The CCN family of proteins is an acronym for
cysteine-rich protein 61 (CYR61), connective tissue growth factor
(CTGF) and nephroblastoma overexpressed (NOV), which were first
identified in mouse, human and chicken in the early 1990s (1–3).
Another three family members exhibiting the same basic structure
domains of the first three CCN members have since been identified.
The latter three members are involved in the Wnt-1 inducible
signalling pathway and consist of Wnt-1-induced secreted protein-1
(WISP-1), WISP-2, and WISP-3 (4).
As each CCN family member has several names associated with its
structures or functions, the official nomenclature has been
recommended (Table I).
CCNs are present in vertebrates, including
zebrafish, poultry such as chickens, rodents including mice and
rats, as well as humans and have been conserved during evolution.
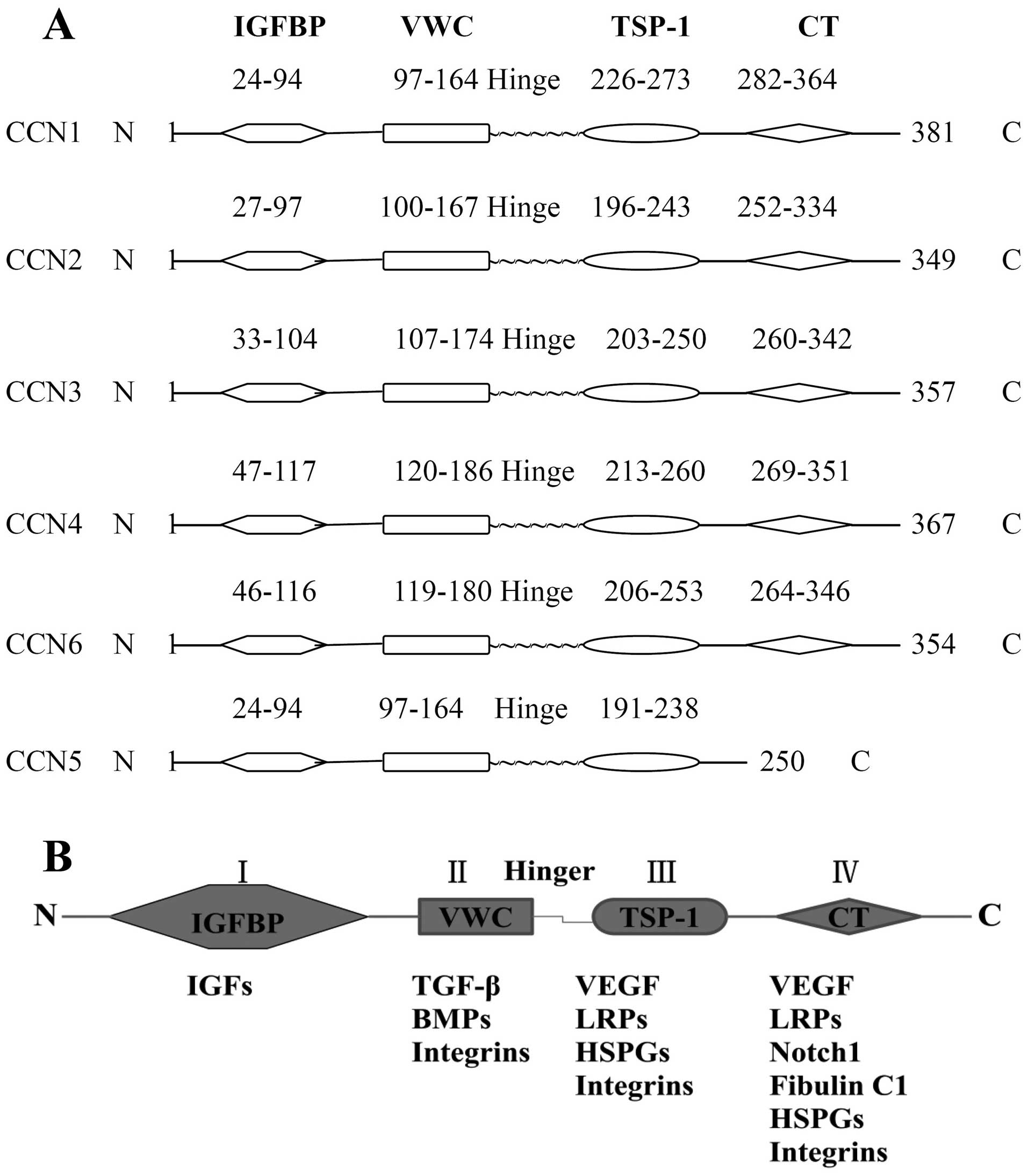
CCNs, with the exception of CCN5, which lacks a cysteine knot
domain (CT) module, comprise an N-terminal secretory signal peptide
and four functional domains: an insulin-like growth factor-binding
protein domain (IGFBP), a Von Willebrand factor domain (VWC), a
thrombospondin type-1 repeat module (TSP-1), and a CT (Fig. 1A). The two N-terminal domains are
separated from the two C-domains by a variable linking sequence of
amino acids (5). According to the
domains CCNs, except CCN5, share five common exons, the first of
which codes the signal sequence, while the other CCNs sequentially
code the four functional domains with corresponding numbers of
amino acids ranging from 349 to 381 (6).
The four discrete functional domains have different
molecular structures that determine the types of binding partners
and ligands with which they interact, resulting in a variety of
biological functions. The known binding partners of each domain are
different: insulin-like growth factors (IGFs) bind with IGFBP;
transforming growth factor β (TGF-β), bone morphogenic proteins
(BMPs) and integrins bind with VWC; vascular endothelial growth
factor (VEGF), LDL receptor proteins (LRPs), heparan sulphate
proteoglycans (HSPGs) and integrins bind with TSP-1; and VEGF,
LRPs, integrins, neurogenic locus notch homolog protein 1 (Notch1),
fibulin C1, HSPGs and integrins bind with CT (7,8)
(Fig. 1B).
CCNs exhibit different expression profiles and
transcript levels in different tissues, organs and tumors (Tables II and III). The different expression levels
of CCNs observed in embryonic tissues compared with that of adult
organs indicates a potential role in development (Table IV). The changing transcript
levels in tumors mean that CCNs may also be important during
tumorigenesis.
The subcellular localization of each of the CCNs is
also different. Immunohistochemical localization of CCN1 protein
has indicated that invasive carcinoma cells show significant
cytoplasmic and perinuclear protein overexpression compared to
non-neoplastic ductal epithelium in invasive ductal carcinoma,
whereas in ductal carcinoma in situ and lobular carcinoma
in situ, CCN1 expression was weaker and heterogeneous
(9). Previous findings have shown
that CCN1 was detected, albeit not abundantly, in culture medium
(10). CCN2 protein was detected
in the nuclei of B16 (F10) cells and at the cell membrane, but was
rarely detectable in the cytoplasm and the cell culture medium
(10,11). CCN3 was detected in the medium,
extracellular matrix (ECM) and at the cell membrane (12–14). A previous study revealed strong
immunohistochemical staining of CCN4, CCN5 and CCN6 in normal
colorectal epithelial cells, which was confined primarily to the
cell membrane with slight staining of stromal tissue. In colorectal
cancer (CRC) tissues, cell membrane and cytoplasmic staining were
assessed. Membrane staining showed a reduction in CCN4, CCN5 and
CCN6, whereas cytoplasmic staining showed a reduction in CCN5 but
an increase in CCN4 and CCN6 (15). Furthermore, CCN5 is mainly
localized to the nucleus in rat and human tissues (16).
