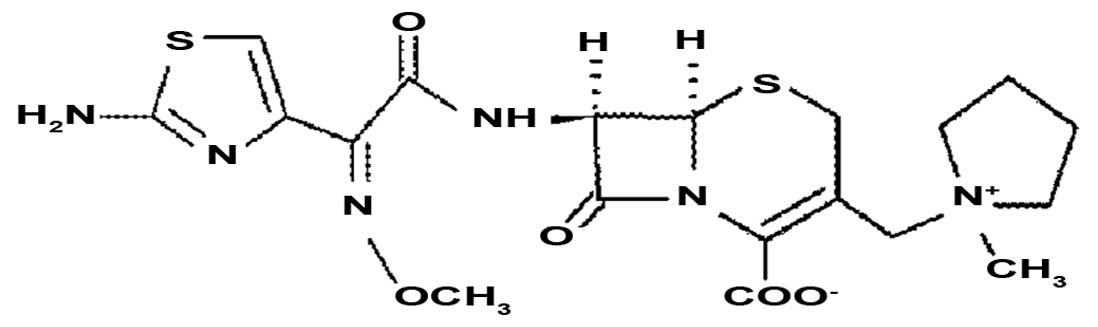
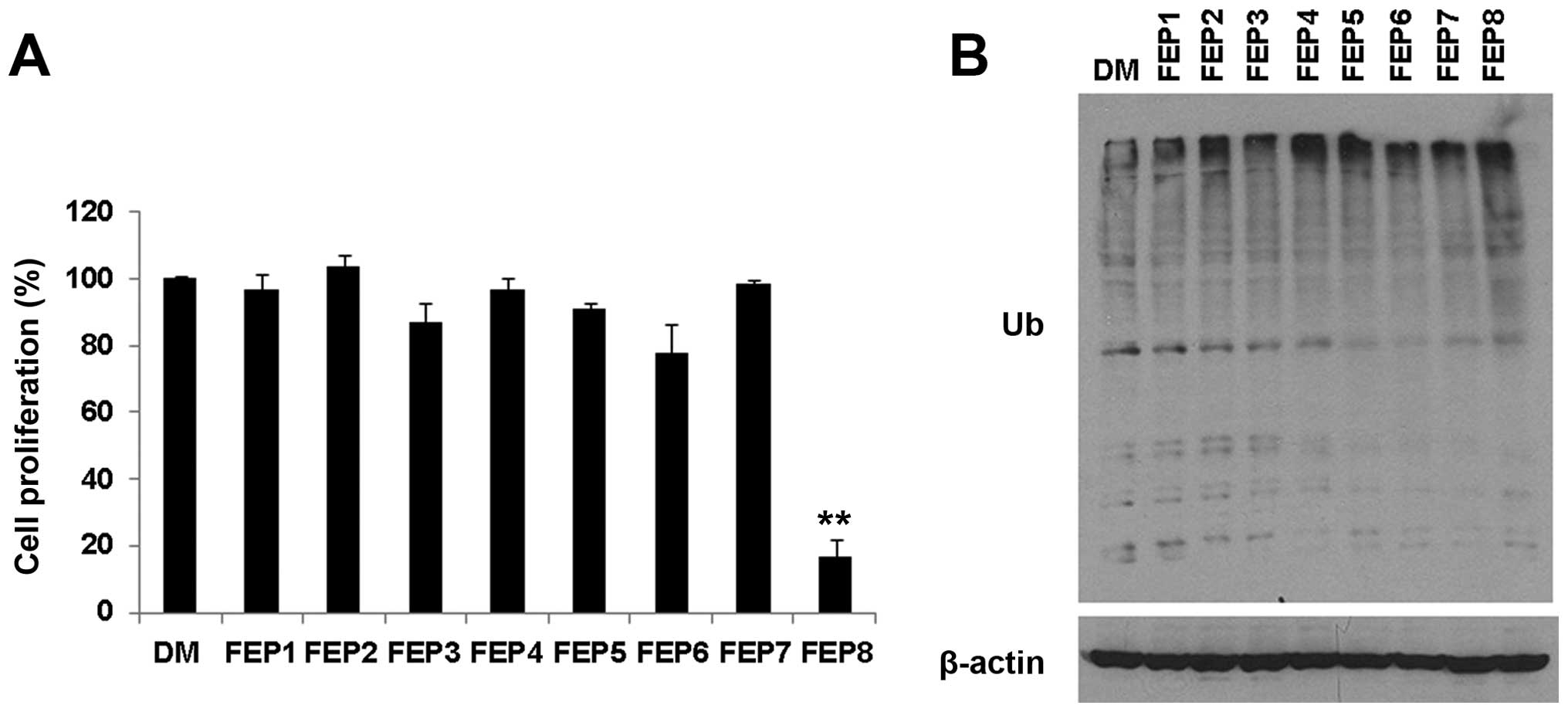
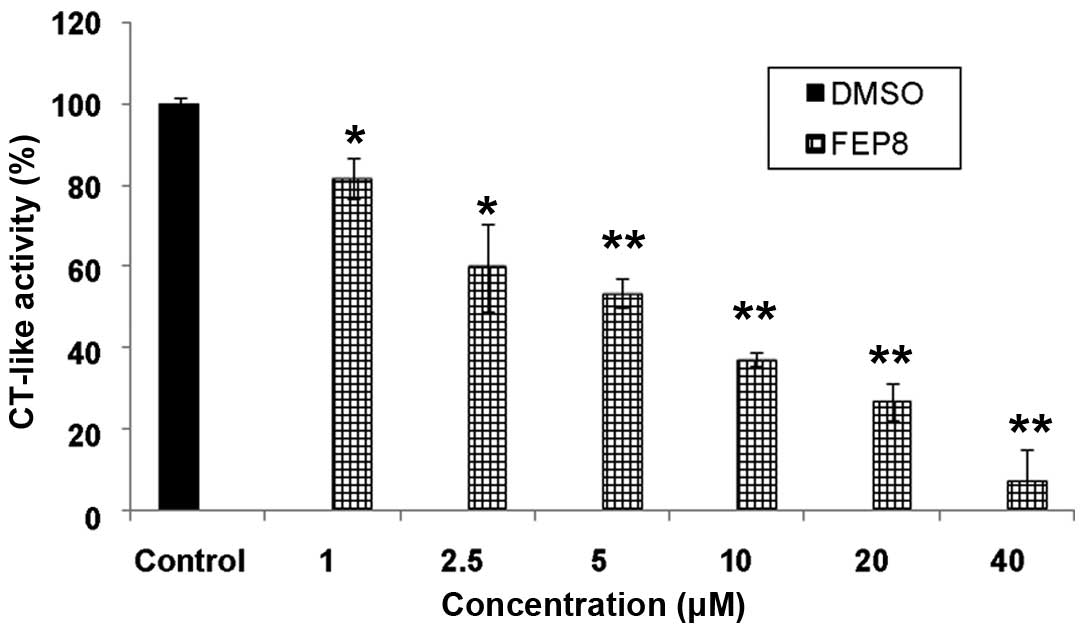
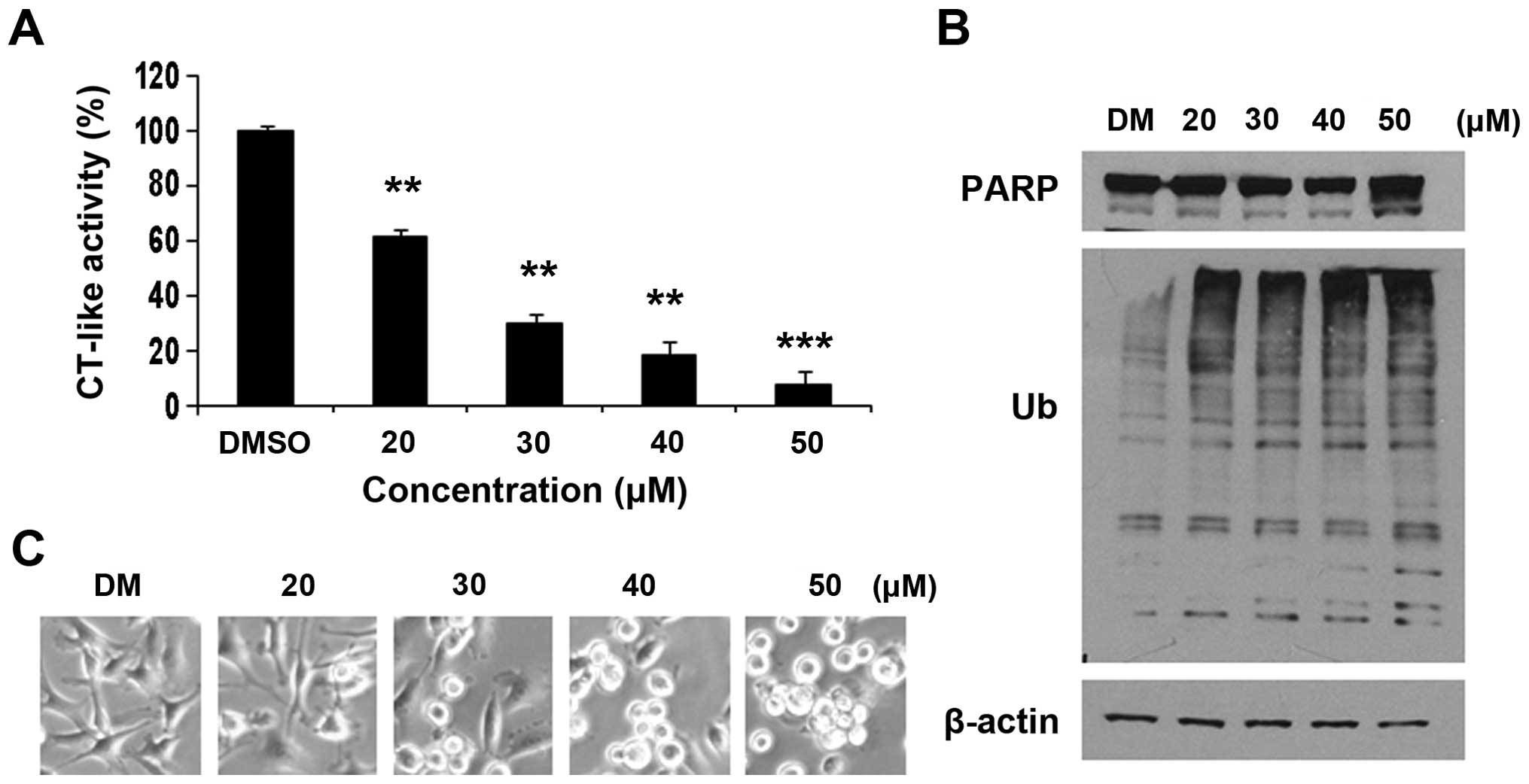
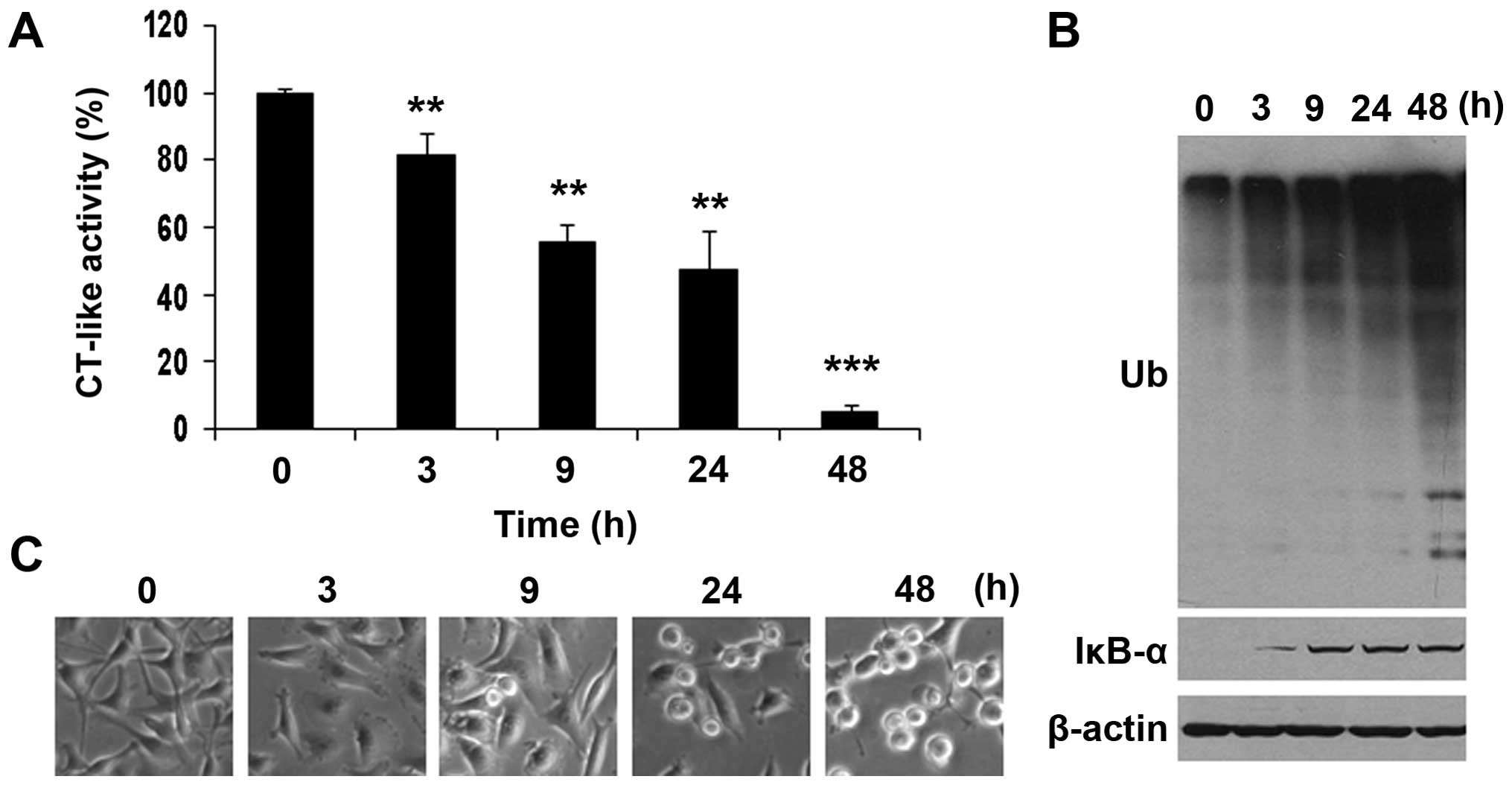
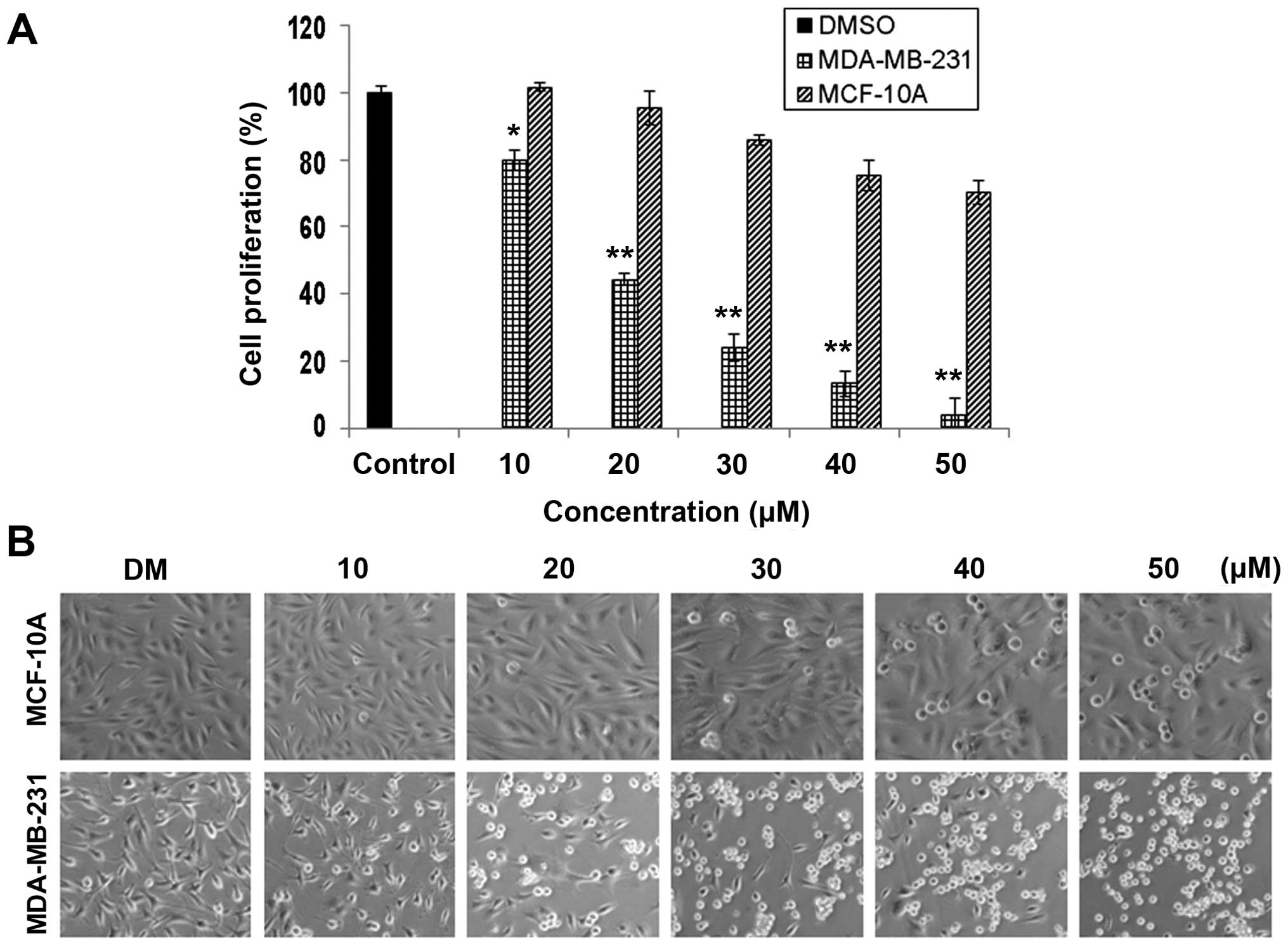
|
1
|
Cunha BA and Gill MV: Cefepime. Med Clin
North Am. 79:721–732. 1995.PubMed/NCBI
|
|
2
|
Jándula BM, Martino R, Gurgi M, Manteiga R
and Sierra J: Treatment of febrile neutropenia with cefepime
monotherapy. Chemotherapy. 47:226–231. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Raad II, Escalante C, Hachem RY, Hanna HA,
Husni R, Afif C, Boktour MR, Whimbey EE, Kontoyiannis D, Jacobson
K, et al: Treatment of febrile neutropenic patients with cancer who
require hospitalization: a prospective randomized study comparing
imipenem and cefepime. Cancer. 98:1039–1047. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh
MJ, Ito JI, Mullen CA, Raad II, Rolston KV, Young J-AH and Wingard
JR: Clinical practice guideline for the use of antimicrobial agents
in neutropenic patients with cancer: 2010 update by the Infectious
Diseases Society of America. Clin Infect Dis. 52:56–93. 2011.
