker for CSOL quality control
and was quantified using an Agilent 1260 HPLC apparatus (Agilent
Technologies, Santa Clara, CA, USA). This sample of CSOL was found
to have an adenosine concentration of 3.94 mg/100 ml (Fig. 1). In the present study, CSOL was
pre-filtered by sterile membrane filtration under aseptic
conditions and stored at 4°C for use.
Fruit fly strain and culture
conditions
The wild-type Oregon-K-strain of the D.
melanogaster fruit fly was kindly provided by Professor Zesheng
Zhang of the Tianjin University of Science and Technology, Tianjin,
China. The fruit flies were housed in 50 ml plastic vials
containing 5 ml of culture medium, and the vials were kept at
25±1°C, 60±5% humidity on a 12:12 h light/dark cycle. The basal
culture medium consisted of 72 g cornmeal, 72 g glucose (63005518),
10 g yeast (Angel Yeast Co., Ltd., Yichang, China), 6 g agar
(10000582), 40 ml antiseptic (1% ethyl 4-hydroxybenzoate in 75%
alcohol) (Sigma-Aldrich, St. Louis, MO, USA) and water to prepare
500 ml of medium. Glucose and agar were purchased from Sinopharm
Chemical Regent Co., Ltd. (Shanghai, China).
The mixture was cooked and poured into vials (5 ml
in each). All relative protocols were approved by the Local
Institutional Committee of Naval Medical Research Institute.
Determination of the effects of CSOL on
fruit flies under physiological conditions
Lifespan assay
A total of 800 male fruit flies (eclosion within 8
h) were randomly divided into 4 groups as follows: the control
group, and the groups treated with 0.02, 0.06 and 0.20 mg/ml CSOL.
The fruit flies in the CSOL-treated groups were fed with culture
medium supplemented with CSOL at final concentrations of 0.02, 0.06
and 0.20 mg/ml, respectively. The fruit flies in the control group
were fed with basal culture medium. The fruit flies were
transferred to fresh culture medium twice a week. The number of
dead fruit flies was recorded every 3 days until all flies died.
The survival time was observed. The lifespan curve was drawn, and
the median and mean lifespans were calculated. Maximum lifespan was
calculated as the average lifespan of the longest surviving 10% of
the fruit fly population.
Enzyme assays
To examine the effects of CSOL on antioxidant
enzymes, male fruit flies (eclosion within 8 h) were randomly
divided into 2 groups: the control group and the 0.06 mg/ml
CSOL-treated group. The daily treatment was as described above. On
days 0, 25 and 45, the fruit flies were collected for the
determination of the activity of SOD and CAT, and the LF content.
Copper-zinc-containing SOD (SOD1) activity, manganese-containing
SOD (SOD2) activity and CAT activity were detected according to the
manufacturer's instructions. The SOD activity assay kit (S0103) was
purchased from Beyotime Biotechnology Co., Ltd. (Haimen, China) and
the CAT activity assay kit (A007-1) was purchased from Nanjing
Jiancheng Bioengineering Institute (Nanjing, China). The LF
concentration was determined as previously described (18). Briefly, the fruit fly homogenate
was extracted with chloroform:methanol (2:1, v/v) and centrifuged
at 3000 x g for 10 min. Chloroform (40064966) and methanol
(40064292) were purchased from Sinopharm Chemical Regent Co., Ltd.
The absorbance (excitation, 360 nm and emission, 430 nm) was
measured using a Fluorospectrophotometer-850 (Hitachi, Tokyo,
Japan). Standardization was carried out with a freshly prepared
solution of quinine sulfate. Quinine sulfate (LQ3281) was purchased
from Hefei Bomei Biotechnology Co., Ltd. (Hefei, China). The LF
concentration was calculated from a calibration curve of quinine
sulfate and expressed as µg/mg body weight.
Measurement of the transcriptional
levels of antioxidant enzymes
This experiment was designed to examine the effect
of CSOL treatment on the mRNA levels of antioxidant genes in fruit
flies. Male fruit flies (eclosion within 8 h) were randomly divided
into 2 groups: the control and 0.06 mg/ml CSOL-treated group, and
housed as described above. The fruit flies were collected on days
0, 15, 25, 35, 45 and 55. The mRNA levels of SOD1, SOD2 and CAT
were quantified by quantitative PCR (qPCR) as described in our
previous study (19). Briefly,
total RNA was extracted using Trizol reagent (Invitrogen, Carlsbad,
CA, USA), 2 µg RNA was reverse transcribed into cDNA using
RNA reverse transcriptase (Promega, Madison, WI, USA), and qPCR was
performed using an Option Monitor 3 Real-Time PCR System (Bio-Rad,
Hercules, CA, USA) on an ABI 7500 PCR instrument (Applied
Biosystems, Carlsbad, CA, USA) with SYBR Premix Ex Taq™ Mixture
(Takara, Otsu, Japan) following the manufacturer's instructions.