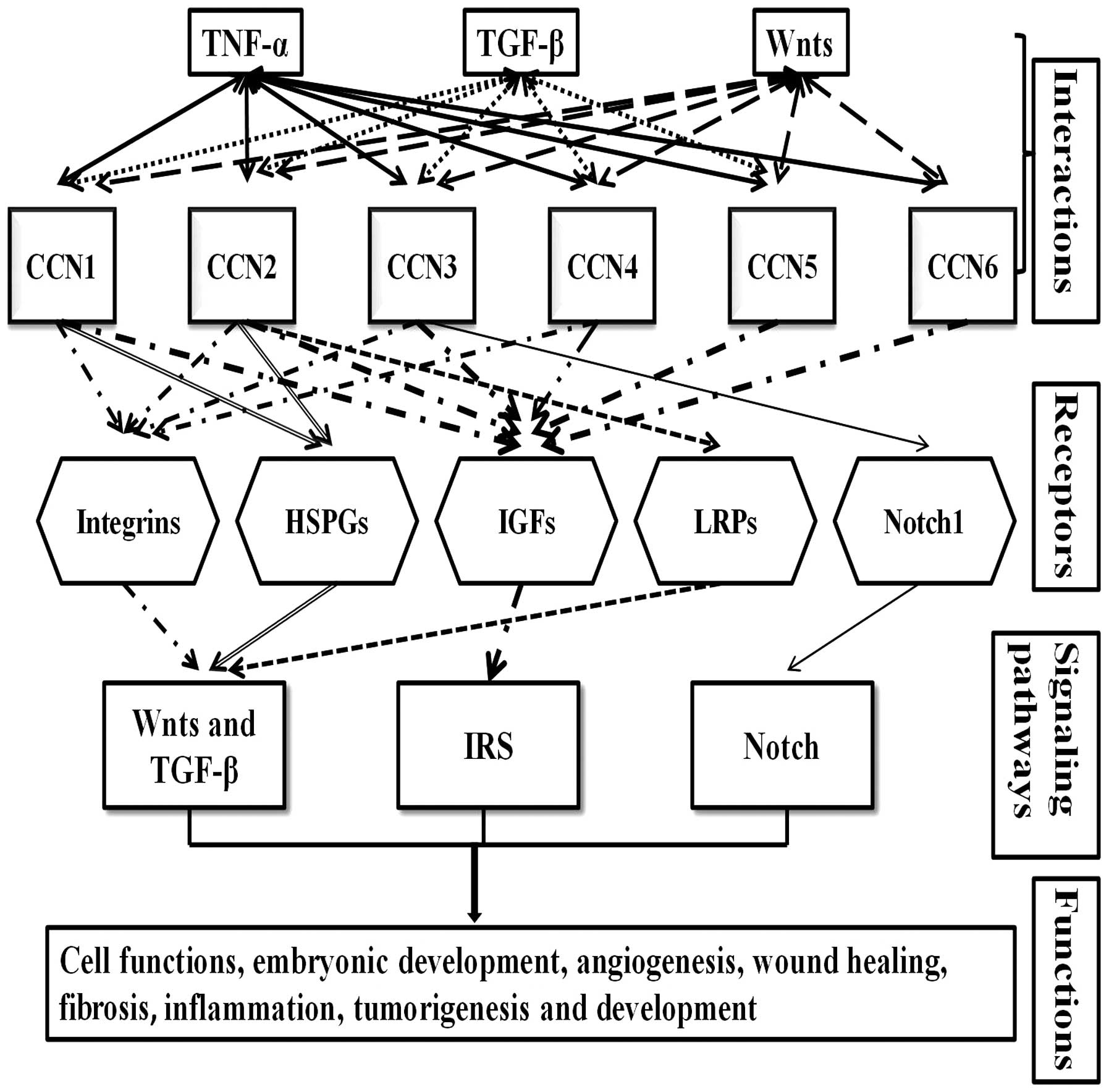
Similar to some ECM proteins, CCNs mediate cell
functions, embryonic development, angiogenesis, wound healing,
fibrosis, inflammation, tumorigenesis and development primarily
through binding and interacting with well-known receptors,
including integrins, HSPGs, IGFs, and lipoprotein receptor-related
proteins (LRPs). Signalling pathways, such as Wnts, TGF-β, insulin
receptor signalling (IRS) and Notch, are involved in the regulation
of these cell functions. The interaction of CCNs with receptors and
other main cytokines has been briefly summarised (Fig. 2).
Integrins, found as heterodimers consisting of α-
and β-subunits are common transmembrane receptors that mediate
cell-to-cell and cell-to-ECM adhesive interactions while also
transducing signals from the ECM to the cell interior and vice
versa. Currently, there are 24 members in the integrin family that
have been identified to have 18 α-subunits and 8 β-subunits in
their structures. In previous decades, it has been shown that
integrins are associated with the different functions of CCNs
(8,17) (Table
V).
HSPGs are known to serve as co-receptors with
integrins under certain circumstances (18). Heparin and HSPGs play important
roles in modulating cell adhesion and fibrosis through TSP or CT
domains (19,20). CCNs are also capable of binding to
HSPGs and mediate cell adhesion and Wnt signalling in some cell
types (21–23). Furthermore, it has been previously
demonstrated that CCN2 binds to fibronectin (HSPG2) through the CT
domain and regulates cell functions (24,25).
The IGF family, which includes the polypeptide
ligands IGF-I and IGF-II, two types of cell membrane receptors
(IGF-IR and IGF-IIR), six binding proteins (IGFBP-1 to IGFBP-6) and
IGFBP proteases play an important role in various types of cancer
(26). The IGFs have interactions
with various molecules that are known to be involved in cancer
development and progression. CCNs may bind IGFs with low affinity
(27), however, the impact on
several cell functions needs to be examined. In previous decades,
the regulative role of CCNs in co-ordinating cell functions has
been a major research focus. Overexpression of CCN2 in chondrocytes
elevates the mRNA transcript levels of IGF-I and IGF-II, resulting
in increased bone growth (28).
Conversely, CCN6 decreases the IGF-1-induced activation of the
IGF-IR, and two of its main downstream signalling molecules,
insulin receptor substrate 1 (IRS1) and extracellular
signal-regulated kinase (ERK)-1/2 in inflammatory breast cancer
cells (29). Downregulation of
CCN6 enhances the effects of IGF-I and increases the growth,
motility and invasiveness of human mammary epithelial cells
(30).
TNF-α regulates CCN1 and CCN2 in a
cell-type-specific manner. TNF-α represses CCN1 and CCN2 expression
in chondrocytes but induces CCN1 expression in osteoblasts and CCN2
expression in synovial cells (37–39). Kular et al identified that
TNF-α stimulated CCN3, CCN4 and CCN6 expression in melanocytes,
cardiac myocytes and fibroblasts and fibroblast-like synoviocytes,
respectively. By contrast, TNF-α stimulated CCN3 expression but
exerted an inhibitory effect on CCN4 expression in cultured
astrocytes (40).
TGF-β has been reported to promote the expression of
CCN1, CCN2, CCN4 and CCN5 but represses the expression of CCN3 in
chondrosarcoma-derived HCS-2/8 and murine osteoblastic cells
(37,41). By contrast, the expression levels
of CCN2, CCN3 and CCN4 were inversely correlated with TGF-β in
leiomyomas (42). Thus, CCN2 is
closely associated with TGF-β as this interaction represses the
expression of TGF-β signalling inhibitors (such as Smad7) through
the VWC domain (43).
CCN2 has been shown to induce chondrocyte
differentiation, through a p38 mitogen-activated protein kinase
(p38/MAPK), and proliferation, through the p44/42 MAPK/ERK
(49).
The functions of CCNs have been revealed in a wide
range of cell types, regulating their cell functions through a
variety of mechanisms. CCN1 increased cell adhesion and migration
through the integrin α6β1-HSPG co-receptors in fibroblasts,
endothelial cells and vascular smooth muscle cells (50,51). In endothelial cells, CCN1 has also
been shown to promote cell adhesion, migration, survival, growth
factor-induced mitogenesis and endothelial tubule formation via
integrin α6β1 (52). CCN2
promoted the adhesion and migration of microvascular endothelial
cells through an integrin-αvβ3-dependent mechanism (53). CCN3 increased the adhesion of
normal melanocytes to collagen type IV (54). However, CCN3 expression was also
decreased immediately after wounding or re-epithelialization
(55), indicating the ability of
CCN3 to negatively regulate fibroblast proliferation. CCN4
stimulated the migration and proliferation through integrin α5β1 in
vascular smooth muscle cells (56). CCN4 has also been verified to
promote the proliferation of hepatic stellate cells in vitro
(57). CCN5 increased cell
proliferation and survival against Streptozotocin in pancreatic
cells (58). However, in vascular
smooth muscle cells, CCN5 negatively regulated smooth muscle cell
proliferation and motility (59).
