Introduction
It has been pointed out that non-alcoholic fatty
liver disease (NAFLD) is the most common cause of chronic liver
injury (1), and in many developed
countries as much as one-third of the population exhibit steatosis
(2,3). The majority of patients with NAFLD
remain asymptomatic, but 20% of NAFLD patients have experienced
non-alcoholic steatohepatitis (NASH) that may progress to cirrhosis
and hepatocellular carcinoma (4).
The rapid pace of modern life, the increasing intake of
high-energy-density foods and reduced physical activity has led to
the increasing prevalence of obesity (5), which affects public health and
results in numerous metabolic disorders, such as NAFLD, type 2
diabetes mellitus, hypertension and hyperlipidemia. Obesity, in
particular, can result in a wide spectrum of liver abnormalities,
ranging from hepatic steatosis to NASH, and even to cirrhosis
(6), implying that obesity is
therefore the main driver of the greater prevalence of NAFLD
(1). Thus, preventing obesity
will benefit patient recovery from NAFLD, and in the present study
obesity was induced by feeding rats a high-fat diet (HFD), which is
one approach to establishing an animal model of NAFLD (7–10).
For the management of NAFLD, medical treatment of
metabolic risk factors such as dyslipidaemia and hypertension is
required, and modifications to diet and physical activity must also
be undertaken (11). However,
antihyperlipidemic and antihypertensive drugs are associated with
an increased risk of myodynia as well as liver and kidney damage.
Thus, an effective strategy for the treatment of NAFLD warrants
urgent investigation.
Regenerative medicine using adipose tissue-derived
stem cells (ADSCs) provides a promising, novel strategy for the
treatment of various intractable diseases, as ADSCs possess
numerous advantages, including the ability to self-renew and
multidifferentiate, their abundant availability, ease of obtainment
and greater immunoregulatory ability (12,13). Previous research has suggested
that ADSCs ameliorate hypertension (14,15), hyperlipemia and obesity (16,17), and alleviate liver damage in an
animal model of acute or chronic liver failure and liver fibrosis
(18–20). Therefore, ADSC transplantation may
be a suitable method of treating NAFLD. In the present study, we
established a rat model of NAFLD by feeding rats a HFD in order to
evaluate the therapeutic effect of ADSC transplantation in
combination with dietary modification. Our results showed that ADSC
transplantation promotes the reversion of NAFLD by improving liver
function and by promoting lipid metabolism as well as
hepatoprotective effects.
Materials and methods
Animals and ethics approval
Fifty adult male Sprague-Dawley rats (weighing
180–200 g) were obtained from the Center for Animal Experiments of
Fujian Medical University (license no. SCXKmin2012-0002). They were
housed at a constant temperature (22±2°C), with 60% relative
humidity, and a 12:12 light-dark cycle. The rats had ad
libitum access to food and autoclaved water. All animal
procedures were approved by the Animal Ethics Committee of Fuzhou
General Hospital (Fuzhu, China).
Isolation and culture of rat ADSCs
Rat ADSCs were harvested as previous described
(21). Briefly, following
anesthetization of the male Sprague-Dawley rats (n=2) using
pentobarbital sodium (40 mg/kg; Merck & Co., Inc., Whitehouse
Station, NJ, USA), adipose tissues (approximately 3×1.5×0.5 cm)
were scraped from the subcutaneous inguinal region, cut into small
pieces (approximately 0.1×0.1×0.1 mm), and digested with 0.1% type
I collagenase (Sigma-Aldrich, St. Louis, MO, USA) at 37°C for 60
min with gentle shaking. Subsequently, the digested tissue was
filtered through a 100-μm cell strainer, centrifuged at 400
× g for 5 min and washed twice with PBS (HyClone, Logan, UT, USA).
The cell pellet was suspended with expanding medium consisting of
α-Modified Eagle's Medium (α-MEM; HyClone) with 20% fetal bovine
serum (FBS; Life Technologies, Scoresby, Australia) supplemented
with penicillin (100 U/ml; Life Technologies) and streptomycin (100
μg/ml; Life Technologies), and then transferred into 6-well
plates (Corning Inc., Acton, MA, USA) at a density of
1×106/ml, and incubated at 37°C with 5% CO2.
Following incubation for 24 h, the non-adherent cells were
discarded, whereas the adherent cells were further expanded in the
complete medium, and medium was changed every 2 days. Once the
cultured cells reached approximately 80% confluence, they were
detached with 0.25% trypsin-0.02% ethylenediaminetetraacetic acid
(EDTA; Life Technologies) and passaged at a ratio of 1:3. Cells
from the third to fifth passages were used in the present
study.
Differentiation of ADSCs
To ensure that the cultured cells were ADSCs, the
ability to differentiate into multi-lineage cells was investigated.