The primers used were as follows: SOD1 sense,
5′-CTGCTCTGCTACGGTCACAC-3′ and antisense,
5′-ACAGCTTTAACCACCATTTCG-3′; SOD2 sense, 5′-CCACATCAACC
ACACCATCT-3′ and antisense, 5′-CAGTTTGCCCGACTTCTTGT-3′; CAT sense,
5′-TTCGATGTCACCAAGGTCTG-3′ and antisense,
5′-TGCTCCACCTCAGCAAAGTA-3′; and RP49 sense,
5′-ACTTCATCCGCCACCAGTC-3′ and antisense, 5′-ATCT CGCCGCAGTAAACG-3′.
RP49 was used as an internal control. The results were analyzed
using the 2−ΔΔCt method.
Determination of the effects of CSOL on
fruit flies exposed to acute oxidative stress
Survival time [hydrogen peroxide
(H2O2) exposure] assay
Oxygen-containing free radicals are considered to be
involved in the mechanisms of aging (20). H2O2 is
usually used to mimic damage induced by oxidative stress (21). The present experiment was designed
to examine the protective effects of CSOL against acute oxidative
stress induced by H2O2 in fruit flies.
H2O2 (lot: 20120601) was purchased from
Sinopharm Chemical Regent Co., Ltd. Male fruit flies (eclosion
within 8 h) were divided into groups and housed as described above.
On day 25, the fruit flies were first starved for 2 h, and then
transferred into new vials containing a filter paper saturated with
1 ml of 30% H2O2. The numbers of dead fruit
flies was recorded every 4 h until all flies died.
Survival time (paraquat exposure)
assay
Paraquat (1,1′-dimethyl-4,4′-bipyridinium
dichloride; Sigma-Aldrich) has been reported to induce
mitochondrial dysfunction and increase reactive oxygen species
(ROS) production (22). The
present experiment was designed to examine the protective effects
of CSOL against acute oxidative stress induced by paraquat in fruit
flies. Male fruit flies (eclosion within 8 h) were grouped and
housed as described above. On day 25, the fruit flies were first
starved for 2 h, and then transferred into new vials containing a
filter paper saturated with 1 ml of 20 mM paraquat in a 6% glucose
solution. The number of dead fruit flies was recorded every 4 h
until all flies died.
Determination of the effects of CSOL
on fruit flies under conditions of pathological aging induced by
D-galactose
The chronic administration of D-galactose has been
reported to accelerate aging (23) and is therefore, regarded as
suitable for use in models of aging. The excessive intake of
D-galactose may contribute to ROS generation through the oxidative
metabolism of D-galactose and through the increased formation of
advanced glycation end products (24). This experiment was carried out to
examine the effects of CSOL on a fruit fly model of
D-galactose-induced aging. D-galactose (SG075003) was purchased
from Sigma-Aldrich.
Lifespan assay
Male fruit flies (eclosion within 8 h) were randomly
divided into 5 groups as follows: the control group, the model
group, and the groups treated with 0.02, 0.06 and 0.20 mg/ml CSOL.
The fruit flies in the model group and the CSOL groups were exposed
to culture medium containing 6.5% D-galactose for the duration of
the experiment. The culture medium of the fruit flies in the
CSOL-treated groups was supplemented with CSOL at a final
concentration of 0.02, 0.06 and 0.20 mg/ml. The fruit flies in the
control group were fed with basal culture medium. The daily
treatment was the same as that described above. The lifespan curve,
the median, mean and maximum lifespans were determined as described
above.
Enzyme assays
The effects of CSOL on pathological aging induced by
D-galactose were further evaluated, and thus, the activity of SOD1,
SOD2 and CAT, and LF accumulation were detected. Male fruit flies
(eclosion within 8 h) were divided into groups and housed as
described above. On day 25, the fruit flies were collected to
detect the enzyme activity. The detection methods were as described
above.
Statistical analysis
Data are expressed as the means ± SD. Comparisons
among groups were made by analysis of variance (ANOVA) followed by
Dunnett's t-test. The Log-rank test was used to evaluate the
equality of the survival curves. P-values <0.05 were considered
to indicate statistically significant differences.
