Introduction
Pelvic organ prolapse (POP) is a highly prevalent
health condition for women in the reproductive and menopausal
years. The lifetime risk of any primary surgery for POP is 12.6%
(1). POP occurs as a result of
the loss of the normal support and suspension normally provided by
the ligaments, endopelvic fascia and levator ani muscle which leads
to the descent of one or more of the following: the anterior or
posterior vaginal wall, the vaginal apex or the uterus (2). Anterior vaginal wall prolapse
(cystocele), with an overall prevalence of 33.8%, is the most
common form of POP (3). Current
treatment options for POP, and specifically for vaginal wall
prolapse, include pelvic floor muscle training, the use of
pessaries, and surgery including native tissue repair and those
with mesh. To achieve improved anatomical outcomes and overcome the
high failure rate following conventional repair, synthetic mesh has
been used since the late 1990s (4,5).
However, severe complications were increasingly reported (6,7),
prompting the FDA to issue two public health notices warning
against mesh use (7,8). Cell-based therapy, using stem cells
for the regeneration of human tissue to restore or establish normal
function, offers a novel approach (9,10).
Smooth muscle (SM) is an integral part of the
vaginal wall and endopelvic structures that support the pelvic
viscera; a deficiency of vaginal SM will lead to inability to
maintain the structural and functional integrity of vaginal support
and consequently pelvic floor support (11). Studies have documented decreases
in the fractional area as well as structural and biochemical
abnormalities in the vaginal SM of patients with POP (12,13). In addition, alterations in the
vaginal SM contractile and regulatory proteins have been reported
(14,15). Therefore, SM may be a target for
cellular therapy. The repair of impaired smooth muscle cells (SMCs)
may promote muscle regeneration in the vaginal wall and endopelvic
structures, thereby improving POP such that there is no need for
further surgery (10).
Stem cells termed endometrial regenerative cells
(ERCs) refer to a population of mesenchymal-like stem cells
isolated from human menstrual blood. Preclinical and clinical
trials have demonstrated the therapeutic potency of ERCs for a
range of clinical applications (16–19). The collection of ERCs utilizes
body waste, and thus, they are easy to obtain with minimal
inconvenience. ERCs are readily expandable in culture and lack
immunogenicity (17). They
possess in vitro pluripotency; cultured under the
appropriate conditions, they may differentiate into nine different
cell lineages from three germ layers (20,21). The myogenic differentiation of
ERCs was demonstrated by coculture with rat cardiomyocytes
(22); however, the direct
differentiation of ERCs into SMCs has not yet been reported, to the
best of our knowledge. In the present study, we examined the role
of transforming growth factor β1 (TGF-β1) in inducing the
differentiation of human ERCs into SMCs as well as the possible
signaling pathways involved, to suggest a potential cell-based
approach for the management of POP.
Materials and methods
Cell isolation and culture
The research proposal for human menstrual blood
collection was approved by the Ethics Committee of Harbin Medical
University, and informed written consent was obtained from each
patient. The investigations were conducted according to the
principles expressed in the Declaration of Helsinki. Thirty females
aged 20–30 were enrolled. The collection of 5 ml menstrual blood
was performed during the first few days of the menstrual cycle with
a urine cup, and then transferred into a 'collection tube'
containing 0.1 ml amphotericin B, 0.1 ml penicillin/streptomycin
(P/S) and 0.1 ml EDTA-Na2 (all from Sigma-Aldrich, St.
Louis, MO, USA) in 5 ml phosphate-buffered saline (PBS).
Mononuclear cells were isolated by Ficoll-Paque (Sigma-Aldrich)
density gradient centrifugation according to the manufacturer's
instructions. The cells were subsequently cultured in a T-25 flask
containing Dulbecco's modified Eagle's medium/F-12 (DMEM/F-12;
Invitrogen, Carlsbad, CA, USA) supplemented with 1% P/S, 1%
amphotericin B and 1% glutamine (Sigma-Aldrich), and 10% fetal
bovine serum (FBS; Invitrogen) (complete DMEM, cDMEM). The medium
was replaced the next day. Once the cells reached 80–90%
confluence, the adherent cells were detached with trypsin
(Sigma-Aldrich); and subcultured at a denisty of 1.5×105
cells in a T25 flask. The cells were passaged twice a week. The
morphology of the cultured cells was examined under a phase
contrast microscope (AX 70; Olympus, Tokyo, Japan).
