Introduction
Folic acid, the fully oxidized monoglutamyl form of
folate, is a water-soluble vitamin found mostly in green
vegetables, peanuts, legumes, strawberries and orange juice,
predominantly as polyglutamates (1). The mammalian system cannot
synthesize folate de novo; therefore, an exogenous dietary
supply of this vitamin is necessary to meet the daily requirements.
Folic acid is the precursor to co-factors which act as one-carbon
donors and are necessary for the synthesis of DNA bases (2). For this reason, folic acid is an
essential dietary nutrient required for healthy cell growth and
division. Folic acid deficiency has been linked to various human
diseases, such as neural tube defects, atherosclerosis and cancers
(3–5). In addition, Li et al
(6) found that folic acid
deficiency during pregnancy affects the skeletal muscle development
of piglets.
Folic acid is able to regulate high levels of
homocysteine, as 5-methyltetrahydrofolate, the predominant form of
dietary folate, functions as a methyl-group donor in the conversion
of homocysteine back to methionine. Elevated levels of plasma
homocysteine have been linked to reduced mobility and muscle
function (7). Betaine, another
methyl donor which is also involved in homocysteine remethylation,
has also been reported to regulate homocysteine levels (8). Recently, betaine was shown to
promote muscle fiber differentiation and increase myotube size
through insulin-like growth factor (IGF)-1 pathway activation
(9). Hyperhomocysteinemia is
associated with ischemic stroke and osteoporotic fractures in
elderly men and women (10). A
double-blind, randomized controlled study with elderly patients who
had suffered a stroke demonstrated that oral treatment with folate
and vitamin B12 decreased the incidence of hip fractures compared
with a placebo control (11).
This treatment may also improve postural stability and/or muscle
function and strength, as folic acid regulates homocysteine levels.
Overall, these aforementioned studies suggest that folic acid
supplementation improves muscle function.
The differentiation of skeletal muscle cells is a
highly organized process which is governed by muscle-specific
transcription factors belonging to the MyoD family, such as MyoD
and myogenin, as well as by the myocyte enhancer factor-2 (MEF2)
family; cell differentiation involves highly complex processes,
including withdrawal from the cell cycle, the expression of
myotube-specific genes and cell fusion to form multinucleate
myotubes (12–14). In addition, the activation of
myogenic regulatory factors (MRFs), including MyoD, myogenic factor
5 (Myf5), MRF4 and myogenin, also regulates the expression of
several muscle-specific genes, such as myosin heavy chain (MyHC,
the major structural protein in myotubes) and creatine kinase (CK),
in muscle fiber-type maturation (15,16). MyoD and Myf5 are essential for
myoblast identity and act early in myogenesis to determine myogenic
fate (17–19). Myogenin is essential for myoblast
differentiation and acts at the late stages of myogenesis to
control the fusion of myoblasts (19). Myoblasts, with controlled
increases in the expression of MyoD, Myf5, myogenin and MRF4, and
decreases in the activity of cell cycle regulatory factors,
terminally differentiate into skeletal myocytes and fuse to form
myotubes (20).
Akt (also known as protein kinase B) is a
serine/threonine Ser/Thr) kinase with key roles in the
proliferation, survival, differentiation and viability of muscle
cells (21,22). Akt controls both protein
synthesis, through the mammalian target of rapamycin (mTOR)
signaling pathway, and protein degradation through the Forkhead Box
O transcription factors. mTOR has also been recognized as an
important player in muscle cell differentiation. Akt/mTOR has been
investigated in studies involving in vivo and in
vitro models of skeletal muscle hypertrophy and atrophy
(23,24). Definitive proof of the myogenic
function of mTOR was provided by a study which revealed that a
rapamycin-resistant mTOR mutant fully rescued C2C12 differentiation
in the presence of rapamycin (25). When the phosphorylation of mTOR by
Akt occurs, the activation of the 70-kDa ribosomal protein S6
kinase 1 (p70S6K1) and eukaryotic translation initiation factor
4E-binding protein-1 (4E-BP1) is then possible; this event is
important for the promotion of muscle growth, as p70S6K1 and 4E-BP1
stimulate protein synthesis (26). Similar to the other members of the
phosphatidylinositol kinase-related kinase family, mTOR is a
Ser/Thr protein kinase that plays an important role in a
nutrient-sensitive signaling pathway which regulates cell growth.
It has been shown that myocytes isolated from
S6K1−/− mice do not exhibit a hypertrophy
response to IGF-1 stimulation, indicating that p70S6K1 is necessary
for myotube hypertrophy (27).
The aim of the present study was to investigate the
effects of folic acid on muscle cell differentiation using C2C12
murine myoblasts. We provide evidence that the supplementation of
folic acid enhances myogenesis and induces the expression of MyoD,
myogenin and MyHC in C2C12 cells. We also demonstrate that folic
acid increases muscle differentiation through the activation of the
Akt/mTOR pathway. Taken together, these data suggest that folic
acid exerts a beneficial effect on muscle cell differentiation.
Materials and methods
Reagents
Folic acid, LY294002 and monoclonal antibody against
β-actin (Cat. no. A5441) were purchased from Sigma-Aldrich (St.
Louis, MO, USA). Folic acid was dissolved in 1 M NaOH to generate
100 mM stock solution and stored at −20°C until use in the
experiments; dilutions were made in culture medium.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
was obtained from Amresco (Solon, OH, USA). Antibodies against MyoD
(sc-760), myogenin (sc-576), MyHC (sc-20641), phos phorylated
(p-)Akt (Ser473; sc-7985-R) and Akt1/2/3 (sc-8312) were all
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Antibodies against p-mTOR (cat. no. 5536), mTOR (cat. no. 2983),
p-p70S6K1 (cat. no. 9234), p70S6K1 (cat. no. 2708), p-4E-BP1 (cat.
no. 2855) and 4E-BP1 (cat. no. 9452) were all purchased from Cell
Signaling Technology (Danvers, MA, USA). Dulbecco's modified
Eagle's medium (DMEM) was purchased from Welgene, Inc. (Daegu,
Korea) and horse serum (HS) was from Invitrogen (Grand Island, NY,
USA). Fetal bovine serum (FBS) and penicillin-streptomycin were
purchased from GE Healthcare Life Sciences (Logan, UT, USA). A
Creatine kinase enzymatic assay kit (MaxDiscovery™ creatine kinase
enzymatic assay kit) was purchased from Bioo Scientific Corp.
(Austin, TX, USA).
Cell culture
Murine C2C12 myoblasts were purchased from the
American Type Culture Collection (ATCC, Manassas, VA, USA). For the
maintenance of the C2C12 myoblasts, the cells were cultured in
growth medium consisting of DMEM supplemented with 10% FBS, 100
U/ml penicillin and 100 µg/ml streptomycin in 5%
CO2 at 37°C. The growth medium was changed every 2
days.
Induction of myogenic
differentiation
For the induction of myogenic differentiation, the
C2C12 myoblasts at 80–90% confluence were transferred to
differentiation medium composed of DMEM supplemented with 2% HS in
order to initiate the differentiation of the myoblasts into
myotubes. The medium was changed with fresh differentiation medium
every 2 days.
