|
1
|
Boix E, Zhang Y, Swaminathan GJ, Brew K
and Acharya KR: Structural basis of ordered binding of donor and
acceptor substrates to the retaining glycosyltransferase,
alpha-1,3-galacto-syltransferase. J Biol Chem. 277:28310–28318.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shao Y, Yu Y, Pei CG, Qu Y, Gao GP, Yang
JL, Zhou Q, Yang L and Liu QP: The expression and distribution of
α-Gal gene in various species ocular surface tissue. Int J
Ophthalmol. 5:543–548. 2012.
|
|
3
|
Galili U: The alpha-gal epitope and the
anti-Gal antibody in xenotransplantation and in cancer
immunotherapy. Immunol Cell Biol. 83:674–686. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Blanken WM and Van den Eijnden DH:
Biosynthesis of terminal Gal alpha 1→3Gal beta 1→4GlcNAc-R
oligosaccharide sequences on glycoconjugates. Purification and
acceptor specificity of a UDP-Gal:N-acetyllactosaminide alpha
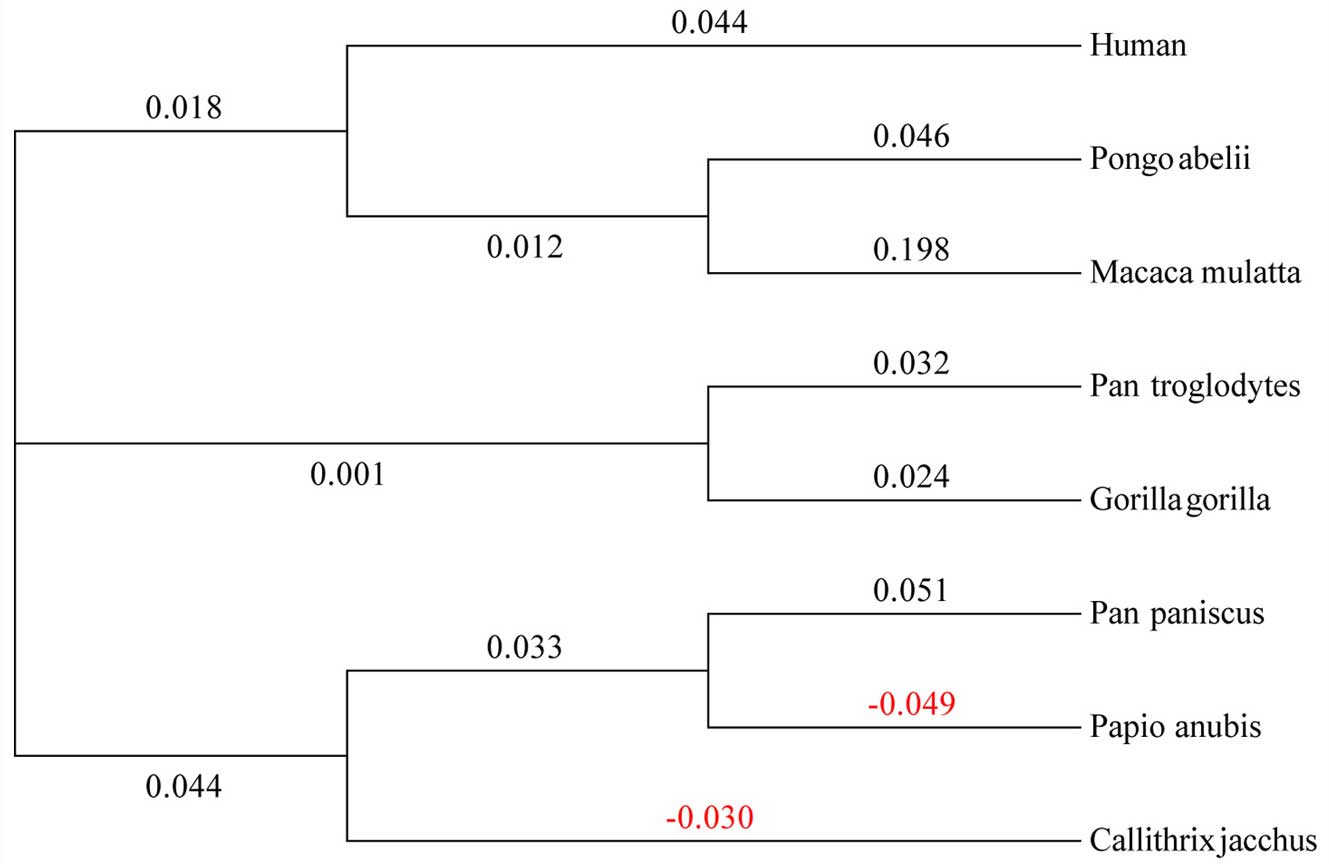
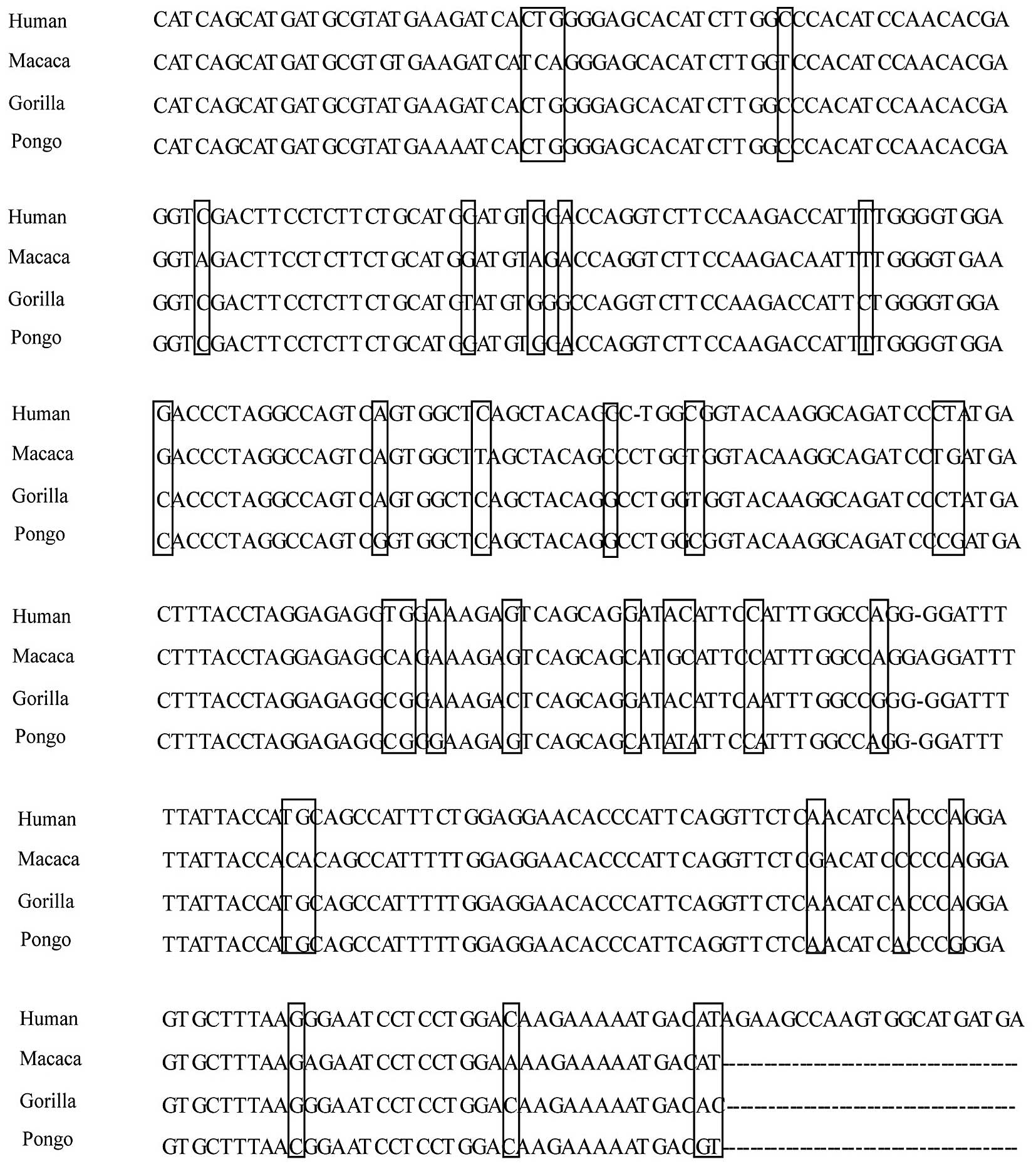
1→3-galactosyltrans-ferase from calf thymus. J Biol Chem.