An inhibitory effect on in vitro growth of the human mammary
epithelial cells function was also assigned to CCN6 (60).
CCN expression profiles appear to be integral to the
development of several key organ systems. CCN1 expression has been
closely associated with the development of skeletal,
cardiovascular, and neuronal systems during mice embryogenesis,
best demonstrated by a CCN1 knockout mice model which exhibited
aberrations in vascular development (61,62). CCN2 knockout mice died at birth,
due to respiratory failure resulting from hypoplastic lungs and
poor thoracic development (63).
A CCN2 knockdown zebrafish model showed bone defects and disruption
in notochord development (64).
CCN3 mutant mice exhibited skeletal and cardiac abnormalities, such
as cardiomyopathy, muscle atrophy, and cataract formation (65). Evidence suggests that CCN4 has an
an important regulatory function in skeletal growth and bone repair
(66). The role of CCN5 remains
unclear; however, it may serve a multifunctional purpose in
developing mice and human embryos (67). CCN6 mutations in humans cause
autosomal recessive skeletal disease progressive pseudorheumatoid
dysplasia, a juvenile-onset joint degenerative disease (68). However, CCN6-null or
CCN6-overexpression mice exhibited no observable phenotype
(69). These findings from CCN
knockout mice models together with their known expression profiles
in the developmental stages (Table
IV) suggest that CCN1 and CCN2 play an essential role, while
the other four members may play a regulatory role, in human
embryonic development.
CCN1 and CCN2 are involved in tissue repair, as the
increased expression of the two CCNs has been observed during
cutaneous wound healing, liver regeneration, in the heart after
myocardial infarction and after bone fracture (70–74). Xu et al showed that CCN2
acted as a downstream effector of TGF-β enhancing the production of
scar tissue indicating that the suppression of CCN2 may prevent a
progressive fibrotic response to TGF-β stimulation (75). Of note, CCN3 transcripts were
decreased during the first three days after wound formation or
re-epithelialization (55).
CCNs, except CCN5, have four highly conservative
functional domains, but play different roles in the same cancer
type. Each CCN member may also play different roles in varying
cancer types through different signalling pathways (Fig. 2 and Table VI). Some CCN members have already
been associated with cancer staging and prognosis as well as
contributing to tumorigenesis or metastasis formation (85–93). Other CCN members have been
considered as diagnostic or prognostic markers and therapeutic
target genes in certain cancer types (46,103,104,119,120).
CCN1 mRNA and protein levels are increased in
ovarian cancer cells and may play an important role in ovarian
carcinogenesis (85). CCN1 is
upregulated in prostate cancer cell lines and tumor tissues and is
associated with the status of the tumor-suppressor gene p53
(86). CCN1 has also been shown
to enhance prostate cancer cell migration via alterations of
function to integrins (87). An
immunohistochemical analysis of 112 human glioma and normal brain
specimens showed that the levels of tumor-associated CCN1 protein
were increased with tumor grade (P<0.001), and this trend was
verified with similar results identified in glioma cells (88). These results have identified a
CCN1-dependent pathway that mediates cell growth, cell migration,
and long-lasting signalling events in glioma cell lines and
possibly astroglial malignancies. CCN1 is overexpressed in U343
glioma cells and has been linked with the integrin-linked
kinase-mediated Akt and β-catenin-TCF/Lef signalling pathways
(89). CCN1 is a transcriptional
target of Hh-GLI signalling leading to increased vascularity and
spontaneous metastasis of breast cancer cells (90). Zuo et al demonstrated that
the overexpression of CCN1 in breast cancer is associated with the
tumorigenesis, migration and invasion of cancer cells (6). CCN1 was expressed in ~30% of
invasive breast cancer biopsies and played a role in breast cancer
progression, possibly through its interactions with the avb3
receptor (91). CCN1 was found to
be overexpressed in patients with endometrial carcinoma and
indicative of a poor prognosis (92). CCN1 has also been shown to be
overexpressed and correlate with invasion and metastasis in CRC
(93).
Other studies, however, have shown different
results. For instance, CCN1 expression was found to be reduced in
endometrial cancer and lung cancer tissues compared to their paired
normal tissues (94,95). Notably, the expression levels of
CCN1 were reduced in high-grade chondrosarcomas and advanced
gastric cancers (96,97).
CCN2 mRNA and protein levels are increased in murine
and human rhabdomyosarcoma cells (98). Overexpression of CCN2 increases
breast cancer cell migration in Boyden chamber assays and promotes
angiogenesis in chorioallantoic membrane assays compared to control
cells in vitro (99). By
contrast, a reduced expression of CCN2 in clinical breast cancer
samples based on a qPCR study is associated with poor prognosis
(P=0.021), metastasis (P=0.012), local recurrence (P=0.0024) and
mortality (P=0.0072) (100).
Similarly, findings in CRC are controversial. CCN2 may play an
oncogenic role in the progression of well-differentiated CRC
(101). However, Lin et
al showed that lower CCN2 expression levels in CRC patients
were associated with a higher peritoneal recurrence rate.
Additionally, CCN2 overexpression decreased the incidence of
peritoneal carcinomatosis and increased the rate of mice survival,
but significantly decreased CRC cell adhesion ability in
vitro (102). CCN2
overexpression was also found to be associated with poor prognosis
in oesophageal squamous cell carcinoma, pancreatic cancer,
high-grade chondro sarcomas and enchondromas (46,100,103,104).
Other cancer types have resulted in inconsistent
results compared to those mentioned above. CCN3-transfected glioma
cells induced tumors to a lesser degree than their parental
counterparts, which did not express detectable amounts of CCN3
(115). In vitro, CCN3
exerted an anti-proliferative effect and interfered with the S/G2
transition of the cell cycle, thereby inducing an artificial
accumulation of glioblastoma cells (G59) at the S phase (116). CCN3 restored cell growth
regulatory properties that were absent in chronic myeloid leukaemia
and sensitized chronic myeloid leukaemia cells to imatinib-induced
apoptosis (117). CCN3 protein
levels were significantly modified in malignant adrenocortical
tumors, but not in benign adrenocortical tumors (118). CCN3 suppressed the cell
proliferation via interaction with the gap junction protein
Connexin43 in glioma cells, and high levels of CCN3 reduced
tumorigenicity, resulting in a lower rate of metastasis (119,120). CCN3 in vitro has been
reported to decrease the transcription and activation of matrix
metal-loproteinases and suppress the invasion of melanoma cells,
indicating that the downregulation of CCN3 expression is a
potential mechanism for melanoma progression (121).
CCN4 is downstream of Wnt-1 signalling and CCN4
overexpression in colon cancer and may play a role in colon
tumorigenesis (4). It has been
revealed that CCN4 transcripts are expressed at higher levels in
tumor samples compared to normal tissue, and are higher in patients
with Dukes' stage B and C compared to Dukes' A. Thus, CCN4 appears
to act as a factor for stimulating aggressiveness in colon cancer
(15). A similar behavior pattern
was observed in oral squamous cell carcinoma cells as CCN4 enhanced
their expression by increasing ICAM-1 expression through the αvβ3
integrin receptor and the ASK1, JNK/p38 and AP-1 signal
transduction pathways (122).
By contrast, CCN4 inhibited the growth and
metastasis of melanoma cells and its expression is increased in low
metastatic cells compared to high metastatic cells (123,124). CCN4 overexpression inhibits the
motility and invasion of lung cancer cells through the inhibition
of Rac activation in vitro (125). Similar results have been
identified in clinical specimens in which CCN4 has been shown to be
reduced in chondrosarcoma and breast cancer with poor prognosis,
suggesting it is a putative tumor suppressor (126,127).
CCN5 has been shown to be increased in
hepatocellular carcinoma compared to paired normal tissues
(128), as well as in
adrenocorticotropic hormone-secreting pituitary tumors compared to
normal pituitaries (129).