Cells were seeded into 12-well plates (Corning Inc.) at a density
of 3×104 cells/well in 1 ml expansion medium. After
reaching 80% confluence, the cells were cultured with specific
induction medium as previously described (22). To induce osteogenic
differentiation, the cells were cultured with osteogenic induction
medium consisting of DMEM, 10% FBS, 100 U/ml penicillin and 100
μg/ml streptomycin (all from Life Technologies), as well as
0.1 μM dexamethasone, 50 μM ascorbate-2-phosphate and
10 mM β-glycerophosphate (all from Sigma-Aldrich). For
adipogenesis, the cells were cultured with adipogenic induction
medium consisting of DMEM, 10% FBS, 100 U/ml penicillin and 100
μg/ml streptomycin (all from Life Technologies), as well as
0.5 mM isobutyl-methylxanthine (IBMX), 1 μM dexamethasone,
10 μM insulin and 200 μM indomethacin (all from
Sigma-Aldrich). For chondrogenesis, the cells were cultured with
chondrogenic induction medium consisting of DMEM, 1% FBS, 100 U/ml
penicillin and 100 μg/ml streptomycin (all from Life
Technologies), as well as 6.25 μg/ml insulin, 10 ng/ml
TGF-β1, 50 nM ascorbate-2-phosphate (all from Sigma-Aldrich). Four
weeks later, the cell population was stained using an oil red O
staining kit (Nanjing Jiancheng Bioengineering Institute, Nanjing,
China), an alizarin red S staining kit (Solarbio, Beijing, China)
and also a toluidine blue staining kit (Nanjing Jiancheng
Bioengineering Institute) respectively, according to the
manufacturers' instructions.
Sphere formation assay
In order to further explore the activity of ADSCs,
sphere cluster formation was analyzed as previously described with
minor modifications (23). The
cells (2×104) were seeded into 24-well
ultralow-attachment culture plates (Thermo Fisher Scientific,
Waltham, MA, USA) and cultured in StemPro® MSC SFM CTS™
medium (Life Technologies). After culture for 24 h, the spheres
were visible under an inverted phase-contrast microscope (Zeiss,
Oberkochen, Germany).
Flow cytometric analysis
The surface biomarkers of ADSCs were characterized
by flow cytometric analysis to ensure cell quality. The adherent
cells were firstly dissociated with 0.25% trypsin-0.02% EDTA, then
re-suspended in α-MEM containing 10% FBS, and further incubated in
PBS containing 5% bovine serum albumin (BSA; Sigma-Aldrich) for 20
min at room temperature. Subsequently, the cells were incubated
with various primary antibodies as indicated for 60 min at room
temperature, including phycoerythrin (PE)-conjugated anti-mouse/rat
CD29 (monoclonal, 1:200) (cat. no. 12-0291-82; purchased from
eBioscience, Inc., San Diego, CA, USA); mouse anti-rat/human CD31
(monoclonal, 1:100) (cat. no. SC-80913), mouse anti-rat/human CD34
(monoclonal, 1:100) (cat. no. SC-7324) (both from Santa Cruz
Biotechnology, Inc., Dallas, TX, USA); PE-conjugated anti-mouse/rat
CD44H (monoclonal, 1:200) (cat. no. 12-0444-82; eBioscience, Inc.),
rabbit anti-rat/human/mouse CD45 (polyclonal, 1:100) (cat. no.
SC-25590), goat anti-rat/human/mouse CD73 (polyclonal, 1:100) (cat.
no. SC-14684), rabbit anti-rat/human/mouse CD90 (polyclonal, 1:100)
(cat. no. SC-9163) (all from Santa Cruz Biotechnology, Inc.); mouse
anti-rat/human CD105 (monoclonal, 1:500) (cat. no. Ab 156756) and
mouse anti-rat human leukocyte antigen - antigen D related (HLA-DR)
(monoclonal, 1:300) (cat. no. Ab 119795) (both from Abcam,
Cambridge, UK), respectively. The cells were then washed twice with
PBS, and further incubated with fluorescent-conjugated secondary
antibodies donkey anti-mouse IgG-Alexa Fluor® 488
(polyclonal, 1:1,000) (cat. no. A-21202) and donkey anti-rabbit
IgG-Alexa Fluor® 647 (polyclonal, 1:1,000) (cat. no.
A-31573) (both from Invitrogen Life Technologies, Carlsbad, CA,
USA) for 30 min at room temperature. Finally, the cells were washed
twice with PBS and characterized using a fluorescence activated
cell sorter (FACS; BD Biosciences, Franklin Lakes, NJ, USA), and
the raw data were further analyzed using FlowJo 7.6 software (Tree
Star, Inc., Ashland, OR, USA).
