Introduction
Cefepime (FEP) is a member of the fourth-generation
cephalosporin class with broad-spectrum activity against both
Gram-positive and Gram-negative bacteria (1) that has been widely used for approved
indications, such as febrile neutropenia, which is a major adverse
effect in patients receiving cancer chemotherapy with or without
radiotherapy (2–5). It is known that several drugs have
better pharmacological properties when in the form of metal
complexes (6). Thus, a number of
cephalosporin metal complexes have been extensively studied as
biochemical and antimicrobial reagents over the past decade
(7–12). However, to date, only a few
studies have investigated the antitumor activity of these metal
complexes.
Manganese (Mn), an essential trace element required
by the human body, has come to be recognized as being significant
in many biological systems (13–15). A few papers have demonstrated that
some Mn-based compounds possess superoxide dismutase (SOD)-like
activity (16,17) and may be considered as potential
anti-Mycobacterium tuberculosis agents (18). In addition, some Mn complexes have
also exhibited antitumor activity against a variety of cancer
cells. For example, Mn(II) complexes of
6,7-dicycanodipyridoquinoxaline and
2H-5-hydroxy-1,2,5-oxadiazo[3,4-f]1,10-phenanthroline have been
found to exhibit antitumor activity due to DNA binding (19,20). The Mn(II) compound
[(Adpa)Mn(Cl)(H2O)] (Adpa = bis(2-pyridylmethyl)
amino-2-propionic acid) was reported to target the mitochondria and
to inhibit the proliferation of U251 human glioma cells (21). Moreover, Mn(II) complexes of
quinoline derivatives have potential as attenuators of
Ca2+ absorption in the mitochondria and can interfere
with the metabolism of O2 for cancer chemotherapy
(22). The novel Mn(II) complexes
of indolecarboxylic acids have been reported to display
cytotoxicity and their antiproliferative activity against some cell
lines (e.g., Jurkat derived from an acute T cell leukemia), is
similar to that of cisplatin (23). However, although many studies have
demonstrated that such Mn(II) complexes have potential for use as
antitumor drugs, their mechanisms of action are not yet fully
understood.
It is known that the ubiquitin-proteasome system
(UPS) maintains protein homestasis in human cells, and it is
involved in highly regulated cellular processes, including cell
cycle progression, proliferation, apoptosis and differentiation
(24,25). It has been proven that the UPS is
an important target in cancer treatment. The 20S proteasome, the
main component of the UPS, possesses multiple peptidase activities,
such as caspase-like or peptidyl-glutamyl peptide-hydrolyzing-like
(PGPH-like) activity (β1 subunit), trypsin like activity (β2
subunit) and chymotrypsin-like (CT-like) activity (β5 subunit)
(26–28). However, only the inhibition of
proteasomal CT-like activity has been shown to be closely
associated with the induction of cancer cell death (29–31). In 2004, Daniel et al
(32) found that mixtures of
copper salts with the ligands (clioquinol, hydroxyquinoline and
dithiocarbamate) inhibited the effect of the 20S proteasome in
vitro and in vivo. Therefore, the inhibition of
proteasomal CT-like activity is associated with the induction of
cancer cell apoptosis (33,34) and is unquestionably linked to the
presence of metal ions (35).
Previously, we found that certain types of
copper(II) complexes,
[Cu(C10H8O2N)2(C12H8N2)]
and [Cu(C11H10O2N)2
(C12H8N2)], cadmium(II) complexes,
[Cd2(C12H12O2N)4
(H2O)2]·2H2O,
[Cd2(C11H10O2N)4(H2O)2]·2H2O
and
[Cd(C7H4N2O2)(C8H6O2)2]·2H2O,
may serve as potent, selective proteasome inhibitors and apoptosis
inducers in cultured human cancer cells (36,37). In this study, we investigated the
effects of FEP-metal complexes on breast cancer cells. We found
that only the FEP-Mn complex inhibited the CT-like activity of the
proteasome in human breast cancer cell cultures and induced cancer
cell death. Furthermore, the MDA-MB-231 breast cancer cells were
more sensitive to the novel candidate FEP-Mn complex than normal
cells. This suggests that the FEP-Mn complex has potential for use
as a novel class of antitumor agents.
Materials and methods
Chemicals and reagents
FEP was purchased from Sinopharm Chemical Reagent
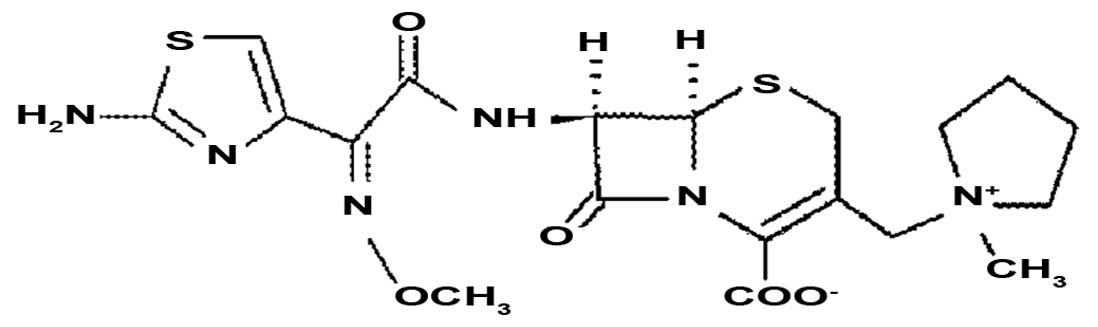
Co., Ltd. (Shanghai, China). The chemical structure of FEP is
illustrated in Fig. 1.
Dimethylsulfoxide (DMSO) and the acetate
[Cu(CH3COO)2•H2O,
Zn(CH3COO)2•2H2O,
Co(CH3COO)2•4H2O,
Ni(CH3COO)2• 4H2O,
Mn(CH3COO)2•4H2O,
Cd(CH3COO)2•2H2O,
Cr(NO3)3• 9H2O and
Fe(NO3)3•9H2O] were all purchased
from Aladdin Reagents Co. Ltd., (Shanghai, China). FEP and various
metal salts were dissolved and formed new complexes [FEP-Cu (FEP1),
FEP-Zn (FEP2), FEP-Co (FEP3), FEP-Ni (FEP4), FEP-Cr (FEP5), FEP-Cd
(FEP6), FEP-Fe (FEP7), FEP-Mn (FEP8)] in DMSO to a final
concentration of 50 mM and stored at 4°C.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT)
was purchased from Sigma-Aldrich (St. Louis, MO, USA). Dulbecco's
modified Eagle's medium (DMEM)/F-12 (1:1) and
penicillin/streptomycin were purchased from Invitrogen Life
Technologies (Carlsbad, CA, USA). Fetal bovine serum (FBS) was
purchased from Aleken Biologicals (Nash, TX, USA). The fluorogenic
peptide substrate Suc-LLVY-AMC (for the proteasomal CT-like
activity) was obtained from Calbiochem (San Diego, CA, USA). Mouse
monoclonal antibody against human poly(ADP-ribose) polymerase
(PARP), mouse monoclonal antibodies against ubiquitin (P4D1) and
IκB-α (H-4), goat polyclonal antibody against β-actin (C-11) and
all secondary antibodies were purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA).