Flow cytometric analysis
The ERCs were stained and labeled with the following
specific anti-human antibodies: CD73-fluorescein isothiocyanate
(FITC; 344016), CD90-FITC (328108), CD34-FITC (343604), CD45-FITC
(368508), CD146-phycoerythrin (PE; 361006) and STRO-1-PE (340106)
(BioLegend, San Diego, CA, USA), and detected by flow cytometric
analysis. Briefly, the cells were trypsinized and
1.0×106 cells were washed and re-suspended in
ice-chilled PBS containing 1% bovine serum albumin (BSA;
Invitrogen). Fluorochrome-conjugated antibodies were added at
concentrations recommended by the respective manufacturer, and
incubated for 30 min in the dark. The cells were then washed twice
in staining buffer, and analyzed under a flow cytometer
(LSRFortessa; BD Biosciences, Franklin Lakes, NJ, USA).
SM cell differentiation
To induce SMC differentiation, ERCs were seeded in
6-well tissue culture plates at a density of 4×104
cells/well in serum-free medium until they reached 30–40%
confluence. The cells were then cultured with the 'SM inducing
medium' which contained cDMEM supplemented with TGF-β1
(Sigma-Aldrich) at different concentrations (0.1, 0.5, 1 and 5
ng/ml) for different periods of time. The medium was replaced every
three days.
Transmission electron microscopy
(TEM)
The ERCs were centrifuged at 2,000 × g for 5 min and
the pellets were fixed with 2.5% glutaraldehyde (Sigma-Aldrich) in
PBS buffer (pH 7.2) at room temperature for 2 h. After several
rinses with PBS, the cells were post-fixed with 1% osmium tetroxide
(Sigma-Aldrich) for 1 h at 4°C, dehydrated in a graded series of
acetone, and embedded in Epon 812. Ultrathin sections were cut
(50–70 nm thick), and then double-stained with uranyl acetate and
lead citrate (both from Amresco, Solon, OH, USA) prior to
examination under a transmission electron microscope (H-7650;
Hitachi, Tokyo, Japan) operating at 80.0 kV.
Western blot analysis
The cultured ERCs were washed twice with pre-cooled
PBS, followed by protein extraction using RIPA buffer. Protein
concentrations were measured using a bicinchoninic acid (BCA)
protein assay kit (Sigma-Aldrich). An automated capillary-based
Simple Western system (Simon; ProteinSimple, Santa Clara, CA, USA),
which allows more accurate and reproducible assessment of protein
levels compared with the traditional system, was used in order to
quantify the protein level of SMC markers and Smads, according to
the manufacturer's instructions. Briefly, the cell lysate samples
were mixed with Fluorescent Standard/4X Master Mix (ProteinSimple)
at a ratio of 1:3, and then heated for denaturation. The samples,
blocking reagent, primary antibodies, HRP-conjugated secondary
antibodies, chemiluminescent substrate, and separation and stacking
matrices were dispensed into a 384-well plate (ProteinSimple).
After loading the plate, the separation electrophoresis and
immunodetection occurred automatically. Primary antibodies for the
target proteins included rabbit anti-human α-smooth muscle actin
[α-SMA; actin, alpha 2, smooth muscle, aorta (ACTA2); 14395-1-AP;
Proteintech, Rosemont, IL, USA], calponin 1 (CNN1; 13938-1-AP;
Proteintech), Smad2 (12570-1-AP; Proteintech), Smad3 (25494-1-AP;
Proteintech), phospho-Smad2 (p-Smad2; sc-135644; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) and phospho-Smad3 anti-bodies
(p-Smad3; sc-130218; Santa Cruz Biotechnology, Inc.); goat
anti-rabbit IgG (A27036; Molecular Probes, Eugene, OR, USA) was
used as the secondary antibody. GAPDH was used as the control. All
antibodies were diluted in antibody diluent (ProteinSimple) with a
1:50 dilution. Luminol (Sigma-Aldrich) and peroxide were then
added. The resulting chemiluminescent signal was captured using a
charge-coupled device (CCD) camera (Simon), and the signal
intensities were quantified and analyzed using Compass software
(ProteinSimple).
RT-PCR and quantitative PCR (qPCR)
Total RNA from the cultured cells was extracted
using a high pure RNA isolation kit (Roche Applied Science,
Indianapolis, IN, USA) according to the manufacturer's
instructions. One microgram of total RNA was used to make cDNA in a
volume of 20 µl using the Transcriptor First Strand cDNA
Synthesis kit (Roche Applied Science). The resulting cDNA was
subjected to PCR analysis using a PCR system (T3000; Biometra,
Göttingen, Germany), and qPCR was performed using the SYBR Premix
Ex Taq II mix (Takara Biotechnology Co., Ltd., Dalian, China) in a
LightCycler 480 Real-Time PCR detection system (Roche Applied
Science). β-actin (ACTB) served as the internal control. The
sequences of the forward and reverse primers for amplifying the SMC
markers [ACTA2, CNN1 and transgelin (TAGLN)], TGF-β1
family receptors [ALK1, ALK5 and transforming growth factor,
beta receptor (TGFBR)2] and Smads (Smad2 and
Smad3) are presented in Table
I.