However, previous findings focusing on the role of CCN5 in breast
cancer remain controversial. Ji et al reported that CCN5
mRNA and protein levels were increased in some breast cancer cells
and in breast tumors from patients with poor prognosis (130). However, CCN5 mRNA and protein
levels were significantly reduced as the cancer progressed from a
non-invasive to invasive type in breast cancer, and CCN5 mRNA and
protein levels were almost undetectable in poorly differentiated
cancers compared to the moderately or well-differentiated samples
(131). In vitro studies
have shown that CCN5 was a negative regulator of growth, migration
and invasion of breast cancer cells (132,133).
CCN6 was overexpressed in 63% of the colon tumors
analyzed and may be downstream of Wnt-1 signalling, thus playing a
role in colon tumorigenesis (4).
A similar result was obtained in a microsatellite instability
subtype of CRC (4,137). However, previous findings
revealed that there is no significant difference in CCN6 mRNA
levels expressed in the majority of CRC in comparison with paired
normal tissues (15). CCN6
transcripts may also play a positive role in the development of
hepatocellular carcinoma (138).
Knockdown of CCN6 expression suppressed gastric cancer cell
proliferation and migration via the Wnt/β-catenin signalling
pathway in vitro, while a high expression of CCN6 indicated
poor prognosis in a gastric cancer clinical cohort (139).
CCN6 mRNA was reduced in 80% of poor outcome cases
of breast cancer, and was found to be essential to induce the
process of epithelial-mesenchymal transition (EMT) in breast cancer
(60). CCN6 overexpression
inhibited cell growth and invasiveness in breast cancer cell lines
(140) and CCN6 expression was
reduced in breast cancer samples compared to paired normal tissues
(141). Taken together, the
evidence suggests CCN6 is a putative tumor suppressor in breast
cancer.
The perturbed expression of CCNs has been observed
in a variety of malignancies. The aberrant expression of certain
CCNs is associated with disease progression and poor prognosis.
Different CCNs may play contrasting roles in the same cancer, while
the same CCN may play different roles in various types of cancer.
Further investigations may highlight their clinical relevance and
application for predicting prognosis. CCNs comprise four functional
domains and exhibit differential expression and functions in
different cells and tissues albeit CCN5 lacks a CT module. CCNs can
regulate cell functions by acting as ligands for integrins,
heparin, and HSPGs, which are regulated by certain growth factors
and cytokines, including IGFs, TGF-α and TGF-β, to fulfil their
role in the consequent physiological and pathological events.
Additionally, CCNs interact with a variety of receptors and
cytokines by modulating downstream signal transduction. Insight
into the detailed mechanisms involved in CCN-mediated regulation
may be useful in understanding their roles and functions in
tumorigenesis and cancer metastasis. This may provide new avenues
for target therapy in certain malignancies.
The authors would like to thank the Cancer Research
Wales and the Cardiff China Medical Research Collaborative (CCMRC)
for supporting their study.
|
1
|
O'Brien TP, Yang GP, Sanders L and Lau LF:
Expression of cyr61, a growth factor-inducible immediate-early
gene. Mol Cell Biol. 10:3569–3577. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bradham DM, Igarashi A, Potter RL and
Grotendorst GR: Connective tissue growth factor: A cysteine-rich
mitogen secreted by human vascular endothelial cells is related to
the SRC-induced immediate early gene product CEF-10. J Cell Biol.
114:1285–1294. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Joliot V, Martinerie C, Dambrine G,
Plassiart G, Brisac M, Crochet J and Perbal B: Proviral
rearrangements and overexpression of a new cellular gene (nov) in
myeloblastosis-associated virus type 1-induced nephroblastomas. Mol
Cell Biol. 12:10–21. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pennica D, Swanson TA, Welsh JW, Roy MA,
Lawrence DA, Lee J, Brush J, Taneyhill LA, Deuel B, Lew M, et al:
WISP genes are members of the connective tissue growth factor
family that are up-regulated in wnt-1-transformed cells and
aberrantly expressed in human colon tumors. Proc Natl Acad Sci USA.
95:14717–14722. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Desnoyers L: Structural basis and
therapeutic implication of the interaction of CCN proteins with
glycoconjugates. Curr Pharm Des. 10:3913–3928. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zuo GW, Kohls CD, He BC, Chen L, Zhang W,
Shi Q, Zhang BQ, Kang Q, Luo J, Luo X, et al: The CCN proteins:
Important signaling mediators in stem cell differentiation and
tumorigenesis. Histol Histopathol. 25:795–806. 2010.PubMed/NCBI
|
|
7
|
Holbourn KP, Acharya KR and Perbal B: The
CCN family of proteins: Structure-function relationships. Trends
Biochem Sci. 33:461–473. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen CC and Lau LF: Functions and
mechanisms of action of CCN matricellular proteins. Int J Biochem
Cell Biol. 41:771–783. 2009. View Article : Google Scholar :
|
|
9
|
Hirschfeld M, zur Hausen A, Bettendorf H,
Jäger M and Stickeler E: Alternative splicing of Cyr61 is regulated
by hypoxia and significantly changed in breast cancer. Cancer Res.
69:2082–2090. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ball DK, Surveyor GA, Diehl JR, Steffen
CL, Uzumcu M, Mirando MA and Brigstock DR: Characterization of 16-
to 20-kilodalton (kDa) connective tissue growth factors (CTGFs) and
demonstration of proteolytic activity for 38-kDa CTGF in pig
uterine luminal flushings. Biol Reprod. 59:828–835. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sha W and Leask A: CCN2 expression and
localization in melanoma cells. J Cell Commun Signal. 5:219–226.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Perbal B: CCN proteins: A centralized
communication network. J Cell Commun Signal. 7:169–177. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Joliot A, Triller A, Volovitch M and
Prochiantz A: Are embryonic forms of NCAM homeobox receptors? C R
Acad Sci III. (Suppl 9): 59–63. 1992.In French.
|
|
14
|
Kyurkchiev S, Yeger H, Bleau AM and Perbal
B: Potential cellular conformations of the CCN3(NOV) protein. Cell
Commun Signal. 2(9)2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Davies SR, Davies ML, Sanders A, Parr C,
Torkington J and Jiang WG: Differential expression of the CCN
family member WISP-1, WISP-2 and WISP-3 in human colorectal cancer
and the prognostic implications. Int J Oncol. 36:1129–1136.
2010.PubMed/NCBI
|
|
16
|
Wiesman KC, Wei L, Baughman C, Russo J,
Gray MR and Castellot JJ: CCN5, a secreted protein, localizes to
the nucleus. J Cell Commun Signal. 4:91–98. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Takada Y, Ye X and Simon S: The integrins.
Genome Biol. 8(215)2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang GP and Lau LF: Cyr61, product of a
growth factor-inducible immediate early gene, is associated with
the extracellular matrix and the cell surface. Cell Growth Differ.
2:351–357. 1991.PubMed/NCBI
|
|
19
|
Chen CC, Young JL, Monzon RI, Chen N,
Todorović V and Lau LF: Cytotoxicity of TNFalpha is regulated by
integrin-mediated matrix signaling. EMBO J. 26:1257–1267. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Holbourn KP, Perbal B and Ravi Acharya K:
Proteins on the catwalk: Modelling the structural domains of the
CCN family of proteins. J Cell Commun Signal. 3:25–41. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Segarini PR, Nesbitt JE, Li D, Hays LG,
Yates JR III and Carmichael DF: The low density lipoprotein
receptor-related protein/alpha2-macroglobulin receptor is a
receptor for connective tissue growth factor. J Biol Chem.
276:40659–40667. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gao R and Brigstock DR: Low density
lipoprotein receptor-related protein (LRP) is a heparin-dependent
adhesion receptor for connective tissue growth factor (CTGF) in rat
activated hepatic stellate cells. Hepatol Res. 27:214–220. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mercurio S, Latinkic B, Itasaki N,
Krumlauf R and Smith JC: Connective-tissue growth factor modulates
WNT signalling and interacts with the WNT receptor complex.
Development. 131:2137–2147. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nishida T, Kubota S, Fukunaga T, Kondo S,
Yosimichi G, Nakanishi T, Takano-Yamamoto T and Takigawa M:
CTGF/Hcs24, hypertrophic chondrocyte-specific gene product,
interacts with perlecan in regulating the proliferation and
differentiation of chondrocytes. J Cell Physiol. 196:265–275. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Abraham S, Riggs MJ, Nelson K, Lee V and
Rao RR: Characterization of human fibroblast-derived extracellular
matrix components for human pluripotent stem cell propagation. Acta
Biomater. 6:4622–4633. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu H and Rohan T: Role of the insulin-like
growth factor family in cancer development and progression. J Natl
Cancer Inst. 92:1472–1489. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hwa V, Oh Y and Rosenfeld RG: The
insulin-like growth factor-binding protein (IGFBP) superfamily.