260:12927–12934. 1985.PubMed/NCBI
|
|
5
|
Taylor SG, McKenzie IF and Sandrin MS:
Characterization of the rat alpha(1,3)galactosyltransferase:
evidence for two independent genes encoding glycosyltransferases
that synthesize Galalpha(1,3)Gal by two separate glycosylation
pathways. Glycobiology. 13:327–337. 2003. View Article : Google Scholar : PubMed/NCBI
|
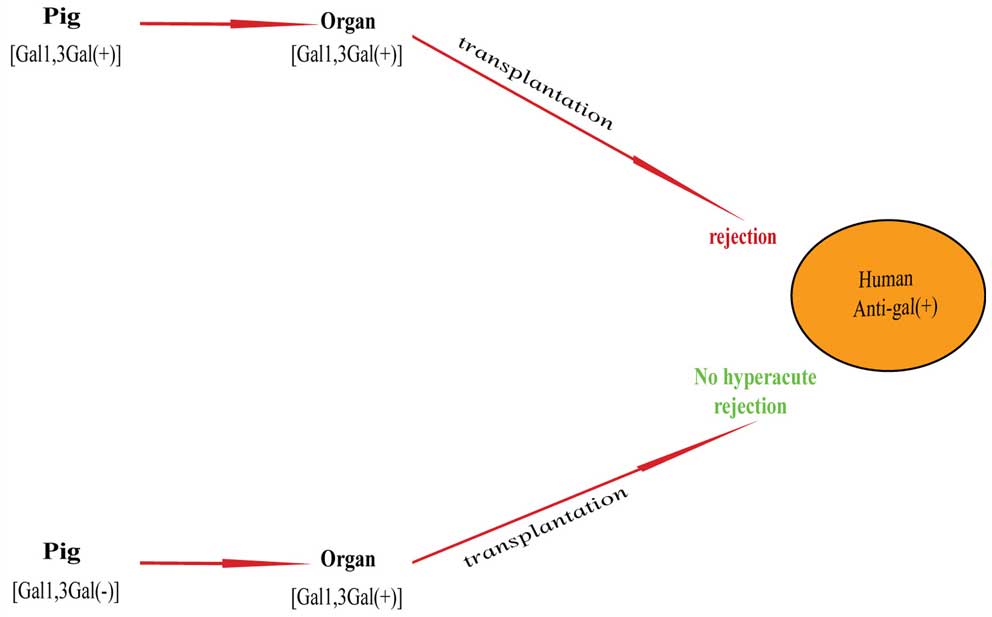
|
6
|
Joziasse DH, Shaper JH, Jabs EW and Shaper
NL: Characterization of an alpha 1→3-galactosyltransferase
homologue on human chromosome 12 that is organized as a processed
pseudogene. J Biol Chem. 266:6991–6998. 1991.PubMed/NCBI
|
|
7
|
Church DM, Goodstadt L, Hillier LW, Zody
MC, Goldstein S, She X, Bult CJ, Agarwala R, Cherry JL, DiCuccio M,
et al: Mouse Genome Sequencing Consortium: Lineage-specific biology
revealed by a finished genome assembly of the mouse. PLoS Biol.
7:e10001122009. View Article : Google Scholar
|
|
8
|
Strahan KM, Gu F, Preece AF, Gustavsson I,
Andersson L and Gustafsson K: cDNA sequence and chromosome
localization of pig alpha 1,3 galactosyltransferase.
