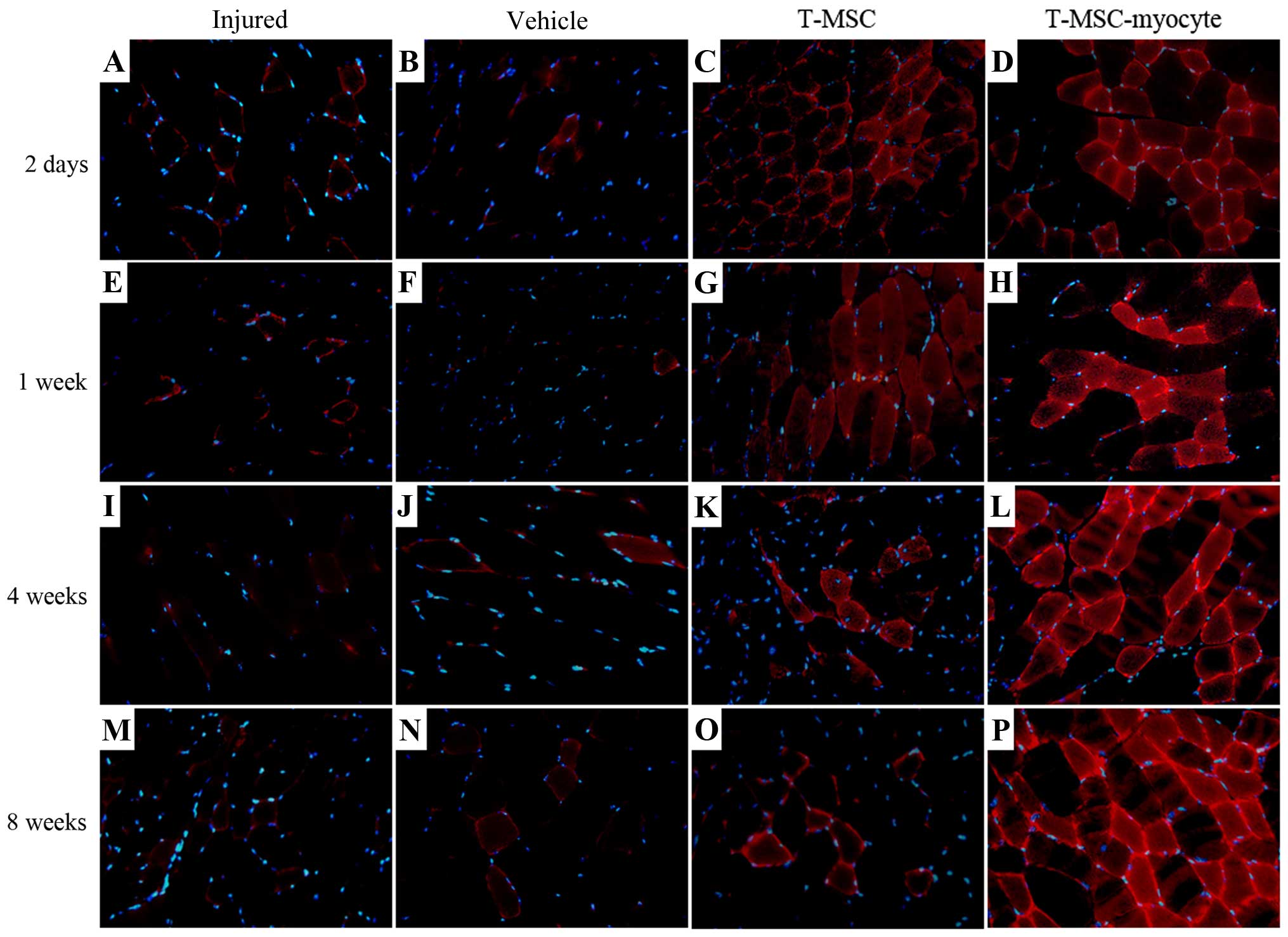
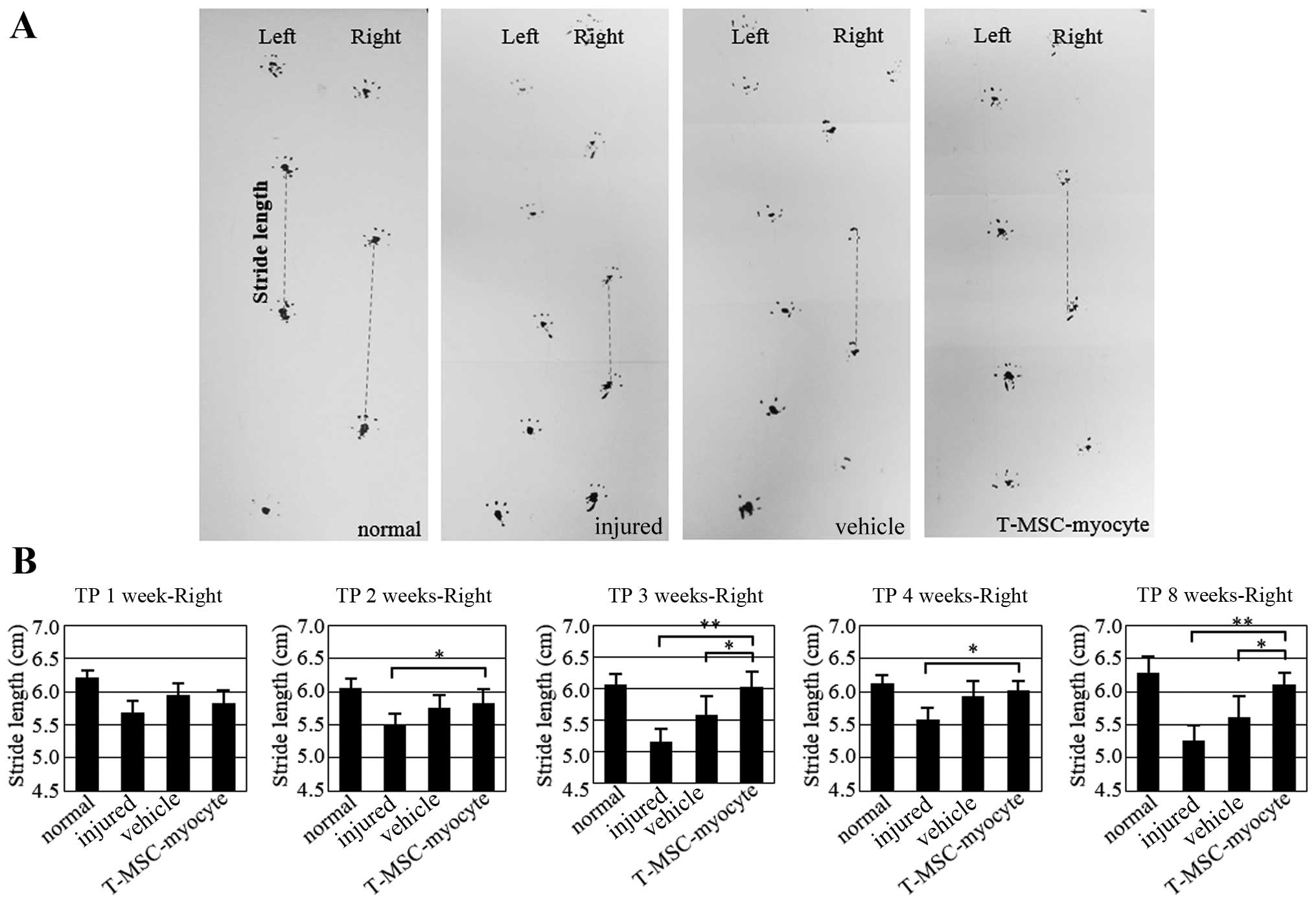
|
1
|
Chen W, Xie M, Yang B, Bharadwaj S, Song
L, Liu G, Yi S, Ye G, Atala A and Zhang Y: Skeletal myogenic
differentiation of human urine-derived cells as a potential source
for skeletal muscle regeneration. J Tissue Eng Regen Med. Jun
19–2014.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Desai VD, Hsia HC and Schwarzbauer JE:
Reversible modulation of myofibroblast differentiation in
adipose-derived mesenchymal stem cells. PLoS One. 9:e868652014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gunetti M, Tomasi S, Giammò A, Boido M,
Rustichelli D, Mareschi K, Errichiello E, Parola M, Ferrero I,
Fagioli F, et al: Myogenic potential of whole bone marrow
mesenchymal stem cells in vitro and in vivo for usage in urinary
incontinence. PLoS One. 7:e455382012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hosoyama T, McGivern JV, Van Dyke JM,
Ebert AD and Suzuki M: Derivation of myogenic progenitors directly
from human pluripotent stem cells using a sphere-based culture.
