Introduction
Lysophosphatidic acid (LPA) is an efficient,
bioactive phospholipid involved in various biological processes,
including cell proliferation, differentiation, apoptosis, adhesion,
chemotaxis, survival and cancer cell invasion (1–6).
LPA is present in mammalian serum at concentrations
of 1–5 µM; newly-formed LPA is rapidly released into the
extracellular environment when platelets are activated (7). It has also been demonstrated that
LPA is produced when P2X7 receptors expressed in osteoblasts are
activated (8). Furthermore, LPA
is released by osteoblasts (8),
which suggests that LPA may be implicated in fracture healing.
LPA is a important signaling molecule exhibiting
bioactive functions through receptors on the cytomembrane. At least
5 G-protein-coupled receptors (GPCRs) have been identified as
LPA-specific receptors: LPA1, LPA2,
LPA3, LPA4 and LPA5 (9,10).
These receptors are expressed in different cell types, including
osteoblasts, epithelial cells and skeletal muscle cells (2,11–13).
Connective tissue growth factor (CTGF/CCN2) is a
member of the CCN (Cyr61/CCN1, CTGF/CCN2 and NOV/CCN3) family,
which also includes CCN4/WISP1, CCN5/WIPS2 and CCN6/WISP3 (14,15). CCN2 promotes the proliferation and
differentiation of osteoblasts; CCN2 is also involved in bone
development and fracture healing (16–18).
LPA directly induces the production of CCN2 in
epithelial cells, myoblasts and human renal fibroblasts (13,19,20) by binding to LPA receptors
expressed in these cells. LPA receptors are also expressed in
osteoblasts (2,3,11).
However, whether LPA is capable of inducing changes in the levels
of CCN2 in osteoblasts remains unclear. Thus, in the present study,
we aimed to investigate the possible regulatory effects of LPA on
CCN2 expression in osteoblasts.
In the present study, we also aimed to elucidate the
mechanisms through which LPA influences CCN2 expression in
osteoblasts. Our results suggest that LPA affects CCN2 expression
in osteoblasts through the GPCR/protein kinase C (PKC) and protein
kinase A (PKA) pathways.
Materials and methods
Reagents
1-Oleoyl lysophosphatidic acid (sodium salt) (LPA;
item no. 62215; Cayman Chemical Co., Ann Arbor, MI, USA) and
Kil16425 (S1315; Selleck Chemicals, Houston, TX, USA) were
dissolved in 4 mM sterile stock solutions and stored at −20°C until
use. Phorbol 12-myristate 13-acetate (PMA; S1819; PKA agonist),
forskolin (S1612; PKA activator), staurosporine (S1882; PKC
inhibitor) and H-89 (S1643; selective inhibitor of PKA) were
purchased from Beyotime Institute of Biotechnology (Shanghai,
China). CCN2 polyclonal rabbit anti-mouse CTGF primary antibody
(ab6992, diluted 1:2,000) and glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) rabbit anti-GAPDH primary antibody (ab181602,
diluted 1:10,000) were purchased from Abcam (Cambridge, UK).
Cell cultures and cell treatment
The MC3T3-E1 cell line (China Center for Type
Culture Collection, Wuhan, China) was cultured in α-modified
minimal essential medium (α-MEM) containing 10% fetal bovine serum
(FBS) (both from HyClone, Logan, UT, USA), 100 U/ml of penicillin
and 100 µg/ml of streptomycin at 37°C in a 5% CO2
atmosphere; the medium was changed to fresh medium at an interval
of 3 days. The cells were seeded on 6-well plates or 6 cm dishes to
examine gene and protein expression. After the cells reached
confluence, the culture medium was replaced with α-MEM and 1%
penicillin-streptomycin without FBS for 12 h. The medium was then
replaced with α-MEM containing 4% bovine seum albumin (BSA) and
varying doses of LPA, PMA or forskolin. Inhibitors, such as
staurosporine and H-89, were used to suppress PKC and PKA activity,
respectively. After co-incubation was performed for the indicated
periods of time (0–12 h), the MC3T3-E1 cells were lysed for the
subsequent analysis of RNA and protein expression.
Measurement of cell viability
The viability of the MC3T3-E1 cells was determined
as previously described (21).
Briefly, following incubation of the cells with increasing
concentrations of LPA (0–40 µM) for different periods of
time (12 to 72 h), cell viability was determined using a cell
viability analyzer (Beckman Coulter, Fullerton, CA, USA).