Cell culture and whole cell extract
preparation
MDA-MB-231 human breast cancer cells were grown in
DMEM/F-12 (1:1) supplemented with 10% FBS, 100 U/ml of penicillin
and 100 µg/ml of streptomycin. MCF-10A cells (immortalized,
but non-tumorigenic) were cultured in 1:1 DMEM/F-12 supplemented
with 5% (v/v) horse serum, 0.029 mol/l sodium bicarbonate, 10 mM
HEPES buffer solution, 100 U/ml of penicillin, 5 mg insulin, 10
µg of epidermal growth factor and 250 µg
hydrocortisone. The cell lines were maintained at 37°C in an
atmosphere containing 5% CO2. The whole cell extracts
were prepared as previously described (38). Briefly, the cells were harvested,
lysed and the supernatants were collected as whole-cell extracts
which were used for western blot analysis.
Cell proliferation assays
The effect of each FEP-metal complex on cell
proliferation was determined by MTT assay. MDA-MB-231 and MCF-10A
cells were seeded in triplicate in a 96-well plate, grown to 70–80%
confluence, and then treated with the indicated concentration of
each FEP-metal complex. After 48 h of incubation at 37°C, the
inhibition of cell proliferation was measured as previously
described (38).
Inhibition of purified 20S proteasome
activity by the FEP-Mn complex
The CT-like activity of the purified human 20S
proteasome was measured as previously described (32). Briefly, 35 ng of purified 20S
proteasome were incubated in 100 µl of assay buffer (20 mM
Tris-HCl, pH 7.5) with or without various concentrations of the
FEP-Mn complex and 20 µM of CT-like substrate Suc-LLVY-AMC
(for the proteasomal CT-like activity) for 2 h at 37°C. Following
incubation, proteasome CT-like activity was measured using the
Wallac Victor 3 multilabel counter with an excitation filter of 365
nm and emission filter of 460 nm.
Analysis of proteasomal CT-like activity
in whole-cell extracts
Whole-cell extracts (10 µg) of MDA-MB-231
breast cancer cells were incubated for 2 h at 37°C in 100 µl
of assay buffer (20 mmol/l Tris-HCl, pH 7.5) with 20 µmol/l
fluorogenic peptide substrate Suc-LLVY-AMC (for the proteasomal
CT-like activity), followed by measurement of AMC group release, as
described above.
Western blot analysis
Breast cancer cells were treated with the different
complexes as indicated in the figure legends. Cells treated with
DMSO served as the controls. After 24 h of treatment, the cells
were harvested and lysed. Protein (40 µg) from whole-cell
extracts was separated by sodium dodecyl sulfate polyacrylamide gel
electrophoresis (SDS-PAGE) and transferred onto a nitrocellulose
membrane. Western blot analysis was performed using specific
antibodies to ubiquitin, IκB-α, PARP, and β-actin, followed by
visualization with an enhanced chemiluminescence reagent, as
previously described (39).
Analysis of cell morphology
Changes in cell morphology were observed using a
Zeiss Axiovert 25 phase contrast microscope (Zeiss, Oberkochen,
Germany). Rounded and detached cells were considered apoptotic.
Statistical analysis
Statistical analysis was performed using Microsoft
Excel software. Differences between groups were analyzed using the
Student's t-test. All data are presented as the means ± SD.
Results
FEP forms new complexes with different
metal salts in solution
Several previous studies have reported that many
complexes, such as clioquinol-copper (40), the ternary complexes,