Immunogenetics. 41:101–105. 1995. View Article : Google Scholar : PubMed/NCBI
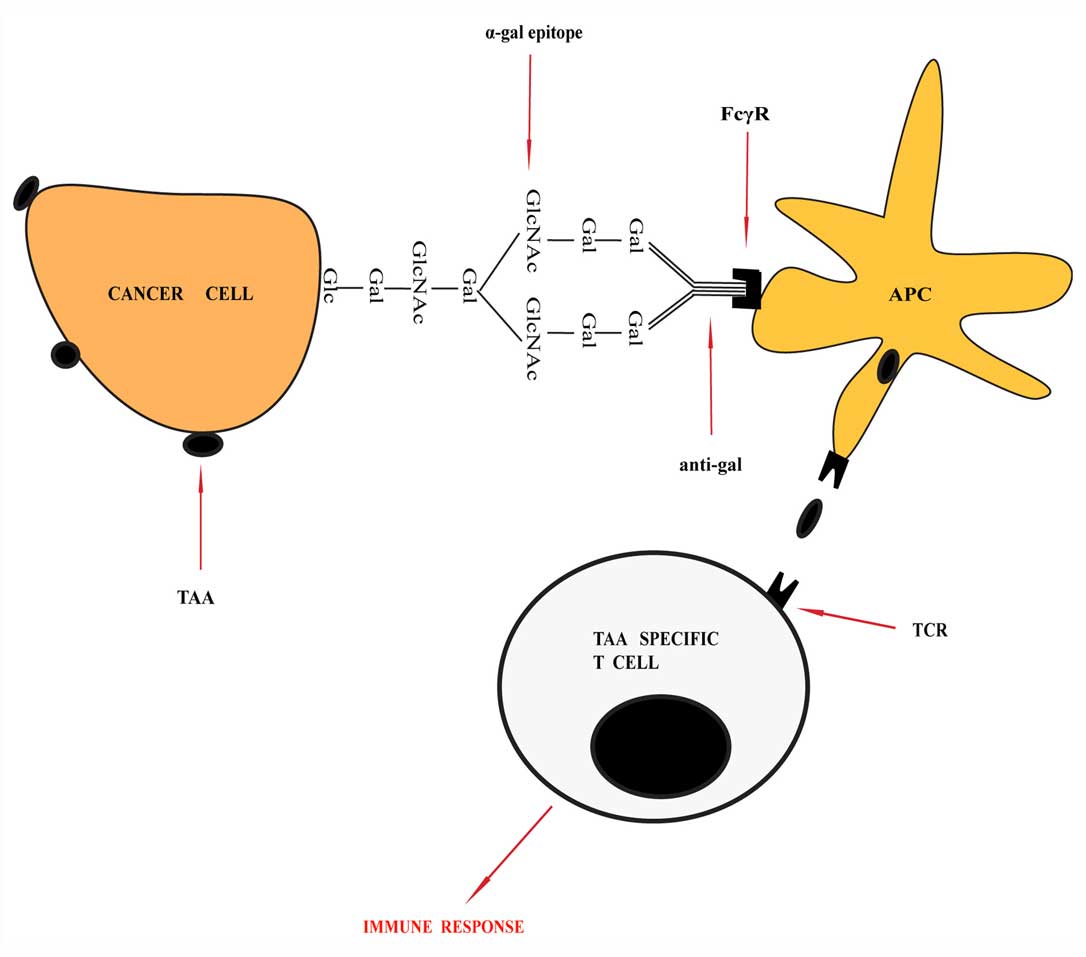
|
|
9
|
Zimin AV, Delcher AL, Florea L, Kelley DR,
Schatz MC, Puiu D, Hanrahan F, Pertea G, Van Tassell CP, Sonstegard
TS, et al: A whole-genome assembly of the domestic cow, Bos taurus.
Genome Biol. 10:R422009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lindblad-Toh K, Wade CM, Mikkelsen TS,
Karlsson EK, Jaffe DB, Kamal M, Clamp M, Chang JL, Kulbokas EJ III,
Zody MC, et al: Genome sequence, comparative analysis and haplotype
structure of the domestic dog. Nature. 438:803–819. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lander ES, Linton LM, Birren B, Nusbaum C,
Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh W, et al:
International Human Genome Sequencing Consortium: Initial
sequencing and analysis of the human genome. Nature. 409:860–921.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Galili U: Evolution and pathophysiology of
the human natural anti-alpha-galactosyl IgG (anti-Gal) antibody.
Springer Semin Immunopathol. 15:155–171. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Larsen RDR-MC, Rivera-Marrero CA, Ernst
LK, Cummings RD and Lowe JB: Frameshift and nonsense mutations in a
human genomic sequence homologous to a murine UDP-Gal:beta-D-G
al(1,4)-D-GlcNAc alpha(1,3)-galactosyltransferase cDNA. J Biol
Chem. 265:7055–7061. 1990.PubMed/NCBI
|
|
14
|
Rodriguez IA and Welsh RM: Possible role
of a cell surface carbohydrate in evolution of resistance to viral
infections in old world primates. J Virol. 87:8317–8326. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hamanova M, Chmelikova M, Nentwich I, Thon
V and Lokaj J: Anti-Gal IgM, IgA and IgG natural antibodies in
childhood. Immunol Lett. 164:40–43. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang L, Radic MZ and Galili U: Human
anti-Gal heavy chain genes. Preferential use of VH3 and the
presence of somatic mutations. J Immunol. 155:1276–1285.
1995.PubMed/NCBI
|
|
17
|
Commins SP and Platts-Mills TA: Delayed
anaphylaxis to red meat in patients with IgE specific for galactose
alpha-1,3-ga-lactose (alpha-gal). Curr Allergy Asthma Rep.