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
MTT assay was performed as described in a previous
study (22). Briefly, the
MC3T3-E1 cells were seeded in 96-well plates (3×103
cells/well) and incubated with increasing concentrations of LPA
(0–40 µM). At the end of the incubation period, 20 µl
of 5 mg/ml MTT were added; the cells were then further incubated
for 4 h. Subsequently, the supernatant was removed, and 150
µl of dimethyl sulfoxide (DMSO) were added to each well. The
absorbance of each well was detected at 490 nm. Data are presented
as percentage of the control group.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
RNA isolation and RT-qPCR were performed as
previously described (23).
Briefly, total RNA was isolated from the ME3T3-E1 cells in each
treatment group using TRIzol reagent (Invitrogen, Carlsbad, CA,
USA) according to the manufacturer's instructions. cDNA was
synthesized from 1 µg of total RNA using a Revert Aid
First-Strand cDNA Synthesis kit (#K1622; Thermo Fisher Scientific,
Waltham, MA, USA). The obtained cDNA was then amplified by
quantitative PCR (qPCR) using an ABI 7900 HT Real-Time PCR system,
(Applied Biosystems, Foster City, CA, USA) and
SYBR®-Green Real-Time PCR Master Mix (Toyobo, Tokyo,
Japan), under the following conditions: stage 1, 95°C for 10 min;
stage 2, 95°C for 10 sec, 55°C for 20 sec, followed by 40 cycles of
72°C for 15 sec; stage 3, melt curve stage. GAPDH was used for the
normalization of gene expression. Relative gene expression was
analyzed using the 2−ΔΔCt method based on normalization
to the endogenous control, GAPDH, and calculation of the threshold
cycle (Ct) value difference. The results were represented as a fold
change of the comparative expression level. The sequences of the
forward and reverse primers were as follows: 5′-GCCTACCGACTGGAA
GACACATTT-3′ and 5′-TTACGCCATGTCTCCGTACA TCTT-3′ for the CCN2 gene;
5′-ACCACAGTCCATGCCA TCAC-3′ and 5′-TCCACCACCCTGTTGCTGTA-3′ for the
internal control GAPDH gene.
Protein extraction and western blot
analysis
The cells were treated with the indicated stimulants
(LPA, LPA + Kil16425, LPA + staurosporine, PMA, LPA + H-89,
forskolin, or LPA + forskolin) for 6 h. Subsequently, the cells
were then washed thrice with phosphate-buffered saline (PBS) and
treated with RIPA lysis buffer. The cell lysates were cleared by
centrifugation; protein concentration in the supernatant was
measured by bicinchoninic acid (BCA) protein assay (Thermo Fisher
Scientific). Total proteins (20 µg) were separated through
10% sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis
and the proteins were then transferred onto PVDF membranes
(Bio-Rad, Hercules, CA, USA). The membranes were blocked with 5%
(w/v) nonfat milk and incubated overnight with the primary
antibodies (CCN2, PKC or GAPDH) at the specified dilutions in TBST
buffer (CW0043; Beijing Conwin Biotech Co., Beijing, China)
according to the manufacturer's instructions. Protein expression
was detected using an enhanced chemiluminescence reagent (Thermo
Fisher Scientific). GADPH was used as an internal control
protein.
PKC activity assay
PKC activity was assessed by determining the
translocation of PKC from the cytosol to the membrane. After the
cells were treated with the indicated stimulants for the indicated
periods of time, PKC in the cytosol and in the membrane of MC3T3-E1
cells was extracted using a Mem-PER Plus Membrane Protein
Extraction kit (Thermo Fisher Scientific). PKC expression in the
cytosol and membrane was then determined by western blot analysis.
The membrane translocation of PKC was also investigated by
immunofluorescence. Briefly, the MC3T3-E1 cells were plated on
cover slips and fixed with 4% paraformaldehyde (PFA) for 30 min at
room temperature. The cells were then washed thrice with PBS
buffer, permeabilized with PBS buffer containing 0.1% Triton X-100
and blocked with 1% BSA. The cells were then incubated with PKC
antibody (P5704; Sigma, St. Louis, MO, USA) for 2 h at 37°C. The
cells were washed again with PBS buffer thrice and incubated with
PE-labeled secondary antibody (CW0113; Beijing Conwin Biotech Co.).