Establishing a rat model of NAFLD, and
transplantation of ADSCs
Forty-eight Sprague-Dawley rats were fed either
normal chow or a HFD consisting of 88% normal chow, 2% cholesterol
and 10% lard, for 6 weeks. After that, the normal rats or those
with NAFLD were sacrificed (n=6). Following the development of
hepatic steatosis, which was verified by gross examination and
pathological assessment, the rats with HFD-induced NAFLD (n=36)
were randomly divided into two groups: the mock group (n=18) which
was treated with PBS (1 ml/rat), and the ADSC therapy group (n=18)
that received intrahepatic transplantation of ADSCs
(2×106 cells/rat). The transplantation procedure was
performed under aseptic conditions as follows: the rats were
temporarily anesthetized via inhalation of ether (Sinopharm
Chemical Reagent Co., Ltd., Shanghai, China), and the abdominal
cavity was then opened under aseptic conditions. The portal vein
was exposed with moistened swabs; subsequently, the ADSCs suspended
in 1 ml of PBS were injected into the portal vein with a 24-gauge
needle. Following transplantation, all rats with HFD-induced were
simultaneously treated with a modified diet (they were fed normal
chow). After rats were sacrificed on weeks 2, 4 and 8 after
transplantation, liver tissues (approximately 400 mg/rat) and sera
(approximately 3 ml/rat) were collected for further
investigation.
Histopathological assessment
After the rats were sacrificed using pentobarbital
sodium (80 mg/kg; Merck & Co., Inc.), the liver samples were
removed and examined using a single lens reflex (SLR) camera
(Nikon, Tokyo, Japan). We also measured the body and liver weight
using an electronic balance (Sartorius, Goettingen, Germany) to
examine the hepatosomatic index (HSI = liver weight/body weight)
×100. Subsequently, fresh liver tissues were fixed in 4%
paraformaldehyde at room temperature for 24 h, and then gradually
dehydrated with ethanol and embedded in paraffin. The paraffin
blocks were subsequently sectioned (5 μm) and stained with a
hematoxylin and eosin (H&E) staining kit (Nanjing Jiancheng
Bioengineering Institute). Double-blind evaluation of hepatic
steatosis was performed by two expert pathologists. To further
clarify the degree of hepatic lipid accumulation, oil red O
staining of the sections was performed using an oil red O staining
kit (Nanjing Jiancheng Bioengineering Institute) following the
manufacturer's instructions. The histopathological examination was
performed using an inverted phase-contrast microscope (Zeiss).
Measuring serum markers of hepatic
damage
To determine whether ADSC transplantation improved
liver function, the sera were separated by centrifugation at 1,000
× g for 10 min at 4°C, and stored at −80°C. Subsequently, alanine
aminotransferase (ALT), aspartate aminotransferase (AST) and total
bilirubin (TBIL) were measured in the rat sera using ALT, AST and
TBIL assay kits respectively, (all from Nanjing Jiancheng
Bioengineering Institute) according to the manufacturer's
instructions.
Measurement of serum markers of lipids
metabolism
To study lipid metabolism after ADSC
transplantation, the serum levels of total cholesterol (TC),
triglycerides (TGs) and fatty acids (FAs) were respectively
measured in rat sera using TC, TG and FA assay kits (Nanjing
Jiancheng Bioengineering Institute), following the manufacturer's
protocols.
Measuring hepatic markers of oxidative
stress
To further explore the protective effects of ADSC
transplantation on hepatic lipid peroxidation, each of the liver
tissue samples were homogenized in stroke-physiological saline
solution to obtain 10% (w/v) liver homogenate, and then centrifuged
at 5,000 × g for 10 min at 4°C. The supernatants were subsequently
collected, and hepatic superoxide dismutase (SOD) activity and
malondialdehyde (MDA) content were measured using SOD and MDA assay
kits (Nanjing Jiancheng Bioengineering Institute) respectively,
according to the manufacturer's instructions. SOD activity and MDA
content were finally normalized to total protein, which was
measured using a bicinchoninic acid (BCA) assay kit (Beijing
TransGen Biotech Co., Ltd., Beijing, China).
Statistical analysis
All quantitative data are expressed as the means ±
standard deviation (SD) and the statistical significance of the
difference between groups was analyzed using the Student's t-test.
A p-value <0.05 was considered to indicate a statistically
significant difference.
Results
Characterization of ADSCs
Flow cytometric analysis revealed that cultured
ADSCs were positive for CD29, CD44, CD73, CD90 and CD105, and
negative for CD31, CD34, CD45 and HLA-DR (Fig. 1A). In relation to the adherent
cells, we noted homogeneous distribution and fibroblastic shape,
which is consistent with the morphological characteristics of ADSCs
from other species (Fig. 1B), as
has been previously described (21). These cells also had the ability to
differentiate into osteogenic, adipogenic and chondrogenic lineages
(Fig. 1C–E), which is a typical
characteristic of mesenchymal stem cells (MSCs). Moreover, the
cultured cells had the potential to form spheres (Fig. 1F), which is consistent with the
results of a previous study (23). Taken together, these findings
indicate that derived cells exhibited typical characteristics of
ADSCs; therefore these ADSCs were subsequently used for cell
transplantation.