13:72–77. 2013. View Article : Google Scholar :
|
|
18
|
Galili U, LaTemple DC and Radic MZ: A
sensitive assay for measuring alpha-Gal epitope expression on cells
by a monoclonal anti-Gal antibody. Transplantation. 65:1129–1132.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
LaTemple DC and Galili U: Adult and
neonatal anti-Gal response in knock-out mice for
alpha1,3galactosyltransferase. Xenotransplantation. 5:191–196.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Park S, Kim WH, Choi SY and Kim YJ:
Removal of alpha-Gal epitopes from porcine aortic valve and
pericardium using recombinant human alpha galactosidase A. J Korean
Med Sci. 24:1126–1131. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Abdel-Motal UM, Guay HM, Wigglesworth K,
Welsh RM and Galili U: Immunogenicity of influenza virus vaccine is
increased by anti-gal-mediated targeting to antigen-presenting
cells. J Virol. 81:9131–9141. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gurtner GCWS, Werner S, Barrandon Y and
Longaker MT: Wound repair and regeneration. Nature. 453:314–321.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
LaTemple DC, Henion TR, Anaraki F and
Galili U: Synthesis of alpha-galactosyl epitopes by recombinant
alpha1,3galactosyl transferase for opsonization of human tumor cell
vaccines by anti-galactose. Cancer Res. 56:3069–3074.
1996.PubMed/NCBI
|
|
24
|
Singer AJ and Clark RA: Cutaneous wound
healing. N Engl J Med. 341:738–746. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sandrin MS, Osman N and McKenzie IF:
Transgenic approaches for the reduction of Galalpha(1,3)Gal for
xenotransplantation. Front Biosci. 2:e1–11. 1997.PubMed/NCBI
|
|
26
|
Osman N, McKenzie IF, Mouhtouris E and
Sandrin MS: Switching amino-terminal cytoplasmic domains of
alpha(1,2) fucosyltransferase and alpha(1,3)galactosyltransferase
alters the expression of H substance and Galalpha(1,3)Gal. J Biol
Chem. 271:33105–33109. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xing L, Xia GH, Fei J, Huang F and Guo LH:
Adenovirus-mediated expression of pig alpha(1,3)
galactosyltransferase reconstructs Gal alpha(1, 3) gal epitope on
the surface of human tumor cells. Cell Res. 11:116–124. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu M, Zhu SM, Zheng H, Wang Y, Wang Z,
Yang YJ, Wu YX, Zeng YZ and Wang YP: Cloning of splicing variants
of alpha1,3-galactosyltransferase cDNA of Chinese Banna Minipig
inbred line and its expression in human cells. Sichuan Da Xue Xue
Bao Yi Xue Ban. 43:145–150. 2012.In Chinese. PubMed/NCBI
|
|
29
|
Joziasse DH, Shaper JH, Van den Eijnden
DH, Van Tunen AJ and Shaper NL: Bovine alpha
1→3-galactosyltransferase: Isolation and characterization of a cDNA
clone. Identification of homologous sequences in human genomic DNA.
J Biol Chem. 264:14290–14297. 1989.PubMed/NCBI
|
|
30
|
Lantéri M, Giordanengo V, Vidal F, Gaudray
P and Lefebvre JC: A complete alpha1,3-galactosyltransferase gene
is present in the human genome and partially transcribed.
Glycobiology. 12:785–792. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Galili U and Swanson K: Gene sequences
suggest inactivation of alpha-1,3-galactosyltransferase in
catarrhines after the divergence of apes from monkeys. Proc Natl
Acad Sci USA. 88:7401–7404. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ma YH, Zhou XG, Hu JH, Fei J, Xia GH and
Guo LH: Human xenoreactivity is reduced in mice bearing porcine
antisense alpha(1,3) galactosyltransferase cDNA. Acta Pharmacol
Sin. 22:231–238. 2001.PubMed/NCBI
|
|
33
|
Galili U and Andrews P: Suppression of
a-galactosyl epitopes synthesis and production of the natural
anti-Gal antibody: A major evolutionary event in ancestral Old
World primates. J Hum Evol. 29:433–442. 1995. View Article : Google Scholar
|
|
34
|
Fang J, Walters A, Hara H, Long C, Yeh P,
Ayares D, Cooper DK and Bianchi J: Anti-gal antibodies in
α1,3-galactosyltransferase gene-knockout pigs. Xenotransplantation.