The fluorochrome dye, 4′,6-diamidino-2-phenylindole (DAPI), was
used to visualize the cell nuclei. Images were captured using a
fluorescence microscope (Leica Microsystems GmbH, Wetzlar,
Germany).
Statistical analysis
Data were analyzed by one-way analysis of variance
(ANOVA) using GraphPad Prism 5.0 software. Data are expressed as
the means ± standard error of the mean (SEM) of 3 independent
experiments. A value of P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of LPA on the viability and
proliferation of MC3T3-E1 cells
We initially examined the effects of LPA on the
viability and proliferation of the MC3T3-E1 cells. The results of
cell viability assay demonstrated that LPA did not exert cytotoxic
effects on the MC3T3-E1 cells (Fig.
1A). A previous study reported that LPA induced an increase in
DNA synthesis in rat osteoblasts in vitro (24). However, our results from MTT assay
revealed that increasing concentrations of LPA did not affect
MC3T3-E1 cell proliferation in vitro (Fig. 1B).
CCN2 expression in LPA-stimulated
MC3T3-E1 cells
The MC3T3-E1 cells were stimulated with 20 µM
LPA and 4% BSA for 0.5, 1, 2, 4, 6, 8 and 12 h, in order to examine
the effects of LPA on CCN2 expression in osteoblasts. The CCN2 mRNA
expression levels were measured by RT-qPCR. The results revealed
that LPA transiently induced the mRNA expression of CCN2; maximum
expression levels were observed 2 h following stimulation. The mRNA
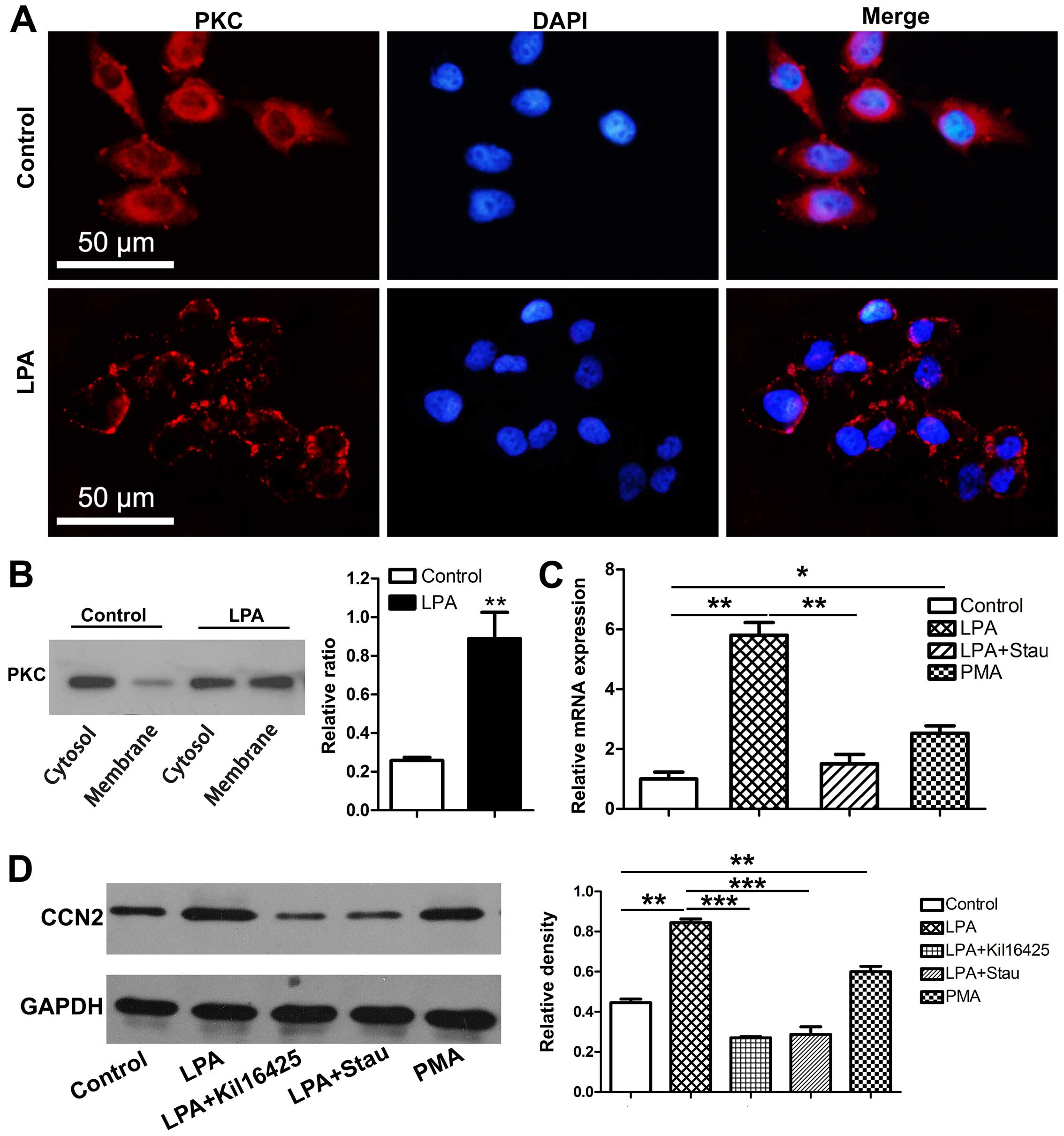
expression levels of CCN2 subsequently decreased (Fig. 2A). The results from western blot
analysis revealed that the CCN2 protein expression levels was
similarly enhanced following 6 h of stimulation with LPA (Fig. 3D).