Stem Cells Transl Med. 3:564–574. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pereira T, Gärtner A, Amorim I,
Armada-da-Silva P, Gomes R, Pereira C, França ML, Morais DM,
Rodrigues MA, Lopes MA, et al: Biomaterials and stem cell therapies
for injuries associated to skeletal muscular tissues. Advances in
Biomaterials Science and Biomedical Applications. Pignatello R:
InTech; Rijeka: pp. 329–355. 2013
|
|
6
|
Kang JS and Krauss RS: Muscle stem cells
in developmental and regenerative myogenesis. Curr Opin Clin Nutr
Metab Care. 13:243–248. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Motohashi N, Asakura Y and Asakura A:
Isolation, culture, and transplantation of muscle satellite cells.
J Vis Exp. 86:e508462014.
|
|
8
|
Shi X and Garry DJ: Muscle stem cells in
development, regeneration, and disease. Genes Dev. 20:1692–1708.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Darabi R, Gehlbach K, Bachoo RM, Kamath S,
Osawa M, Kamm KE, Kyba M and Perlingeiro RC: Functional skeletal
muscle regeneration from differentiating embryonic stem cells. Nat
Med. 14:134–143. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mizuno Y, Chang H, Umeda K, Niwa A, Iwasa
T, Awaya T, Fukada S, Yamamoto H, Yamanaka S, Nakahata T and Heike
T: Generation of skeletal muscle stem/progenitor cells from murine
induced pluripotent stem cells. FASEB J. 24:2245–2253. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dezawa M, Ishikawa H, Itokazu Y, Yoshihara
T, Hoshino M, Takeda S, Ide C and Nabeshima Y: Bone marrow stromal
cells generate muscle cells and repair muscle degeneration.
Science. 309:314–317. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Di Rocco G, Iachininoto MG, Tritarelli A,
Straino S, Zacheo A, Germani A, Crea F and Capogrossi MC: Myogenic
potential of adipose-tissue-derived cells. J Cell Sci.
119:2945–2952. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nunes VA, Cavaçana N, Canovas M, Strauss
BE and Zatz M: Stem cells from umbilical cord blood differentiate
into myotubes and express dystrophin in vitro only after exposure
to in vivo muscle environment. Biol Cell. 99:185–196. 2007.
View Article : Google Scholar
|
|
14
|
Kim JA, Shon YH, Lim JO, Yoo JJ, Shin HI
and Park EK: MYOD mediates skeletal myogenic differentiation of
human amniotic fluid stem cells and regeneration of muscle injury.
Stem Cell Res Ther. 4:1472013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Park S, Kim E, Koh SE, Maeng S, Lee WD,
Lim J, Shim I and Lee YJ: Dopaminergic differentiation of neural
progenitors derived from placental mesenchymal stem cells in the
brains of Parkinson's disease model rats and alleviation of
asymmetric rotational behavior. Brain Res. 1466:158–166. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kerkis I, Kerkis A, Dozortsev D,
Stukart-Parsons GC, Gomes Massironi SM, Pereira LV, Caplan AI and
Cerruti HF: Isolation and characterization of a population of
immature dental pulp stem cells expressing OCT-4 and other
embryonic stem cell markers. Cells Tissues Organs. 184:105–116.