Measurement of cell viability
Cell viability was determined by MTT assay. The
cells were seeded in 6-well plates and incubated in culture medium
until they reached 80–90% confluence. The medium was then switched
to differentiation medium, and the cells were treated with or
without folic acid (0, 10, 20, 50 and 100 µM) and observed
after 6 days. The cells were incubated in the dark with MTT reagent
(0.5 mg/ml) at 37°C for 2 h. The medium was removed, the formazan
was dissolved in dimethyl sulfoxide (DMSO), and the absorbance at
540 nm was measured using an enzyme-linked immuno-sorbent assay
(ELISA) plate reader (Thermo Fisher Scientific, Vantaa,
Finland).
Measurement of CK activity
The cells were washed with phosphate-buffered saline
(PBS) and then lysed with lysis buffer [40 mM Tris (pH 8.0), 120 mM
NaCl, 0.5% NP-40, 2 µg/ml aprotinin, 2 µg/ml
leupeptin and 100 µg/ml phenymethylsulfonyl fluoride (PMSF)]
and complete protease inhibitor and stored at −70°C until use. CK
activity was determined using a CK enzymatic assay kit (Bioo
Scientific Corp.), according to the manufacturer's instructions.
Briefly, 250 µl CK reagent were added to 5 µl cell
lysate in a microplate. CK activity was immediately measured 2
times at 5-min intervals at 340 nm. Each assay was performed in
duplicate. The average 5-min absorbance increase was multiplied by
2,186 (conversion factor) to obtain the CK activity (IU/l).
Immunofluorescence staining and
determination of the fusion index
The C2C12 myoblasts were cultured in 6-well plates
and cell differentiation was induced with the use of
differentiation medium with or without folic acid (0, 2.5, 5, 10
and 20 µM) for 6 days. For immunofluorescence microscopy,
the cells were fixed in 4% paraformaldehyde for 20 min at room
temperature. After the cells were blocked in 2% normal goat serum,
they were then incubated with primary antibody at 4°C for 24 h.
mouse anti-MyHC antibody was used at a 1:200 dilution. MyHC was
detected by incubating the cells with goat anti-mouse Rhodamine
Red-X (1:1,000; Jackson ImmunoResearch, Bar Harbor, ME, USA) at 4°C
for 1 h, and 4′-6-diamidino-2-phenylindol (DAPI) was then used to
label the nuclei. All images were taken using a confocal microscope
(FV10C-W), using the same exposure time, and were analyzed in
VS-FlexGrid Pro 8.0J (both from Olympus, Tokyo, Japan).
Differentiated myotubes in a specific microscopic field were
observed under ×20 magnification. Either the total number of nuclei
or the number of nuclei within MyHC-positive myotubes was counted
in 5 fields/sample. The fusion index was calculated as follows: (%)
= (number of nuclei within MyHC-stained myotubes/total number of
nuclei) ×100. All experiments were performed 3 times.
Western blot analysis
The C2C12 myoblasts were cultured in 6-well plates
and differentiation was induced with the use of differentiation
medium with or without folic acid (0, 2.5, 5, 10 and 20 µM)
for 6 days. For western blot analysis, the myoblasts and
differentiated myotubes were washed with PBS and homogenized in
lysis buffer. Following centrifugation (14,240 × g) at 4°C for 15