View Article : Google Scholar
|
|
5
|
Saito H, Takahashi K, Okuno M, Saka H,
Imaizumi K, Hasegawa Y, Tanikawa Y, Yamamoto M, Taniguchi H,
Shindoh J, et al Central Japan Lung Study Group: Cefepime
monotherapy for febrile neutropenia in patients with lung cancer. J
Infect Chemother. 20:365–369. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Reiss A, Chifiriuc MC, Amzoiu E and Spînu
CI: Transition metal (II) complexes with cefotaxime-derived Schiff
base: Synthesis, characterization, and antimicrobial studies.
Bioinorg Chem Appl. 2014:9262872014. View Article : Google Scholar
|
|
7
|
Anacona JR and Estacio J: Synthesis and
antibacterial activity of cefixime metal complexes. Transit Metab
Chem. 31:227–231. 2006. View Article : Google Scholar
|
|
8
|
Anacona JR and Lopez M: Mixed-ligand
nickel (II) complexes containing sulfathiazole and cephalosporin
antibiotics: Synthesis, characterization, and antibacterial
activity. Int J Inorg Chem. 2012:1–8. 2012. View Article : Google Scholar
|
|
9
|
Anacona JR and Patino C:
Metalloantibiotics: synthesis and antibacterial activity of
ceftazidime metal complexes. J Coord Chem. 62:613–621. 2009.
View Article : Google Scholar
|
|
10
|
Ali AE: Synthesis, spectral, thermal and
antimicrobial studies of some new tri metallic biologically active
ceftriaxone complexes. Spectrochim Acta A Mol Biomol Spectrosc.
78:224–230. 2011. View Article : Google Scholar
|
|
11
|
Sultana N, Arayne MS and Afzal M:
Synthesis and antibacterial activity of cephradine metal complexes:
part II complexes with cobalt, copper, zinc and cadmium. Pak J
Pharm Sci. 18:36–42. 2005.
|
|
12
|
Anacona JR and Rodriguez H:
Metalloantibiotics: synthesis and antibacterial activity of
cefepime metal complexes. J Coord Chem. 62:2212–2219. 2009.
View Article : Google Scholar
|
|
13
|
Guo Z and Sadler SP: Metals in medicine.
Angew Chem Int. 38:1512–1531. 1999. View Article : Google Scholar
|
|
14
|
Orvig C and Abrams MJ: Medicinal inorganic
chemistry: Introduction. Chem Rev. 99:2201–2204. 1999. View Article : Google Scholar
|
|
15
|
Gasser G, Ott I and Metzler-Nolte N:
Organometallic anticancer compounds. J Med Chem. 54:3–25. 2011.
View Article : Google Scholar :
|
|
16
|
Faulkner KM, Liochev SI and Fridovich I:
Stable Mn(III) porphyrins mimic superoxide dismutase in vitro and
substitute for it in vivo. J Biol Chem. 269:23471–23476.
1994.PubMed/NCBI
|
|
17
|
Szabó C, Day BJ and Salzman AL: Evaluation
of the relative contribution of nitric oxide and peroxynitrite to
the suppression of mitochondrial respiration in immunostimulated
macrophages using a manganese mesoporphyrin superoxide dismutase
mimetic and peroxynitrite scavenger. FEBS Lett. 381:82–86. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Oliveira CG, da S Maia PI, Souza PC, Pavan
FR, Leite CQ, Viana RB, Batista AA, Nascimento OR and Deflon VM:
Manganese(II) complexes with thiosemicarbazones as potential
anti-Mycobacterium tuberculosis agents. J Inorg Biochem. 132:21–29.
2014. View Article : Google Scholar
|
|
19
|
Xu ZD, Liu H, Wang M, Xiao SL, Yang M and
Bu XH: Manganese(II) complex of 6,7-dicycanodipyridoquinoxaline
with antitumor activities: synthesis, crystal structure and binding
with DNA. J Inorg Biochem. 92:149–155. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu ZD, Liu H, Xiao SL, Yang M and Bu XH:
Synthesis, crystal structure, antitumor activity and DNA-binding
study on the Mn(II) complex of
2H-5-hydroxy-1,2,5-oxadiazo[3,4-f]1,10-phenanthroline. J Inorg
Biochem. 90:79–84. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qiu-Yun C, Dong-Fang Z, Juan H, Wen-Jie G
and Jing G: Synthesis, anticancer activities, interaction with DNA
and mitochondria of manganese complexes. J Inorg Biochem.