 | Table IPrimer sequences for RT-PCR and
quantitative PCR. |
Table I
Primer sequences for RT-PCR and
quantitative PCR.
| Gene (NCBI ID) | Primer
sequence | Product size
(bp) |
|---|
| ACTA2
(NM_001613.2) | Sense:
5′-CCTTGAGAAGAGTTACGAGTTGC-3′
Antisense: 5′-ATGATGCTGTTGTAGGTGGTTT-3′ | 144 |
| CNN1
(NM_001299.4) | Sense:
5′-AACCACCACGCACACAACTACTA-3′
Antisense: 5′-TGCTCTCTCCAAACTCTAACCCT-3′ | 187 |
| TAGLN
(NM_003186.3) | Sense:
5′-GGTCTTCAAGCAGATGGAGCAG-3′
Antisense: 5′-TTGCCTTCAAAGAGGTCAACAGT-3′ | 105 |
| ALK1
(NM_001077401.1) | Sense:
5′-AAACCCCTCTGCCCGACTCAC-3′
Antisense: 5′-CCAGCACACACCACACTCACACTAC-3′ | 196 |
| ALK5
(NM_004612.2) | Sense:
5′-GACAACGTCAGGTTCTGGCTCAG-3′
Antisense: 5′-TCCTCTCCAAACTTCTCCAAATCG-3′ | 115 |
| TGFBR2
(NM_001024847.2) | Sense:
5′-AAGATTCCTGAAGACGGCTCCCTA-3′
Antisense: 5′-CCTGCTGCTGTTGTTTCTGCTTATC-3′ | 199 |
| Smad2
(NM_001003652.3) | Sense:
5′-TGAAAGGGTGGGGAGCAGAATA-3′
Antisense: 5′-GAGCAACGCACTGAAGGGGAT-3′ | 136 |
| Smad3
(NM_001145102.1) | Sense:
5′-GAGTGAAGATGGAGAAACCAGTGAC-3′
Antisense: 5′-GTAGTAGGAGATGGAGCACCAGAAG-3′ | 160 |
Immunocytofluorescence
The cells cultured on sterile coverslips in six-well
plates were fixed with 4% paraformaldehyde for 20 min at room
temperature, permeabilized with 0.1% Triton X-100 in PBS (pH 7.4)
for 15 min and blocked with 3% BSA in PBS (pH 7.4) for 20 min. They
were then incubated with monoclonal primary antibodies against
α-SMA (mouse IgG, 1:100) and calponin (rabbit IgG, 1:100) (both
from Cell Signaling Technology, Inc., Danvers, MA, USA) in 1% BSA
overnight at 4°C. Excessive antibody was removed which was followed
by incubation with a secondary antibody (FITC-conjugated goat
anti-mouse antibody, PE-conjugated goat anti-rabbit antibody,
1:1000; Molecular Probes) at 37°C for 1 h in the dark. The cell
nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI;
1:500; Sigma-Aldrich). Then, the slides were washed with PBS and
visualized using a fluorescence microscope (BX51; Olympus).
Statistical analysis
All experiments were performed at least in
triplicate. Data processing and statistical analysis were performed
using Microsoft Excel and GraphPad Prism software. Data are
presented as the means ± standard deviation (SD). Statistical
analysis was performed using a Student's t-test or one-way analysis
of variance followed by a Bonferroni's multiple-comparison post hoc
test to determine differences between the groups. A P-value
<0.05 was considered to indicate a statistically significant
difference.