Results
Effects of CSOL on fruit flies under
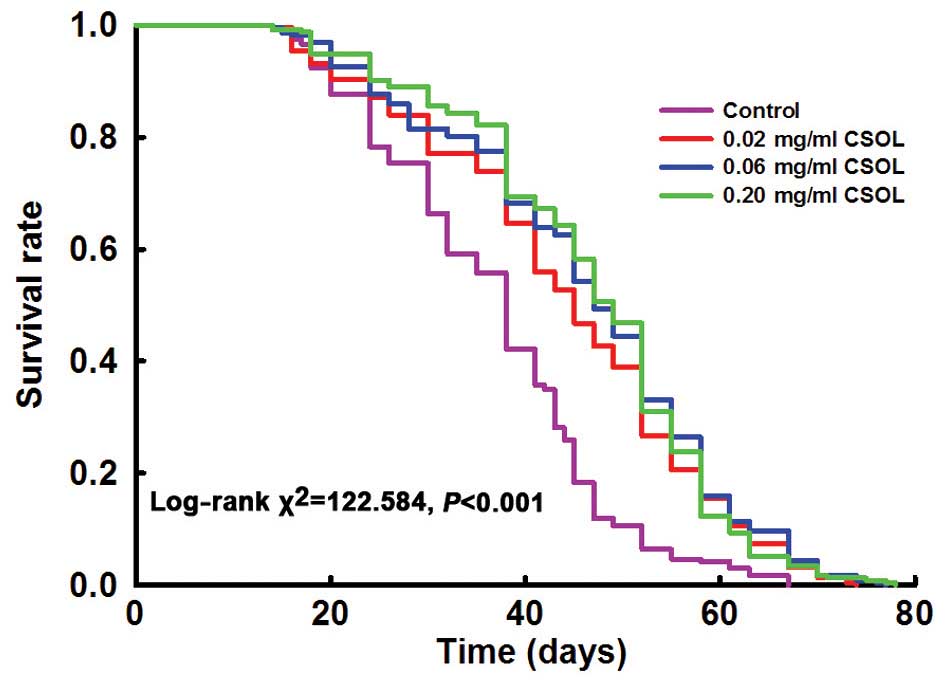
physiological conditions Lifelong treatment with CSOL prolongs the
lifespan of fruit flies
Lifelong treatment with CSOL at concentrations of
0.02, 0.06 and 0.20 mg/ml significantly prolonged the lifespan of
the fruit flies (Fig. 2). The
median and mean lifespan parameters shown in Table I confirmed these results.
Treatment with CSOL at 0.02, 0.06 and 0.20 mg/ml prolonged the mean
lifespan of the fruit flies (vs. the control) by 25, 31 and 32%,
respectively (Table I).
 | Table ILifespan parameters in fruit flies
following lifelong treatment with CSOL. |
Table I
Lifespan parameters in fruit flies
following lifelong treatment with CSOL.
| Group (mg/ml) | Median lifespan
(days) | Mean lifespan
(days) | Change from control
(%) | Log-rank (vs.
control) | Maximum lifespan
(days) |
|---|
| Control | 38.0±1.0 | 35.3±0.8 | – | – | 57.8±5.8 |
| 0.02 | 45.0±1.5 | 44.2±1.0 | 25 |
χ2=53.167, P<0.001 |
67.3±3.5a |
| 0.06 | 47.0±1.1 | 46.1±1.0 | 31 |
χ2=79.677, P<0.001 |
69.0±3.4a |
| 0.20 | 49.0±0.8 | 46.6±0.9 | 32 |
χ2=87.063, P<0.001 |
66.7±5.0a |