104:1141–1147. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang ZW, Chen QY and Liu QS: Manganese
(II) complexes of quinoline derivatives: characterization, catalase
activity, interaction with mitochondria and anticancer activity.
Transit Metab Chem. 39:917–924. 2014. View Article : Google Scholar
|
|
23
|
Barbara MO, Mariusz K, Ewa RS, Krystyna
GK, Beata FP, Joanna W and Danuta M: Crystal structure, infrared
and EPR spectra and anticancer activity in vitro of the novel
manganese (II) complexes of indolecarboxylic acids. Polyhedron.
67:464–470. 2014. View Article : Google Scholar
|
|
24
|
Nalepa G, Rolfe M and Harper JW: Drug
discovery in the ubiquitin-proteasome system. Nat Rev Drug Discov.
5:596–613. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Orlowski RZ and Dees EC: The role of the
ubiquitination-proteasome pathway in breast cancer: applying drugs
that affect the ubiquitin-proteasome pathway to the therapy of
breast cancer. Breast Cancer Res. 5:1–7. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Seemüller E, Lupas A, Stock D, Löwe J,
Huber R and Baumeister W: Proteasome from Thermoplasma acidophilum:
a threonine protease. Science. 268:579–582. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Glickman MH and Ciechanover A: The
ubiquitin-proteasome proteolytic pathway: Destruction for the sake
of construction. Physiol Rev. 82:373–428. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gallastegui N and Groll M: The 26S
proteasome: assembly and function of a destructive machine. Trends
Biochem Sci. 35:634–642. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kisselev AF and Goldberg AL: Proteasome
inhibitors: from research tools to drug candidates. Chem Biol.
8:739–758. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Adams J, Palombella VJ, Sausville EA,
Johnson J, Destree A, Lazarus DD, Maas J, Pien CS, Prakash S and
Elliott PJ: Proteasome inhibitors: a novel class of potent and
effective antitumor agents. Cancer Res. 59:2615–2622.
1999.PubMed/NCBI
|
|
31
|
Shinohara K, Tomioka M, Nakano H, Toné S,
Ito H and Kawashima S: Apoptosis induction resulting from
proteasome inhibition. Biochem J. 317:385–388. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Daniel KG, Gupta P, Harbach RH, Guida WC
and Dou QP: Organic copper complexes as a new class of proteasome
inhibitors and apoptosis inducers in human cancer cells. Biochem
Pharmacol. 67:1139–1151. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
An B, Goldfarb RH, Siman R and Dou QP:
Novel dipeptidyl proteasome inhibitors overcome Bcl-2 protective
function and selectively accumulate the cyclin-dependent kinase
inhibitor p27 and induce apoptosis in transformed, but not normal,
human fibroblasts. Cell Death Differ. 5:1062–1075. 1998. View Article : Google Scholar
|
|
34
|
Lopes UG, Erhardt P, Yao R and Cooper GM:
p53-dependent induction of apoptosis by proteasome inhibitors. J
Biol Chem. 272:12893–12896. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Verani CN: Metal complexes as inhibitors
of the 26S proteasome in tumor cells. J Inorg Biochem. 106:59–67.
