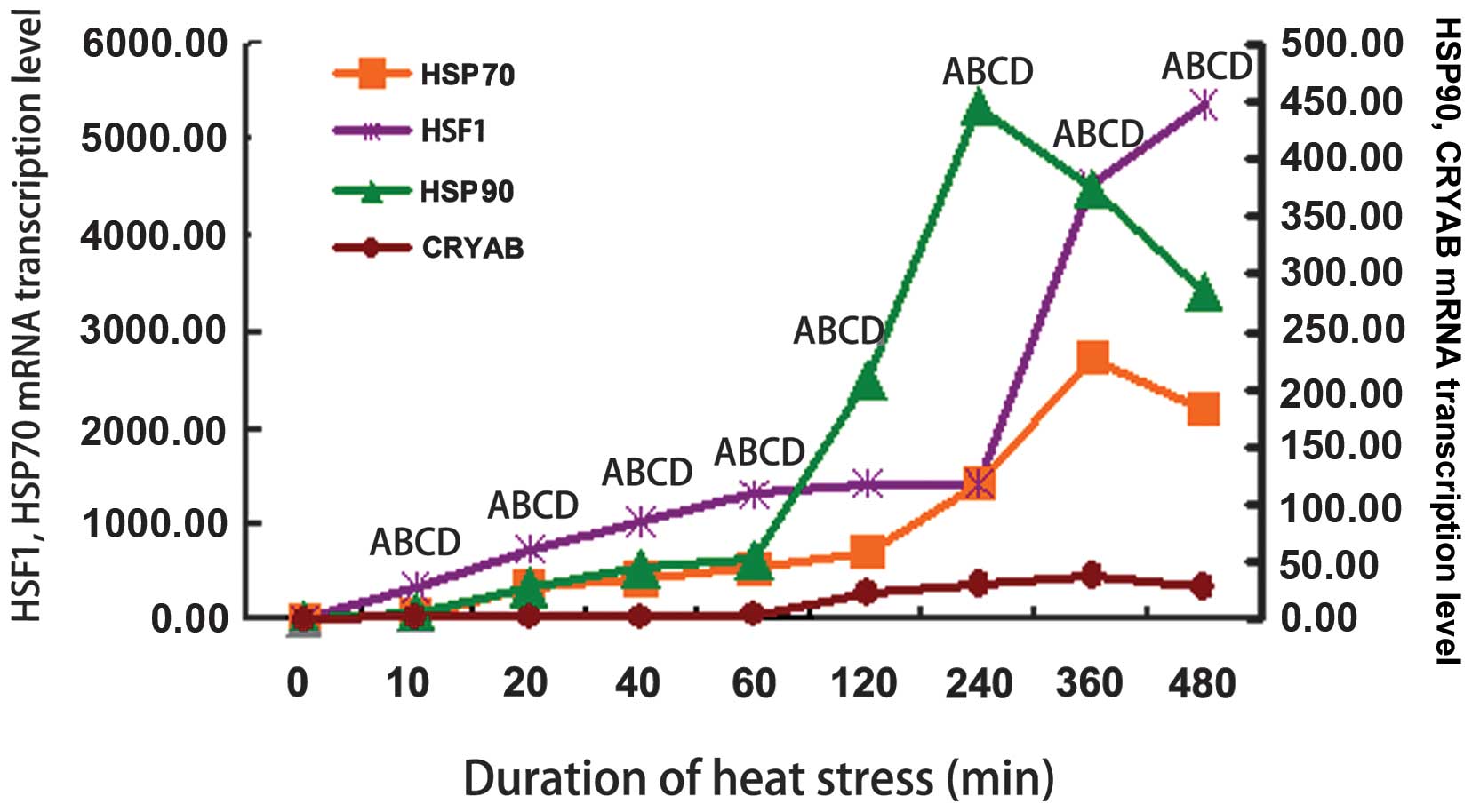
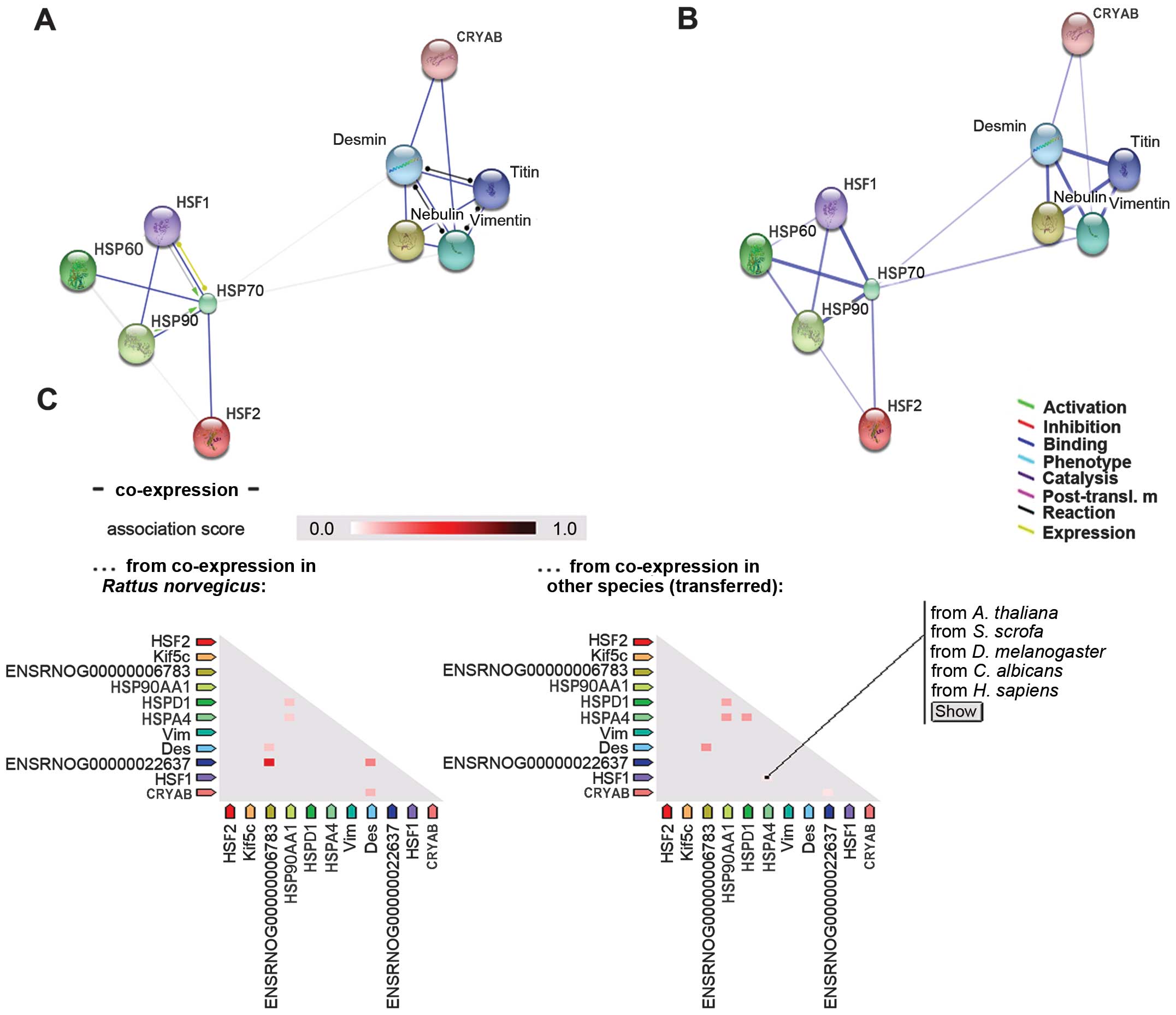
|
1
|
Gisolfi CV, Matthes RD, Kregel KC and
Oppliger R: Splanchnic sympathetic nerve activity and circulating
catecholamines in the hyperthermic rat. J Appl Physiol (1985).