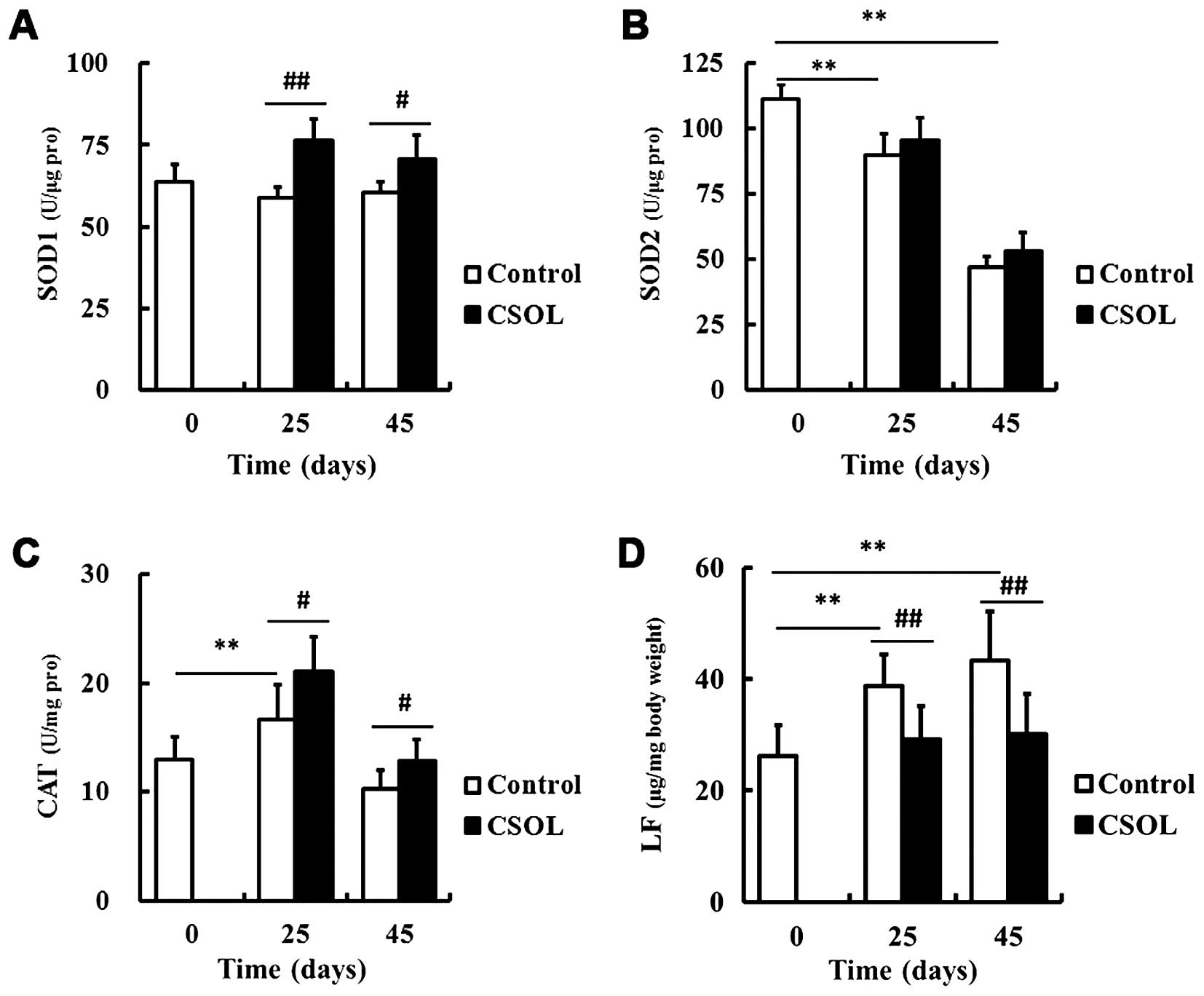
CSOL enhances the activity of most
antioxidant enzymes
To eluciate the mechanisms responsible for the
lifespan-prolonging effect of CSOL on fruit flies, we examined the
effects of CSOL on the activity of the antioxidant enzymes, SOD1,
SOD2 and CAT. Compared with the control group at the same time
point, treatment with CSOL significantly enhanced the activity of
SOD1 (Fig. 3A) and CAT (Fig. 3C), and inhibited LF accumulation
(Fig. 3D). CSOL had no
significant effect on the activity of SOD2, although its activity
was found to significantly decrease with age (Fig. 3B).
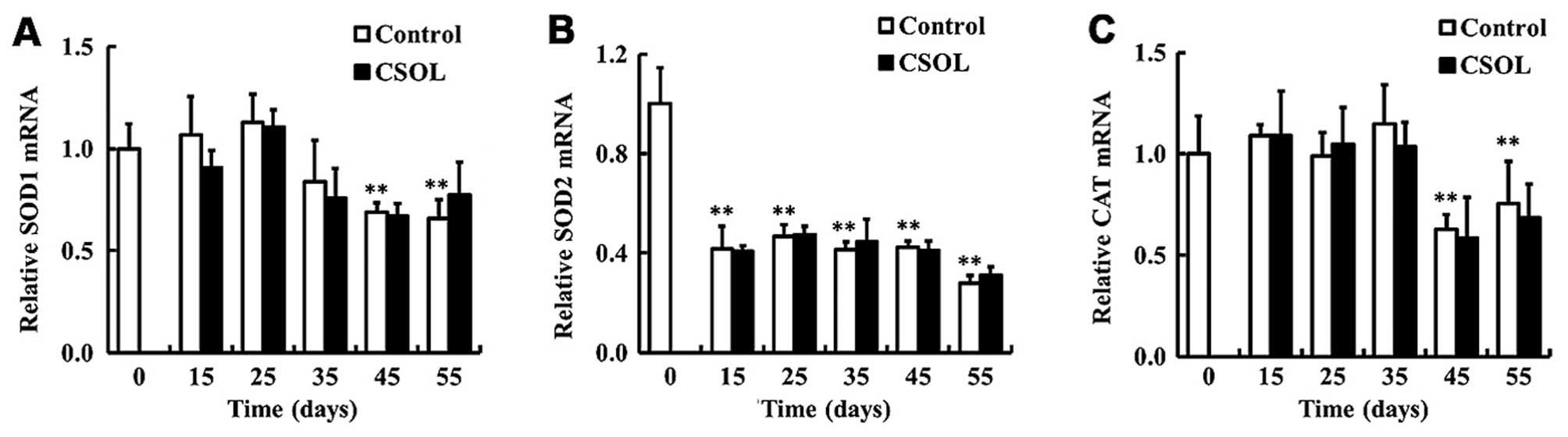
CSOL has no effect on the transcriptional
levels of antioxidant enzymes
To determine the effects of CSOL on the
transcriptional levels of antioxidant enzymes, we measured the mRNA
levels of SOD1, SOD2 and CAT in the fruit flies. Treatment with
CSOL was found to have no effect on the mRNA levels of SOD1
(Fig. 4A), SOD2 (Fig. 4B) and CAT (Fig. 4C). In the control group, the mRNA
level of SOD2 was highest at eclosion, and then decreased to a
relative low level from day 15 (Fig.