 | Figure 1Characterization of rat adipose
tissue-derived stem cells (ADSCs). (A) Flow cytometric analysis
revealed that rat ADSCs positively expressed CD29, CD44, CD73, CD90
and CD105, and negatively expressed CD31, CD34, CD45 and human
leukocyte antigen - antigen D related (HLA-DR). Orange lines
indicate the negative control, while black lines indicate surface
biomarker expression. (B) Fibroblastic morphology of ADSCs
(magnification, ×50); scale bar, 50 μm. (C) Osteogenic, (D)
adipogenic and (E) chondrogenic differentiation of ADSCs
(magnification, ×200); scale bar, 20 μm. (F) ADSCs had the
potential to form spheres (magnification, ×100); scale bar, 50
μm. |
ADSC transplantation affects gross liver
morphology and the hepatosomatic index (HSI)
Images of the liver samples isolated from the normal
(control) group and the rats with HFD-induced NAFLD were captured
to investigate the extent of liver injury, using an SLR camera
(Nikon). The livers from the rats with NAFLD exhibited typical
signs of hepatic steatosis, with more yellow and rough surfaces
compared with the livers from the control rats. However, following
dietary modification and ADSC transplantation, the gross hepatic
morphology of the ADSC-treated rats showed obvious signs of
recovery (Fig. 2A). Even in the
mock group (rats with NAFLD treated with PBS), the signs of hepatic
steatosis were alleviated, although not as markedly as the
ADSC-treated group. Although dietary modification contributed to
recovery from NAFLD, ADSC transplantation was an efficient strategy
to accelerate liver recovery.
Furthermore, the HSI index of the rats with
HFD-induced NAFLD was analyzed (the percentage of wet liver
weight/body weight). The HSI of the NAFLD group was significantly
higher than of the normal (control) rats, whereas the HIS decreased
slightly following dietary modification, which suggested that
dietary modification promoted recovery from NAFLD. Furthermore, in
the ADSC-treated groups we noted a significant decrease compared to
the mock groups, which revealed that ADSC transplantation
effectively reduces the liver weight of rats with NAFLD(Fig. 2B).
ADSC transplantation improves liver
function
The serum levels of ALT, AST and TBIL were measured
to evaluate hepatic damage in rats with HFD-induced NAFLD. Compared
with the normal rats, the rats in the NAFLD group exhibited
markedly increased levels of ALT and TBIL, which was an indicator
of serious hepatic damage; while the AST level was slightly higher
although the difference was not statistically significant. Two
weeks after ADSC transplantation, the serum levels of ALT and TBIL
were significantly lower than the rats in the mock group
(PBS-treated rats). Furthermore, the serum levels of ALT and TBIL
of the PBS-treated and ADSC-transplanted rats continuously declined
when the rats were fed for another 2 (a total of 4 weeks) or 6
weeks (a total of 8 weeks), and the ADSC-transplanted rats
exhibited an even lower ALT level compared with the mock rats
(Fig. 3). The improvement in
liver function suggested that the ADSC transplantation accelerated
the recovery of the liver from NAFLD progression.
ADSC transplantation promotes lipid
metabolism
In order to evaluate whether ADSCs affected lipid
metabolism, serum levels of FAs, TGs and TC were measured. Compared
with the normal rats, markedly higher levels of FAs, TGs and TC
were observed in the NAFLD group, which means that the rats with
HFD-induced NAFLD suffered from an imbalance of lipid metabolism.
However, after feeding them with normal chow and administering
ADSCs, the serum levels of FAs, TGs and TC in the ADSC-treated
groups were significantly decreased compared to the rats treated
with PBS; however, even in the mock groups improvement in the lipid
metabolism was noted (Fig. 4).
Thus, ADSC transplantation and dietary modification in combination
affected the lipid metabolism of rats with NAFLD to the extent that
levels were close to normal. Of note, at 2 and 4 weeks
post-transplantation, marked improvements in lipid metablism were
noted between the ADSC-treated groups and the mock groups.
Furthermore, the serum levels of FAs and TGs in the
ADSC-transplanted rats continued to decline when the rats were fed
for another 4 weeks (a total of 8 weeks). It should be noted that
there were no significant differences in the TC levels between the
ADSC-transplanted group and the mock group, and the TC levels of
these two groups reverted to almost normal levels (Fig. 4C). Thus, ADSC transplantation
constitutes a more effective method of improving the lipid
metabolism of rats with HFD-induced NAFLD.
ADSC transplantation reverses hepatic
pathological changes
To further examine whether ADSC transplantation
reverses the progression of NAFLD, histological examination of
liver tissues was performed. The liver tissues of the normal rats
exhibited no evidence of steatosis, while typical steatosis was
clearly observed in the samples of rats with HFD-induced NAFLD,
which means that the rat model of NAFLD was successfully
established using the HFD; after dietary modification, there was
less evidence of steatosis in both the PBS-treated and the
ADSC-treated groups compared with the NAFLD group. Although dietary
modification ameliorated steatosis in rats with HFD-induced NAFLD,
fewer fat vacuoles were observed in the ADSC-treated rats compared
with the mock group, which indicates that the ADSCs accelerated the
reversion of NAFLD (Fig. 5A).