Results
Isolation, culture and characterization
of ERCs
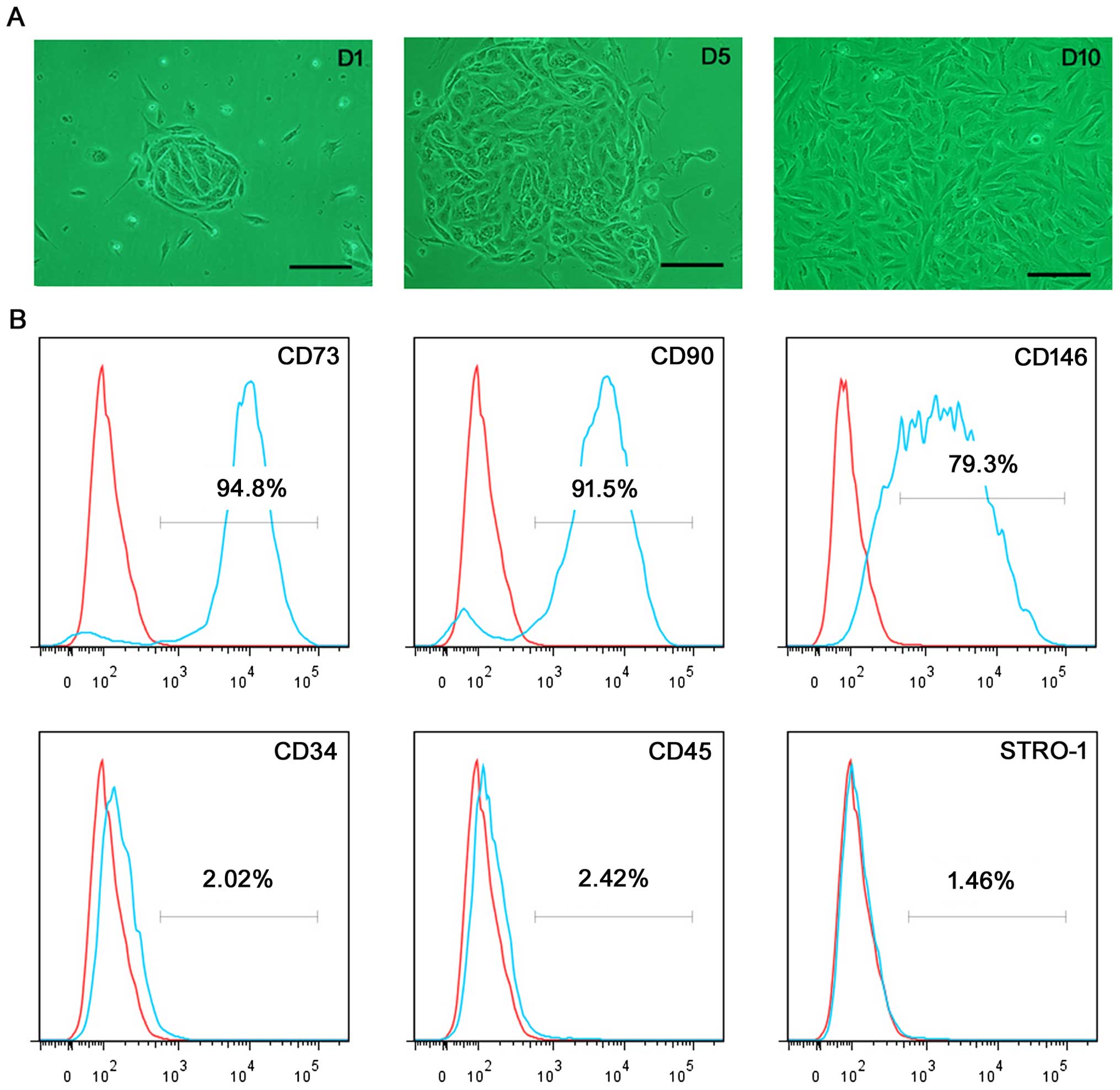
Single, small, compact cells were revealed 1–2 days
after initial seeding; some small colony-like morphology was
observed. Within an additional 3–6 days, these cells formed more
large colonies, and then stretched into an outgrowth of adherent
cells with a fibroblast-like short spindle-shaped morphology upon
the completion of 9–12 days of culture (Fig. 1A). Flow cytometric analysis
revealed that the majority of ERCs showed strong expression levels
for mesenchymal stem cell (MSC) markers CD73 (94.8%), CD90 (91.5%),
and CD146 (79.3%), and were negative for the hematopoietic stem
cell markers CD34 (2.02%), and CD45 (2.42%). In addition, the MSC
marker STRO-1 (1.46%) was negatively expressed (Fig. 1B).
TGF-β1 induces the differentiation of
ERCs into SMCs
To examine the TGF-β1-induced SM differentiation of
ERCs and to optimize the induction conditions, the ERCs were
cultured with the inducing medium and different concentrations of
TGF-β1 for different periods of time. SMC-specific marker
expression was evaluated by automated western blot analysis
performed on Simon, a Simple Western system. Simple Western is a
gel-free, blot-free, capillary-based, automated technique. This
rapid procedure allows the more accurate, stable and reproducible
assessment of protein levels, and greatly reduces the variability
caused by the labor-intensive and time-consuming manual processes
of the traditional western blot assay (23–28). The image generated by Simple
Western analysis is quite different from the conventional one.
Chemiluminescence signals were collected using a CCD camera, and
the collected signal intensity was subsequently processed,
converted to an electropherogram, and integrated using the Compass
software included on Simon (23).