Ki16425 antagonizes the LPA-induced
increase in CCN2 expression in osteoblasts
LPA elicits its functions through receptors on
plasma membranes; MC3T3-E1 cells express LPA-specific receptors
with the following relative abundance pattern: LPA1 >
LPA4 > LPA2 > LPA3 (2). Thus, in this study, we wished to
determine whether Kil16425, a specific inhibitor of
LPA1/3 (25), is was
capable of antagonizing the effects of LPA on CCN2 expression in
MC3T3-E1 cells. We found that pre-treatment with Ki16425 (20
µM) significantly reduced the LPA-induced increase in the
mRNA expression of CCN2 in the MC3T3-E1 cells (Fig. 2B). Kil16425 also blocked the
LPA-induced increase in the protein expression of CCN2 in the
MC3T3-E1 cells (Fig. 3D).
The effects of LPA on CCN2 expression may be
attributed to LPA1 due to the very low expression of
LPA3 (2). Thus, LPA
induces an increase in CCN2 expression through the LPA1
receptor in MC3T3-E1 cells.
Effects of LPA on PKC activity
LPA receptors belong to the GPCR family (9). The LPA-induced activation of GPCR
induces an increase in a variety of second messengers, such as
Ca2+ in the cytoplasm of osteoblasts (2,3,8,26).
Ca2+ is essential for PKC membrane translocation and
activation (27). The activation
PKC is manifested by the membrane translocation of PKC (28) and PKC activity may be thus
enhanced by LPA. In this study, the MC3T3-E1 cells were stimulated
with LPA for 15 min; PKC membrane translocation was observed by
immunofluorescence staining (Fig.
3A). Western blot analysis revealed that LPA increased the
ratio between membrane-derived PKC and cytosolic PKC (Fig. 3B). These results indicate that LPA
induces PKC membrane translocation and enhances PKC activity in
osteoblasts.
Induction of CCN2 in response to LPA
requires the activation of PKC
Staurosporine and PMA, an inhibitor and an agonist
of PKC, respectively, were used to determine whether PKC is
involved in the effects of LPA on CCN2 expression. Staurosporine
(20 nM) markedly impaired the ability of LPA to enhance CCN2
expression in the MC3T3-E1 cells, whereas PMA (1 µM)
mimicked LPA and induced the expression of CCN2 (Fig. 3C and D).
Effects of PKA on LPA-induced CCN2
expression
Cyclic AMP (cAMP), another second messenger,
accumulates in cells expressing LPA4,5 receptors
(29,30) in the presence of LPA; the
accumulation of cAMP increases PKA activity. We thus examined the
role of PKA in the expression of CCN2 in LPA-treated MC3T3-E1
cells. The MC3T3-E1 cells were pre-incubated with H-89 (10
µM), a selective inhibitor of PKA, for 30 min; LPA (20
µM) was then added or the cells were treated with the PKA
activator, forskolin, alone for 2 and 6 h. The mRNA and protein
expression levels of CCN2 in the LPA-H-89 group were enhanced
compared with those of the LPA group (Fig. 4). Pre-treatment with forskolin (10
µM) significantly decreased the mRNA and protein expression
levels of of CCN2 which had been increased by LPA in the MC3T3-E1
cells. No significant effects were observed in the cells treated
with forskolin alone compared to the cells also treated with LPA
(Fig. 4).