Endocr Rev. 20:761–787. 1999.PubMed/NCBI
|
|
28
|
Tomita N, Hattori T, Itoh S, Aoyama E, Yao
M, Yamashiro T and Takigawa M: Cartilage-specific over-expression
of CCN family member 2/connective tissue growth factor (CCN2/CTGF)
stimulates insulin-like growth factor expression and bone growth.
PLoS One. 8:e592262013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kleer CG, Zhang Y, Pan Q and Merajver SD:
WISP3 (CCN6) is a secreted tumor-suppressor protein that modulates
IGF signaling in inflammatory breast cancer. Neoplasia. 6:179–185.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang Y, Pan Q, Zhong H, Merajver SD and
Kleer CG: Inhibition of CCN6 (WISP3) expression promotes neoplastic
progression and enhances the effects of insulin-like growth
factor-1 on breast epithelial cells. Breast Cancer Res.
7:R1080–R1089. 2005. View Article : Google Scholar
|
|
31
|
Abreu JG, Ketpura NI, Reversade B and De
Robertis EM: Connective-tissue growth factor (CTGF) modulates cell
signalling by BMP and TGF-beta. Nat Cell Biol. 4:599–604.
2002.PubMed/NCBI
|
|
32
|
Inoki I, Shiomi T, Hashimoto G, Enomoto H,
Nakamura H, Makino K, Ikeda E, Takata S, Kobayashi K and Okada Y:
Connective tissue growth factor binds vascular endothelial growth
factor (VEGF) and inhibits VEGF-induced angiogenesis. FASEB J.
16:219–221. 2002.
|
|
33
|
Sakamoto K, Yamaguchi S, Ando R, Miyawaki
A, Kabasawa Y, Takagi M, Li CL, Perbal B and Katsube K: The
nephro-blastoma overexpressed gene (NOV/ccn3) protein associates
with Notch1 extracellular domain and inhibits myoblast
differentiation via Notch signaling pathway. J Biol Chem.
277:29399–29405. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Perbal B, Martinerie C, Sainson R, Werner
M, He B and Roizman B: The C-terminal domain of the regulatory
protein NOVH is sufficient to promote interaction with fibulin 1C:
A clue for a role of NOVH in cell-adhesion signaling. Proc Natl
Acad Sci USA. 96:869–874. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li CL, Martinez V, He B, Lombet A and
Perbal B: A role for CCN3 (NOV) in calcium signalling. Mol Pathol.
55:250–261. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Perbal B: NOV (nephroblastoma
overexpressed) and the CCN family of genes: Structural and
functional issues. Mol Pathol. 54:57–79. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Moritani NH, Kubota S, Sugahara T and
Takigawa M: Comparable response of ccn1 with ccn2 genes upon
arthritis: An in vitro evaluation with a human chondrocytic cell
line stimulated by a set of cytokines. Cell Commun Signal.
3(6)2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kok SH, Hou KL, Hong CY, Wang JS, Liang
PC, Chang CC, Hsiao M, Yang H, Lai EH and Lin SK: Simvastatin
inhibits cytokine-stimulated Cyr61 expression in osteoblastic
cells: A therapeutic benefit for arthritis. Arthritis Rheum.
63:1010–1020. 2011. View Article : Google Scholar
|
|
39
|
Nozawa K, Fujishiro M, Kawasaki M, Kaneko
H, Iwabuchi K, Yanagida M, Suzuki F, Miyazawa K, Takasaki Y, Ogawa
H, et al: Connective tissue growth factor promotes articular damage
by increased osteoclastogenesis in patients with rheumatoid
arthritis. Arthritis Res Ther. 11:R1742009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kular L, Pakradouni J, Kitabgi P, Laurent
M and Martinerie C: The CCN family: A new class of inflammation
modulators? Biochimie. 93:377–388. 2011. View Article : Google Scholar
|
|
41
|
Parisi MS, Gazzerro E, Rydziel S and
Canalis E: Expression and regulation of CCN genes in murine
osteoblasts. Bone. 38:671–677. 2006. View Article : Google Scholar
|
|
42
|
Luo X, Ding L and Chegini N: CCNs,
fibulin-1C and S100A4 expression in leiomyoma and myometrium:
Inverse association with TGF-beta and regulation by TGF-beta in
leiomyoma and myometrial smooth muscle cells. Mol Hum Reprod.
12:245–256. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mason RM: Connective tissue growth
factor(CCN2), a pathogenic factor in diabetic nephropathy. What
does it do? How does it do it? J Cell Commun Signal. 3:95–104.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Si W, Kang Q, Luu HH, Park JK, Luo Q, Song
WX, Jiang W, Luo X, Li X, Yin H, et al: CCN1/Cyr61 is regulated by
the canonical Wnt signal and plays an important role in
Wnt3A-induced osteoblast differentiation of mesenchymal stem cells.
Mol Cell Biol. 26:2955–2964. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Latinkic BV, Mercurio S, Bennett B, Hirst
EM, Xu Q, Lau LF, Mohun TJ and Smith JC: Xenopus Cyr61 regulates
gastrulation movements and modulates Wnt signalling. Development.
130:2429–2441. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Deng YZ, Chen PP, Wang Y, Yin D, Koeffler
HP, Li B, Tong XJ and Xie D: Connective tissue growth factor is
overexpressed in esophageal squamous cell carcinoma and promotes
tumorigenicity through beta-catenin-T-cell factor/Lef signaling. J
Biol Chem. 282:36571–36581. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Smerdel-Ramoya A, Zanotti S, Deregowski V
and Canalis E: Connective tissue growth factor enhances
osteoblastogenesis in vitro. J Biol Chem. 283:22690–22699. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Rydziel S, Stadmeyer L, Zanotti S, Durant
D, Smerdel-Ramoya A and Canalis E: Nephroblastoma overexpressed
(Nov) inhibits osteoblastogenesis and causes osteopenia. J Biol
Chem. 282:19762–19772. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yosimichi G, Nakanishi T, Nishida T,
Hattori T, Takano-Yamamoto T and Takigawa M: CTGF/Hcs24 induces
chondrocyte differentiation through a p38 mitogen-activated protein
kinase (p38MAPK), and proliferation through a p44/42
MAPK/extracellular-signal regulated kinase (ERK). Eur J Biochem.
268:6058–6065. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chen N, Chen CC and Lau LF: Adhesion of
human skin fibroblasts to Cyr61 is mediated through integrin alpha
6beta 1 and cell surface heparan sulfate proteoglycans. J Biol
Chem. 275:24953–24961. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Grzeszkiewicz TM, Lindner V, Chen N, Lam
SC and Lau LF: The angiogenic factor cysteine-rich 61 (CYR61, CCN1)
supports vascular smooth muscle cell adhesion and stimulates
chemotaxis through integrin alpha(6)beta(1) and cell surface
heparan sulfate proteoglycans. Endocrinology. 143:1441–1450.
2002.PubMed/NCBI
|
|
52
|
Leu SJ, Lam SC and Lau LF: Pro-angiogenic
activities of CYR61 (CCN1) mediated through integrins alphavbeta3
and alpha6beta1 in human umbilical vein endothelial cells. J Biol
Chem. 277:46248–46255. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Babic AM, Chen CC and Lau LF: Fisp12/mouse
connective tissue growth factor mediates endothelial cell adhesion
and migration through integrin alphavbeta3, promotes endothelial
cell survival, and induces angiogenesis in vivo. Mol Cell Biol.