19:305–310. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Koike C, Fung JJ, Geller DA, Kannagi R,
Libert T, Luppi P, Nakashima I, Profozich J, Rudert W, Sharma SB,
et al: Molecular basis of evolutionary loss of the alpha
1,3-galac-tosyltransferase gene in higher primates. J Biol Chem.
277:10114–10120. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sandrin MS, Vaughan HA, Dabkowski PL and
McKenzie IF: Anti-pig IgM antibodies in human serum react
predominantly with Gal(alpha 1–3) Gal epitopes. Proc Natl Acad Sci
USA. 90:11391–11395. 1993. View Article : Google Scholar
|
|
37
|
Galili U, Rachmilewitz EA, Peleg A and
Flechner I: A unique natural human IgG antibody with
anti-a-galactosyl specificity. J Exp Med. 160:1519–1581. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Galili U: Anti-Gal: An abundant human
natural antibody of multiple pathogeneses and clinical benefits.
Immunology. 140:1–11. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Galili U: Significance of the evolutionary
α1,3-galacto-syltransferase (GGTA1) gene inactivation in preventing
extinction of apes and old world monkeys. J Mol Evol. 80:1–9. 2015.
View Article : Google Scholar
|
|
40
|
Ma YH1, Zhou XG, Hu JH, Fei J, Xia GH and
Guo LH: Human xeno-reactivity is reduced in mice bearing porcine
antisense alpha(1,3) galactosyltransferase cDNA. Acta Pharmacol
Sin. 22:231–238. 2001.PubMed/NCBI
|
|
41
|
Celis E, Abraham KG and Miller RW:
Modulation of the immunological response to hepatitis B virus by
antibodies. Hepatology. 7:563–568. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Byrne GW, Stalboerger PG, Du Z, Davis TR
and McGregor CG: Identification of new carbohydrate and membrane
protein antigens in cardiac xenotransplantation. Transplantation.
91:287–292. 2011. View Article : Google Scholar
|
|
43
|
Galili U: Interaction of the natural
anti-Gal antibody with alpha-galactosyl epitopes: A major obstacle
for xenotransplan-tation in humans. Immunol Today. 14:480–482.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Anderson M: Xenotransplantation: A
bioethical evaluation. J Med Ethics. 32:205–208. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lee C, Ahn H, Kim SH, Choi SY and Kim YJ:
Immune response to bovine pericardium implanted into
α1,3-galactosyltransferase knockout mice: feasibility as an animal
model for testing efficacy of anticalcification treatments of
xenografts. Eur J Cardiothorac Surg. 42:164–172. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kim YG, Gil GC, Harvey DJ and Kim BG:
Structural analysis of alpha-Gal and new non-Gal carbohydrate
epitopes from specific pathogen-free miniature pig kidney.
Proteomics. 8:2596–2610. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Park CS, Oh SS, Kim YE, Choi SY, Lim HG,
Ahn H and Kim YJ: Anti-alpha-Gal antibody response following
xenogeneic heart valve implantation in adults. J Heart Valve Dis.
22:222–229. 2013.PubMed/NCBI
|
|
48
|
Wilczek P, Lesiak A, Niemiec-Cyganek A,
Kubin B, Slomski R, Nozynski J, Wilczek G, Mzyk A and Gramatyka M:
Biomechanical properties of hybrid heart valve prosthesis utilizing
the pigs that do not express the galactose-α-1,3-galactose (α-Gal)
antigen derived tissue and tissue engineering technique. J Mater
Sci Mater Med. 26(5329)2015. View Article : Google Scholar
|
|
49
|
Choi SY, Jeong HJ, Lim HG, Park SS, Kim SH
and Kim YJ: Elimination of alpha-gal xenoreactive epitope:
Alpha-galactosidase treatment of porcine heart valves. J Heart
Valve Dis. 21:387–397. 2012.PubMed/NCBI
|
|
50
|
Konakci KZ, Bohle B, Blumer R,
Hoetzenecker W, Roth G, Moser B, Boltz-Nitulescu G, Gorlitzer M,
Klepetko W, Wolner E, et al: Alpha-Gal on bioprostheses: Xenograft
immune response in cardiac surgery. Eur J Clin Invest. 35:17–23.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Collins BH, Cotterell AH, McCurry KR,
Alvarado CG, Magee JC, Parker W and Platt JL: Cardiac xenografts
between primate species provide evidence for the importance of the
alpha-galactosyl determinant in hyperacute rejection. J Immunol.