Discussion
LPA is a small, bioactive phospholipid that mediates
multiple cellular processes (1–6).
Previous studies have demonstrated that LPA enhances CCN2
expression in epithelial cells, myoblasts and human renal
fibroblasts (13,19,20). However, the mechanisms through
which LPA influences CCN2 expression in these cells remain unclear,
and it is unknown whether LPA is capable of enhancing CCN2
expression in osteoblasts. In this study, we demonstrated that LPA
induced CCN2 expression in MC3T3-E1 cells. To the best of our
knowledge, our study provides preliminary data on LPA-induced CCN2
expression in MC3T3-E1 cells.
LPA receptors are GPCRs expressed in MC3T3-E1 cells
(2). Given the dominant
expression of LPA1, we used Kil16425, a specific
inhibitor of LPA1/3 (25) to identify which LPA receptor
subtype is involved in the LPA-induced increase in CCN2 expression.
The results revealed that the LPA-induced increase in CCN2
expression in osteoblasts was antagonized by Kil16425.
The activation of LPA receptors by LPA increases the
amount of various second messengers, such as Ca2+, which
are required for PKC activation and membrane translocation in
osteoblasts (31). Thus, in this
study, we evaluated PKC activity in LPA-stimulated MC3T3-E1 cells.
The membrane translocation of PKC in the MC3T3-E1 cells was induced
by LPA following 15 min of stimulation. This finding indicated that
LPA enhanced PKC activity in osteoblasts.
We subsequently examined the role of PKC in the
LPA-induced increase in CCN2 expression. Pre-treatment with
staurosporine significantly reduced the LPA-induced increase in
CCN2 expression. Treatment with PMA alone enhanced the effects of
LPA on CCN2 expression. Taken together, these findings demonstrate
that the PKC pathway is involved in the LPA-induced increase in
CCN2 expression, and thus a positive association between CCN2
expression and PKC activity was established herein. To the best of
our knowledge, the present study is the first to demonstrate that
the PKC pathway is involved in the promoting effects of LPA on CCN2
expression in MC3T3-E1 cells.
The cells pre-treated with the PKA inhibitor, H-89,
and subsequently stimulated with LPA exhibited higher expression
levels of CCN2 than the cells stimulated with LPA alone. The PKA
activator, forskolin, had no effect on CCN2 expression compared to
the cells stimulated with LPA alone. Our results are similar to
those of other studies which concluded that cAMP stimulated CCN2
degradation in microvessel cells and decreased CCN2 expression in
human renal fibroblasts; moreover, the results demonstrated that
elevated intracellular cAMP levels activated PKA which in turn
prevented the induction of CCN2 (20,32). Those results suggested that the
PKA pathway reduced the LPA-induced increase in CCN2 expression in
MC3T3-E1 cells.
The present study revealed the mechanisms
responsible for the LPA-induced increase in CCN2 expression in
MC3T3-E1 cells. We identified LPA1 as an important
receptor in this process. Moreover, the PKC and PKA pathways were
shown to be involved in the LPA-induced increase in CCN2
expression. The hypothetical signaling pathway of the LPA-induced
increase in CCN2 expression in osteoblasts is illustrated in
Fig. 5. These findings elucidated
the mechanisms responsible for the increase in CCN2 expression in
LPA-stimulated cells.
Abbreviations:
|
LPA
|
lysophosphatidic acid
|
|
CTGF
|
connective tissue growth factor
|
|
GPCRs
|
G-protein-coupled receptors
|
|
PKC
|
protein kinase C
|
|
PKA
|
protein kinase A
|
|
PMA
|
phorbol 12-myristate 13-acetate
|
|
α-MEM
|
α-modified minimal essential
medium
|
|
FBS
|
fetal bovine serum
|
|
DMSO
|
dimethyl sulfoxide
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
GAPDH
|
glyceraldehyde-3-phosphate
dehydrogenase
|
|
PBS
|
phosphate-buffered saline
|
|
PFA
|
paraformaldehyde
|
|
cAMP
|
cyclic AMP
|
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (grant nos. 81271107 and 81470718).