19:2958–2966. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Fukunaga-Kalabis M, Martinez G, Liu ZJ,
Kalabis J, Mrass P, Weninger W, Firth SM, Planque N, Perbal B and
Herlyn M: CCN3 controls 3D spatial localization of melanocytes in
the human skin through DDR1. J Cell Biol. 175:563–569. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lin CG, Leu SJ, Chen N, Tebeau CM, Lin SX,
Yeung CY and Lau LF: CCN3 (NOV) is a novel angiogenic regulator of
the CCN protein family. J Biol Chem. 278:24200–24208. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Liu H, Dong W, Lin Z, Lu J, Wan H, Zhou Z
and Liu Z: CCN4 regulates vascular smooth muscle cell migration and
proliferation. Mol Cells. 36:112–118. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Jian YC, Wang JJ, Dong S, Hu JW, Hu LJ,
Yang GM, Zheng YX and Xiong WJ: Wnt-induced secreted protein 1/CCN4
in liver fibrosis both in vitro and in vivo. Clin Lab. 60:29–35.
2014.PubMed/NCBI
|
|
58
|
Chowdhury S, Wang X, Srikant CB, Li Q, Fu
M, Gong YJ, Ning G and Liu JL: IGF-I stimulates CCN5/WISP2 gene
expression in pancreatic β-cells, which promotes cell proliferation
and survival against streptozotocin. Endocrinology. 155:1629–1642.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Lake AC and Castellot JJ Jr: CCN5
modulates the antiproliferative effect of heparin and regulates
cell motility in vascular smooth muscle cells. Cell Commun Signal.
1(5)2003. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kleer CG, Zhang Y and Merajver SD: CCN6
(WISP3) as a new regulator of the epithelial phenotype in breast
cancer. Cells Tissues Organs. 185:95–99. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
O'Brien TP and Lau LF: Expression of the
growth factor-inducible immediate early gene cyr61 correlates with
chondrogenesis during mouse embryonic development. Cell Growth
Differ. 3:645–654. 1992.PubMed/NCBI
|
|
62
|
Mo FE, Muntean AG, Chen CC, Stolz DB,
Watkins SC and Lau LF: CYR61 (CCN1) is essential for placental
development and vascular integrity. Mol Cell Biol. 22:8709–8720.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Baguma-Nibasheka M and Kablar B: Pulmonary
hypoplasia in the connective tissue growth factor (Ctgf) null
mouse. Dev Dyn. 237:485–493. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Chiou MJ, Chao TT, Wu JL, Kuo CM and Chen
JY: The physiological role of CTGF/CCN2 in zebrafish notochond
development and biological analysis of the proximal promoter
region. Biochem Biophys Res Commun. 349:750–758. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Heath E, Tahri D, Andermarcher E,
Schofield P, Fleming S and Boulter CA: Abnormal skeletal and
cardiac development, cardiomyopathy, muscle atrophy and cataracts
in mice with a targeted disruption of the Nov (Ccn3) gene. BMC Dev
Biol. 8(18)2008. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
French DM, Kaul RJ, D'Souza AL, Crowley
CW, Bao M, Frantz GD, Filvaroff EH and Desnoyers L: WISP-1 is an
osteoblastic regulator expressed during skeletal development and
fracture repair. Am J Pathol. 165:855–867. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Jones JA, Gray MR, Oliveira BE, Koch M and
Castellot JJ Jr: CCN5 expression in mammals: I. Embryonic and fetal
tissues of mouse and human. J Cell Commun Signal. 1:127–143. 2007.
View Article : Google Scholar
|
|
68
|
Hurvitz JR, Suwairi WM, Van Hul W,
El-Shanti H, Superti-Furga A, Roudier J, Holderbaum D, Pauli RM,
Herd JK, Van Hul EV, et al: Mutations in the CCN gene family member
WISP3 cause progressive pseudorheumatoid dysplasia. Nat Genet.
23:94–98. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
69
|
Kutz WE, Gong Y and Warman ML: WISP3, the
gene responsible for the human skeletal disease progressive
pseudorheumatoid dysplasia, is not essential for skeletal function
in mice. Mol Cell Biol. 25:414–421. 2005. View Article : Google Scholar :
|
|
70
|
Hilfiker-Kleiner D, Kaminski K, Kaminska
A, Fuchs M, Klein G, Podewski E, Grote K, Kiian I, Wollert KC,
Hilfiker A, et al: Regulation of proangiogenic factor CCN1 in
cardiac muscle: Impact of ischemia, pressure overload, and
neurohumoral activation. Circulation. 109:2227–2233. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Lau LF and Lam SC: The CCN family of
angiogenic regulators: The integrin connection. Exp Cell Res.
248:44–57. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Grotendorst GR: Connective tissue growth
factor: A mediator of TGF-beta action on fibroblasts. Cytokine
Growth Factor Rev. 8:171–179. 1997. View Article : Google Scholar
|
|
73
|
Ujike K, Shinji T, Hirasaki S, Shiraha H,
Nakamura M, Tsuji T and Koide N: Kinetics of expression of
connective tissue growth factor gene during liver regeneration
after partial hepatectomy and D-galactosamine-induced liver injury
in rats. Biochem Biophys Res Commun. 277:448–454. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Hadjiargyrou M, Ahrens W and Rubin CT:
Temporal expression of the chondrogenic and angiogenic growth
factor CYR61 during fracture repair. J Bone Miner Res.
15:1014–1023. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Xu S-W, Leask A and Abraham D: Regulation
and function of connective tissue growth factor/CCN2 in tissue
repair, scarring and fibrosis. Cytokine Growth Factor Rev.
19:133–144. 2008. View Article : Google Scholar
|
|
76
|
Takehara K: Hypothesis: Pathogenesis of
systemic sclerosis. J Rheumatol. 30:755–759. 2003.PubMed/NCBI
|
|
77
|
Leask A: Targeting the TGFbeta,
endothelin-1 and CCN2 axis to combat fibrosis in scleroderma. Cell
Signal. 20:1409–1414. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Mori T, Kawara S, Shinozaki M, Hayashi N,
Kakinuma T, Igarashi A, Takigawa M, Nakanishi T and Takehara K:
Role and interaction of connective tissue growth factor with
transforming growth factor-beta in persistent fibrosis: A mouse
fibrosis model. J Cell Physiol. 181:153–159. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Lipson KE, Wong C, Teng Y and Spong S:
CTGF is a central mediator of tissue remodeling and fibrosis and
its inhibition can reverse the process of fibrosis. Fibrogenesis
Tissue Repair. 5(Suppl 1): S242012. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Zhang L, Li Y, Liang C and Yang W: CCN5
overexpression inhibits profibrotic phenotypes via the PI3K/Akt
signaling pathway in lung fibroblasts isolated from patients with
idiopathic pulmonary fibrosis and in an in vivo model of lung
fibrosis. Int J Mol Med. 33:478–486. 2014.
|
|
81
|
Wiedmaier N, Müller S, Köberle M, Manncke
B, Krejci J, Autenrieth IB and Bohn E: Bacteria induce CTGF and
CYR61 expression in epithelial cells in a lysophosphatidic acid
receptor- dependent manner. Int J Med Microbiol. 298:231–243. 2008.
View Article : Google Scholar
|
|
82
|
Kim SM, Park JH, Chung SK, Kim JY, Hwang
HY, Chung KC, Jo I, Park SI and Nam JH: Coxsackievirus B3 infection
induces cyr61 activation via JNK to mediate cell death. J Virol.
78:13479–13488. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Muehlich S, Schneider N, Hinkmann F,
Garlichs CD and Goppelt-Struebe M: Induction of connective tissue
growth factor (CTGF) in human endothelial cells by lysophosphatidic
acid, sphingosine-1-phosphate, and platelets. Atherosclerosis.
175:261–268. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Sakamoto S, Yokoyama M, Zhang X, Prakash
K, Nagao K, Hatanaka T, Getzenberg RH and Kakehi Y: Increased
expression of CYR61, an extracellular matrix signaling protein, in
human benign prostatic hyperplasia and its regulation by
lysophosphatidic acid. Endocrinology. 145:2929–2940. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Gery S, Xie D, Yin D, Gabra H, Miller C,
Wang H, Scott D, Yi WS, Popoviciu ML, Said JW, et al: Ovarian
carcinomas: CCN genes are aberrantly expressed and CCN1 promotes
proliferation of these cells. Clin Cancer Res. 11:7243–7254. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Lv H, Fan E, Sun S, Ma X, Zhang X, Han DM
and Cong YS: Cyr61 is up-regulated in prostate cancer and
associated with the p53 gene status. J Cell Biochem. 106:738–744.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Schmitz P, Gerber U, Jüngel E, Schütze N,
Blaheta R and Bendas G: Cyr61/CCN1 affects the integrin-mediated
migration of prostate cancer cells (PC-3) in vitro. Int J Clin
Pharmacol Ther. 51:47–50. 2013. View Article : Google Scholar
|
|
88
|
Goodwin CR, Lal B, Zhou X, Ho S, Xia S,
Taeger A, Murray J and Laterra J: Cyr61 mediates hepatocyte growth
factor-dependent tumor cell growth, migration, and Akt activation.