2012. View Article : Google Scholar
|
|
36
|
Zhang Z, Bi C, Schmitt SM, Fan Y, Dong L,
Zuo J and Dou QP: 1,10-Phenanthroline promotes copper complexes
into tumor cells and induces apoptosis by inhibiting the proteasome
activity. J Biol Inorg Chem. 17:1257–1267. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang Z, Bi C, Buac D, Fan Y, Zhang X, Zuo
J, Zhang P, Zhang N, Dong L and Dou QP: Organic cadmium complexes
as proteasome inhibitors and apoptosis inducers in human breast
cancer cells. J Inorg Biochem. 123:1–10. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Daniel KG, Chen D, Orlu S, Cui QC, Miller
FR and Dou QP: Clioquinol and pyrrolidine dithiocarbamate complex
with copper to form proteasome inhibitors and apoptosis inducers in
human breast cancer cells. Breast Cancer Res. 7:R897–R908. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen D, Peng F, Cui QC, Daniel KG, Orlu S,
Liu J and Dou QP: Inhibition of prostate cancer cellular proteasome
activity by a pyrrolidine dithiocarbamate-copper complex is
associated with suppression of proliferation and induction of
apoptosis. Front Biosci. 10:2932–2939. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen D, Cui QC, Yang H, Barrea RA, Sarkar
FH, Sheng S, Yan B, Reddy GPV and Dou QP: Clioquinol, a therapeutic
agent for Alzheimer's disease, has proteasome-inhibitory, androgen
receptor-suppressing, apoptosis-inducing, and antitumor activities
in human prostate cancer cells and xenografts. Cancer Res.
67:1636–1644. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xiao Y, Bi C, Fan Y, Cui C, Zhang X and
Dou QP: L-glutamine Schiff base copper complex as a proteasome
inhibitor and an apoptosis inducer in human cancer cells. Int J
Oncol. 33:1073–1079. 2008.PubMed/NCBI
|
|
42
|
Zhang X, Bi C, Fan Y, Cui Q, Chen D, Xiao
Y and Dou QP: Induction of tumor cell apoptosis by taurine Schiff
base copper complex is associated with the inhibition of
proteasomal activity. Int J Mol Med. 22:677–682. 2008.PubMed/NCBI
|
|
43
|
Li L, Yang H, Chen D, Cui C and Dou QP:
Disulfiram promotes the conversion of carcinogenic cadmium to a
proteasome inhibitor with pro-apoptotic activity in human cancer
cells. Toxicol Appl Pharmacol. 229:206–214. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cvek B, Milacic V, Taraba J and Dou QP:
Ni(II), Cu(II), and Zn(II) diethyldithiocarbamate complexes show
various activities against the proteasome in breast cancer cells. J
Med Chem. 51:6256–6258. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li B and Dou QP: Bax degradation by the
ubiquitin/proteasome-dependent pathway: Involvement in tumor
survival and progression. Proc Natl Acad Sci USA. 97:3850–3855.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Dou QP and Li B: Proteasome inhibitors as
potential novel anticancer agents. Drug Resist Updat. 2:215–223.
1999. View Article : Google Scholar
|
|
47
|
Goldberg AL: Functions of the proteasome:
the lysis at the end of the tunnel. Science. 268:522–523. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hindo SS, Frezza M, Tomco D, Heeg MJ,
Hryhorczuk L, McGarvey BR, Dou QP and Verani CN: metals in
anticancer therapy: Copper(II) complexes as inhibitors of the 20S
proteasome. Eur J Med Chem. 44:4353–4361. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chen D, Milacic V, Frezza M and Dou QP:
Metal complexes, their cellular targets and potential for cancer
therapy. Curr Pharm Des. 15:777–791. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Li X, Zhao K, Guo W, Liu X, Liu J, Gao J,
Chen Q and Bai Y: A novel manganese complex LMnAc selectively kills
cancer cells by induction of ROS-triggered and
mitochondrial-mediated cell death. Sci China Life Sci. 57:998–1010.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Aschner M, Ruilarte TR, Schneider JS and
Zheng W: Manganese: recent advances in understanding its transport
and neurotoxicity. Toxicol Appl Pharmacol. 221:131–147. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhou DF, Chen QY, Qi Y, Fu HJ, Li Z, Zhao
KD and Gao J: Anticancer activity, attenuation on the absorption of
calcium in mitochondria, and catalase activity for manganese
complexes of N-substituted di(picolyl)amine. Inorg Chem.
50:6929–6937. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Das S, Banerjee K, Roy S, Majumder S,
Chatterjee M, Majumdar S and Choudhuri SK: Mn complex-mediated
enhancement of antitumor response through modulating
myeloid-derived suppressor cells in drug-resistant tumor. In Vivo.
28:909–918. 2014.PubMed/NCBI
|