In order to confirm whether ADSC transplantation
decreased lipid accumulation in the liver tissues of rats with
NAFLD, oil red O staining was performed. Compared with the normal
rats, greater lipid accumulation in the liver tissues of the NAFLD
group was observed, which indicates an imbalance of lipid
metabolism. Lipid accumulation was significantly decreased by
dietary modification alone or together with ADSC transplantation,
compared with the NAFLD group. Thus, we suggest that lifestyle
modification alone reverses the development of NAFLD; however, we
suggest that ADSC transplantation has the potential to further
enhance the reversion of NAFLD since there was a marked decrease in
lipid accumulation in the ADSC-treated rats compared with the mock
group: lipid accumulation almost returned to normal levels in the
ADSC-treated group (Fig. 5B).
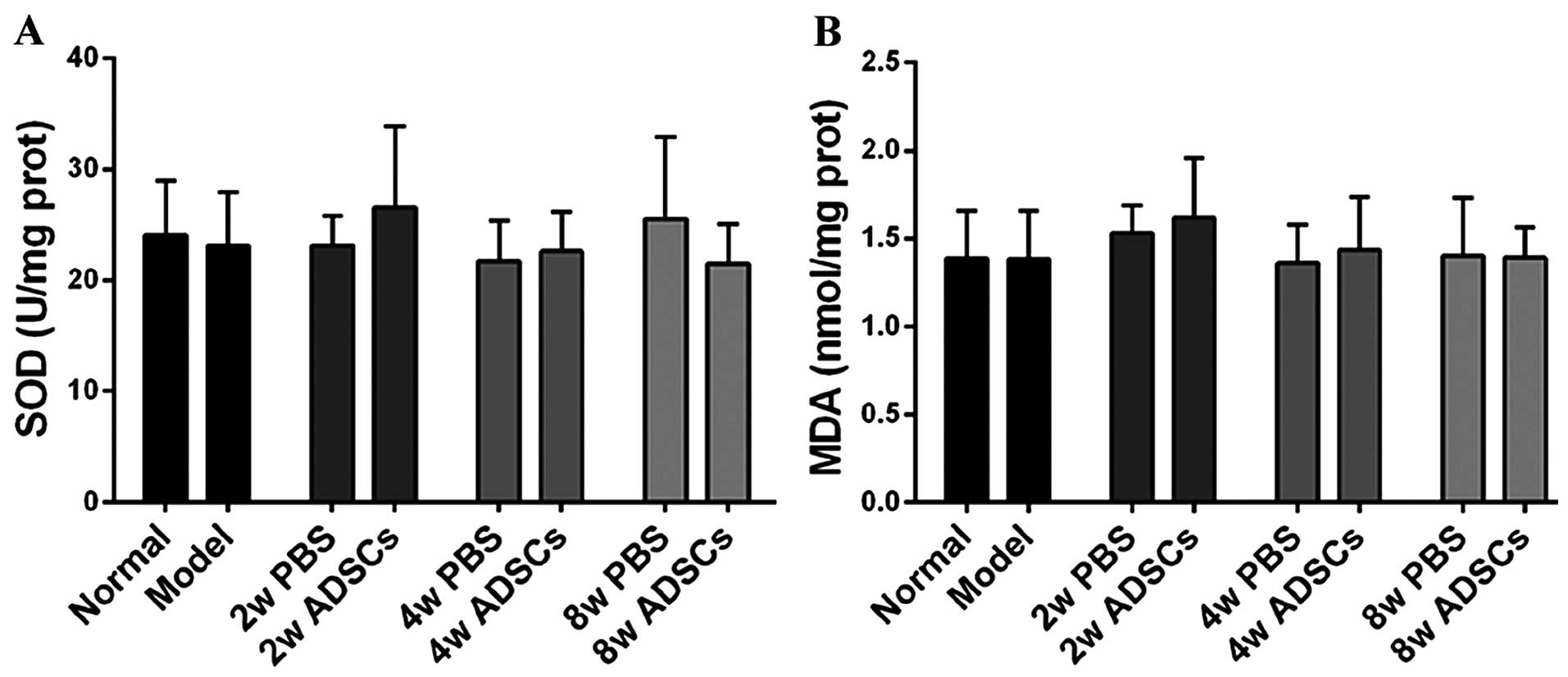
Absence of oxidative stress
Excessive lipid accumulation is capable of
increasing oxidative stress, which is usually characterized by low
SOD activity and increased MDA content in the liver, during the
progression of NAFLD. However, there was no significant difference
in SOD and MDA levels in our harvested liver tissues, between all
groups (Fig. 6), which indicated
the absence of oxidative stress.
Discussion
NAFLD is the most common chronic liver disease in
the world, and it is seriously harmful to human public health
(1,2). However, little is known about its
pathomechanism, particularly at a cellular and a molecular level.