min, the supernatant was collected and the protein concentration
was determined using protein assay reagents (Bio-Rad, Hercules, CA,
USA). Equal amounts of protein extracts were denatured by boiling
them at 100°C for 5 min in sample buffer (Bio-Rad). The proteins
were separated by 6–15% sodium dodecyl sulfate polyacrylamide gel
electrophoresis (SDS-PAGE) and transferred onto polyvinylidene
difluoride (PVDF) membranes (Merck Millipore, Berlin, Germany). The
membranes were blocked with 5% non-fat dry milk in Tris-buffered
saline with Tween-20 buffer (TBST; 20 mM Tris, 100 mM NaCl, pH 7.5
and 0.1% Tween-20) for 1 h at room temperature followed by
incubation with primary antibodies specific for each protein at 4°C
for 24 h: MyHC (1:800), MyoD (1:1,000), myogenin (1:1,000), β-actin
(1:50,000), p-Akt (Ser473) (1:1,000), Akt1/2/3 (1:1,000), p-mTOR
(1:3,000), mTOR (1:3,000), p-p70S6K1 (1:3,000), p70S6K1 (1:3,000),
p-4E-BP1 (1:3,000) and 4E-BP1 (1:3,000). The blots were washed with
TBST buffer and then incubated with horseradish
peroxidase-conjugated secondary antibodies (1:3,000; Santa Cruz
Biotechnology, Inc.) for 1 h at room temperature. Immunolabeling
was carried out using an enhanced chemiluminescence (ECL) detection
system (GE Healthcare, Piscataway, NJ, USA).
Statistical analysis
Statistical software (version 10.0; StatSoft, Inc.,
Tulsa, OK, USA) was used for statistical analysis. Data are
presented as the means ± SD. Data were analyzed using the Student's
t-test. A P-value <0.05 was considered to indicate a
statistically significant difference.
Results
Folic acid enhances the myogenic
differentiation of C2C12 cells and CK activity
To investigate the role of folic acid in the process
of muscle differentiation, we first determined the effects of folic
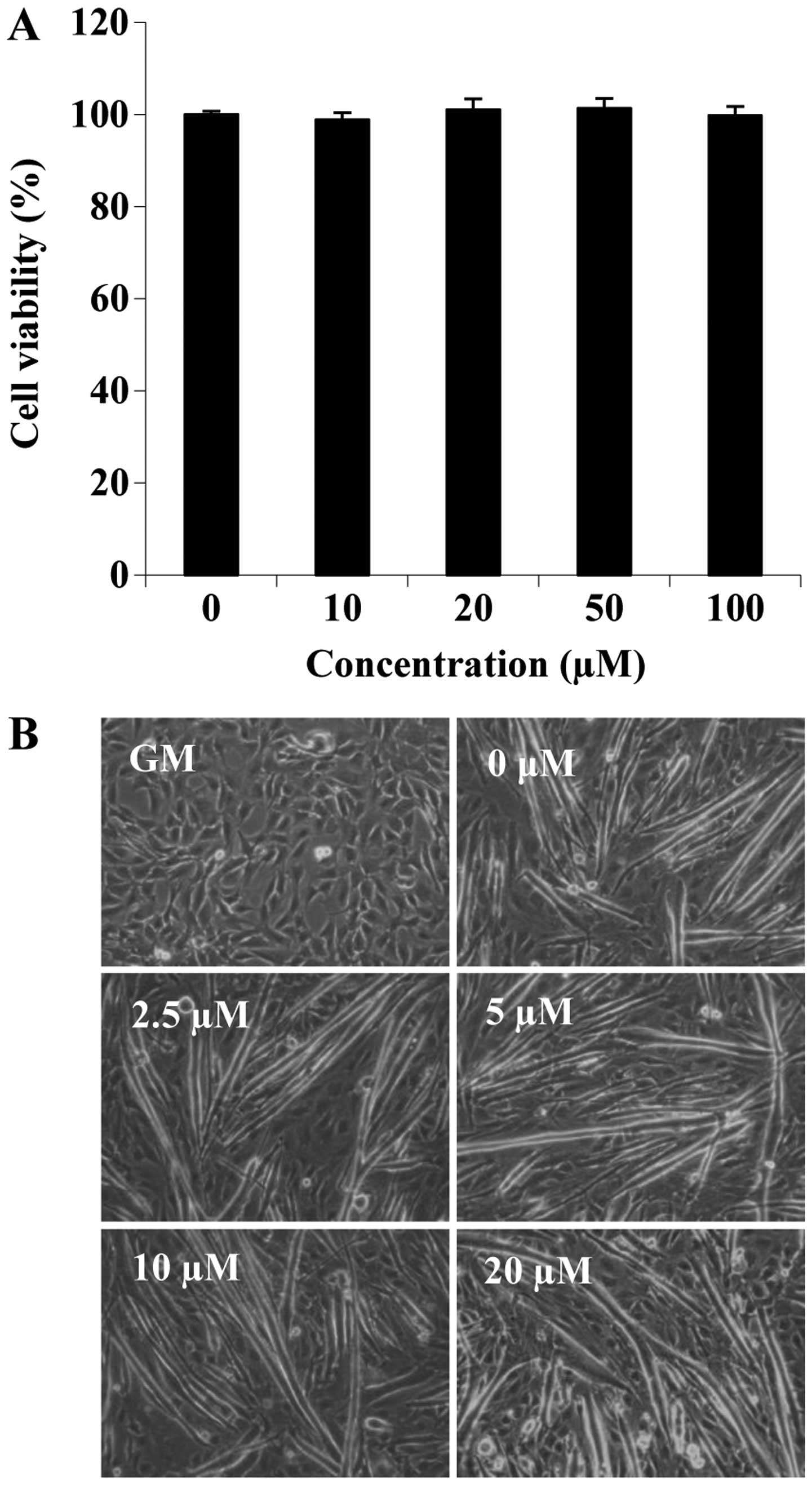
acid on cell viability during differentiation. C2C12 cells cultured
in differentiation medium were treated with folic acid (0, 10, 20,
50 and 100 µM) for 6 days. No significant differences were
observed with the addition of up to 100 µM folic acid
(Fig. 1A). Subsequently, we
examined the effects of folic acid on the morphological changes of
C2C12 cells (such as the loss of their typical triangular
morphology and the cell shape gradually changed into a new
elongated shape) that are associated with the differentiation
process (Fig. 1B). On the 6th day
of differentiation, myotube formation was increased by folic acid
treatment in a concentration-dependent manner (Fig. 1B). These data suggested that folic
acid promoted the myogenic differentiation of C2C12 cells. To
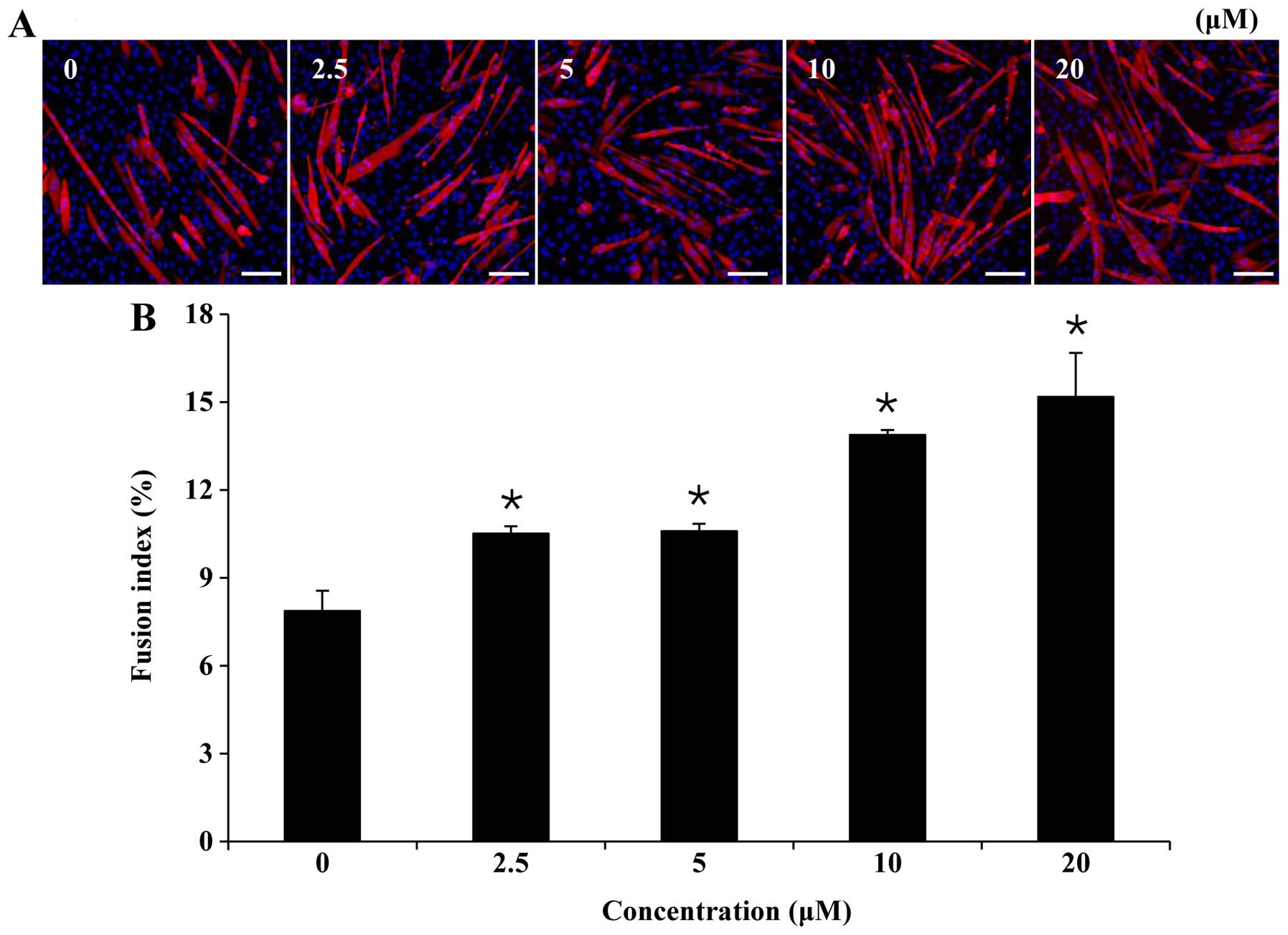
confirm this, we examined the effects of folic acid on myogenic
differentiation by measuring the fraction of nuclei incorporated
into MyHC-stained myotubes on the 6th day of myogenic
differentiation (Fig. 2A). The
results, as indicated in Fig. 2B,
demonstrated that the fusion index for each group was, in
increasing order: control (7.9±0.7%), 2.5 µM folic acid
(10.5±0.2%), 5 µM folic acid (10.6±0.2%), 10 µM folic
acid (13.9±0.2%) and 20 µM folic acid (15.2±1.5%).