70:1821–1826. 1991.
|
|
2
|
Mirchandani HG, McDonald G, Hood IC and
Fonseca C: Heat-related deaths in Philadelphia - 1993. Am J
Forensic Med Pathol. 17:106–108. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Scheers-Masters JR, Schootman M and Thach
BT: Heat stress and sudden infant death syndrome incidence: A
United States population epidemiologic study. Pediatrics.
113:e586–e592. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yu J, Bao E, Yan J and Lei L: Expression
and localization of Hsps in the heart and blood vessel of
heat-stressed broilers. Cell Stress Chaperones. 13:327–335. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang M, Lv Y, Yue Z, Islam A, Rehana B,
Bao E and Hartung J: Effects of transportation on expression of
Hsp90, Hsp70, Hsp27 and αB-crystallin in the pig stomach. Vet Rec.
169:3122011. View
Article : Google Scholar
|
|
6
|
Li Z and Srivastava P: Heat-shock
proteins. Curr Protoc Immunol (Appendix 1). 58:A.1T.1–A.1T.6.
2004.
|
|
7
|
Adhikari AS, Sridhar Rao K, Rangaraj N,
Parnaik VK and Mohan Rao Ch: Heat stress-induced localization of
small heat shock proteins in mouse myoblasts: Intranuclear lamin
A/C speckles as target for alphaB-crystallin and Hsp25. Exp Cell
Res. 299:393–403. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Borges JC and Ramos CH: Protein folding
assisted by chaperones. Protein Pept Lett. 12:257–261. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Walter S and Buchner J: Molecular
chaperones - cellular machines for protein folding. Angew Chem Int
Ed Engl. 41:1098–1113. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guisbert E, Yura T, Rhodius VA and Gross
CA: Convergence of molecular, modeling, and systems approaches for
an understanding of the Escherichia coli heat shock response.
Microbiol Mol Biol Rev. 72:545–554. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Locke M and Tanguay RM: Diminished heat
shock response in the aged myocardium. Cell Stress Chaperones.
1:251–260. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Abravaya K, Myers MP, Murphy SP and
Morimoto RI: The human heat shock protein hsp70 interacts with HSF,
the transcription factor that regulates heat shock gene expression.
Genes Dev. 6:1153–1164. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Baler R, Zou J and Voellmy R: Evidence for
a role of Hsp70 in the regulation of the heat shock response in
mammalian cells. Cell Stress Chaperones. 1:33–39. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nathan DF, Vos MH and Lindquist S: In vivo
functions of the Saccharomyces cerevisiae Hsp90 chaperone. Proc
Natl Acad Sci USA. 94:12949–12956. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jakob U, Meyer I, Bügl H, André S,
Bardwell JC and Buchner J: Structural organization of procaryotic
and eucaryotic Hsp90. Influence of divalent cations on structure
and function. J Biol Chem. 270:14412–14419. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Young JC, Moarefi I and Hartl FU: Hsp90: A
specialized but essential protein-folding tool. J Cell Biol.
154:267–273. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nadeau K, Das A and Walsh CT: Hsp90
chaperonins possess ATPase activity and bind heat shock
transcription factors and peptidyl prolyl isomerases. J Biol Chem.
268:1479–1487. 1993.PubMed/NCBI
|
|
18
|
Arya R, Mallik M and Lakhotia SC: Heat
shock genes - integrating cell survival and death. J Biosci.
32:595–610. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kirchhoff SR, Gupta S and Knowlton AA:
Cytosolic heat shock protein 60, apoptosis, and myocardial injury.
Circulation. 105:2899–2904. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Veereshwarayya V, Kumar P, Rosen KM,
Mestril R and Querfurth HW: Differential effects of mitochondrial
heat shock protein 60 and related molecular chaperones to prevent
intracellular β-amyloid-induced inhibition of complex IV and limit
apoptosis. J Biol Chem. 281:29468–29478. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Malik ZA, Kott KS, Poe AJ, Kuo T, Chen L,
Ferrara KW and Knowlton AA: Cardiac myocyte exosomes: Stability,
HSP60, and proteomics. Am J Physiol Heart Circ Physiol.