indole-3-acetic acid-copper-phenanthroline (phen) and
indole-3-propionic-copper-phen (36), taurine and L-glutamine Schiff base
copper complexes (41,42), zinc, nickel, cadmium complexes and
so on, are capable of inhibiting cell proliferation and proteasome
activity, and inducing the apoptosis of human cancer cells
(37–43,44). However, to the best of our
knowledge, the effects of Mn(II) complexes on cancer cells have not
been reported to date. In the current study, FEP was mixed with
various metal salts, dissolved in DMSO at a concentration of 50 mM,
in a 1:1 molar ratio. The reaction of FEP with the different metal
salts resulted in a marked change in color, which indicated that a
chemical reaction had occurred and the complexes had formed (data
not shown). In this study, we focused on the FEP-metal complex
solution and investigated the effects of this complex on human
breast cancer cells.
Antiproliferative and
proteasome-inhibitory effects of FEP1-FEP8 in MDA-MB-231 breast
cancer cells
For this portion of the study, the FEP1-FEP8
complexes were investigated for their growth inhibitory effects on
MDA-MB-231 breast cancer cells at the concentration of at 40
µM of each compound for 24 h. Cells treated with DMSO were
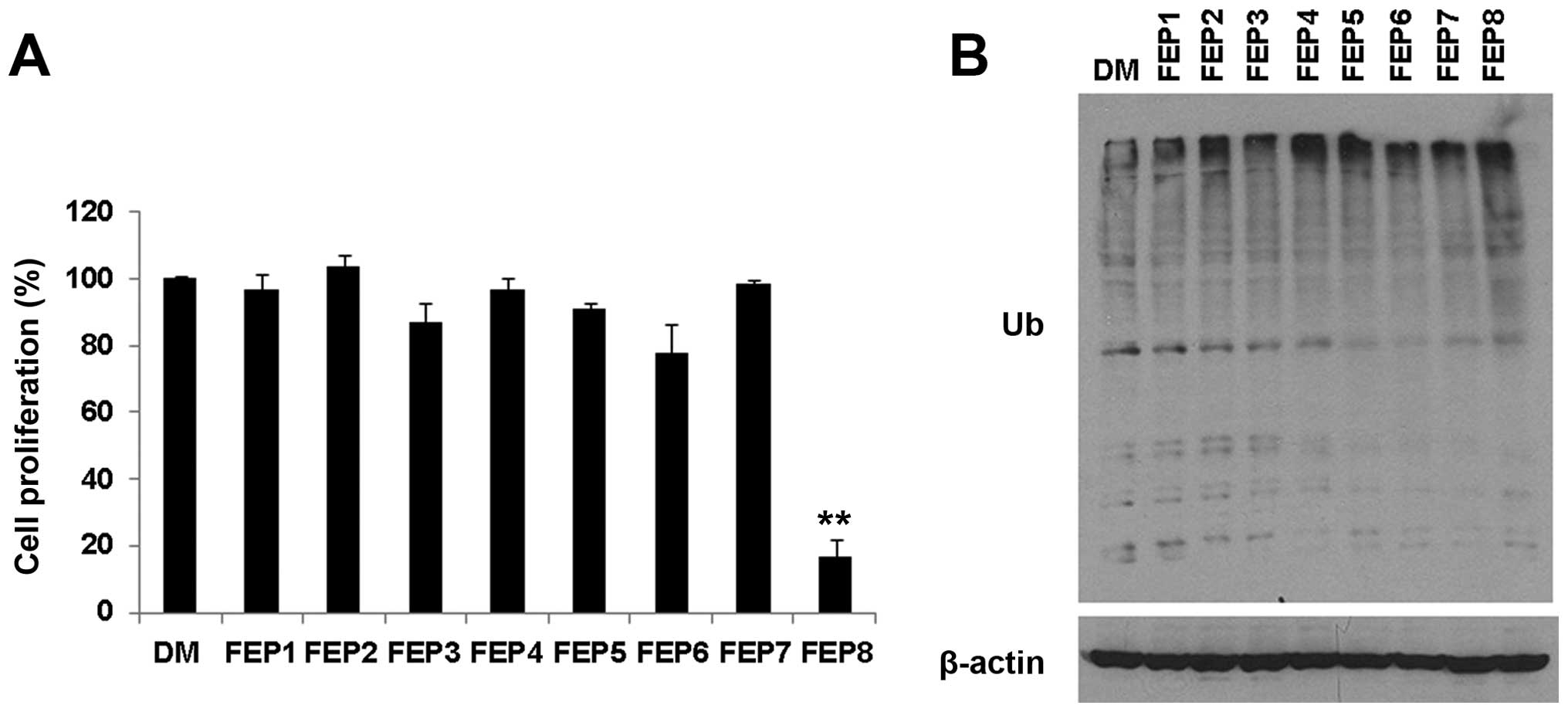
used as a control. Of note, the most effective compound was found
to be FEP8, the FEP-Mn complex, which induced the greatest decrease
in the proliferation of MDA-MB-231 cells, causing a >83% growth
inhibition after 24 h of treatment (Fig. 2A). However, all the other
FEP-metal complexes caused little or no growth inhibition of
MDA-MB-231 breast cancer cells (Fig.
2A).
We then measured the ability of complexes FEP1-FEP8
to inhibit proteasome activity. The MDA-MB-231 cells were treated
with 40 µM of each compound for 24 h and the effect on
accumulated ubiquitinated proteins was assessed. The results of
western blot analysis confirmed that the FEP-Mn complex was a
potent proteasome inhibitor. The accumulation of ubiquitinated
proteins was observed in the cells treated with FEP8, but not in
the cells treated with the other complexes (Fig. 2B). Thus, our results suggest that
FEP8 is more potent in its ability to inhibit cell proliferation
and proteasome activity than the other compounds tested
(FEP1-FEP7).
In our previous studies, we demonstrated that a
number of organic copper- and cadmium-based complexes were capable
of inhibiting the tumor cell proteasome and thus, cell
proliferation, thereby inhibiting cancer cell growth (36,37). It was of interest to determine
whether this was also an effect of the Mn complex.
Inhibition of purified 20S proteasome
activity by FEP8
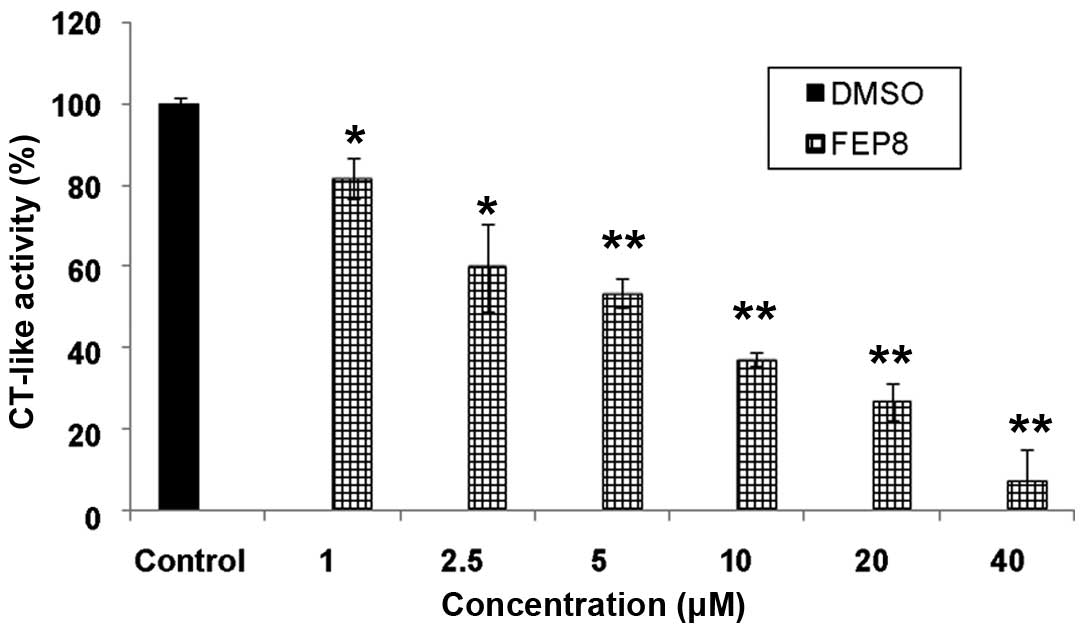
To investigate the effects of the Mn complex, we
also carried out in vitro assays using the purified 20S
proteasome, which was treated with various concentrations of FEP8
and with DMSO as a control. The results revealed that this Mn
complex is capable of inhibiting the CT-like activity of the
purified 20S proteasome (Fig. 3).
This indicated that the FEP-Mn complex targeted the 20S proteasomal
catalytic β5 subunit. This is consistent with the results of our
previous studies (36,37), which demonstrated that copper and
cadmium complexes have the potential to serve as potent, selective
proteasome inhibitors.