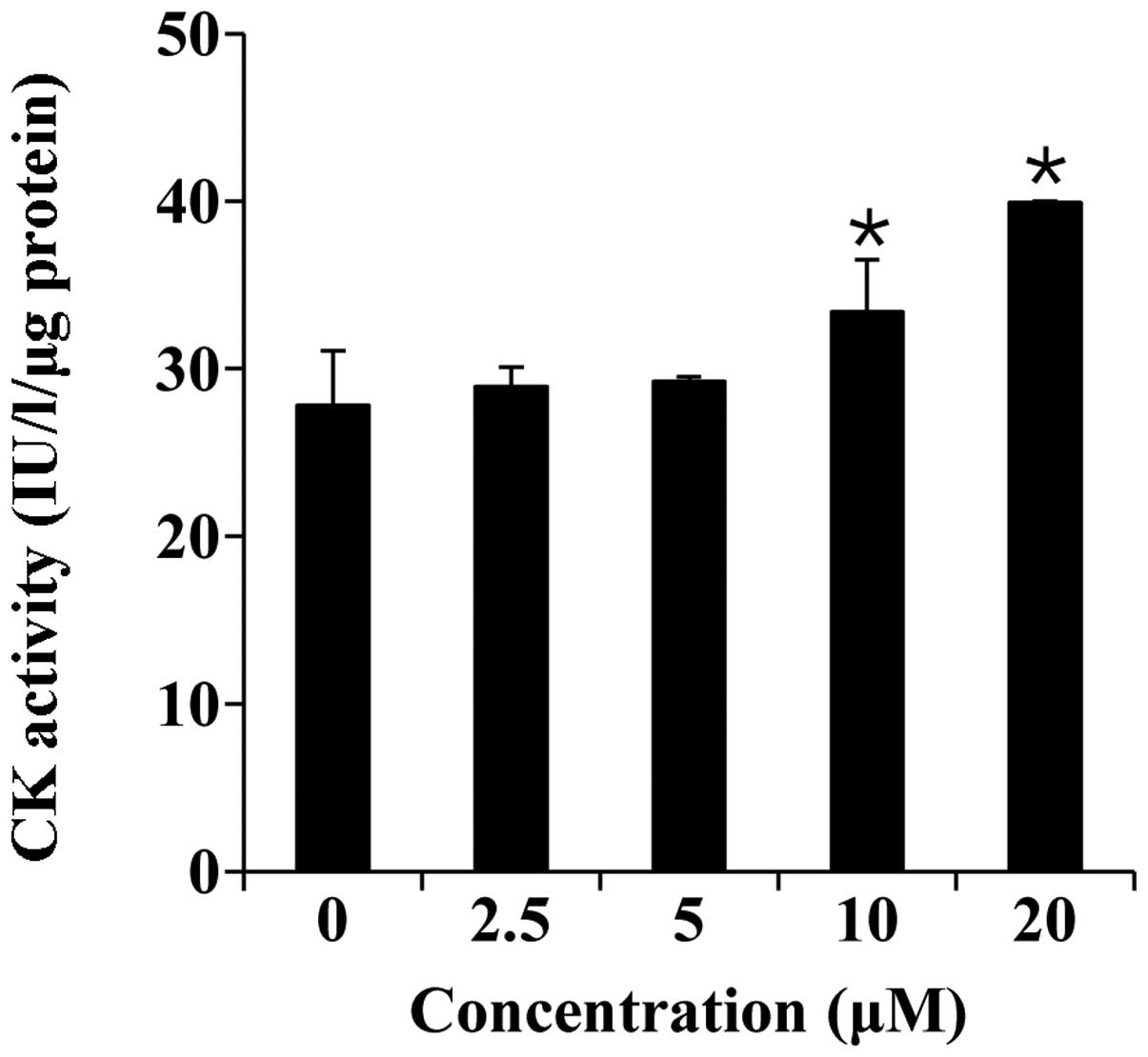
Thereafter, we measured CK activity, which is a well-described
marker of C2C12 cell differentiation (28). As shown in Fig. 3, folic acid (0–20 µM)
significantly increased CK activity in a concentration-dependent
manner.
Folic acid increases the expression of
MyoD, myogenin and MyHC
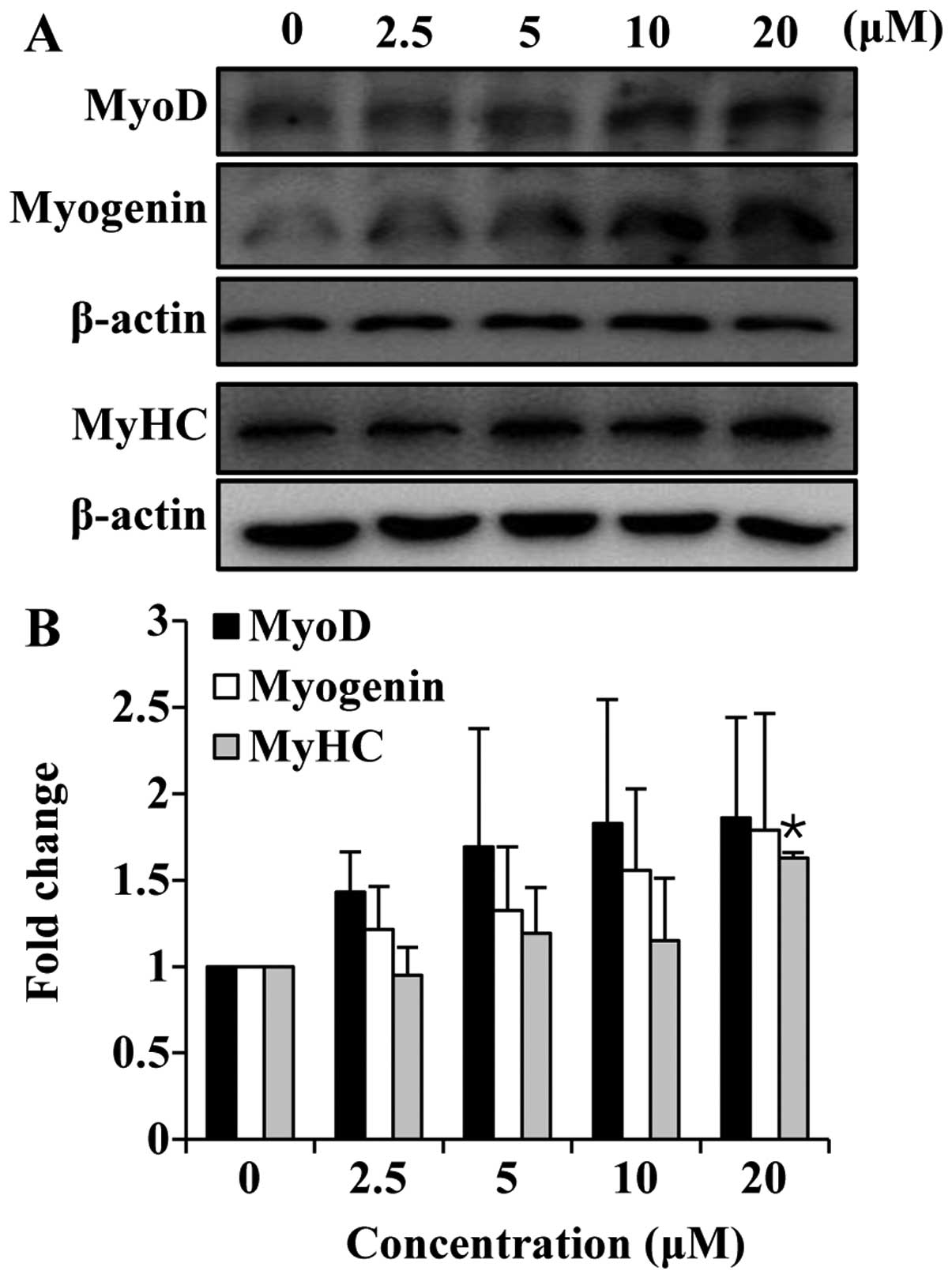
Since myotube formation was affected by folic acid,
we wished to determine the effects of folic acid on the expression
of myogenic markers. MyoD is a muscle-specific transcription factor
that is expressed in myogenic cells during late proliferation and
early differentiation. It is commonly assumed that MyoD is upstream
of myogenin during the differentiation process (29,30). The C2C12 cells were cultured in
growth medium until they reached 80–90% confluence, and the medium
was then switched to differentiation medium and the cells treated
with or without folic acid; the cells were then harvested after 1
day of differentiation in order to measure the MyoD and myogenin
expression levels. To determine the effects of folic acid on the
late-stage differentiation marker, MyHC, the cells were cultured
for 6 days. The results revealed a marked increase in MyoD
expression in the cells treated with folic acid compared to the
cells not treated with folic acid (Fig. 4). Myogenin is part of a second
wave of regulators expressed in cells that have ceased mitosis and
are committed to terminal differentiation (31). As expected, the expression of
myogenin was also increased in a concentration-dependent manner
following treatment with folic acid (Fig. 4). Finally, the expression of MyHC,
a specific marker of myotubes, was also increased by treatment with
folic acid (Fig. 4). Taken
together, these data demonstrate that treatment with folic acid
increases the expression of myogenic markers in C2C12 cells.