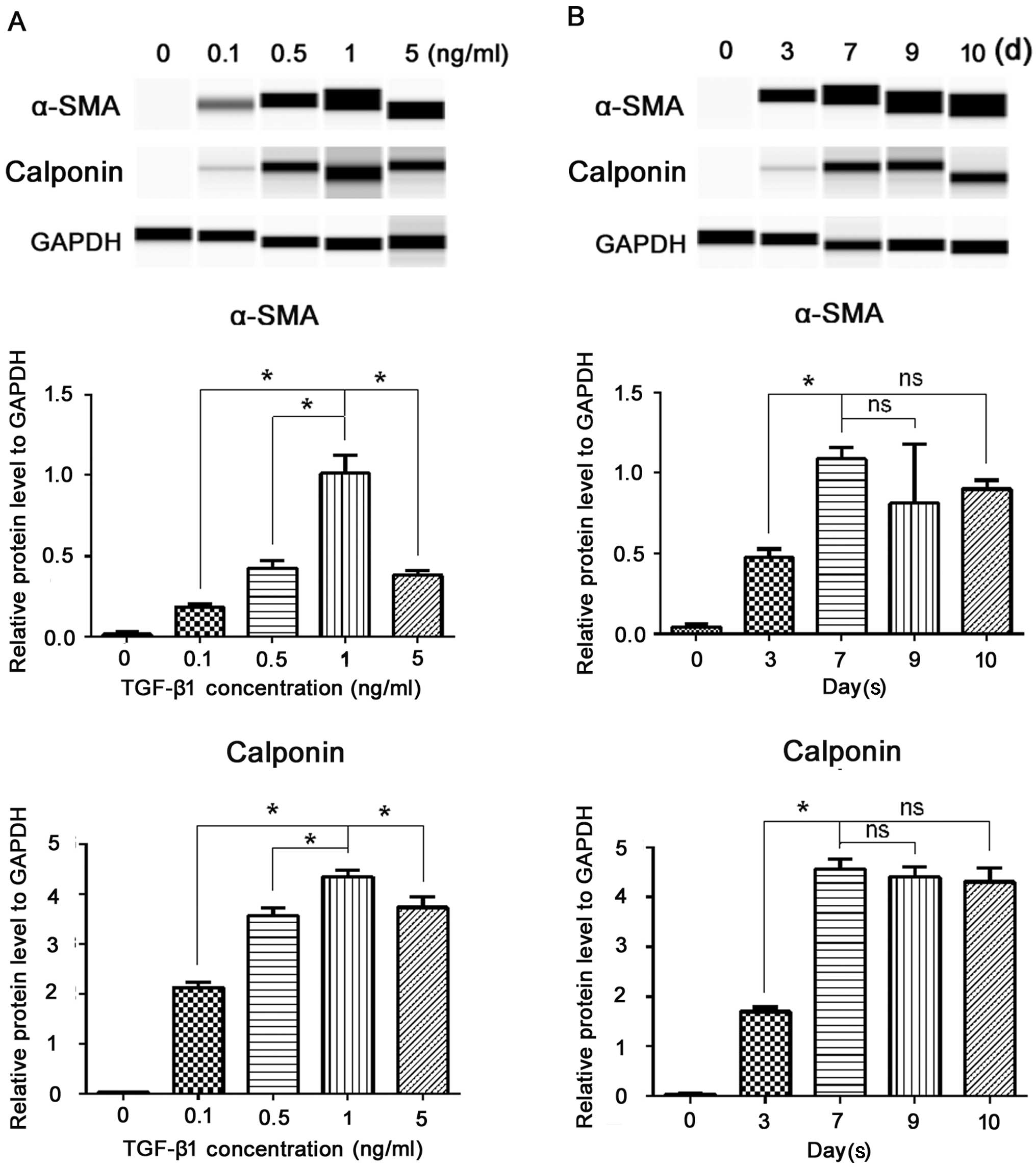
As shown in the gel-like image of Simple Western analysis (Fig. 2), at various concentrations of
TGF-β1 (0.1, 0.5, 1 and 5 ng/ml), the protein levels of α-SMA and
calponin significantly increased; among the different
concentrations, 1 ng/ml produced the greatest SM differentiation
(P<0.05) (Fig. 2A). Thus, from
the viewpoint of marker expression, we considered that 1 ng/ml
TGF-β1 performed most efficiently. The fluorescent intensity of the
SMC markers increased in a time-dependent manner. As shown in
Fig. 2B, three days after the
addition of TGF-β1, α-SMA and calponin became detectable; they
increased on the 7th day, and then remained at a relative stable
level on the 9th day (the longest time we examined), suggesting
that 7 days may be the most efficient duration for the induction.