Inhibition of CT-like activity and
induction of apoptosis by FEP8 in MDA-MB-231 breast cancer
cells
In the previous section, we have discussed the
inhibition of proteasomal CT-like activity under cell-free
conditions. We then sought to determine whether this effect is dose
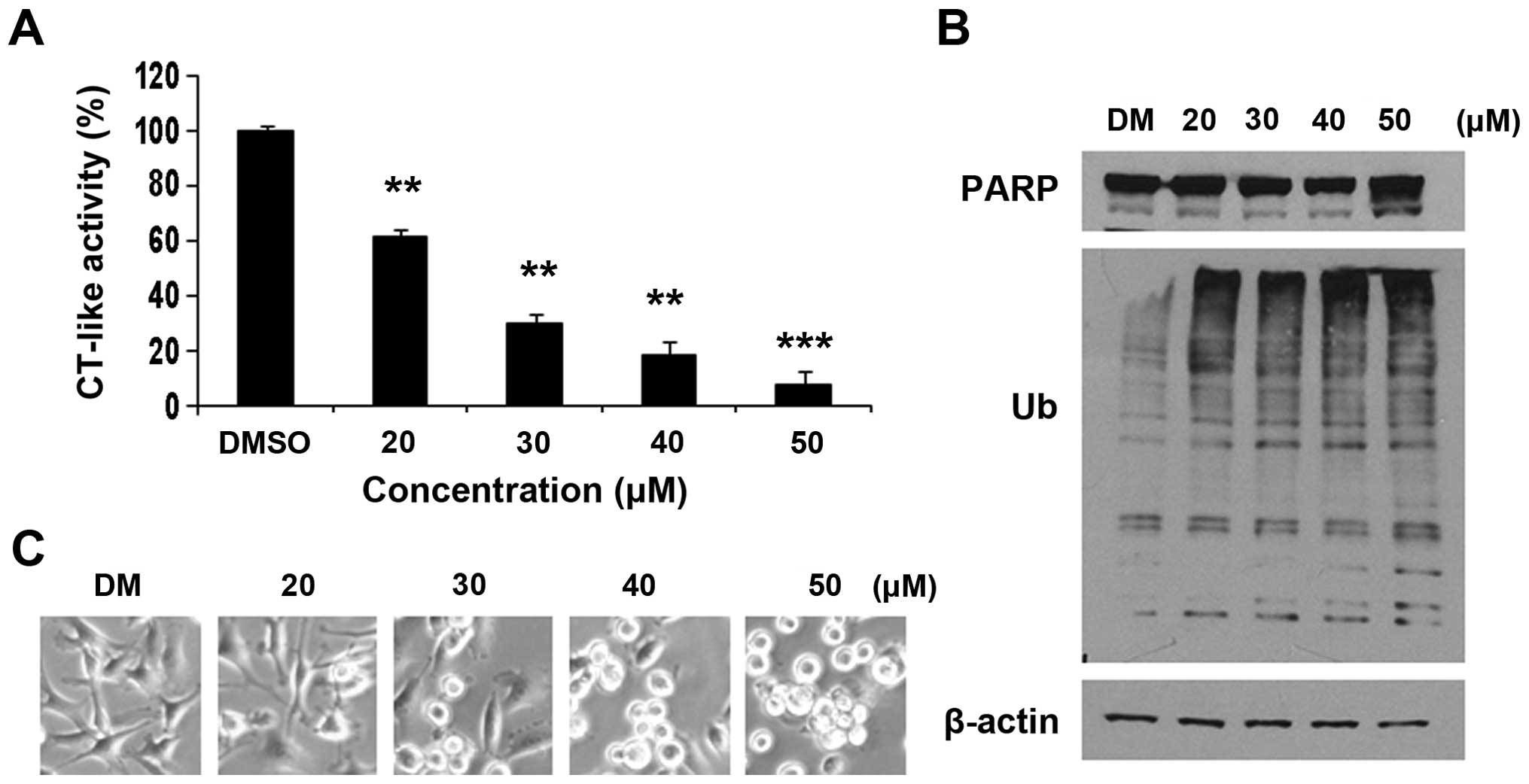
dependent in cancer cells. The MDA-MB-231 cells were treated with
FEP8 at concentrations of 20, 30, 40 and 50 µM. Cells
treated with DMSO were used as a vehicle control. After 24 h of
treatment, the cells were collected and cell extracts were prepared
for the determination of the extent of proteasome inhibition. The
inhibition of cellular proteasome activity was measured as the
decreased levels of CT-like activity and the accumulation of
ubiquitinated proteins, as previously described (45).
The results revealed that FEP8 inhibited the
proteasomal CT-like activity in a dose-dependent manner by
approximately 40% at 20 µM, 70% at 30 µM, 82% at 40
µM and 92% at 50 µM (Fig. 4A). Consistently, the
dose-dependent accumulation of ubiquitinated proteins was also
observed in the MDA-MB-231 breast cancer cells treated with FEP8
(Fig. 4B). It has been reported
that the inhibition of the proteasomal CT-like activity is
associated with the apoptosis of cancer cells (34).
In the same experiment, we then evaluated the
apoptosis-inducing effect of FEP8 in breast cancer cells. The
cleaved p85 fragment of PARP was observed at an FEP8 concentration
of 50 µM (Fig. 4B),
indicating the induction of cell apoptosis. To further investigate
the apoptosis-inducing effect of FEP8, we examined changes in cell
morphology. Treatment with FEP8 induced the rounding and shrinkage
of the MDA-MB-231 cells (Fig. 4C)
in a concentration-dependent manner. These results demonstrated
that FEP8 possesses the ability to inhibit proteasome activity and
is able of inducing the apoptosis of MDA-MB-231 human breast cancer
cells in a dose-dependent manner.