4B).
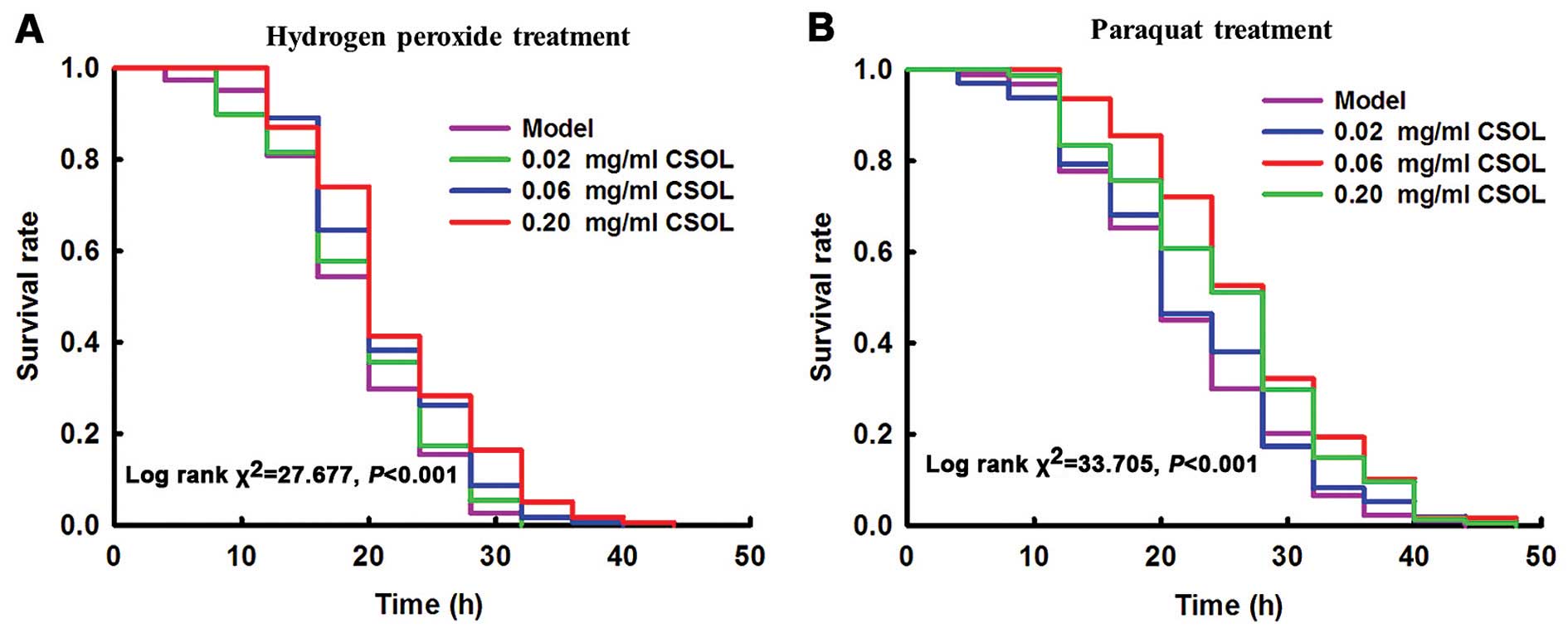
CSOL prolongs the survival time of fruit
flies subjected to H2O2- or paraquat-induced
oxidative stress
Exposure to
H2O2
The results revealed that treatment with CSOL
significantly prolonged the survival time of the fruit flies
exposed to H2O2 (Fig. 5A). Treatment with CSOL at
concentrations of 0.06 and 0.20 mg/ml significantly prolonged the
mean survival time by 13 and 16%, respectively, vs. the model group
(Table II). Treatment with CSOL
at a concentration of 0.02 mg/ml also prolonged the mean survival
time of the fruit flies exposed to H2O2;
however, no statistically significant difference was observed.