154:5500–5510. 1995.PubMed/NCBI
|
|
52
|
Manji RA, Menkis AH, Ekser B and Cooper
DK: Porcine bioprosthetic heart valves: The next generation. Am
Heart J. 164:177–185. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Tazelaar HD, Byrne GW and McGregor CG:
Comparison of Gal and non-Gal-mediated cardiac xenograft rejection.
Transplantation. 91:968–975. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lim HG, Choi SY, Yoon EJ, Kim SH and Kim
YJ: In vivo efficacy of alpha-galactosidase as possible promise for
prolonged durability of bioprosthetic heart valve using
alpha1,3-galactosyl-transferase knockout mouse. Tissue Eng Part A.
19:2339–2348. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
McGregor CG, Carpentier A, Lila N, Logan
JS and Byrne GW: Cardiac xenotransplantation technology provides
materials for improved bioprosthetic heart valves. J Thorac
Cardiovasc Surg. 141:269–275. 2011. View Article : Google Scholar
|
|
56
|
Mangold A, Szerafin T, Hoetzenecker K,
Hacker S, Lichtenauer M, Niederpold T, Nickl S, Dworschak M, Blumer
R, Auer J, et al: Alpha-Gal specific IgG immune response after
implantation of bioprostheses. Thorac Cardiovasc Surg. 57:191–195.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Nam J, Choi SY, Sung SC, Lim HG, Park SS,
Kim SH and Kim YJ: Changes of the structural and biomechanical
properties of the bovine pericardium after the removal of α-Gal
epitopes by decellularization and α-galactosidase treatment. Korean
J Thorac Cardiovasc Surg. 45:380–389. 2012. View Article : Google Scholar :
|
|
58
|
Kasimir MT, Rieder E, Seebacher G, Wolner
E, Weigel G and Simon P: Presence and elimination of the
xenoantigen gal (alpha1, 3) gal in tissue-engineered heart valves.
Tissue Eng. 11:1274–1280. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
McGregor CG, Davies WR, Oi K, Teotia SS,
Schirmer JM, Risdahl JM, Tazelaar HD, Kremers WK, Walker RC, Byrnew
GW, et al: Cardiac xenotransplantation: Recent preclinical progress
with 3-month median survival. J Thorac Cardiovasc Surg.
130:844–851. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Milland J, Christiansen D, Lazarus BD,
Taylor SG, Xing PX and Sandrin MS: The molecular basis for
galalpha(1,3)gal expression in animals with a deletion of the
alpha1,3galactosyltransferase gene. J Immunol. 176:2448–2454. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Puga Yung G, Schneider MK and Seebach JD:
Immune responses to alpha1,3 galactosyltransferase knockout pigs.
Curr Opin Organ Transplant. 14:154–160. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Stone KR, Abdel-Motal UM, Walgenbach AW,
Turek TJ and Galili U: Replacement of human anterior cruciate
ligaments with pig ligaments: A model for anti-non-gal antibody
response in long-term xenotransplantation. Transplantation.
83:211–219. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Park HM, Kim YW, Kim KJ, Kim YJ, Yang YH,
Jin JM, Kim YH, Kim BG, Shim H and Kim YG: Comparative N-linked
glycan analysis of wild-type and α1,3-galactosyltransferase gene
knock-out pig fibroblasts using mass spectrometry approaches. Mol
Cells. 38:65–74. 2015.
|
|
64
|
Macher BA and Galili U: The
Galalpha1,3Galbeta1,4GlcNAc-R (alpha-Gal) epitope: A carbohydrate
of unique evolution and clinical relevance. Biochim Biophys Acta.
1780:75–88. 2008. View Article : Google Scholar
|
|
65
|
Deriy L, Chen ZC, Gao GP and Galili U:
Expression of alpha-gal epitopes on HeLa cells transduced with
adenovirus containing alpha1,3galactosyltransferase cDNA.
Glycobiology. 12:135–144. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Naso F, Gandaglia A, Bottio T, Tarzia V,
Nottle MB, d'Apice AJ, Cowan PJ, Cozzi E, Galli C, Lagutina I, et
al: First quantification of alpha-Gal epitope in current
glutaraldehyde-fixed heart valve bioprostheses.
Xenotransplantation. 20:252–261. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Galili U: Conversion of tumors into
autologous vaccines by intra-tumoral injection of α-Gal glycolipids
that induce anti-Gal/α-Gal epitope interaction. Clin Dev Immunol.
2011(134020)2011. View Article : Google Scholar
|
|
68
|
Huang AY, Golumbek P, Ahmadzadeh M, Jaffee
E, Pardoll D and Levitsky H: Role of bone marrow-derived cells in
presenting MHC class I-restricted tumor antigens. Science.