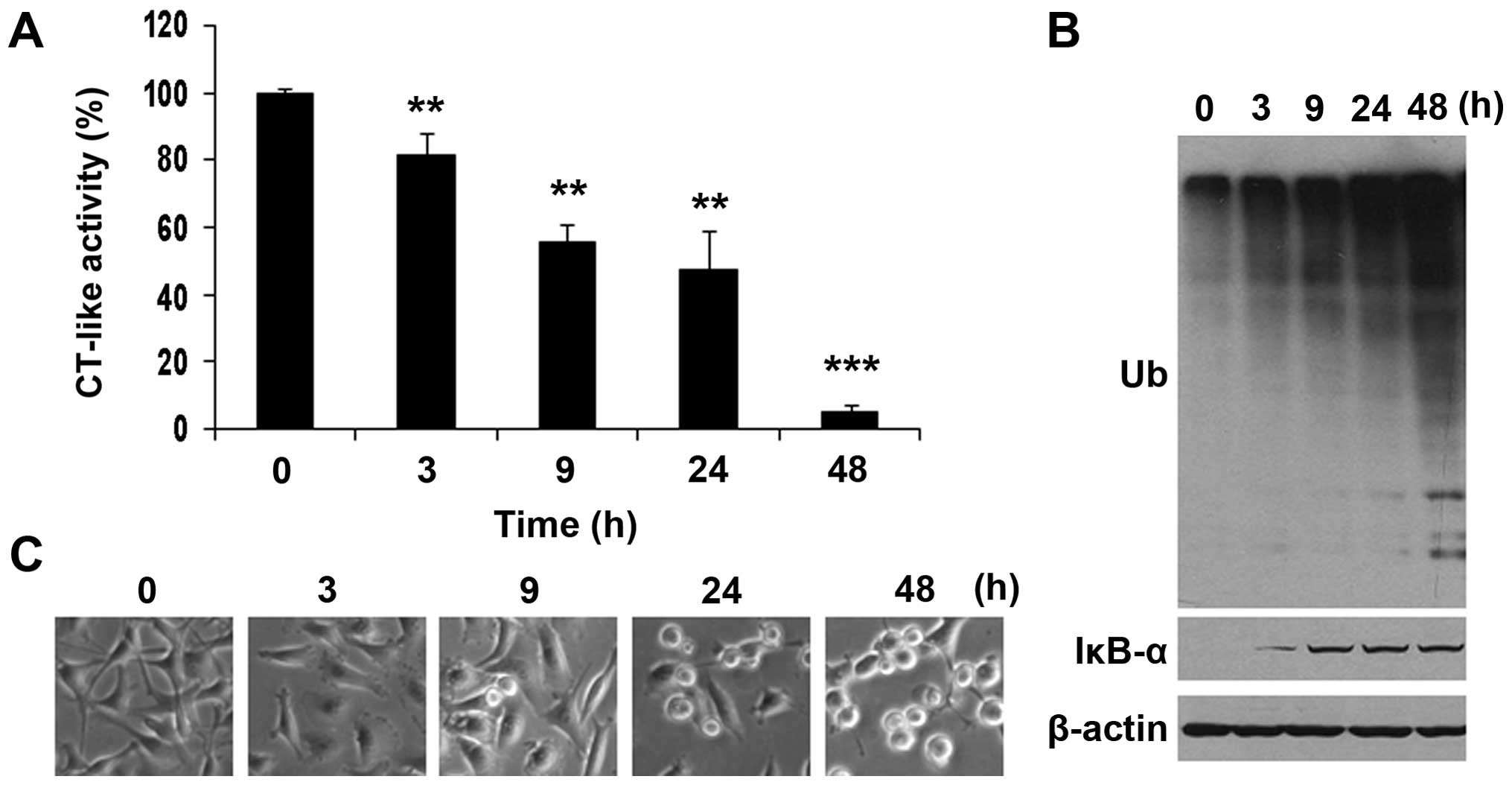
Time-dependet effect of FEP8 on
proteasome inhibition and induction of apoptosis in MDA-MB-231
breast cancer cells
In order to examine the time-dependent effect of
FEP8-induced proteasome inhibition, the MDA-MB-231 cells were
treated with 20 µM of FEP8 for 3, 9, 24 or 48 h
(DMSO-treated cells were used as a vehicle control), followed by
the measurement of proteasome activity (Fig. 5). Proteasomal CT-like activity was
inhibited by FEP8 in a time-dependent manner, as early as 3 h, with
~20% inhibition (Fig. 5A). The
CT-like activity was further decreased at subsequent time-points,
with 45, 55 or 95% inhibition observed after 9, 24 and 48 h,
respectively (Fig. 5A).
Furthermore, the inhibition of proteasomal CT-like activity was
associated with the gradual accumulation of ubiquitinated proteins,
starting at 3 h and peaking at 48 h (Fig. 5B). Additionally, higher levels of
the proteasomal target protein, IκB-α, were also observed at 3 h
post-treatment and later time-points (Fig. 5B). In the same time-dependent
experiment, abnormal morphological changes were not detected until
9 h post-treatment with FEP8. Moreover, the appearance of rounded
and detached cells, indicative of apoptosis, gradually increased as
time progressed (Fig. 5C). These
results clearly indicte that FEP8 induces proteasome inhibition,
followed by the induction of apoptosis in breast cancer cells.
Therefore, the apoptosis induced by FEP8 is a consequence of
proteasome inhibition.
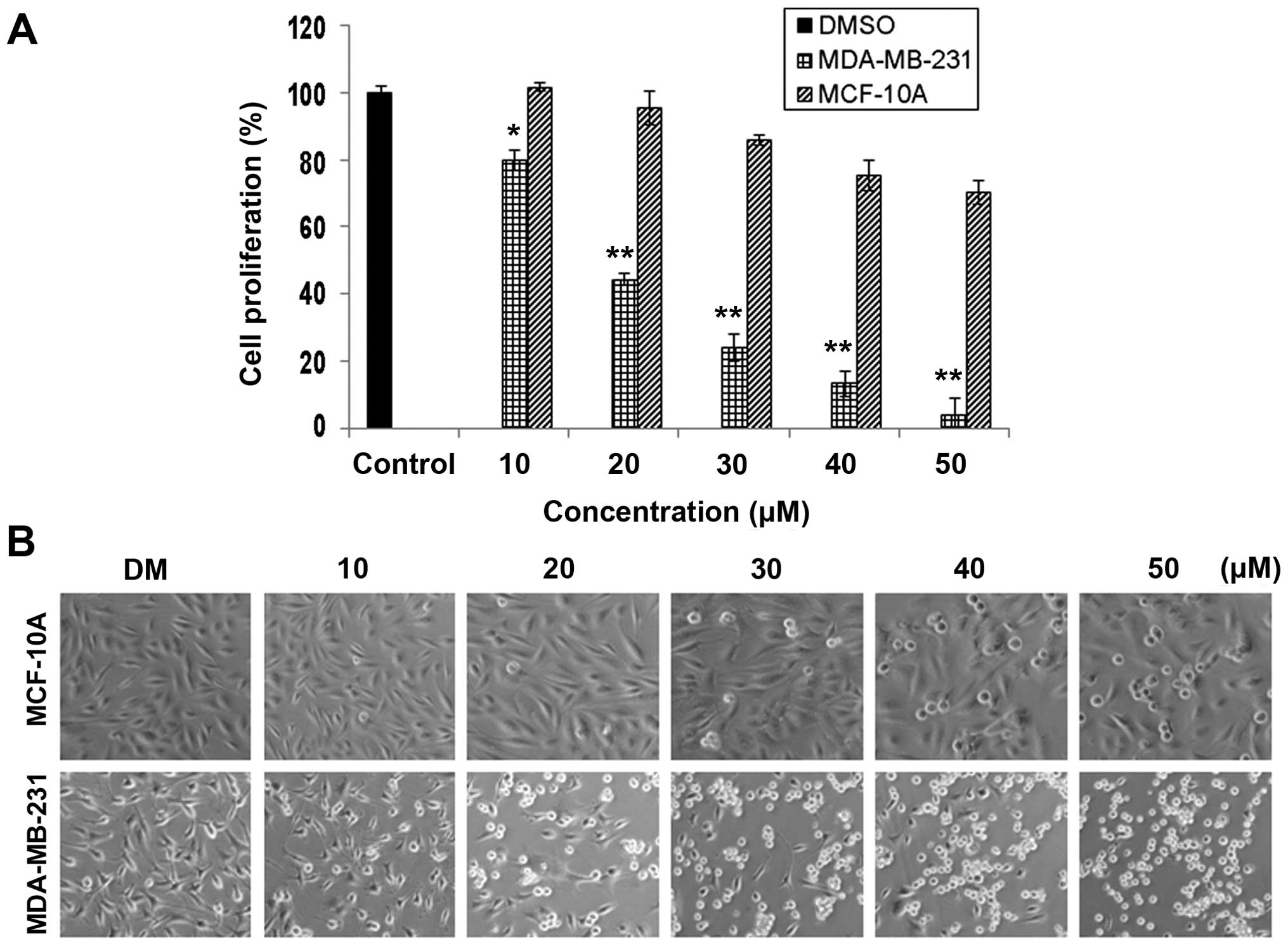
Less toxic effect of FEP8 on
immortalized, non-tumorigenic MCF-10A breast cells compared with
MDA-MB-231 breast cancer cells
It is imperative for effective anticancer drugs to
have the ability to distinguish between normal and cancer cells
(46–48). Thus, to investigate whether FEP8
exhibited selectivity toward cancer cells, but not normal cells, we
used the MDA-MB-231 breast cancer cells and normal immortalized
MCF-10A human breast cells. The two cell lines were treated with
various concentrations of FEP8 for 24 h, and with DMSO as a
control, followed by the measurement of cell proliferation and the
observation of changes in cell morphology. When FEP8 was used at a
concentration of 10 µM, no inhibition of cell proliferation
was observed in the MCF-10A cells, whereas ~20% inhibition of
proliferation was observed in the MDA-MB-231 cells (Fig. 6A). FEP8 exhibited markedly
different effects on cell proliferation when the two cell lines
were treated with FEP8 at concentrations ranging from 20 to 50
µM, causing 56, 76, 87 and 95% growth inhibition of
MDA-MB-231 breast cancer cells and 5, 14, 25 and 30% inhibition of
non-tumorigenic MCF-10A cells, respectively (Fig. 6A). In this regard, it may be
concluded that FEP8 is a potent inhibitor of MDA-MB-231 cell
proliferation, but that it is less toxic to non-tumorigenic MCF-10A
breast cells, thus rendering our novel Mn complexes more favorable
for further preclinical studies.
Additionally, under the same conditions,
morphological changes were meaningful in determining whether FEP8
induces less apoptosis in normal MCF-10A cells compared with
MDA-MB-231 breast cancer cells (Fig.
6B). Shrunken and rounded MDA-MB-231 cells were observed
following treatment with various concentrations of FEP8, whereas
little or nearly no morphological changes were observed in the
MCF-10A cells (Fig. 6B), which
was consistent with the results of MTT assay. Taken together, our
results suggest that FEP8 inhibits cell proliferation and induces
apoptosis selectively in human cancer cells and exerts less toxic
effects on normal immortalized breast cells.
Discussion
Since the discovery of the antitumor activity of
cisplatin and its analogs, novel metal complexes have been studied
extensively in chemotherapy (49). Mn is important in the biochemical
and physiological processes of many living organisms (50). Mn is also highly expressed in some
tumor tissues (51). Based on the
biological function of Mn, many Mn complexes have been reported to