Therefore, we selected a TGF-β1 concentration of 1 ng/ml and an
incubation period of 7 days for the subsequent experiments. As we
are seeking an ideal cell source for cellular therapy, we then
examined whether the induced cells display a stable phenotype after
passaging. After 7 days of induction, the ERCs were passaged and
cultured in TGF-β1-free cDMEM; SMC markers were then examined by
western blot analysis. As shown in Fig. 2B, the cells continued to express
SMC markers after one passage (the 10th day) without significant
changes in the expression level (P>0.05). Taken together, these
data demonstrated that TGF-β1 was necessary and sufficient for the
induction of an independent and long-lasting differentiation of
ERCs into the SMC phenotype, and it occurred in a dose and
time-dependent manner.
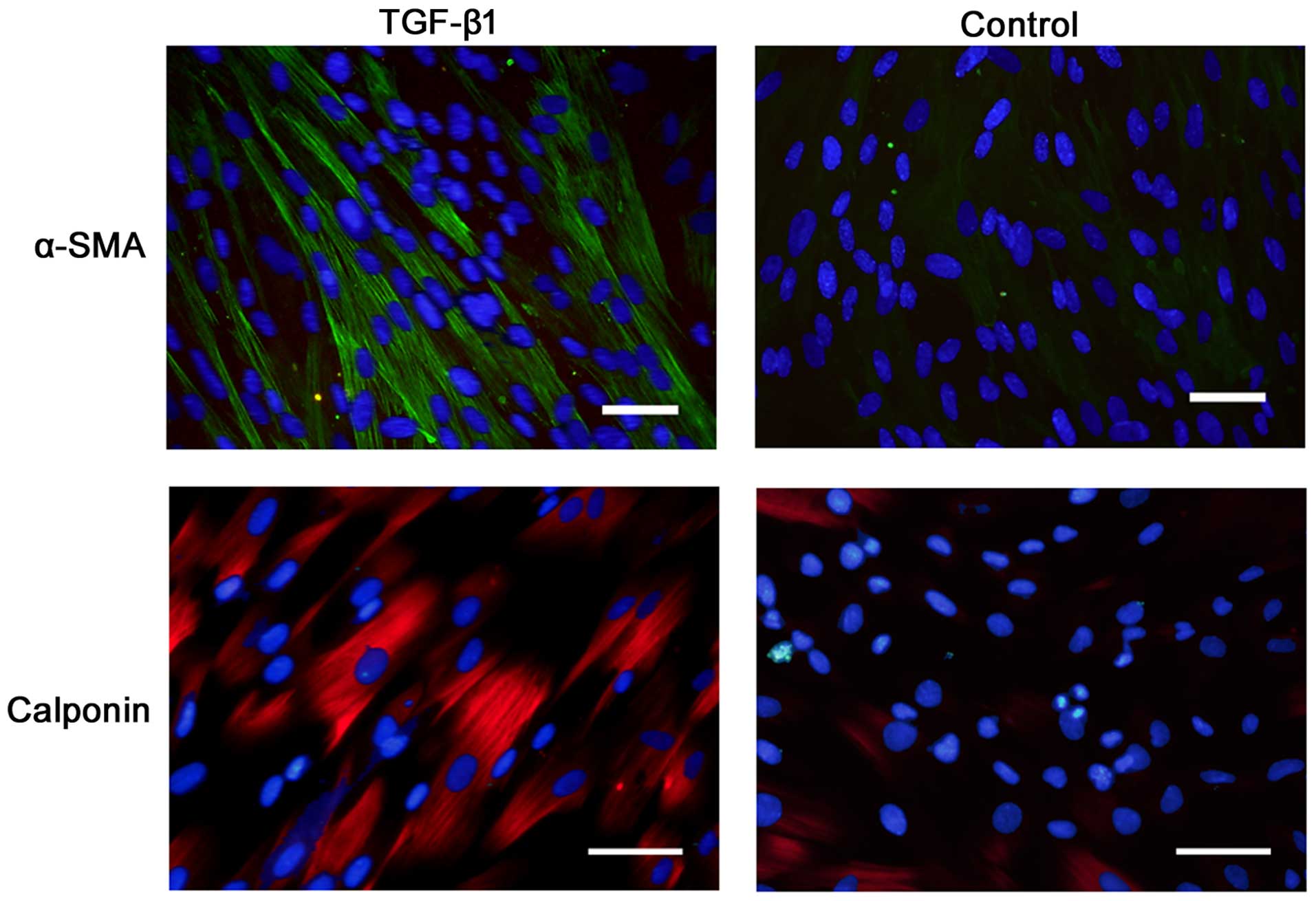
Immunostaining revealed that α-SMA and calponin were
positively expressed on the cells after 7 days of incubation in the
presence of TGF-β1 (Fig. 3).