264:961–965. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Manca F, Fenoglio D, Li Pira G, Kunkl A
and Celada F: Effect of antigen/antibody ratio on macrophage
uptake, processing, and presentation to T cells of antigen
complexed with polyclonal antibodies. J Exp Med. 173:37–48. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Abdel-Motal UM, Wigglesworth K and Galili
U: Intratumoral injection of alpha-gal glycolipids induces a
protective anti-tumor T cell response which overcomes Treg
activity. Cancer Immunol Immunother. 58:1545–1556. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Schweighoffer T, Schmidt W, Buschle M and
Birnstiel ML: Depletion of naive T cells of the peripheral lymph
nodes abrogates systemic antitumor protection conferred by IL-2
secreting cancer vaccines. Gene Ther. 3:819–824. 1996.PubMed/NCBI
|
|
72
|
Liu C1, Gosselin EJ and Guyre PM: Fc gamma
RII on human B cells can mediate enhanced antigen presentation.
Cell Immunol. 167:188–194. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
LaTemple DC, Abrams JT, Zhang SY and
Galili U: Increased immunogenicity of tumor vaccines complexed with
anti-Gal: studies in knockout mice for
alpha1,3galactosyltransferase. Cancer Res. 59:3417–3423.
1999.PubMed/NCBI
|
|
74
|
Tanida T, Tanemura M, Miyoshi E, Nagano H,
Furukawa K, Nonaka Y, Akita H, Hama N, Wada H, Kawamoto K, et al:
Pancreatic cancer immunotherapy using a tumor lysate vaccine,
engineered to express α-gal epitopes, targets pancreatic cancer
stem cells. Int J Oncol. 46:78–90. 2015.
|
|
75
|
Tanemura M, Miyoshi E, Nagano H, Eguchi H,
Taniyama K, Kamiike W, Mori M and Doki Y: Role of α-gal
epitope/anti-Gal antibody reaction in immunotherapy and its
clinical application in pancreatic cancer. Cancer Sci. 104:282–290.
2013. View Article : Google Scholar
|
|
76
|
Deguchi T, Tanemura M, Miyoshi E, Nagano
H, Machida T, Ohmura Y, Kobayashi S, Marubashi S, Eguchi H, Takeda
Y, et al: Increased immunogenicity of tumor-associated antigen,
mucin 1, engineered to express alpha-gal epitopes: A novel approach
to immunotherapy in pancreatic cancer. Cancer Res. 70:5259–5269.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Whalen GF, Sullivan M, Piperdi B, Wasseff
W and Galili U: Cancer immunotherapy by intratumoral injection of
α-gal glyco-lipids. Anticancer Res. 32:3861–3868. 2012.PubMed/NCBI
|
|
78
|
Galili U, Repik PM, Anaraki F,
Mozdzanowska K, Washko G and Gerhard W: Enhancement of antigen
presentation of influenza virus hemagglutinin by the natural human
anti-Gal antibody. Vaccine. 14:321–328. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Henion TR, Gerhard W, Anaraki F and Galili
U: Synthesis of alpha-gal epitopes on influenza virus vaccines, by
recombinant alpha 1,3galactosyltransferase, enables the formation
of immune complexes with the natural anti-Gal antibody. Vaccine.
15:1174–1182. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Abdel-Motal U, Wang S, Lu S, Wigglesworth
K and Galili U: Increased immunogenicity of human immunodeficiency
virus gp120 engineered to express Galalpha1-3Galbeta1-4GlcNAc-R
epitopes. J Virol. 80:6943–6951. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Hurwitz ZIR, Lalikos J and Galili U:
Accelerated porcine wound healing with a-Gal nanoparticles. Plast
Reconstr Surg. 129:242–251. 2012. View Article : Google Scholar
|
|
82
|
Galili U: Discovery of the natural
anti-Gal antibody and its past and future relevance to medicine.
Xenotransplantation. 20:138–147. 2013.PubMed/NCBI
|
|
83
|
Wigglesworth KM, Racki WJ, Mishra R,
Szomolanyi-Tsuda E, Greiner DL and Galili U: Rapid recruitment and
activation of macrophages by anti-Gal/α-Gal liposome interaction
accelerates wound healing. J Immunol. 186:4422–4432. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Galili U, Wigglesworth K and Abdel-Motal
UM: Accelerated healing of skin burns by anti-Gal/alpha-gal
liposomes interaction. Burns. 36:239–251. 2010. View Article : Google Scholar
|