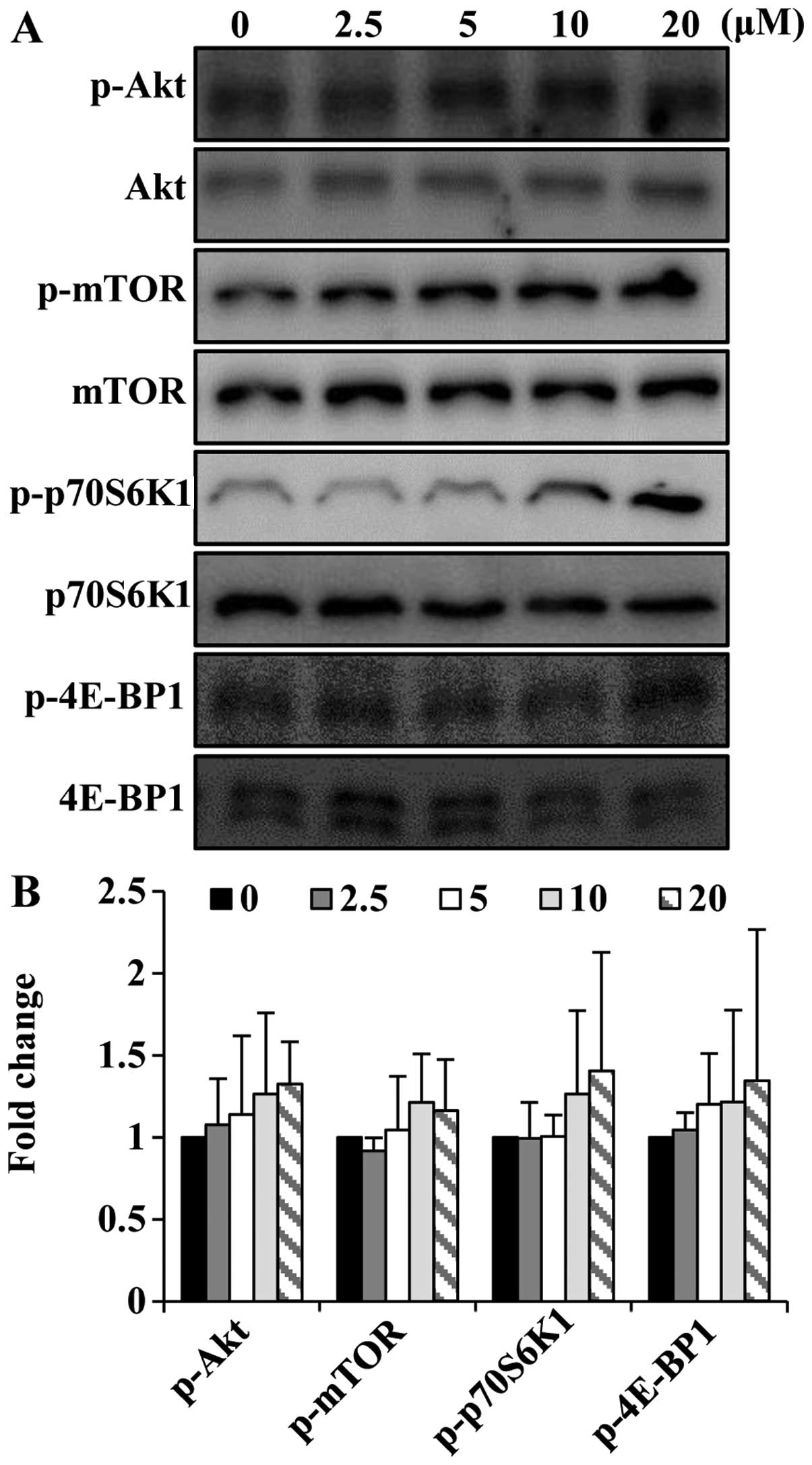
Folic acid activates the Akt/mTOR pathway
in C2C12 cells
As Akt and mTOR are required for skeletal muscle
development (23,24), we wished to determine whether
folic acid modulates the activation of the Akt/mTOR pathway in
C2C12 cells. To determine the ability of folic acid to affect the
Akt/mTOR signaling pathway, short-term (8 h) stimulation
experiments were performed. Folic acid increased the
phosphorylation of Akt and mTOR in the C2C12 cells (Fig. 5). In addition to increasing the
phosphorylation of Akt and mTOR, folic acid activated p70S6K1 and
4E-BP1, which are key downstream targets of the Akt/mTOR signaling
cascade.
Akt activation is required for the
induction of C2C12 cell differentiation by folic acid
To confirm whether Akt is necessary for the folic
acid-induced differentiation of C2C12 cells, the cells were
cultured in the presence or absence of the Akt inhibitor, LY294002,
and then treated (or not) with folic acid. Treatment with folic
acid for 8 h induced the phosphorylation of Akt; however, LY294002
reversed the effects of folic acid on Akt (Fig. 6A). In addition, pre-treatment with
LY294002 abolished the effects of folic acid on MyoD and myogenin
expression (Fig. 6B). This result
was further confirmed by examining C2C12 myoblast differentiation.
The results revealed that the blockade of Akt completely inhibited
the differentiation of C2C12 myoblasts into myotubes in both the
presence and absence of folic acid, as shown by the decrease in
MyHC expression (late-stage differentiation marker) in the cells
pre-treated with LY294002 (Fig.
6C). Under differentiation conditions, treatment with LY294002
did not alter cell viability (data not shown). We also conducted
western blot analyses to confirm the effects of LY294002 on the
folic acid-induced expression of MyHC. Consistent with the
morphological changes of the C2C12 cells, the induction of MyHC
expression by folic acid was markedly reversed in the
LY294002-treated cells (Fig. 6D).
Taken together, these data demonstrate that folic acid enhances the
myogenic differentiation of C2C12 cells through the Akt signaling
pathway.