exert anticancer activity in some cancer cell lines (19–22,52,53). Therefore, combining Mn(II) with
several functional molecules has aroused considerable interest for
the development of potent anticancer drugs.
We have previously reported that several organic
copper and cadmium complexes possess the ability to inhibit
proteasome activity and induce apoptosis in human cancer cells
(36,37). However, in the present study, we
found that only the FEP-Mn complex (FEP8) had the ability to
inhibit cell proliferation and proteasome activity (Fig. 2). Therefore, FEP8 was selected for
further study. Further experiments indicated that FEP8 inhibited
the CT-like activity of the purified 20S proteasome (Fig. 3). Moreover, the inhibition of
cellular proteasome activity was measured as the decreased levels
of CT-like activity and the accumulation of ubiquitinated proteins
(Fig. 4). With respect to
proteasome-inhibitory activity, this FEP-Mn complex exhibited high
activity toward not only the purified 20S proteasome, but also the
proteasome from whole-cell extracts. Cellular morphological changes
(shrinkage and rounding) were observed in a concentration-dependent
manner (Fig. 4C). In line with
these observations, FEP8 also induced the cleavage of PARP to form
the p85 fragment (Fig. 4B),
indicative of the induction of cell apoptosis. Thus, these results
suggest that FEP8 has the potential for development as a novel
anticancer agent.
The mechanism of action for the Mn complexes is
suggested as follows: i) a Mn complex interacts with DNA and causes
DNA damage, ultimately blocking the division of cancer cells and
resulting in cell death (19,20); ii) a Mn(II) complex attenuates the
absorption of calcium(II) in the mitochondria and interferes with
the metabolite of O2 formed by
H2O2 or ROS involved signaling, causing the
apoptosis of cancer cells (21–22,52); iii) a Mn complex reduces the
number of tumor-associated myeloid-derived suppressor cells,
leading to the modulation of the immunosuppressive tumor
microenvironment (TME) (53).
However, our investigation revealed that the Mn complex (FEP8)
inhibited 20S proteasome activity and directly inhibited CT-like
activity, which is primarily associated with the β5 active site
(26). In addition, it has been
reported that the decreased proteasomal CT-like activity is
associated with the loss of cancer cell availability (35). In this study, it is interesting to
note that the Mn complex inhibited proteasomal CT-like activity as
early as 3 h (Fig. 5A), which was
consistent with our results demonstrating the accumulation of
ubiquitinated proteins and the target protein, IκB-α (Fig. 5B). In addition, changes to cell
morphology occurred 9 h after treatment began (Fig. 5C). In summary, our findings
indicate that FEP8 has the potential to act as a specific inhibitor
of proteasomal CT-like activity and thus to induce cell
apoptosis.
A significant challenge in the development of novel
anticancer drugs is the safety of metal compounds. Therefore, in
this study, we examined whether FEP8 selectively inhibits cell
proliferation and induces the apoptosis of MDA-MB-231 breast cancer
cells, but not that of normal human MCF-10A breast cells. Indeed,
our results demonstrated that the FEP-Mn complex had little effect
on MCF-10A cells in contrast to its effect on MDA-MB-231 breast
cancer cells (Fig. 6). We noted
that the FEP-Mn complex is a potent inhibitor of cell
proliferation, which is specific to MDA-MB-231 breast cancer cells
(Fig. 6A). Also noted was the
fact that the MDA-MB-231 cells became detached and rounded when
treated with the FEP-Mn complex and almost no toxicity was observed
in the MCF-10A cells (Fig.
6B).
In conclusion, the data presented herein suggest
that the FEP-Mn complex is a ligand could act as a potent
proteasome inhibitor and inducer of apoptosis, and may be a
potential anticancer agent targeting breast cancer cells, rather
than normal cells. Therefore, this FEP-Mn complex has great
potential for development into a drug for cancer treatment.