To explain the pathological changes of NAFLD, a classical
hypothesis, the 'two-hit hypothesis', was widely accepted. The
'first hit' is initiated with hepatic accumulation of high levels
of free fatty acids, resulting in steatosis; this makes the liver
more prone to the 'second hit', involving factors such as oxidative
stress, mitochondrial dysfunction and inflammation, which lead to
steatohepatitis and/or fibrosis (24,25). According to this 'two-hit
hypothesis', excessive lipid accumulation may cause peroxidation,
followed by increasing oxidative stress (26–28). In the present study, we therefore
evaluated the SOD activity and the MDA content of the rat liver
samples. However, no significant difference was observed among all
the groups (Fig. 6), indicating
the absence of excessive oxidative stress in our rat model;
additionally, no significant difference was observed in the serum
level of AST among all the groups (Fig. 3). Collectively, these data suggest
that the animal model used in this study evolved in the early
stages of NAFLD.
It should be noted that lifestyle modification is
the key for NAFLD patients to maintain weight loss. A recent
systematic review assessing the effect of diet, physical activity,
and/or exercise modification in adult populations with NAFLD
suggested that lifestyle modifications lead to weight reduction and
consistently reduce liver fat (29). Consistent with these results, our
study revealed that dietary modification moderated gross hepatic
morphology and HSI index (Fig.
2), reducing hepatic damage (Fig.
3), balancing lipid metabolism and even reversing the
pathological changes in the liver (Fig. 4 and 5). These results demonstrate that
lifestyle modification through dietary intervention should be used
to prevent the progression of NAFLD.
Although important benefits result from dietary
modification, other metabolic risk factors, such as hyperlipidemia
and hypertension, also require further treatment. MSC
transplantation was identified as one of the choices to reduce
these risk factors (14–17). Therefore, MSC transplantation
presents a promising strategy for the treatment of NAFLD; previous
studies have noted this in relation to bone marrow-derived stem
cells (BMSCs) (30,31).
As well as the common features of other MSCs, ADSCs
possess the same abilities in terms of tissue repair and immune
regulation (32–35), and also have many advantages,
including abundant availability, ease of obtainment, better
immunoregulatory ability and the fact that they are more suitable
for autologous transplantation (36,37). As described in this study, ADSC
transplantation in combination with dietary modification was more
effective at improving liver function of rats with HFD-induced
NAFLD (Fig. 3), regulating lipid
metabolism (Fig. 4), and
ameliorating changes to the hepatic pathological morphology
(Fig. 5) than treatment with
dietary modification alone, which indicated that ADSC
transplantation is another promising strategy for the treatment of
NAFLD.
Although ADSC transplantation presents a promising
therapeutic approach for the treatment of NAFLD, the mechanisms and
the safety of ADSC transplantation have not yet been elucidated.
Therefore, further research is necessary to ascertain this
information, particularly the safety of ADSC transplantation, prior
to clinical application.
In conclusion, we suggest that ADSC transplantation
significantly improves liver function, promotes lipid metabolism
and decreases the intrahepatic content of lipids, thereby reversing
the progression of NAFLD. Therefore, ADSC transplantation presents
a potential therapeutic approach for NAFLD.
Acknowledgments
This study was supported by the Key Clinical
Specialty Discipline Construction Program of Fujian, China; the key
project of National Science and Technology of China (grant nos.
2012ZX10002010-001-006 and 2012ZX10002016-013), the National
Natural Science Foundation of China (grant no. 31201008), the Key
Project of Fujian Province (grant no. 2013YZ0002-3), the Science
and Technology Infrastructure Construction Program of Fujian
Province (grant no. 2014Y2005), the Natural Science Foundation of
Fujian Province (grant nos. 2015J05175 and 2016J01592), the Project
of Nanjing Military Region (grant no. 15MS136), the Scientific
Foundation of Fuzhou Health Department (grant nos. 2013-S-wq15,
2013-S-wp1, 2014-S-wq-17, 2015-S-wq13 and 2014-S-wq20), and the
Project of Fuzhou Science and Technology Department (grant no.
2014-S-139-3).
References
|
1
|
Sattar N, Forrest E and Preiss D:
Non-alcoholic fatty liver disease. BMJ. 349:g45962014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Browning JD, Szczepaniak LS, Dobbins R,
Nuremberg P, Horton JD, Cohen JC, Grundy SM and Hobbs HH:
Prevalence of hepatic steatosis in an urban population in the
United States: impact of ethnicity. Hepatology. 40:1387–1395. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wong VW, Chu WC, Wong GL, Chan RS, Chim
AM, Ong A, Yeung DK, Yiu KK, Chu SH, Woo J, et al: Prevalence of
nonalcoholic fatty liver disease and advanced fibrosis in Hong Kong
Chinese: a population study using proton-magnetic resonance
spectroscopy and transient elastography. Gut. 61:409–415. 2012.