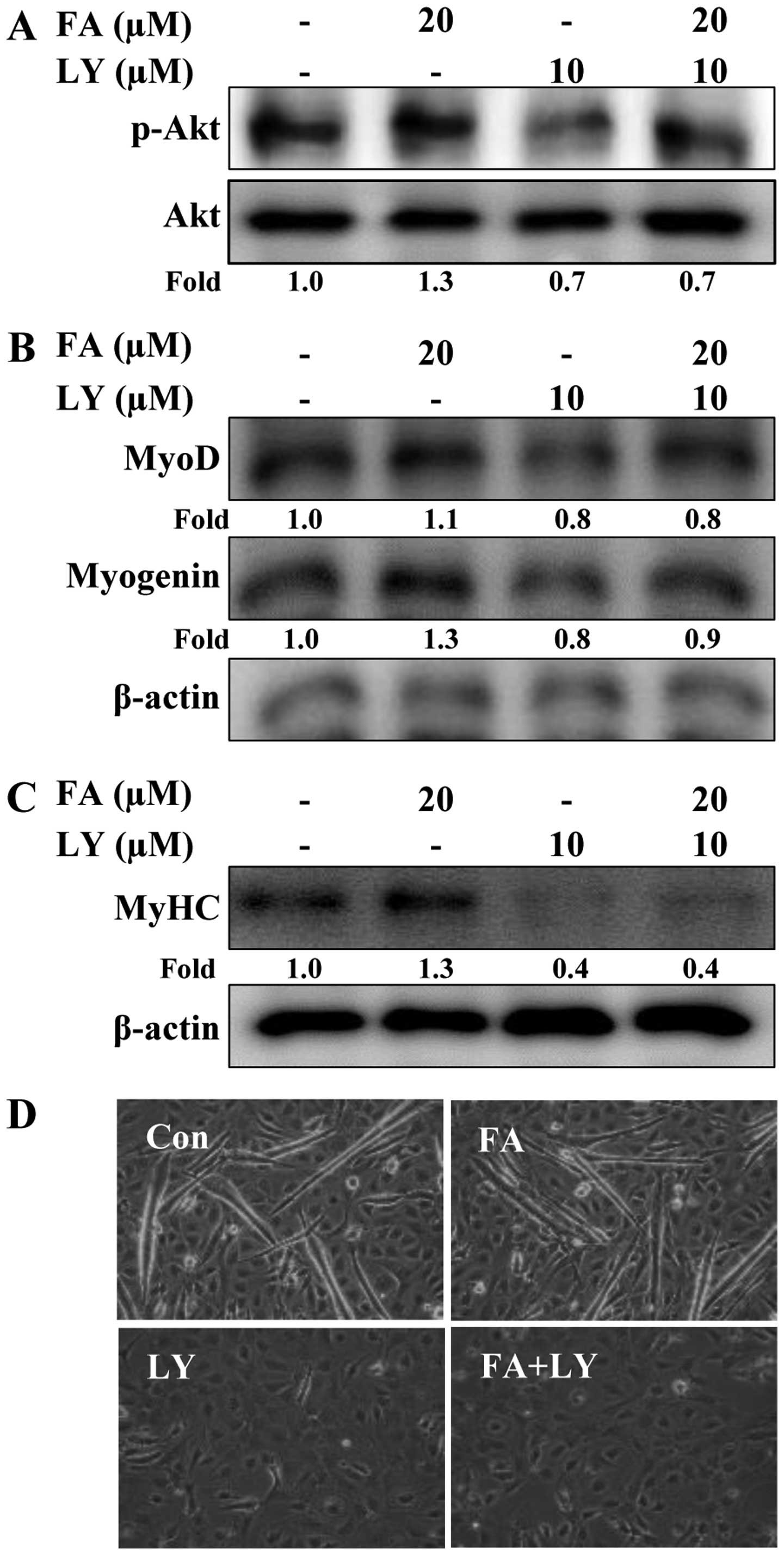 | Figure 6Effects of Akt inhibition on folic
acid-induced C2C12 cell differentiation. Cells were cultured in
differentiation medium with or without folic acid FA following
pre-treatment with or without LY294002 for 30 min. Cells were
incubated for a further (A) 8 h (p-Akt and Akt), (B) 1 day (MyoD
and myogenin) and (C) 6 days [myosin heavy chain (MyHC)]. The
expression of p-Akt, Akt, MyoD, myogenin and MyHC measured by
western blot analysis. Densitometric analysis of 3 independent
experiments is shown. The values were normalized to total Akt (for
p-Akt) and those of β-actin (for MyoD, myogenin and MyHC), and the
fold change relative to the untreated cells is presented. (D)
Representative phase contrast photomicrographs showing myotube
formation after 6 days. The experiments were performed 3 times, and
a representative result is shown. FA, folic acid; LY, LY294002;
Con, control. |
Discussion
C2C12 cells are murine myoblasts derived from
satellite cells, which can spontaneously differentiate into
myotubes when moved from high-serum medium to low-serum medium. The
C2C12 cells are a useful tool for studying the differentiation of
myoblasts, the expression of various proteins, and also exploring
mechanistic pathways (32). In
the present study, we used C2C12 cells to examine the effects of
folic acid on myogenic differentiation.
First, we observed that folic acid increased
neo-myotube formation, compared to the untreated control cells,
without affecting cell viability. The results revealed that folic
acid increased the fusion of myoblasts into multinucleated
myotubes, as determined by examining cell morphology and by MyHC
staining. No significant difference in cell viability was observed
in the cells treated with 10 and up to 100 µM folic acid
(Fig. 1). Therefore, following
this result, we expected folic acid to increase myogenic
differentiation.
Secondly, we observed that folic acid increased the
expression of MyoD and myogenin and of the muscle-specific
structural genes, MyHC and muscle CK during the differentiation
phase (Figs. 3 and 4). These data suggest that treatment
with folic acid increased the differentiation of C2C12 cells. The
myogenic differentiation program is known to be largely controlled
by the myogenic basic helix-loop-helix family of transcription
factors (MyoD, myogenin, Myf5 and MRF4) and MEF2, which regulate
the expression of several muscle-specific genes, such as MyHC, and
p21, a cyclin-dependent kinase inhibitor (33,34). Notably, similar transcriptional
programs regulate skeletal muscle satellite cell
proliferation/differentiation in vivo and the maturation of
isolated myoblasts in vitro. Expression during myogenesis is
distinct among the MRF members; undifferentiated myoblasts express
Myf5 and MyoD, but not myogenin (35). By contrast, previous studies have
demonstrated that multinucleated myotubes are all positive for
Myf5, MyoD and myogenin, although the expression level of Myf5 is
higher in mononuclear myoblasts and MyoD and myogenin is
predominantly expressed in myotubes (36,37). MyoD directly binds to MEF2 and
enhances MEF2-dependent transcription through its own
transcriptional activities, which results in the cooperative
enhancement of MEF2- and MyoD-dependent myogenic differentiation
(38). Myogenin has been proven
to play a critical role in the transcriptional regulation of the
multiple epidermal growth factor-like-domains 10 (MEGF10) gene, a
well-known myogenic regulator of satellite cells (39). Both MyoD and myogenin bind to
E-box elements in the promoter region of muscle-specific genes and
regulate their expression in skeletal muscle (40). Cornelison et al (41) reported that MyoD deficient
(MyoD−/−) satellite cells displayed aberrant
morphology during the later phases of proliferation and
differentiation, and exhibited major differences in myogenic gene
expression and efficiency of terminal differentiation compared to
wild-type satellite cells. Taken together, these data indicate that
MyoD and myogenin regulate myogenic differentiation through
transcriptional activities and protein-protein interactions. Our
results suggest that folic acid promotes myogenic differentiation
by increasing the expression of muscle-specific factors, such as
MyoD, myogenin and MyHC.