Cancer Res. 70:2932–2941. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Xie D, Yin D, Tong X, O'Kelly J, Mori A,
Miller C, Black K, Gui D, Said JW and Koeffler HP: Cyr61 is
overexpressed in gliomas and involved in integrin-linked
kinase-mediated Akt and beta-catenin-TCF/Lef signaling pathways.
Cancer Res. 64:1987–1996. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Harris LG, Pannell LK, Singh S, Samant RS
and Shevde LA: Increased vascularity and spontaneous metastasis of
breast cancer by hedgehog signaling mediated upregulation of cyr61.
Oncogene. 31:3370–3380. 2012. View Article : Google Scholar :
|
|
91
|
Tsai MS, Hornby AE, Lakins J and Lupu R:
Expression and function of CYR61, an angiogenic factor, in breast
cancer cell lines and tumor biopsies. Cancer Res. 60:5603–5607.
2000.PubMed/NCBI
|
|
92
|
Watari H, Xiong Y, Hassan MK and Sakuragi
N: Cyr61, a member of ccn (connective tissue growth
factor/cysteine-rich 61/nephroblastoma overexpressed) family,
predicts survival of patients with endometrial cancer of
endometrioid subtype. Gynecol Oncol. 112:229–234. 2009. View Article : Google Scholar
|
|
93
|
Monnier Y, Farmer P, Bieler G, Imaizumi N,
Sengstag T, Alghisi GC, Stehle JC, Ciarloni L, Andrejevic-Blant S,
Moeckli R, et al: CYR61 and alphaVbeta5 integrin cooperate to
promote invasion and metastasis of tumors growing in preirradiated
stroma. Cancer Res. 68:7323–7331. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Chen PP, Li WJ, Wang Y, Zhao S, Li DY,
Feng LY, Shi XL, Koeffler HP, Tong XJ and Xie D: Expression of
Cyr61, CTGF, and WISP-1 correlates with clinical features of lung
cancer. PLoS One. 2:e5342007. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Chien W, Kumagai T, Miller CW, Desmond JC,
Frank JM, Said JW and Koeffler HP: Cyr61 suppresses growth of human
endometrial cancer cells. J Biol Chem. 279:53087–53096. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Brigstock DR: The connective tissue growth
factor/cysteine-rich 61/nephroblastoma overexpressed (CCN) family.
Endocr Rev. 20:189–206. 1999.PubMed/NCBI
|
|
97
|
Maeta N, Osaki M, Shomori K, Inaba A,
Kidani K, Ikeguchi M and Ito H: CYR61 downregulation correlates
with tumor progression by promoting MMP-7 expression in human
gastric carcinoma. Oncology. 73:118–126. 2007. View Article : Google Scholar
|
|
98
|
Croci S, Landuzzi L, Nicoletti G,
Palladini A, Antognoli A, De Giovanni C, Nanni P and Lollini PL:
Expression of connective tissue growth factor (CTGF/CCN2) in a
mouse model of rhabdomyosarcomagenesis. Pathol Oncol Res.
13:336–339. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Chien W, O'Kelly J, Lu D, Leiter A, Sohn
J, Yin D, Karlan B, Vadgama J, Lyons KM and Koeffler HP: Expression
of connective tissue growth factor (CTGF/CCN2) in breast cancer
cells is associated with increased migration and angiogenesis. Int
J Oncol. 38:1741–1747. 2011.PubMed/NCBI
|
|
100
|
Jiang WG, Watkins G, Fodstad O,
Douglas-Jones A, Mokbel K and Mansel RE: Differential expression of
the CCN family members Cyr61, CTGF and Nov in human breast cancer.
Endocr Relat Cancer. 11:781–791. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Jacobson A and Cunningham JL: Connective
tissue growth factor in tumor pathogenesis. Fibrogenesis Tissue
Repair. 5(Suppl 1): S82012. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Lin BR, Chang CC, Chen RJ, Jeng YM, Liang
JT, Lee PH, Chang KJ and Kuo ML: Connective tissue growth factor
acts as a therapeutic agent and predictor for peritoneal
carcinomatosis of colorectal cancer. Clin Cancer Res. 17:3077–3088.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Bennewith KL, Huang X, Ham CM, Graves EE,
Erler JT, Kambham N, Feazell J, Yang GP, Koong A and Giaccia AJ:
The role of tumor cell-derived connective tissue growth factor
(CTGF/CCN2) in pancreatic tumor growth. Cancer Res. 69:775–784.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Shakunaga T, Ozaki T, Ohara N, Asaumi K,
Doi T, Nishida K, Kawai A, Nakanishi T, Takigawa M and Inoue H:
Expression of connective tissue growth factor in cartilaginous
tumors. Cancer. 89:1466–1473. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Chien W, Yin D, Gui D, Mori A, Frank JM,
Said J, Kusuanco D, Marchevsky A, McKenna R and Koeffler HP:
Suppression of cell proliferation and signaling transduction by
connective tissue growth factor in non-small cell lung cancer
cells. Mol Cancer Res. 4:591–598. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Kikuchi R, Tsuda H, Kanai Y, Kasamatsu T,
Sengoku K, Hirohashi S, Inazawa J and Imoto I: Promoter
hypermethylation contributes to frequent inactivation of a putative
conditional tumor suppressor gene connective tissue growth factor
in ovarian cancer. Cancer Res. 67:7095–7105. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Li MH, Sanchez T, Pappalardo A, Lynch KR,
Hla T and Ferrer F: Induction of antiproliferative connective
tissue growth factor expression in Wilms' tumor cells by
sphingosine-1-phosphate receptor 2. Mol Cancer Res. 6:1649–1656.
2008.PubMed/NCBI
|
|
108
|
Gardini A, Corti B, Fiorentino M, Altimari
A, Ercolani G, Grazi GL, Pinna AD, Grigioni WF and D'Errico
Grigioni A: Expression of connective tissue growth factor is a
prognostic marker for patients with intrahepatic
cholangiocarcinoma. Dig Liver Dis. 37:269–274. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Lin BR, Chang CC, Che TF, Chen ST, Chen
RJ, Yang CY, Jeng YM, Liang JT, Lee PH, Chang KJ, et al: Connective
tissue growth factor inhibits metastasis and acts as an independent
prognostic marker in colorectal cancer. Gastroenterology. 128:9–23.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Maillard M, Cadot B, Ball RY, Sethia K,
Edwards DR, Perbal B and Tatoud R: Differential expression of the
ccn3 (nov) proto-oncogene in human prostate cell lines and tissues.
Mol Pathol. 54:275–280. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Manara MC, Perbal B, Benini S, Strammiello
R, Cerisano V, Perdichizzi S, Serra M, Astolfi A, Bertoni F, Alami
J, et al: The expression of ccn3(nov) gene in musculoskeletal
tumors. Am J Pathol. 160:849–859. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Gluk hova L, Angevin E, Lavialle C, Cadot
B, Terrier-Lacombe MJ, Perbal B, Bernheim A and Goguel AF: Patterns
of specific genomic alterations associated with poor prognosis in
high-grade renal cell carcinomas. Cancer Genet Cytogenet.
130:105–110. 2001. View Article : Google Scholar
|
|
113
|
Perbal B: CCN3: Doctor Jekyll and Mister
Hyde. J Cell Commun Signal. 2:3–7. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Zhang T, Zhao C, Luo L, Xiang J, Sun Q,
Cheng J and Chen D: The clinical and prognostic significance of
CCN3 expression in patients with cervical cancer. Adv Clin Exp Med.