References
|
1
|
Goetzl EJ: Pleiotypic mechanisms of
cellular responses to biologically active lysophospholipids.
Prostaglandins. 64:11–20. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Masiello LM, Fotos JS, Galileo DS and
Karin NJ: Lysophosphatidic acid induces chemotaxis in MC3T3-E1
osteoblastic cells. Bone. 39:72–82. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu YB, Kharode Y, Bodine PV, Yaworsky PJ,
Robinson JA and Billiard J: LPA induces osteoblast differentiation
through interplay of two receptors: LPA1 and
LPA4. J Cell Biochem. 109:794–800. 2010.PubMed/NCBI
|
|
4
|
Peyruchaud O, Leblanc R and David M:
Pleiotropic activity of lysophosphatidic acid in bone metastasis.
Biochim Biophys Acta. 1831:99–104. 2013. View Article : Google Scholar
|
|
5
|
Sims SM, Panupinthu N, Lapierre DM,
Pereverzev A and Dixon SJ: Lysophosphatidic acid: a potential
mediator of osteoblast-osteoclast signaling in bone. Biochim
Biophys Acta. 1831:109–116. 2013. View Article : Google Scholar
|
|
6
|
Hurst-Kennedy J, Boyan BD and Schwartz Z:
Lysophosphatidic acid signaling promotes proliferation,
differentiation, and cell survival in rat growth plate
chondrocytes. Biochim Biophys Acta. 1793:836–846. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Eichholtz T, Jalink K, Fahrenfort I and
Moolenaar WH: The bioactive phospholipid lysophosphatidic acid is
released from activated platelets. Biochem J. 291:677–680. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Panupinthu N, Rogers JT, Zhao L,
Solano-Flores LP, Possmayer F, Sims SM and Dixon SJ: P2X7 receptors
on osteoblasts couple to production of lysophosphatidic acid: a
signaling axis promoting osteogenesis. J Cell Biol. 181:859–871.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Noguchi K, Herr D, Mutoh T and Chun J:
Lysophosphatidic acid (LPA) and its receptors. Curr Opin Pharmacol.
9:15–23. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fukushima N and Chun J: The LPA receptors.
Prostaglandins Other Lipid Mediat. 64:21–32. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dziak R, Yang BM, Leung BW, Li S, Marzec
N, Margarone J and Bobek L: Effects of sphingosine-1-phosphate and
lysophosphatidic acid on human osteoblastic cells. Prostaglandins
Leukot Essent Fatty Acids. 68:239–249. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vial C, Zúñiga LM, Cabello-Verrugio C,
Cañón P, Fadic R and Brandan E: Skeletal muscle cells express the
profibrotic cytokine connective tissue growth factor (CTGF/CCN2),
which induces their dedifferentiation. J Cell Physiol. 215:410–421.
2008. View Article : Google Scholar
|
|
13
|
Wiedmaier N, Müller S, Köberle M, Manncke
B, Krejci J, Autenrieth IB and Bohn E: Bacteria induce CTGF and
CYR61 expression in epithelial cells in a lysophosphatidic acid
receptor-dependent manner. Int J Med Microbiol. 298:231–243. 2008.
View Article : Google Scholar
|
|
14
|
Brigstock DR: The CCN family: a new
stimulus package. J Endocrinol. 178:169–175. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brigstock DR, Goldschmeding R, Katsube KI,
Lam SC, Lau LF, Lyons K, Naus C, Perbal B, Riser B, Takigawa M and
Yeger H: Proposal for a unified CCN nomenclature. Mol Pathol.
56:127–128. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kawaki H, Kubota S, Suzuki A, Yamada T,
Matsumura T, Mandai T, Yao M, Maeda T, Lyons KM and Takigawa M:
Functional requirement of CCN2 for intramembranous bone formation
in embryonic mice. Biochem Biophys Res Commun. 366:450–456. 2008.
View Article : Google Scholar
|
|
17
|
Nakanishi T, Nishida T, Shimo T, Kobayashi
K, Kubo T, Tamatani T, Tezuka K and Takigawa M: Effects of
CTGF/Hcs24, a product of a hypertrophic chondrocyte-specific gene,
on the proliferation and differentiation of chondrocytes in
culture. Endocrinology. 141:264–273. 2000.
|
|
18
|
Arnott JA, Lambi AG, Mundy C, Hendesi H,
Pixley RA, Owen TA, Safadi FF and Popoff SN: The role of connective
tissue growth factor (CTGF/CCN2) in skeletogenesis. Crit Rev
Eukaryot Gene Expr. 21:43–69. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cabello-Verrugio C, Córdova G, Vial C,
Zúñiga LM and Brandan E: Connective tissue growth factor induction
by lysophosphatidic acid requires transactivation of transforming
growth factor type β receptors and the JNK pathway. Cell Signal.