View Article : Google Scholar
|
|
4
|
Henao-Mejia J, Elinav E, Jin C, Hao L,
Mehal WZ, Strowig T, Thaiss CA, Kau AL, Eisenbarth SC, Jurczak MJ,
et al: Inflammasome-mediated dysbiosis regulates progression of
NAFLD and obesity. Nature. 482:179–185. 2012.PubMed/NCBI
|
|
5
|
Lavallard VJ and Gual P: Autophagy and
non-alcoholic fatty liver disease. Biomed Res Int. 2014:1201792014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hassanian M, Al-Mulhim A, Al-Sabhan A,
Al-Amro S, Bamehriz F, Abdo A and Al Khalidi H: The effect of
bariatric surgeries on nonalcoholic fatty liver disease. Saudi J
Gastroenterol. 20:270–278. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lu Y, Ma Z, Zhang Z, Xiong X, Wang X,
Zhang H, Shi G, Xia X, Ning G and Li X: Yin Yang 1 promotes hepatic
steatosis through repression of farnesoid X receptor in obese mice.
Gut. 63:170–178. 2014. View Article : Google Scholar
|
|
8
|
Dhibi M, Brahmi F, Mnari A, Houas Z,
Chargui I, Bchir L, Gazzah N, Alsaif MA and Hammami M: The intake
of high-fat diet with different trans fatty acid levels
differentially induces oxidative stress and non alcoholic fatty
liver disease (NAFLD) in rats. Nutr Metab (Lond). 8:652011.
View Article : Google Scholar
|
|
9
|
Wei J, Sun X, Chen Y, Li Y, Song L, Zhou
Z, Xu B, Lin Y and Xu S: Perinatal exposure to bisphenol A
exacerbates nonalcoholic steatohepatitis-like phenotype in male rat
offspring fed on a high-fat diet. J Endocrinol. 222:313–325. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu J, Zhang H, Zheng H and Jiang Y:
Hepatic inflammation scores correlate with common carotid
intima-media thickness in rats with NAFLD induced by a high-fat
diet. BMC Vet Res. 10:1622014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dyson JK, Anstee QM and McPherson S:
Non-alcoholic fatty liver disease: a practical approach to
treatment. Frontline Gastroenterol. 5:277–286. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Meyerrose TE, De Ugarte DA, Hofling AA,
Herrbrich PE, Cordonnier TD, Shultz LD, Eagon JC, Wirthlin L, Sands
MS, Hedrick MA and Nolta JA: In vivo distribution of human
adipose-derived mesenchymal stem cells in novel xenotransplantation
models. Stem Cells. 25:220–227. 2007. View Article : Google Scholar
|
|
13
|
Philippe B, Luc S, Valérie PB, Jérôme R,
Alessandra BR and Louis C: Culture and use of mesenchymal stromal
cells in phase I and II clinical trials. Stem Cells Int.
2010:5035932010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu K, Liu R, Cao G, Sun H and Wangxand Wu
S: Adipose-derived stromal cell autologous transplantation
ameliorates pulmonary arterial hypertension induced by shunt flow
in rat models. Stem Cells Dev. 20:1001–1010. 2011. View Article : Google Scholar
|
|
15
|
Eirin A, Zhu XY, Krier JD, Tang H, Jordan
KL, Grande JP, Lerman A, Textor SC and Lerman LO: Adipose
tissue-derived mesenchymal stem cells improve revascularization
outcomes to restore renal function in swine atherosclerotic renal
artery stenosis. Stem Cells. 30:1030–1041. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Okura H, Saga A, Fumimoto Y, Soeda M,
Moriyama M, Moriyama H, Nagai K, Lee CM, Yamashita S, Ichinose A,
et al: Transplantation of human adipose tissue-derived multilineage
progenitor cells reduces serum cholesterol in hyperlipidemic
Watanabe rabbits. Tissue Eng Part C Methods. 17:145–154. 2011.
View Article : Google Scholar :
|
|
17
|
Ji AT, Chang YC, Fu YJ, Lee OK and Ho JH:
Niche-dependent regulations of metabolic balance in high-fat
diet-induced diabetic mice by mesenchymal stromal cells. Diabetes.
64:926–936. 2015. View Article : Google Scholar
|
|
18
|
Zhang Y, Chen XM and Sun DL: Effects of
coencapsulation of hepatocytes with adipose-derived stem cells in
the treatment of rats with acute-on-chronic liver failure. Int J
Artif Organs. 37:133–141. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Saito Y, Shimada M, Utsunomiya T, Ikemoto
T, Yamada S, Morine Y, Imura S, Mori H, Sugimoto K, Iwahashi S and
Asanoma M: The protective effect of adipose-derived stem cells
against liver injury by trophic molecules. J Surg Res. 180:162–168.