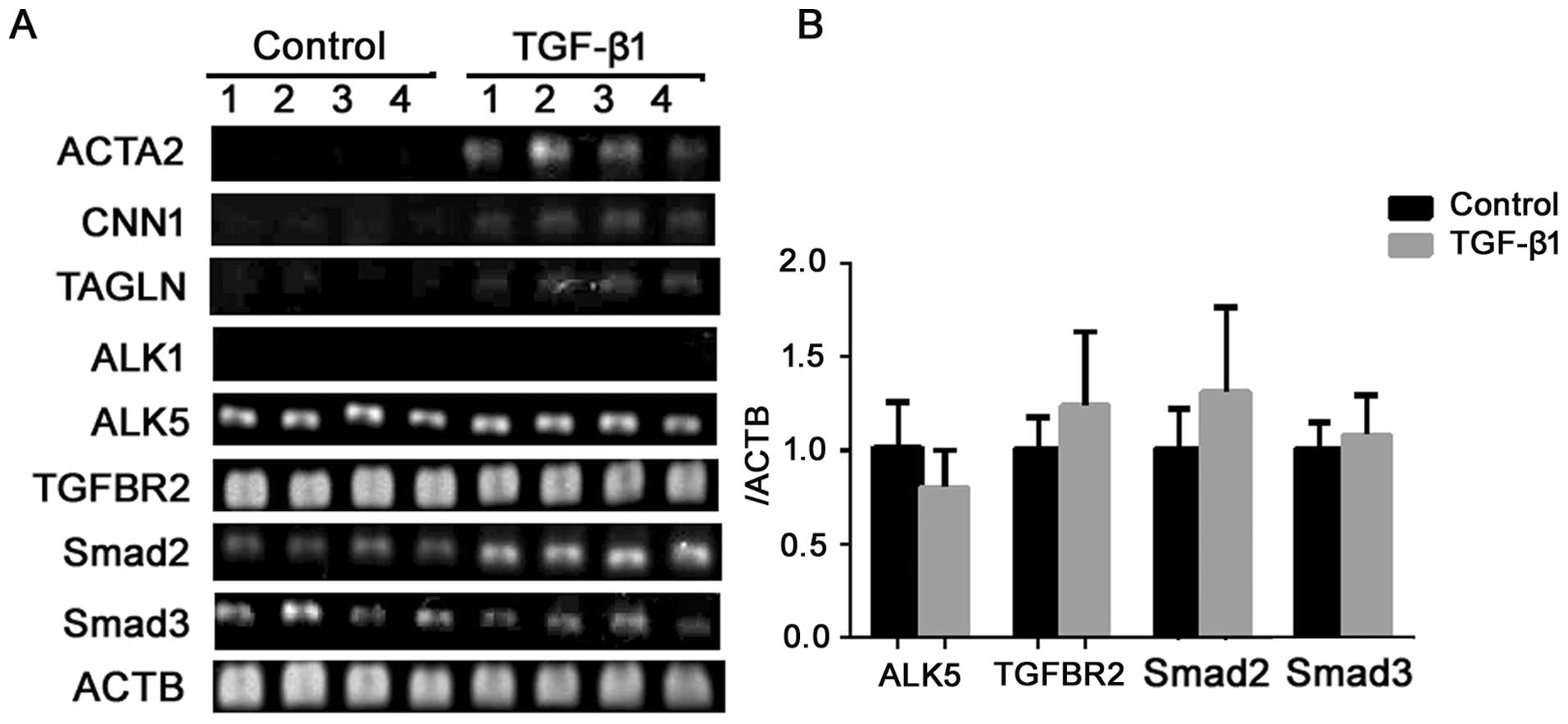
To characterize the induced cells at the genetic
level, we examined the expression of several common markers before
and after the induction by semiquantitative RT-PCR. As seen in
Fig. 4, TGF-β1 induced the
expression of ACTA2, CNN1 and SM22α (TAGLN) expression.
These data further suggest that the induction of α-SMA and calponin
detected by western blot analysis and immunocytofluorescence were
not an isolated phenomenon, but part of the differentiation process
into the SMC lineage.