These results suggest an obvious dose-effect relationship.
 | Table IISurvival parameters in fruit flies
exposed to H2O2 or paraquat and treated with
CSOL. |
Table II
Survival parameters in fruit flies
exposed to H2O2 or paraquat and treated with
CSOL.
| Group (mg/ml) | Median survival
time (h) | Mean survival time
(h) | Change from model
(%) | Log-rank (vs.
model) |
|---|
|
H2O2 | | | | |
| Model | 20.0±0.5 | 18.9±0.5 | – | – |
| 0.02 | 20.0±0.7 | 19.5±0.5 | 3 |
χ2=1.107, P=0.293 |
| 0.06 | 20.0±0.6 | 21.3±0.5 | 13 |
χ2=11.170, P<0.001 |
| 0.20 | 20.0±0.5 | 22.0±0.5 | 16 |
χ2=21.605, P<0.001 |
| Paraquat | | | | |
| Model | 20.0±0.8 | 21.7±0.6 | – | – |
| 0.02 | 20.0±0.9 | 22.3±0.6 | 3 |
χ2=0.756, P=0.385 |
| 0.06 | 28.0±0.7 | 26.8±0.6 | 24 |
χ2=27.739, P<0.001 |
| 0.20 | 28.0±0.9 | 25.0±0.7 | 15 |
χ2=13.091, P<0.001 |
Exposure to paraquat
The results revealed that treatment with CSOL
significantly prolonged the survival time of the fruit flies
exposed to paraquat (Fig. 5B).
Treatment with CSOL at concentrations of 0.06 and 0.20 mg/ml,
significantly prolonged the mean survival time by 24 and 15%,
respectively, vs. the model group (Table II). Treatment with CSOL at a
concentration of 0.02 mg/ml prolonged the mean survival time of the
fruit flies exposed to paraquat; however, no statistically
significant difference was observed.
CSOL prolongs the lifespan and
inhibits oxidative stress in patholgically-aged fruit flies
(induced by D-galactose)
The addition of D-galactose to the culture medium
significantly shortened the maximum lifespan of the fruit flies
from 72.2±5.7 days in the control group to 57.7±2.6 days in the
model group (Fig. 6A and Table III). However, treatment with
CSOL at concentrations of 0.02, 0.06 and 0.20 mg/ml, modestly
inhibited the lifespan shortening effect by 6, 8 and 12%,
respectively (Table III). These
results were supported by the parameters of the mean and maximum
lifespans (Table III).
 | Table IIILifespan parameters in
pathologically-aged fruit flies (exposed to D-galactose) following
lifelong treatment with CSOL. |
Table III
Lifespan parameters in
pathologically-aged fruit flies (exposed to D-galactose) following
lifelong treatment with CSOL.
| Group (mg/ml) | Median lifespan
(day) | Mean lifespan
(day) | Change from model
(%) | Log-rank (vs.
model) | Maximum lifespan
(day) |
|---|
| Control | 55.0±1.7 | 52.5±1.1 | 25 |
χ2=74.033, P<0.001 |
72.2±5.7a |
| Model | 44.0±1.4 | 42.1±0.8 | – | – | 57.7±2.6 |
| 0.02 | 44.0±1.8 | 44.7±1.0 | 6 |
χ2=13.968, P<0.001 |
64.4±3.4a |
| 0.06 | 50.0±1.8 | 45.6±1.0 | 8 |
χ2=23.662, P<0.001 |
65.2±4.5a |
| 0.20 | 50.0±2.5 | 47.2±1.2 | 12 |
χ2=34.615, P<0.001 |
67.0±2.3a |
Since we found that CSOL enhanced the activity of
SOD1 and CAT, inhibited LF accumulation, and had no effect on the
activity of SOD2, we measured the levels of these enzymes in fruit
flies pathologically-aged by D-galactose in order to further
confirm the effect of CSOL on oxidative stress under these
conditions. SOD1 (Fig. 6B) and
CAT (Fig. 6D) activity in the
fruit fly model group decreased significantly compared to the
control group. The content of LF (Fig. 6E) increased significantly in the
model group compared with the control group. Consistent with the
results of our other experiments (described above), treatment with
CSOL inhibited the decrease in SOD1 (Fig. 6B) and CAT (Fig. 6D) activity induced by D-galactose,
and inhibited LF accumulation (Fig.
6E). CSOL had no significant effect on the activity of SOD2
(Fig. 6C).
Discussion
A suitable animal model is required for
investigation of the anti-aging effects of CSOL. In the present
study, we focused on the fruit fly D. melanogaster. It has
been widely used as a model of aging for basic and applied research
due to its advantages over mammalian models, such as high genus
purity, strong reproductive capacity and a short lifespan (25). For these reasons, D.
melanogaster was selected for use as a model organism in the
study of the physiological and pathological processes affecting
lifespan.
Our findings demonstrated that treatment with CSOL
significantly prolonged the lifespan of the fruit flies. A previous
study indicated that Cordyceps militaris extract possesses
ROS scavenging activity which protects against premature senescence
induced by oxidative stress (26). However, to the best of our
knowledge, the present study used a model of physiological aging
for the first time. This suggests that CSOL may have the potential
to be exploited for anti-aging applications.