2013. View Article : Google Scholar
|
|
20
|
Harn HJ, Lin SZ, Hung SH, Subeq YM, Li YS,
Syu WS, Ding DC, Lee RP, Hsieh DK, Lin PC and Chiou TW:
Adipose-derived stem cells can abrogate chemical-induced liver
fibrosis and facilitate recovery of liver function. Cell
Transplant. 21:2753–2764. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Banas A, Teratani T, Yamamoto Y, Tokuhara
M, Takeshita F, Quinn G, Okochi H and Ochiya T: Adipose
tissue-derived mesenchymal stem cells as a source of human
hepatocytes. Hepatology. 46:219–228. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell
JW, Katz AJ, Benhaim P, Lorenz HP and Hedrick MH: Multilineage
cells from human adipose tissue: implications for cell-based
therapies. Tissue Eng. 7:211–228. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Takahashi H, Haraguchi N, Nishikawa S,
Miyazaki S, Suzuki Y, Mizushima T, Nishimura J, Takemasa I,
Yamamoto H, Mimori K, et al: Biological and clinical availability
of adipose-derived stem cells for pelvic dead space repair. Stem
Cells Transl Med. 1:803–810. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ibrahim MA, Kelleni M and Geddawy A:
Nonalcoholic fatty liver disease: current and potential therapies.
Life Sci. 92:114–118. 2013. View Article : Google Scholar
|
|
25
|
Shaker M, Tabbaa A, Albeldawi M and
Alkhouri N: Liver transplantation for nonalcoholic fatty liver
disease: new challenges and new opportunities. World J
Gastroenterol. 20:5320–5330. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mollica MP, Lionetti L, Moreno M, Lombardi
A, De Lange P, Antonelli A, Lanni A, Cavaliere G, Barletta A and
Goglia F: 3,5-diiodo-l-thyronine, by modulating mitochondrial
functions, reverses hepatic fat accumulation in rats fed a high-fat
diet. J Hepatol. 51:363–370. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zou Y, Li J, Lu C, Wang J, Ge J, Huang Y,
Zhang L and Wang Y: High-fat emulsion-induced rat model of
nonalcoholic steatohepatitis. Life Sci. 79:1100–1107. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Takaki A, Kawai D and Yamamoto K:
Molecular mechanisms and new treatment strategies for non-alcoholic
steatohepatitis (NASH). Int J Mol Sci. 15:7352–7379. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Thoma C, Day CP and Trenell MI: Lifestyle
interventions for the treatment of non-alcoholic fatty liver
disease in adults: a systematic review. J Hepatol. 56:255–266.
2012. View Article : Google Scholar
|
|
30
|
Ezquer M, Ezquer F, Ricca M, Allers C and
Conget P: Intravenous administration of multipotent stromal cells
prevents the onset of non-alcoholic steatohepatitis in obese mice
with metabolic syndrome. J Hepatol. 55:1112–1120. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Winkler S, Borkham-Kamphorst E, Stock P,
Brückner S, Dollinger M, Weiskirchen R and Christ B: Human
mesenchymal stem cells towards non-alcoholic steatohepatitis in an
immunodeficient mouse model. Exp Cell Res. 326:230–239. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gutiérrez-Fernández M, Rodríguez-Frutos B,
Ramos-Cejudo J, Teresa Vallejo-Cremades M, Fuentes B, Cerdán S and
Díez-Tejedor E: Effects of intravenous administration of allogenic
bone marrow- and adipose tissue-derived mesenchymal stem cells on
functional recovery and brain repair markers in experimental
ischemic stroke. Stem Cell Res Ther. 4:112013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xie X, Wang Y, Zhao C, Guo S, Liu S, Jia
W, Tuan RS and Zhang C: Comparative evaluation of MSCs from bone
marrow and adipose tissue seeded in PRP-derived scaffold for
cartilage regeneration. Biomaterials. 33:7008–7018. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Valina C, Pinkernell K, Song YH, Bai X,
Sadat S, Campeau RJ, Le Jemtel TH and Alt E: Intracoronary
administration of autologous adipose tissue-derived stem cells
improves left ventricular function, perfusion, and remodelling
after acute myocardial infarction. Eur Heart J. 28:2667–2677. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Niemeyer P, Kornacker M, Mehlhorn A,
Seckinger A, Vohrer J, Schmal H, Kasten P, Eckstein V, Südkamp NP
and Krause U: Comparison of immunological properties of bone marrow
stromal cells and adipose tissue-derived stem cells before and
after osteogenic differentiation in vitro. Tissue Eng. 13:111–121.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sun CK, Chang CL, Lin YC, Kao YH, Chang
LT, Yen CH, Shao PL, Chen CH, Leu S and Yip HK: Systemic
administration of autologous adipose-derived mesenchymal stem cells
alleviates hepatic ischemia-reperfusion injury in rats. Crit Care
Med. 40:1279–1290. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Seki T, Yokoyama Y, Nagasaki H, Kokuryo T
and Nagino M: Adipose tissue-derived mesenchymal stem cell
transplantation promotes hepatic regeneration after hepatic
ischemia-reperfusion and subsequent hepatectomy in rats. J Surg
Res. 178:63–70. 2012. View Article : Google Scholar : PubMed/NCBI
|