2006. View Article : Google Scholar
|
|
17
|
Ryu KH, Cho KA, Park HS, Kim JY, Woo SY,
Jo I, Choi YH, Park YM, Jung SC, Chung SM, et al: Tonsil-derived
mesenchymal stromal cells: evaluation of biologic, immunologic and
genetic factors for successful banking. Cytotherapy. 14:1193–1202.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ting CH, Ho PJ and Yen BL: Age-related
decreases of serum-response factor levels in human mesenchymal stem
cells are involved in skeletal muscle differentiation and
engraftment capacity. Stem Cells Dev. 23:1206–1216. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Djouad F, Jackson WM, Bobick BE, Janjanin
S, Song Y, Huang GT and Tuan RS: Activin A expression regulates
multipotency of mesenchymal progenitor cells. Stem Cell Res Ther.
1:112010. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Park M, Kim YH, Woo SY, Lee HJ, Yu Y, Kim
HS, Park YS, Jo I, Park JW, Jung SC, et al: Tonsil-derived
mesenchymal stem cells ameliorate CCI4-induced liver fibrosis in
mice via autophagy activation. Sci Rep. 5:86162015. View Article : Google Scholar
|
|
21
|
Yu Y, Park YS, Kim HS, Kim HY, Jin YM,
Jung SC, Ryu KH and Jo I: Characterization of long-term in vitro
culture-related alterations of human tonsil-derived mesenchymal
stem cells: role for CCN1 in replicative senescence-associated
increase in osteogenic differentiation. J Anat. 225:510–518. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Park M, Kim YH, Ryu JH, Woo SY and Ryu KH:
Immune suppressive effects of tonsil-derived mesenchymal stem cells
on mouse bone-marrow-derived dendritic cells. Stem Cells Int.
2015:1065402015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ryu KH, Kim SY, Kim YR, Woo SY, Sung SH,
Kim HS, Jung SC, Jo I and Park JW: Tonsil-derived mesenchymal stem
cells alleviate concanavalin A-induced acute liver injury. Exp Cell
Res. 326:143–154. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Park YS, Hwang S, Jin YM, Yu Y, Jung SA,
Jung SC, Ryu KH, Kim HS and Jo I: CCN1 secreted by tonsil-derived
mesenchymal stem cells promotes endothelial cell angiogenesis via
integrin αvβ3 and AMPK. J Cell Physiol.
230:140–149. 2015. View Article : Google Scholar
|
|
25
|
Mauro A: Satellite cell of skeletal muscle
fibers. J Biophys Biochem Cytol. 9:493–495. 1961. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chargé SBP and Rudnicki MA: Cellular and
molecular regulation of muscle regeneration. Physiol Rev.
84:209–238. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guide for the Care and Use of Laboratory
Animals. 8th edition. National Research Council. The National
Academies Press; Washington, DC: 2011
|
|
28
|
Cho KA, Kim JY, Kim HS, Ryu KH and Woo SY:
Tonsil-derived mesenchymal progenitor cells acquire a follicular
dendritic cell phenotype under cytokine stimulation. Cytokine.
59:211–214. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
D'Hooge R, Hartmann D, Manil J, Colin F,
Gieselmann V and De Deyn PP: Neuromotor alterations and cerebellar
deficits in aged arylsulfatase A-deficient transgenic mice.
Neurosci Lett. 273:93–96. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fernagut PO, Diguet E, Labattu B and Tison
F: A simple method to measure stride length as an index of
nigrostriatal dysfunction in mice. J Neurosci Methods. 113:123–130.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Beattie GM, Lopez AD, Bucay N, Hinton A,
Firpo MT, King CC and Hayek A: Activin A maintains pluripotency of
human embryonic stem cells in the absence of feeder layers. Stem
Cells. 23:489–495. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Carpenter MK, Rosler ES, Fisk GJ,
Brandenberger R, Ares X, Miura T, Lucero M and Rao MS: Properties
of four human embryonic stem cell lines maintained in a feeder-free
culture system. Dev Dyn. 229:243–258. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nakatake Y, Fukui N, Iwamatsu Y, Masui S,
Takahashi K, Yagi R, Yagi K, Miyazaki J, Matoba R, Ko MS and Niwa
H: Klf4 cooperates with Oct3/4 and Sox2 to activate the Lefty1 core
promoter in embryonic stem cells. Mol Cell Biol. 26:7772–7782.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ropka-Molik K, Eckert R and Piórkowska K:
The expression pattern of myogenic regulatory factors MyoD, Myf6
and Pax7 in postnatal porcine skeletal muscles. Gene Expr Patterns.