22:839–845. 2013.
|
|
115
|
Gupta N, Wang H, McLeod TL, Naus CC,
Kyurkchiev S, Advani S, Yu J, Perbal B and Weichselbaum RR:
Inhibition of glioma cell growth and tumorigenic potential by CCN3
(NOV). Mol Pathol. 54:293–299. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Bleau AM, Planque N, Lazar N, Zambelli D,
Ori A, Quan T, Fisher G, Scotlandi K and Perbal B:
Antiproliferative activity of CCN3: Involvement of the C-terminal
module and post-translational regulation. J Cell Biochem.
101:1475–1491. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
McCallum L, Lu W, Price S, Lazar N, Perbal
B and Irvine AE: CCN3 suppresses mitogenic signalling and
reinstates growth control mechanisms in chronic myeloid leukaemia.
J Cell Commun Signal. 6:27–35. 2012. View Article : Google Scholar :
|
|
118
|
Thibout H, Martinerie C, Créminon C,
Godeau F, Boudou P, Le Bouc Y and Laurent M: Characterization of
human NOV in biological fluids: An enzyme immunoassay for the
quantification of human NOV in sera from patients with diseases of
the adrenal gland and of the nervous system. J Clin Endocrinol
Metab. 88:327–336. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Xin LW, Martinerie C, Zumkeller W,
Westphal M and Perbal B: Differential expression of novH and CTGF
in human glioma cell lines. Clin Mol Pathol. 49:M91–M97. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Sin WC, Bechberger JF, Rushlow WJ and Naus
CC: Dose-dependent differential upregulation of CCN1/Cyr61 and
CCN3/NOV by the gap junction protein Connexin43 in glioma cells. J
Cell Biochem. 103:1772–1782. 2008. View Article : Google Scholar
|
|
121
|
Fukunaga-Kalabis M, Martinez G, Telson SM,
Liu ZJ, Balint K, Juhasz I, Elder DE, Perbal B and Herlyn M:
Downregulation of CCN3 expression as a potential mechanism for
melanoma progression. Oncogene. 27:2552–2560. 2008. View Article : Google Scholar
|
|
122
|
Chuang JY, Chang AC, Chiang IP, Tsai MH
and Tang CH: Apoptosis signal-regulating kinase 1 is involved in
WISP-1-promoted cell motility in human oral squamous cell carcinoma
cells. PLoS One. 8:e780222013. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Hashimoto Y, Shindo-Okada N, Tani M,
Takeuchi K, Toma H and Yokota J: Identification of genes
differentially expressed in association with metastatic potential
of K-1735 murine melanoma by messenger RNA differential display.
Cancer Res. 56:5266–5271. 1996.PubMed/NCBI
|
|
124
|
Hashimoto Y, Shindo-Okada N, Tani M,
Nagamachi Y, Takeuchi K, Shiroishi T, Toma H and Yokota J:
Expression of the Elm1 gene, a novel gene of the CCN (connective
tissue growth factor, Cyr61/Cef10, and neuroblastoma overexpressed
gene) family, suppresses in vivo tumor growth and metastasis of
K-1735 murine melanoma cells. J Exp Med. 187:289–296. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Soon LL, Yie TA, Shvarts A, Levine AJ, Su
F and Tchou-Wong KM: Overexpression of WISP-1 down-regulated
motility and invasion of lung cancer cells through inhibition of
Rac activation. J Biol Chem. 278:11465–11470. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Yu C, Le AT, Yeger H, Perbal B and Alman
BA: NOV (CCN3) regulation in the growth plate and CCN family member
expression in cartilage neoplasia. J Pathol. 201:609–615. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Davies SR, Watkins G, Mansel RE and Jiang
WG: Differential expression and prognostic implications of the CCN
family members WISP-1, WISP-2, and WISP-3 in human breast cancer.
Ann Surg Oncol. 14:1909–1918. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Tomimaru Y, Koga H, Yano H, de la Monte S,
Wands JR and Kim M: Upregulation of T-cell factor-4
isoform-responsive target genes in hepatocellular carcinoma. Liver
Int. 33:1100–1112. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Colli LM, Saggioro F, Serafini LN, Camargo
RC, Machado HR, Moreira AC, Antonini SR and de Castro M: Components
of the canonical and non-canonical Wnt pathways are not
mis-expressed in pituitary tumors. PLoS One. 8:e624242013.
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Ji J, Jia S, Ji K and Jiang WG: Wnt1
inducible signalling pathway protein-2 (WISP 2/CCN5): Roles and
regulation in human cancers (Review). Oncol Rep. 31:533–539.
2014.
|
|
131
|
Banerjee S, Dhar G, Haque I, Kambhampati
S, Mehta S, Sengupta K, Tawfik O, Phillips TA and Banerjee SK:
CCN5/WISP-2 expression in breast adenocarcinoma is associated with
less frequent progression of the disease and suppresses the
invasive phenotypes of tumor cells. Cancer Res. 68:7606–7612. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Dhar K, Banerjee S, Dhar G, Sengupta K and
Banerjee SK: Insulin-like growth factor-1 (IGF-1) induces
WISP-2/CCN5 via multiple molecular cross-talks and is essential for
mitogenic switch by IGF-1 axis in estrogen receptor-positive breast
tumor cells. Cancer Res. 67:1520–1526. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Banerjee SK and Banerjee S: CCN5/WISP-2: A
micromanager of breast cancer progression. J Cell Commun Signal.
6:63–71. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Yang Z, Yang Z, Zou Q, Yuan Y, Li J, Li D,
Liang L, Zeng G and Chen S: A comparative study of
clinicopathological significance, FGFBP1, and WISP-2 expression
between squamous cell/adenosquamous carcinomas and adenocarcinoma
of the gallbladder. Int J Clin Oncol. 19:325–335. 2014. View Article : Google Scholar
|
|
135
|
Dhar G, Mehta S, Banerjee S, Gardner A,
McCarty BM, Mathur SC, Campbell DR, Kambhampati S and Banerjee SK:
Loss of WISP-2/CCN5 signaling in human pancreatic cancer: A
potential mechanism for epithelial-mesenchymal-transition. Cancer
Lett. 254:63–70. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Kouzu Y, Uzawa K, Kato M, Higo M, Nimura
Y, Harada K, Numata T, Seki N, Sato M and Tanzawa H: WISP-2
expression in human salivary gland tumors. Int J Mol Med.
17:567–573. 2006.PubMed/NCBI
|
|
137
|
Thorstensen L, Diep CB, Meling GI, Aagesen
TH, Ahrens CH, Rognum TO and Lothe RA: WNT1 inducible signaling
pathway protein 3, WISP-3, a novel target gene in colorectal
carcinomas with microsatellite instability. Gastroenterology.
121:1275–1280. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Cervello M, Giannitrapani L, Labbozzetta
M, Notarbartolo M, D'Alessandro N, Lampiasi N, Azzolina A and
Montalto G: Expression of WISPs and of their novel alternative
variants in human hepatocellular carcinoma cells. Ann NY Acad Sci.
1028:432–439. 2004. View Article : Google Scholar
|
|
139
|
Fang F, Zhao WY, Li RK, Yang XM, Li J, Ao
JP, Jiang SH, Kong FZ, Tu L, Zhuang C, et al: Silencing of WISP3
suppresses gastric cancer cell proliferation and metastasis and
inhibits Wnt/β-catenin signaling. Int J Clin Exp Pathol.
7:6447–6461. 2014.
|
|
140
|
Huang W, Zhang Y, Varambally S, Chinnaiyan
AM, Banerjee M, Merajver SD and Kleer CG: Inhibition of CCN6
(Wnt-1-induced signaling protein 3) down-regulates E-cadherin in
the breast epithelium through induction of snail and ZEB1. Am J
Pathol. 172:893–904. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
van Golen KL, Davies S, Wu ZF, Wang Y,
Bucana CD, Root H, Chandrasekharappa S, Strawderman M, Ethier SP
and Merajver SD: A novel putative low-affinity insulin-like growth
factor-binding protein, LIBC (lost in inflammatory breast cancer),
and RhoC GTPase correlate with the inflammatory breast cancer
phenotype. Clin Cancer Res. 5:2511–2519. 1999.PubMed/NCBI
|