This prompted us to elucidate the mechanisms
responsible for the lifespan-prolonging effects of CSOL. Oxidative
stress has been implicated in aging and degenerative diseases
(27). It is the result of
increased ROS production (28),
and causes progressive structural and functional alterations of
cellular organelles. Antioxidants inhibit the process of aging
(29). The continuous generation
of ROS is also closely related to organism aging (30). There are two main assumptions of
the free radical hypothesis of aging. Firstly, there are
antioxidant defenses; organisms endogenously produce a group of
antioxidant enzymes including SOD and CAT, which serve as the first
line of defense against ROS. Secondly, a fraction of ROS escape
elimination and are able to inflict molecular damage that
accumulates with age, thereby causing the functional attrition
associated with aging (31,32).
Since oxidative stress is one of the mechanisms
responsible for aging, we investigated the anti-oxidative stress
effect of CSOL in fruit flies to further elucidate the mechanisms
through which CSOL exerts a lifespan-prolonging effect. The
upregulation of SOD1 and CAT activity, and the inhibition of LF
accumulation suggested that the anti-aging effects of CSOL in fruit
flies were associated, at least in part, with the upregulation of
endogenous antioxidant enzymes, as well as the inhibition of LF
accumulation. However, the unaltered mRNA levels of SOD1, SOD2 and
CAT implied that CSOL had no effect at the transcriptional level of
antioxidant genes in fruit flies. Thus, subsequent experiments
mainly focused on the anti-oxidative stress effect of CSOL on the
enzymes rather than on their transcriptional levels.
To confirm the anti-oxidative stress effect of CSOL,
fruit flies were exposed to acute oxidative stress induced by
either H2O2 or paraquat. The results,
including the parameter of the mean survival time, further verified
this effect.
To further examine the anti-oxidative stress effect
of CSOL under pathological conditions, we selected a model of
pathological aging induced by D-galactose in fruit flies. The
results, including lifespan curves, and the mean and maximum
lifespan parameters, confirmed the effects of CSOL. Additionally,
the examination of SOD1 activity, CAT activity and the LF content
in fruit flies, pathologically aged by D-galactose, further
supported the theory that CSOL exerts an anti-aging effect through
the anti-oxidative stress pathway under pathological
conditions.
Taking into account these results, the anti-aging
effect of CSOL, exerted through the anti-oxidative stress pathway,
has been confirmed in both under physiological and pathological
conditions in fruit flies.
In conclusion, our data demonstrated that CSOL
prolonged the lifespan of fruit flies through an anti-oxidative
stress pathway which involved the upregulation of the activity of
SOD1 and CAT activity and the inhibition of LF accumulation. If the
association between CSOL and anti-aging could be confirmed and the
detailed mechanisms could be further clarified in future studies,
it may provide a novel strategy for slowing the human aging
process.
Acknowledgments
This study was supported by grants from the Natural
Science Foundation of Shanghai City of China (no. 12ZR1444600 to
Y.Z.) and the National Natural Science Foundation of China (no.
81402922 to Y.Z.).
References
|
1
|
Fontana L, Kennedy BK, Longo VD, Seals D
and Melov S: Medical research: treat ageing. Nature. 511:405–407.
2014. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hung WW, Ross JS, Boockvar KS and Siu AL:
Recent trends in chronic disease, impairment and disability among
older adults in the United States. BMC Geriatr. 11(47)2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dröge W: Oxidative stress and ageing: is
ageing a cysteine deficiency syndrome? Philos Trans R Soc Lond B
Biol Sci. 360:2355–2372. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pham-Huy LA, He H and Pham-Huy C: Free
radicals, antioxidants in disease and health. Int J Biomed Sci.
4:89–96. 2008.PubMed/NCBI
|
|
5
|
López-Otín C, Blasco MA, Partridge L,
Serrano M and Kroemer G: The hallmarks of aging. Cell.
153:1194–1217. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Höhn A and Grune T: Lipofuscin: formation,
effects and role of macroautophagy. Redox Biol. 1:140–144. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pincus Z, Smith-Vikos T and Slack FJ:
MicroRNA predictors of longevity. Caenorhabditis elegans PLoS
Genet. 7:e10023062011. View Article : Google Scholar
|
|
8
|
Xiao JH, Xiao DM, Sun ZH, Xiao Y and Zhong
IJ: Antioxidative potential of polysaccharide fractions produced
from traditional Chinese medicinal macrofungus Cordyceps
jiangxiensis in vitro. Afr J Biotechnol. 10:6607–6615. 2011.
|
|
9
|
Xiao JH, Xiao DM, Sun ZH, Xiong Q, Liang
ZQ and Zhong JJ: Chemical compositions and antimicrobial property
of three edible and medicinal Cordyceps species. J Food Agric
Environ. 7:91–100. 2009.
|
|
10
|
Panda AK and Swain KC: Traditional uses
and medicinal potential of Cordyceps sinensis of Sikkim. J Ayurveda
Integr Med. 2:9–13. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yamaguchi Y, Kagota S, Nakamura K,
Shinozuka K and Kunitomo M: Antioxidant activity of the extracts
from fruiting bodies of cultured Cordyceps sinensis. Phytother Res.