However, further biological analysis and preclinical testing are
warranted using animal tumor models.
Acknowledgments
The authors thank Mr. Maocai Yan for his critical
reading of the manuscript. This study was supported by grants from
the National Science Foundation of China (no. 21371161 to C.B.) and
the Doctoral Foundation of Jining Medical University (no. JY14QD06
to Z.Z).
References
|
1
|
Cunha BA and Gill MV: Cefepime. Med Clin
North Am. 79:721–732. 1995.PubMed/NCBI
|
|
2
|
Jándula BM, Martino R, Gurgi M, Manteiga R
and Sierra J: Treatment of febrile neutropenia with cefepime
monotherapy. Chemotherapy. 47:226–231. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Raad II, Escalante C, Hachem RY, Hanna HA,
Husni R, Afif C, Boktour MR, Whimbey EE, Kontoyiannis D, Jacobson
K, et al: Treatment of febrile neutropenic patients with cancer who
require hospitalization: a prospective randomized study comparing
imipenem and cefepime. Cancer. 98:1039–1047. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh
MJ, Ito JI, Mullen CA, Raad II, Rolston KV, Young J-AH and Wingard
JR: Clinical practice guideline for the use of antimicrobial agents
in neutropenic patients with cancer: 2010 update by the Infectious
Diseases Society of America. Clin Infect Dis. 52:56–93. 2011.
View Article : Google Scholar
|
|
5
|
Saito H, Takahashi K, Okuno M, Saka H,
Imaizumi K, Hasegawa Y, Tanikawa Y, Yamamoto M, Taniguchi H,
Shindoh J, et al Central Japan Lung Study Group: Cefepime
monotherapy for febrile neutropenia in patients with lung cancer. J
Infect Chemother. 20:365–369. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Reiss A, Chifiriuc MC, Amzoiu E and Spînu
CI: Transition metal (II) complexes with cefotaxime-derived Schiff
base: Synthesis, characterization, and antimicrobial studies.
Bioinorg Chem Appl. 2014:9262872014. View Article : Google Scholar
|
|
7
|
Anacona JR and Estacio J: Synthesis and
antibacterial activity of cefixime metal complexes. Transit Metab
Chem. 31:227–231. 2006. View Article : Google Scholar
|
|
8
|
Anacona JR and Lopez M: Mixed-ligand
nickel (II) complexes containing sulfathiazole and cephalosporin
antibiotics: Synthesis, characterization, and antibacterial
activity. Int J Inorg Chem. 2012:1–8. 2012. View Article : Google Scholar
|
|
9
|
Anacona JR and Patino C:
Metalloantibiotics: synthesis and antibacterial activity of
ceftazidime metal complexes. J Coord Chem. 62:613–621. 2009.
View Article : Google Scholar
|
|
10
|
Ali AE: Synthesis, spectral, thermal and
antimicrobial studies of some new tri metallic biologically active
ceftriaxone complexes. Spectrochim Acta A Mol Biomol Spectrosc.
78:224–230. 2011. View Article : Google Scholar
|
|
11
|
Sultana N, Arayne MS and Afzal M:
Synthesis and antibacterial activity of cephradine metal complexes:
part II complexes with cobalt, copper, zinc and cadmium. Pak J
Pharm Sci. 18:36–42. 2005.
|
|
12
|
Anacona JR and Rodriguez H:
Metalloantibiotics: synthesis and antibacterial activity of
cefepime metal complexes. J Coord Chem. 62:2212–2219. 2009.
View Article : Google Scholar
|
|
13
|
Guo Z and Sadler SP: Metals in medicine.
Angew Chem Int. 38:1512–1531. 1999. View Article : Google Scholar
|
|
14
|
Orvig C and Abrams MJ: Medicinal inorganic
chemistry: Introduction. Chem Rev. 99:2201–2204. 1999. View Article : Google Scholar
|
|
15
|
Gasser G, Ott I and Metzler-Nolte N:
Organometallic anticancer compounds. J Med Chem. 54:3–25. 2011.
View Article : Google Scholar :
|
|
16
|
Faulkner KM, Liochev SI and Fridovich I:
Stable Mn(III) porphyrins mimic superoxide dismutase in vitro and
substitute for it in vivo. J Biol Chem. 269:23471–23476.
1994.PubMed/NCBI
|
|
17
|
Szabó C, Day BJ and Salzman AL: Evaluation
of the relative contribution of nitric oxide and peroxynitrite to
the suppression of mitochondrial respiration in immunostimulated
macrophages using a manganese mesoporphyrin superoxide dismutase
mimetic and peroxynitrite scavenger. FEBS Lett. 381:82–86. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Oliveira CG, da S Maia PI, Souza PC, Pavan
FR, Leite CQ, Viana RB, Batista AA, Nascimento OR and Deflon VM:
Manganese(II) complexes with thiosemicarbazones as potential
anti-Mycobacterium tuberculosis agents. J Inorg Biochem. 132:21–29.
2014. View Article : Google Scholar
|
|
19
|
Xu ZD, Liu H, Wang M, Xiao SL, Yang M and
Bu XH: Manganese(II) complex of 6,7-dicycanodipyridoquinoxaline
with antitumor activities: synthesis, crystal structure and binding
with DNA. J Inorg Biochem. 92:149–155. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu ZD, Liu H, Xiao SL, Yang M and Bu XH:
Synthesis, crystal structure, antitumor activity and DNA-binding
study on the Mn(II) complex of
2H-5-hydroxy-1,2,5-oxadiazo[3,4-f]1,10-phenanthroline. J Inorg
Biochem. 90:79–84. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qiu-Yun C, Dong-Fang Z, Juan H, Wen-Jie G
and Jing G: Synthesis, anticancer activities, interaction with DNA
and mitochondria of manganese complexes. J Inorg Biochem.
104:1141–1147. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang ZW, Chen QY and Liu QS: Manganese
(II) complexes of quinoline derivatives: characterization, catalase
activity, interaction with mitochondria and anticancer activity.
Transit Metab Chem. 39:917–924. 2014. View Article : Google Scholar
|
|
23
|
Barbara MO, Mariusz K, Ewa RS, Krystyna
GK, Beata FP, Joanna W and Danuta M: Crystal structure, infrared
and EPR spectra and anticancer activity in vitro of the novel
manganese (II) complexes of indolecarboxylic acids. Polyhedron.
67:464–470. 2014. View Article : Google Scholar
|
|
24
|
Nalepa G, Rolfe M and Harper JW: Drug
discovery in the ubiquitin-proteasome system. Nat Rev Drug Discov.