Finally, we found that treatment with folic acid
activated the Akt/mTOR signaling pathway (Figs. 5 and 6). By inhibiting the Akt pathway with a
specific inhibitor (LY294002), we demonstrated that this pathway is
required for the stimulatory effects of folic acid on muscle cell
differentiation. MyHC expression displayed a differential
sensitivity toward Akt inhibition, revealing an Akt
activity-dependent induction by folic acid. The crucial role of Akt
in myogenic differentiation and hypertrophy has been previously
demonstrated (42,43). In skeletal muscle, Akt signaling
plays a role in hypertrophy and contributes to increase in the size
of C2C12 myotubes (24). In
dystrophic muscle, elevated Akt signaling has been associated with
advanced dystrophy and peak stages of muscle hypertrophy (44). Izumiya et al (45) demonstrated that muscle-specific
Akt transgene expression led to muscle hypertrophy, whereas
decreasing adipose mass was due to the growth of type IIb muscle
fibers, which was accompanied by an increase in strength.
Furthermore, the IGF-phosphoinositide 3-kinase (PI3K)-Akt signaling
pathway has been shown to stimulate myogenic differentiation by
inducing myogenin gene expression (46). Also, the IGF-PI3K-Akt signaling
pathway can target MyoD and MEF2 by enhancing the transcriptional
activity of MyoD and MEF2 in normal myogenic cells (47). Taken together, these data support
the theory that the activation of Akt in muscle plays a role as a
key mediator of the hypertrophic response by promoting myogenic
gene expression. Therefore, we hypothesize that folic acid promotes
myogenic differentiation by increasing myogenin gene expression
through Akt signaling.
The loss of skeletal muscle mass and reduced
contractive force are common and disabling features of various
human diseases, including neuromuscular disorders, cancer, AIDS and
diabetes (48,49). The decrease in muscle function has
also been shown to be associated with aging, a sedentary lifestyle
and immobilization (50).
Furthermore, aging- and disease-related skeletal muscle wasting has
been shown to be associated with inflammatory and homocysteine
levels (50). Folic acid was
found to exert an anti-inflammatory effect (51) and to inhibit inflammation-related
signaling molecule nuclear factor-κB (NF-κB) and its upstream
mitogen-activated protein kinases (52); thus folic acid may exert
beneficial effects on skeletal muscle function. Moreover, the
metabolism of homocysteine and folic acid are closely linked to
skeletal muscle weakness through mitochondrial dysfunction that
involves epigenetic changes (53,54).
In conclusion, the findings of our study indicate
that treatment with folic acid promotes skeletal muscle myoblast
differentiation, particularly affecting the progression of the
differentiation process and myotube morphology. This suggests that
the Akt-dependent regulation by folic acid controls the expression
of MyoD, myogenin and MyHC.
Acknowledgments
The present study was supported by the R&D
program of MOTIF/KEIT (10040391, Development of Functional Food
Materials and Device for Prevention of Aging-associated Muscle
Function Decrease). This study was also supported by the National
Research Foundation of Korea (NRF) grant, funded by the Korean
Government (Ministry of Science, ICT and Future Planning, no.
2009-0083538). We thank the Aging Tissue Bank for providing
research information.
References
|
1
|
Iyer R and Tomar SK: Folate: a functional
food constituent. J Food Sci. 74:R114–R122. 2009. View Article : Google Scholar
|
|
2
|
Lucock M: Is folic acid the ultimate
functional food component for disease prevention? BMJ. 328:211–214.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Czeizel AE, Dudás I, Vereczkey A and
Bánhidy F: Folate deficiency and folic acid supplementation: the
prevention of neural-tube defects and congenital heart defects.
Nutrients. 5:4760–4775. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hou TC, Lin JJ, Wen HC, Chen LC, Hsu SP
and Lee WS: Folic acid inhibits endothelial cell migration through
inhibiting the RhoA activity mediated by activating the folic acid
receptor/cSrc/p190RhoGAP-signaling pathway. Biochem Pharmacol.
85:376–384. 2013. View Article : Google Scholar
|
|
5
|
Kim YI: Will mandatory folic acid
fortification prevent or promote cancer? Am J Clin Nutr.
80:1123–1128. 2004.PubMed/NCBI
|
|
6
|
Li Y, Zhang X, Sun Y, Feng Q, Li G, Wang
M, Cui X, Kang L and Jiang Y: Folate deficiency during early-mid
pregnancy affects the skeletal muscle transcriptome of piglets from
a reciprocal cross. PLoS One. 8:e826162013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Swart KM, Enneman AW, van Wijngaarden JP,
van Dijk SC, Brouwer-Brolsma EM, Ham AC, Dhonukshe-Rutten RA, van
der Velde N, Brug J, van Meurs JB, et al: Homocysteine and the
methylenetetrahydrofolate reductase 677C–>T polymorphism in
relation to muscle mass and strength, physical performance and
postural sway. Eur J Clin Nutr. 67:743–748. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Olthof MR and Verhoef P: Effects of
betaine intake on plasma homocysteine concentrations and
consequences for health. Curr Drug Metab. 6:15–22. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Senesi P, Luzi L, Montesano A, Mazzocchi N
and Terruzzi I: Betaine supplement enhances skeletal muscle
differentiation in murine myoblasts via IGF-1 signaling activation.
J Transl Med. 11:1742013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
van Meurs JB, Dhonukshe-Rutten RA, Pluijm
SM, van der Klift M, de Jonge R, Lindemans J, de Groot LC, Hofman
A, Witteman JC, van Leeuwen JP, et al: Homocysteine levels and the
risk of osteoporotic fracture. N Engl J Med. 350:2033–2041. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sato Y, Honda Y, Iwamoto J, Kanoko T and
Satoh K: Effect of folate and mecobalamin on hip fractures in
patients with stroke: a randomized controlled trial. JAMA.
293:1082–1088. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Buckingham M, Bajard L, Chang T, Daubas P,
Hadchouel J, Meilhac S, Montarras D, Rocancourt D and Relaix F: The
formation of skeletal muscle: from somite to limb. J Anat.
202:59–68. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Parker MH, Seale P and Rudnicki MA:
Looking back to the embryo: defining transcriptional networks in
adult myogenesis. Nat Rev Genet. 4:497–507. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Weintraub H: The MyoD family and
myogenesis: redundancy, networks, and thresholds. Cell.
75:1241–1244. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lassar AB, Skapek SX and Novitch B:
Regulatory mechanisms that coordinate skeletal muscle
differentiation and cell cycle withdrawal. Curr Opin Cell Biol.
6:788–794. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Olson EN, Perry M and Schulz RA:
Regulation of muscle differentiation by the MEF2 family of MADS box
transcription factors. Dev Biol. 172:2–14. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rudnicki MA, Braun T, Hinuma S and
Jaenisch R: Inactivation of MyoD in mice leads to up-regulation of
the myogenic HLH gene Myf-5 and results in apparently normal muscle
development. Cell. 71:383–390. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rudnicki MA, Schnegelsberg PN, Stead RH,
Braun T, Arnold HH and Jaenisch R: MyoD or Myf-5 is required for
the formation of skeletal muscle. Cell. 75:1351–1359. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Perry RL and Rudnick MA: Molecular
mechanisms regulating myogenic determination and differentiation.