304:H954–H965. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Campanella C, Cappello F, Bucchieri F, et
al: Hsp60 secretion and migration from cancer cells: A proposal for
a multistage pathway. FASEB J. 26:521–526. 2012.
|
|
23
|
Fink AL: Chaperone-mediated protein
folding. Physiol Rev. 79:425–449. 1999.PubMed/NCBI
|
|
24
|
Singh BN, Rao KS, Ramakrishna T, Rangaraj
N and Rao ChM: Association of αB-crystallin, a small heat shock
protein, with actin: Role in modulating actin filament dynamics in
vivo. J Mol Biol. 366:756–767. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ashby RS, Megaw PL and Morgan IG: Changes
in retinal alphaB-crystallin (cryab) RNA transcript levels during
periods of altered ocular growth in chickens. Exp Eye Res.
90:238–243. 2010. View Article : Google Scholar
|
|
26
|
McCully JD, Lotz MM, Krukenkamp IB and
Levitsky S: A brief period of retrograde hyperthermic perfusion
enhances myocardial protection from global ischemia: Association
with accumulation of Hsp 70 mRNA and protein. J Mol Cell Cardiol.
28:231–241. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Morimoto RI, Sarge KD and Abravaya K:
Transcriptional regulation of heat shock genes. A paradigm for
inducible genomic responses. J Biol Chem. 267:21987–21990.
1992.PubMed/NCBI
|
|
28
|
Wu C: Heat shock transcription factors:
Structure and regulation. Annu Rev Cell Dev Biol. 11:441–469. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Westerheide SD, Raynes R, Powell C, Xue B
and Uversky VN: HSF transcription factor family, heat shock
response, and protein intrinsic disorder. Curr Protein Pept Sci.
13:86–103. 2012. View Article : Google Scholar
|
|
30
|
Wu AM, Amdams LG and Pugh R:
Immunochemical and partial chemical characterization of fractions
of membrane-bound smooth lipopolysaccharide-protein complex from
Brucella abortus. Mol Cell Biochem. 75:93–102. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Franceschini A, Szklarczyk D, Frankild S,
et al: STRING v9.1: Protein-protein interaction networks, with
increased coverage and integration. Nucleic Acids Res.
41:D808–D815. 2013. View Article : Google Scholar :
|
|
32
|
Ritossa FM: Experimental activation of
specific loci in polytene chromosomes of Drosophila. Exp Cell Res.
35:601–607. 1964. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ashburner M and Bonner JJ: The induction
of gene activity in drosophilia by heat shock. Cell. 17:241–254.
1979. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Spradling A, Pardue ML and Penman S:
Messenger RNA in heat-shocked Drosophila cells. J Mol Biol.
109:559–587. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Trinklein ND, Chen WC, Kingston RE and
Myers RM: Transcriptional regulation and binding of heat shock
factor 1 and heat shock factor 2 to 32 human heat shock genes
during thermal stress and differentiation. Cell Stress Chaperones.
9:21–28. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Buriro R, Lv YJ, Ali I, Tang S, Liu ZJ,
Zhang M, Adem A, Hartung J and Bao ED: Temporal variations of Hsp60
and HSF-1 in primary rat myocardial cells in vitro under heat
stress. Genet Mol Res. 12:3003–3016. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Colett MS, Larson R, Gold C, Strick D,
Anderson DK and Purchio AF: Molecular cloning and nucleotide
sequence of the pestivirus bovine viral diarrhea virus. Virology.
165:191–199. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cotto C, Berille J, Souquet PJ, Riou R,
Croisile B, Turjman F, Giroux B, Brune J and Trillet-Lenoir V: A
phase II trial of fote-mustine and cisplatin in central nervous
system metastases from non-small cell lung cancer. Eur J Cancer.