23:449–457. 2011. View Article : Google Scholar
|
|
20
|
Heusinger-Ribeiro J, Eberlein M, Wahab NA
and Goppelt-Struebe M: Expression of connective tissue growth
factor in human renal fibroblasts: regulatory roles of RhoA and
cAMP. J Am Soc Nephrol. 12:1853–1861. 2001.PubMed/NCBI
|
|
21
|
Chen G, Zhu JY, Zhang ZL, Zhang W, Ren JG,
Wu M, Hong ZY, Lv C, Pang DW and Zhao YF: Transformation of
cell-derived microparticles into quantum-dot-labeled nanovectors
for antitumor siRNA delivery. Angew Chem Int Ed Engl. 54:1036–1040.
2015. View Article : Google Scholar
|
|
22
|
Li YG, Zhu W, Tao JP, Xin P, Liu MY, Li JB
and Wei M: Resveratrol protects cardiomyocytes from oxidative
stress through SIRT1 and mitochondrial biogenesis signaling
pathways. Biochem Biophys Res Commun. 438:270–276. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu Q, Wan Q, Yang R, Zhou H and Li Z:
Effects of intermittent versus continuous parathyroid hormone
administration on condylar chondrocyte proliferation and
differentiation. Biochem Biophys Res Commun. 424:182–188. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Grey A, Banovic T, Naot D, Hill B, Callon
K, Reid I and Cornish J: Lysophosphatidic acid is an osteoblast
mitogen whose proliferative actions involve G(i) proteins and
protein kinase C, but not P42/44 mitogen-activated protein kinases.
Endocrinology. 142:1098–1106. 2001.PubMed/NCBI
|
|
25
|
Ohta H, Sato K, Murata N, Damirin A,
Malchinkhuu E, Kon J, Kimura T, Tobo M, Yamazaki Y, Watanabe T, et
al: Ki16425, a subtype-selective antagonist for EDG-family
lysophosphatidic acid receptors. Mol Pharmacol. 64:994–1005. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Aki Y, Kondo A, Nakamura H and Togari A:
Lysophosphatidic acid-stimulated interleukin-6 and -8 synthesis
through LPA1 receptors on human osteoblasts. Arch Oral
Biol. 53:207–213. 2008. View Article : Google Scholar
|
|
27
|
Escribá PV, Wedegaertner PB, Goñi FM and
Vögler O: Lipid-protein interactions in GPCR-associated signaling.
Biochim Biophys Acta. 1768:836–852. 2007. View Article : Google Scholar
|
|
28
|
Yuan X, Chen H, Li X, Dai M, Zeng H, Shan
L, Sun Q and Zhang W: Inhibition of protein kinase C by
isojacareubin suppresses hepatocellular carcinoma metastasis and
induces apoptosis in vitro and in vivo. Sci Rep. 5:128892015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee CW, Rivera R, Gardell S, Dubin AE and
Chun J: GPR92 as a new G12/13 - and Gq -
coupled lysophosphatidic acid receptor that increases cAMP,
LPA5. J Biol Chem. 281:23589–23597. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee CW, Rivera R, Dubin AE and Chun J:
LPA(4)/GPR23 is a lysophosphatidic acid (LPA) receptor
utilizing G(s)-,
G(q)/G(i)-mediated calcium signaling and
G(12/13)-mediated Rho activation. J Biol Chem.
282:4310–4317. 2007. View Article : Google Scholar
|
|
31
|
Lin ME, Herr DR and Chun J:
Lysophosphatidic acid (LPA) receptors: signaling properties and
disease relevance. Prostaglandins Other Lipid Mediat. 91:130–138.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Boes M, Dake BL, Booth BA, Erondu NE, Oh
Y, Hwa V, Rosenfeld R and Bar RS: Connective tissue growth factor
(IGFBP-rP2) expression and regulation in cultured bovine
endothelial cells. Endocrinology. 140:1575–1580. 1999.PubMed/NCBI
|