Front Biosci. 5:D750–D767. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Moncaut N, Rigby PW, Carvajal JJ and Dial
M: Dial M(RF) for myogenesis. FEBS J. 280:3980–3990. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ceci M, Ross J Jr and Condorelli G:
Molecular determinants of the physiological adaptation to stress in
the cardiomyocyte: a focus on AKT. J Mol Cell Cardiol. 37:905–912.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guttridge DC: Signaling pathways weigh in
on decisions to make or break skeletal muscle. Curr Opin Clin Nutr
Metab Care. 7:443–450. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bodine SC, Stitt TN, Gonzalez M, Kline WO,
Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC,
Glass DJ and Yancopoulos GD: Akt/mTOR pathway is a crucial
regulator of skeletal muscle hypertrophy and can prevent muscle
atrophy in vivo. Nat Cell Biol. 3:1014–1019. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim M, Sung B, Kang YJ, Kim DH, Lee Y,
Hwang SY, Yoon JH, Yoo MA, Kim CM, Chung HY and Kim ND: The
combination of ursolic acid and leucine potentiates the
differentiation of C2C12 murine myoblasts through the mTOR
signaling pathway. Int J Mol Med. 35:755–762. 2015.
|
|
25
|
Erbay E and Chen J: The mammalian target
of rapamycin regulates C2C12 myogenesis via a kinase-independent
mechanism. J Biol Chem. 276:36079–36082. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gingras AC, Raught B and Sonenberg N:
Regulation of translation initiation by FRAP/mTOR. Genes Dev.
15:807–826. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ohanna M, Sobering AK, Lapointe T, Lorenzo
L, Praud C, Petroulakis E, Sonenberg N, Kelly PA, Sotiropoulos A
and Pende M: Atrophy of S6K1(−/−) skeletal muscle cells reveals
distinct mTOR effectors for cell cycle and size control. Nat Cell
Biol. 7:286–294. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lawson MA and Purslow PP: Differentiation
of myoblasts in serum-free media: effects of modified media are
cell line-specific. Cells Tissues Organs. 167:130–137. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nabeshima Y, Hanaoka K, Hayasaka M, Esumi
E, Li S, Nonaka I and Nabeshima Y: Myogenin gene disruption results
in perinatal lethality because of severe muscle defect. Nature.
364:532–535. 1993. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hasty P, Bradley A, Morris JH, Edmondson
DG, Venuti JM, Olson EN and Klein WH: Muscle deficiency and
neonatal death in mice with a targeted mutation in the myogenin
gene. Nature. 364:501–506. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tidball JG and Villalta SA: Regulatory
interactions between muscle and the immune system during muscle
regeneration. Am J Physiol Regul Integr Comp Physiol.
298:R1173–R1187. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yaffe D and Saxel O: Serial passaging and
differentiation of myogenic cells isolated from dystrophic mouse
muscle. Nature. 270:725–727. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tapscott SJ: The circuitry of a master
switch: Myod and the regulation of skeletal muscle gene
transcription. Development. 132:2685–2695. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hawke TJ and Garry DJ: Myogenic satellite
cells: physiology to molecular biology. J Appl Physiol (1985).
91:534–551. 2001.
|
|
35
|
Yoshida N, Yoshida S, Koishi K, Masuda K
and Nabeshima Y: Cell heterogeneity upon myogenic differentiation:
down-regulation of MyoD and Myf-5 generates 'reserve cells'. J Cell
Sci. 111:769–779. 1998.PubMed/NCBI
|
|
36
|
Ferri P, Barbieri E, Burattini S, Guescini
M, D'Emilio A, Biagiotti L, Del Grande P, De Luca A, Stocchi V and
Falcieri E: Expression and subcellular localization of myogenic
regulatory factors during the differentiation of skeletal muscle
C2C12 myoblasts. J Cell Biochem. 108:1302–1317. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kitzmann M, Carnac G, Vandromme M, Primig
M, Lamb NJ and Fernandez A: The muscle regulatory factors MyoD and
myf-5 undergo distinct cell cycle-specific expression in muscle
cells. J Cell Biol. 142:1447–1459. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Molkentin JD, Black BL, Martin JF and
Olson EN: Cooperative activation of muscle gene expression by MEF2
and myogenic bHLH proteins. Cell. 83:1125–1136. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Park SY, Yun Y, Kim MJ and Kim IS:
Myogenin is a positive regulator of MEGF10 expression in skeletal
muscle. Biochem Biophys Res Commun. 450:1631–1637. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ludolph DC and Konieczny SF: Transcription
factor families: muscling in on the myogenic program. FASEB J.
9:1595–1604. 1995.PubMed/NCBI
|
|
41
|
Cornelison DD, Olwin BB, Rudnicki MA and
Wold BJ: MyoD(−/−) satellite cells in single-fiber culture are
differentiation defective and MRF4 deficient. Dev Biol.
224:122–137. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jiang BH, Aoki M, Zheng JZ, Li J and Vogt
PK: Myogenic signaling of phosphatidylinositol 3-kinase requires
the serine-threonine kinase Akt/protein kinase B. Proc Natl Acad
Sci USA. 96:2077–2081. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sumitani S, Goya K, Testa JR, Kouhara H
and Kasayama S: Akt1 and Akt2 differently regulate muscle creatine
kinase and myogenin gene transcription in insulin-induced
differentiation of C2C12 myoblasts. Endocrinology. 143:820–828.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Peter AK and Crosbie RH: Hypertrophic
response of Duchenne and limb-girdle muscular dystrophies is
associated with activation of Akt pathway. Exp Cell Res.
312:2580–2591. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Izumiya Y, Hopkins T, Morris C, Sato K,
Zeng L, Viereck J, Hamilton JA, Ouchi N, LeBrasseur NK and Walsh K:
Fast/Glycolytic muscle fiber growth reduces fat mass and improves
metabolic parameters in obese mice. Cell Metab. 7:159–172. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Florini JR, Ewton DZ and Roof SL:
Insulin-like growth factor-I stimulates terminal myogenic
differentiation by induction of myogenin gene expression. Mol
Endocrinol. 5:718–724. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Xu Q and Wu Z: The insulin-like growth
factor-phosphatidylinositol 3-kinase-Akt signaling pathway
regulates myogenin expression in normal myogenic cells but not in
rhabdomyo-sarcoma-derived RD cells. J Biol Chem. 275:36750–36757.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jagoe RT and Goldberg AL: What do we
really know about the ubiquitin-proteasome pathway in muscle
atrophy? Curr Opin Clin Nutr Metab Care. 4:183–190. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Glass DJ: Signalling pathways that mediate
skeletal muscle hypertrophy and atrophy. Nat Cell Biol. 5:87–90.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
No authors listed. Epidemiologic and
methodologic problems in determining nutritional status of older
persons. Proceedings of a conference Albuquerque, New Mexico,
October 19–21, 1988. Am J Clin Nutr. 50(Suppl): 1121–1235.
1989.
|
|
51
|
Solini A, Santini E and Ferrannini E:
Effect of short-term folic acid supplementation on insulin
sensitivity and inflammatory markers in overweight subjects. Int J
Obes. 30:1197–1202. 2006. View Article : Google Scholar
|
|
52
|
Feng D, Zhou Y, Xia M and Ma J: Folic acid
inhibits lipopolysaccharide-induced inflammatory response in
RAW264.7 macrophages by suppressing MAPKs and NF-κB activation.
Inflamm Res. 60:817–822. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Veeranki S, Winchester LJ and Tyagi SC:
Hyperhomocysteinemia associated skeletal muscle weakness involves
mitochondrial dysfunction and epigenetic modifications. Biochim
Biophys Acta. 1852:732–741. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Moat SJ, Doshi SN, Lang D, McDowell IF,
Lewis MJ and Goodfellow J: Treatment of coronary heart disease with
folic acid: is there a future? Am J Physiol Heart Circ Physiol.
287:H1–H7. 2004. View Article : Google Scholar : PubMed/NCBI
|