14:647–649. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou X, Gong Z, Su Y, Lin J and Tang K:
Cordyceps fungi: Natural products, pharmacological functions and
developmental products. J Pharm Pharmacol. 61:279–291. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li SP, Yang FQ and Tsim KW: Quality
control of Cordyceps sinensis, a valued traditional Chinese
medicine. J Pharm Biomed Anal. 41:1571–1584. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ji DB, Ye J, Li CL, Wang YH, Zhao J and
Cai SQ: Antiaging effect of Cordyceps sinensis extract. Phytother
Res. 23:116–122. 2009. View
Article : Google Scholar
|
|
15
|
Xiao JH, Xiao DM, Chen DX, Xiao Y, Liang
ZQ and Zhong JJ: Polysaccharides from the medicinal mushroom
Cordyceps taii show antioxidant and immunoenhancing activities in a
D-galactose-induced aging mouse model. Evid Based Complement
Alternat Med. 2012(273435)2012.
|
|
16
|
Sun YH: Naval Medical Research Institute
manufactured the 'immunomodulatory mixtures'. Navy Med.
2(11)1993.
|
|
17
|
Wu JR, Chen DM, Sun YH, Ding JM and Zhu
ZY: Study of Cordyceps capsules therapy on chronic obstructive lung
disease. Chinese Patent Med Study. (8): 26–27. 1986.
|
|
18
|
Falfushynska HI, Gnatyshyna LL, Osadchuk
OY, Farkas A, Vehovszky A, Carpenter DO, Gyori J and Stoliar OB:
Diversity of the molecular responses to separate wastewater
effluents in freshwater mussels. Comp Biochem Physiol C Toxicol
Pharmacol. 164:51–58. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zou YX, Zhang XH, Su FY and Liu X:
Importance of riboflavin kinase in the pathogenesis of stroke. CNS
Neurosci Ther. 18:834–840. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee KW and Lee HJ: Biphasic effects of
dietary antioxidants on oxidative stress-mediated carcinogenesis.
Mech Ageing Dev. 127:424–431. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Goncalves RL, Rothschild DE, Quinlan CL,
Scott GK, Benz CC and Brand MD: Sources of
superoxide/H2O2 during mitochondrial proline
oxidation. Redox Biol. 2:901–909. 2014. View Article : Google Scholar
|
|
22
|
Dagda RK, Das Banerjee T and Janda E: How
Parkinsonian toxins dysregulate the autophagy machinery. Int J Mol
Sci. 14:22163–22189. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lei M, Hua X, Xiao M, Ding J, Han Q and Hu
G: Impairments of astrocytes are involved in the
D-galactose-induced brain aging. Biochem Biophys Res Commun.
369:1082–1087. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lu J, Zheng YL, Luo L, Wu DM, Sun DX and
Feng YJ: Quercetin reverses D-galactose induced neurotoxicity in
mouse brain. Behav Brain Res. 171:251–260. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim SI, Jung JW, Ahn YJ, Restifob LL and
Kwon HW: Drosophila as a model system for studying lifespan and
neuroprotective activities of plant-derived compounds. J Asia Pac
Entomol. 14:509–517. 2011. View Article : Google Scholar
|
|
26
|
Park JM, Lee JS, Lee KR, Ha SJ and Hong
EK: Cordyceps militaris extract protects human dermal fibroblasts
against oxidative stress-induced apoptosis and premature
senescence. Nutrients. 6:3711–3726. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ma Q: Advances in mechanisms of
anti-oxidation. Discov Med. 17:121–130. 2014.PubMed/NCBI
|
|
28
|
Apel K and Hirt H: Reactive oxygen
species: Metabolism, oxidative stress, and signal transduction.
Annu Rev Plant Biol. 55:373–399. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sadowska-Bartosz I and Bartosz G: Effect
of antioxidants supplementation on aging and longevity. Biomed Res
Int. 2014:4046802014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Di DF, Barone E, Perluiqi M and
Butterfield DA: Strategy to reduce free radical species in
Alzheimer's disease: An update of selected antioxidants. Expert Rev
Neurother. 22:1–22. 2014.
|
|
31
|
Hou S: Introduction to Aging: A positive,
interdisciplinary approach. Health Promot Pract. 16:12–14. 2014.
View Article : Google Scholar
|
|
32
|
Zarrouk A, Vejux A, Mackrill J,
O'Callaghan Y, Hammami M, O'Brien N and Lizard G: Involvement of
oxysterols in age-related diseases and ageing processes. Ageing Res
Rev. 18:148–162. 2014. View Article : Google Scholar : PubMed/NCBI
|