32A:69–71. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sorger PK: Heat shock factor and the heat
shock response. Cell. 65:363–366. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Islam A, Lv YJ, Abdelnasir A, et al: The
role of Hsp90α in heat-induced apoptosis and cell damage in primary
myocardial cell cultures of neonatal rats. Genet Mol Res.
12:6080–6091. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hutter JJ, Mestril R, Tam EK, Sievers RE,
Dillmann WH and Wolfe CL: Overexpression of heat shock protein 72
in transgenic mice decreases infarct size in vivo. Circulation.
94:1408–1411. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tang S, Buriro R, Liu Z, Zhang M, Ali I,
Adam A, Hartung J and Bao E: Localization and expression of Hsp27
and αB-crystallin in rat primary myocardial cells during heat
stress in vitro. PLoS One. 8:e690662013. View Article : Google Scholar
|
|
43
|
Ananthan J, Goldberg AL and Voellmy R:
Abnormal proteins serve as eukaryotic stress signals and trigger
the activation of heat shock genes. Science. 232:522–524. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tulapurkar ME, Asiegbu BE, Singh IS and
Hasday JD: Hyperthermia in the febrile range induces HSP72
expression proportional to exposure temperature but not to HSF-1
DNA-binding activity in human lung epithelial A549 cells. Cell
Stress Chaperones. 14:499–508. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Santoro MG: Heat shock factors and the
control of the stress response. Biochem Pharmacol. 59:55–63. 2000.
View Article : Google Scholar
|
|
46
|
Madrigano J, Mittleman MA, Baccarelli A,
Goldberg R, Melly S, von Klot S and Schwartz J: Temperature,
myocardial infarction, and mortality: effect modification by
individual and area-level characteristics. Epidemiology.
24:439–446. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Locke M, Noble EG, Tanguay RM, Feild MR,
Ianuzzo SE and Ianuzzo CD: Activation of heat-shock transcription
factor in rat heart after heat shock and exercise. Am J Physiol.
268:C1387–C1394. 1995.PubMed/NCBI
|
|
48
|
Zou J, Guo Y, Guettouche T, Smith DF and
Voellmy R: Repression of heat shock transcription factor HSF1
activation by HSP90 (HSP90 complex) that forms a stress-sensitive
complex with HSF1. Cell. 94:471–480. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Le Masson F and Christians E: HSFs and
regulation of Hsp70.1 (Hspa1b) in oocytes and preimplantation
embryos: New insights brought by transgenic and knockout mouse
models. Cell Stress Chaperones. 16:275–285. 2011. View Article : Google Scholar :
|
|
50
|
Erlejman AG, Lagadari M, Toneatto J,
Piwien-Pilipuk G and Galigniana MD: Regulatory role of the
90-kDa-heat-shock protein (Hsp90) and associated factors on gene
expression. Biochim Biophys Acta. 1839:71–87. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Acunzo J, Katsogiannou M and Rocchi P:
Small heat shock proteins HSP27 (HspB1), αB-crystallin (HspB5) and
HSP22 (HspB8) as regulators of cell death. Int J Biochem Cell Biol.
44:1622–1631. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Garrido C, Paul C, Seigneuric R and
Kampinga HH: The small heat shock proteins family: The long
forgotten chaperones. Int J Biochem Cell Biol. 44:1588–1592. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ray PS, Martin JL, Swanson EA, Otani H,
Dillmann WH and Das DK: Transgene overexpression of alphaB
crystallin confers simultaneous protection against cardiomyocyte
apoptosis and necrosis during myocardial ischemia and reperfusion.
FASEB J. 15:393–402. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Haskin CL, Athanasiou KA, Klebe R and
Cameron IL: A heat-shock-like response with cytoskeletal disruption
occurs following hydrostatic pressure in MG-63 osteosarcoma cells.
Biochem Cell Biol. 71:361–371. 1993. View Article : Google Scholar : PubMed/NCBI
|