5:596–613. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Orlowski RZ and Dees EC: The role of the
ubiquitination-proteasome pathway in breast cancer: applying drugs
that affect the ubiquitin-proteasome pathway to the therapy of
breast cancer. Breast Cancer Res. 5:1–7. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Seemüller E, Lupas A, Stock D, Löwe J,
Huber R and Baumeister W: Proteasome from Thermoplasma acidophilum:
a threonine protease. Science. 268:579–582. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Glickman MH and Ciechanover A: The
ubiquitin-proteasome proteolytic pathway: Destruction for the sake
of construction. Physiol Rev. 82:373–428. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gallastegui N and Groll M: The 26S
proteasome: assembly and function of a destructive machine. Trends
Biochem Sci. 35:634–642. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kisselev AF and Goldberg AL: Proteasome
inhibitors: from research tools to drug candidates. Chem Biol.
8:739–758. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Adams J, Palombella VJ, Sausville EA,
Johnson J, Destree A, Lazarus DD, Maas J, Pien CS, Prakash S and
Elliott PJ: Proteasome inhibitors: a novel class of potent and
effective antitumor agents. Cancer Res. 59:2615–2622.
1999.PubMed/NCBI
|
|
31
|
Shinohara K, Tomioka M, Nakano H, Toné S,
Ito H and Kawashima S: Apoptosis induction resulting from
proteasome inhibition. Biochem J. 317:385–388. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Daniel KG, Gupta P, Harbach RH, Guida WC
and Dou QP: Organic copper complexes as a new class of proteasome
inhibitors and apoptosis inducers in human cancer cells. Biochem
Pharmacol. 67:1139–1151. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
An B, Goldfarb RH, Siman R and Dou QP:
Novel dipeptidyl proteasome inhibitors overcome Bcl-2 protective
function and selectively accumulate the cyclin-dependent kinase
inhibitor p27 and induce apoptosis in transformed, but not normal,
human fibroblasts. Cell Death Differ. 5:1062–1075. 1998. View Article : Google Scholar
|
|
34
|
Lopes UG, Erhardt P, Yao R and Cooper GM:
p53-dependent induction of apoptosis by proteasome inhibitors. J
Biol Chem. 272:12893–12896. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Verani CN: Metal complexes as inhibitors
of the 26S proteasome in tumor cells. J Inorg Biochem. 106:59–67.
2012. View Article : Google Scholar
|
|
36
|
Zhang Z, Bi C, Schmitt SM, Fan Y, Dong L,
Zuo J and Dou QP: 1,10-Phenanthroline promotes copper complexes
into tumor cells and induces apoptosis by inhibiting the proteasome
activity. J Biol Inorg Chem. 17:1257–1267. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang Z, Bi C, Buac D, Fan Y, Zhang X, Zuo
J, Zhang P, Zhang N, Dong L and Dou QP: Organic cadmium complexes
as proteasome inhibitors and apoptosis inducers in human breast
cancer cells. J Inorg Biochem. 123:1–10. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Daniel KG, Chen D, Orlu S, Cui QC, Miller
FR and Dou QP: Clioquinol and pyrrolidine dithiocarbamate complex
with copper to form proteasome inhibitors and apoptosis inducers in
human breast cancer cells. Breast Cancer Res. 7:R897–R908. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen D, Peng F, Cui QC, Daniel KG, Orlu S,
Liu J and Dou QP: Inhibition of prostate cancer cellular proteasome
activity by a pyrrolidine dithiocarbamate-copper complex is
associated with suppression of proliferation and induction of
apoptosis. Front Biosci. 10:2932–2939. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen D, Cui QC, Yang H, Barrea RA, Sarkar
FH, Sheng S, Yan B, Reddy GPV and Dou QP: Clioquinol, a therapeutic
agent for Alzheimer's disease, has proteasome-inhibitory, androgen
receptor-suppressing, apoptosis-inducing, and antitumor activities
in human prostate cancer cells and xenografts. Cancer Res.
67:1636–1644. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xiao Y, Bi C, Fan Y, Cui C, Zhang X and
Dou QP: L-glutamine Schiff base copper complex as a proteasome
inhibitor and an apoptosis inducer in human cancer cells. Int J
Oncol. 33:1073–1079. 2008.PubMed/NCBI
|
|
42
|
Zhang X, Bi C, Fan Y, Cui Q, Chen D, Xiao
Y and Dou QP: Induction of tumor cell apoptosis by taurine Schiff
base copper complex is associated with the inhibition of
proteasomal activity. Int J Mol Med. 22:677–682. 2008.PubMed/NCBI
|
|
43
|
Li L, Yang H, Chen D, Cui C and Dou QP:
Disulfiram promotes the conversion of carcinogenic cadmium to a
proteasome inhibitor with pro-apoptotic activity in human cancer
cells. Toxicol Appl Pharmacol. 229:206–214. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cvek B, Milacic V, Taraba J and Dou QP:
Ni(II), Cu(II), and Zn(II) diethyldithiocarbamate complexes show
various activities against the proteasome in breast cancer cells. J
Med Chem. 51:6256–6258. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li B and Dou QP: Bax degradation by the
ubiquitin/proteasome-dependent pathway: Involvement in tumor
survival and progression. Proc Natl Acad Sci USA. 97:3850–3855.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Dou QP and Li B: Proteasome inhibitors as
potential novel anticancer agents. Drug Resist Updat. 2:215–223.
1999. View Article : Google Scholar
|
|
47
|
Goldberg AL: Functions of the proteasome:
the lysis at the end of the tunnel. Science. 268:522–523. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hindo SS, Frezza M, Tomco D, Heeg MJ,
Hryhorczuk L, McGarvey BR, Dou QP and Verani CN: metals in
anticancer therapy: Copper(II) complexes as inhibitors of the 20S
proteasome. Eur J Med Chem. 44:4353–4361. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chen D, Milacic V, Frezza M and Dou QP:
Metal complexes, their cellular targets and potential for cancer
therapy. Curr Pharm Des. 15:777–791. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Li X, Zhao K, Guo W, Liu X, Liu J, Gao J,
Chen Q and Bai Y: A novel manganese complex LMnAc selectively kills
cancer cells by induction of ROS-triggered and
mitochondrial-mediated cell death. Sci China Life Sci. 57:998–1010.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Aschner M, Ruilarte TR, Schneider JS and
Zheng W: Manganese: recent advances in understanding its transport
and neurotoxicity. Toxicol Appl Pharmacol. 221:131–147. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhou DF, Chen QY, Qi Y, Fu HJ, Li Z, Zhao
KD and Gao J: Anticancer activity, attenuation on the absorption of
calcium in mitochondria, and catalase activity for manganese
complexes of N-substituted di(picolyl)amine. Inorg Chem.
50:6929–6937. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Das S, Banerjee K, Roy S, Majumder S,
Chatterjee M, Majumdar S and Choudhuri SK: Mn complex-mediated
enhancement of antitumor response through modulating
myeloid-derived suppressor cells in drug-resistant tumor. In Vivo.
28:909–918. 2014.PubMed/NCBI
|