11:79–83. 2011. View Article : Google Scholar
|
|
35
|
García-Pelagio KP, Bloch RJ, Ortega A and
González-Serratos H: Biomechanics of the sarcolemma and costameres
in single skeletal muscle fibers from normal and dystrophin-null
mice. J Muscle Res Cell Motil. 31:323–336. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hass R, Kasper C, Böhm S and Jacobs R:
Different populations and sources of human mesenchymal stem cells
(MSC): a comparison of adult and neonatal tissue-derived MSC. Cell
Commun Signal. 9:122011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Böttcher-Haberzeth S, Biedermann T, Klar
AS, Pontiggia L, Rac J, Nadal D, Schiestl C, Reichmann E and Meuli
M: Tissue engineering of skin: human tonsil-derived mesenchymal
cells can function as dermal fibroblasts. Pediatr Surg Int.
30:213–222. 2014. View Article : Google Scholar
|
|
38
|
Choy L, Skillington J and Derynck R: Roles
of autocrine TGF-beta receptor and Smad signaling in adipocyte
differentiation. J Cell Biol. 149:667–682. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ignotz RA and Massagué J: Type beta
transforming growth factor controls the adipogenic differentiation
of 3T3 fibroblasts. Proc Natl Acad Sci USA. 82:8530–8534. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Centrella M, Horowitz MC, Wozney JM and
McCarthy TL: Transforming growth factor-beta gene family members
and bone. Endocr Rev. 15:27–39. 1994.PubMed/NCBI
|
|
41
|
Olson EN: Proto-oncogenes in the
regulatory circuit for myogenesis. Semin Cell Biol. 3:127–136.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Massagué J, Cheifetz S, Endo T and
Nadal-Ginard B: Type beta transforming growth factor is an
inhibitor of myogenic differentiation. Proc Natl Acad Sci USA.
83:8206–8210. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Olson EN, Sternberg E, Hu JS, Spizz G and
Wilcox C: Regulation of myogenic differentiation by type beta
transforming growth factor. J Cell Biol. 103:1799–1805. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sordella R, Jiang W, Chen GC, Curto M and
Settleman J: Modulation of Rho GTPase signaling regulates a switch
between adipogenesis and myogenesis. Cell. 113:147–158. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Schiaffino S and Mammucari C: Regulation
of skeletal muscle growth by the IGF1-Akt/PKB pathway: insights
from genetic models. Skelet Muscle. 1:42011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zdanowicz MM, Moyse J, Wingertzahn MA,
O'Connor M, Teichberg S and Slonim AE: Effect of insulin-like
growth factor I in murine muscular dystrophy. Endocrinology.
136:4880–4886. 1995.PubMed/NCBI
|
|
47
|
Jones JI and Clemmons DR: Insulin-like
growth factors and their binding proteins: biological actions.
Endocr Rev. 16:3–34. 1995.PubMed/NCBI
|
|
48
|
Bassaglia Y and Gautron J: Fast and slow
rat muscles degenerate and regenerate differently after whole crush
injury. J Muscle Res Cell Motil. 16:420–429. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Dedkov EI, Kostrominova TY, Borisov AB and
Carlson BM: Reparative myogenesis in long-term denervated skeletal
muscles of adult rats results in a reduction of the satellite cell
population. Anat Rec. 263:139–154. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Politi PK, Havaki S, Manta P and Lyritis
G: Bupivacaine-induced regeneration of rat soleus muscle:
ultrastructural and immunohistochemical aspects. Ultrastruct
Pathol. 30:461–469. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Merritt EK, Hammers DW, Tierney M, Suggs
LJ, Walters TJ and Farrar RP: Functional assessment of skeletal
muscle regeneration utilizing homologous extracellular matrix as
scaffolding. Tissue Eng Part A. 16:1395–1405. 2010. View Article : Google Scholar
|