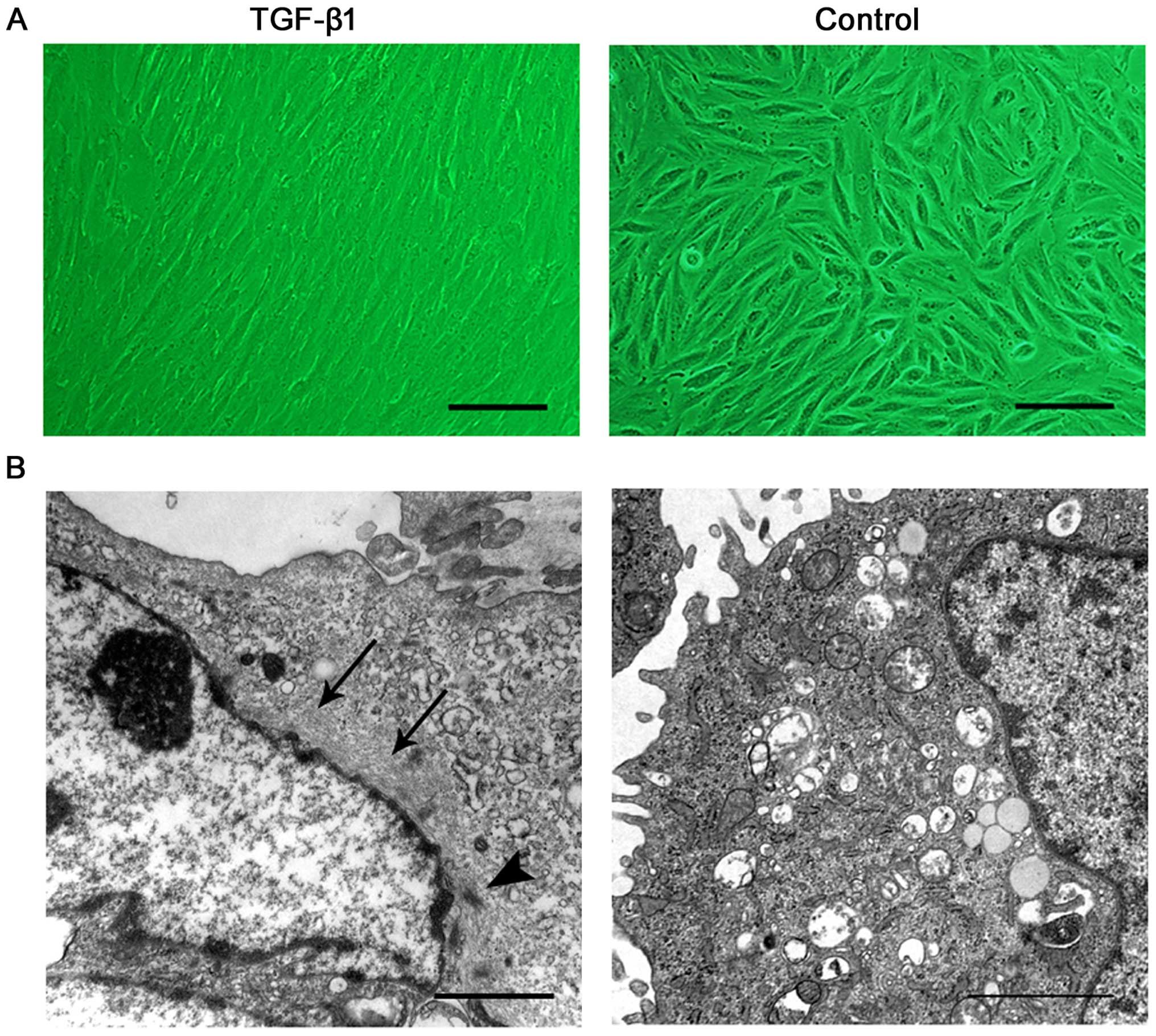
Morphologically, after 7 days of treatment, the
cultured cells changed from a short spindle shape to a large,
spindle shape (Fig. 5A).
Ultrastructure was revealed by TEM. Prior to the induction, the
ERCs contained free polyribosome, profiles of rough endoplasmic
reticulum and a number of mitochondria. Following the addition of
TGF-β1, the cells exhibited bundles of thin filaments including
'dense body' like structures (Fig.
5B), which are representative of typical ultrastructural
characteristics of SMCs.
Signaling pathway involved in the
TGF-β1-induced differ-entiation of ERCs into SMCs
TGF-β family members signal through transmembrane
type I and type II serine/threonine kinase receptors (TGFBR1 and
TGFBR2) and intracellular Smad transcriptional effector proteins
(45). To examine whether TGF-β
signaling components are present in ERCs, the gene expression of
TGFBR1/ALK1, ALK5 and TGFBR2 were examined by
RT-PCR. ALK5 and TGFBR2 were positively expressed in
the ERCs, and the expression level did not alter after TGF-β1
involvement (P>0.05) (Fig. 4A and
B). Negative expression of ALK1 (Fig. 4A) demonstrated that the ERCs we
obtained were not of endothelial origin. TGF signaling is
predominantly transduced by the Smads; Smad2 and Smad3 have shown
to play crucial roles in SMC differentiation. Gene expression
analysis by RT-PCR and qPCR (Fig. 4A
and B) revealed that both Smad2 and Smad3 were expressed in the
ERCs prior to TGF-β1 involvement, and no significant differences
were revealed following the induction (P>0.05). Thus, it appears
that ERCs contain all of the components necessary for transducing
the TGF signal from the cell surface to the nucleus.
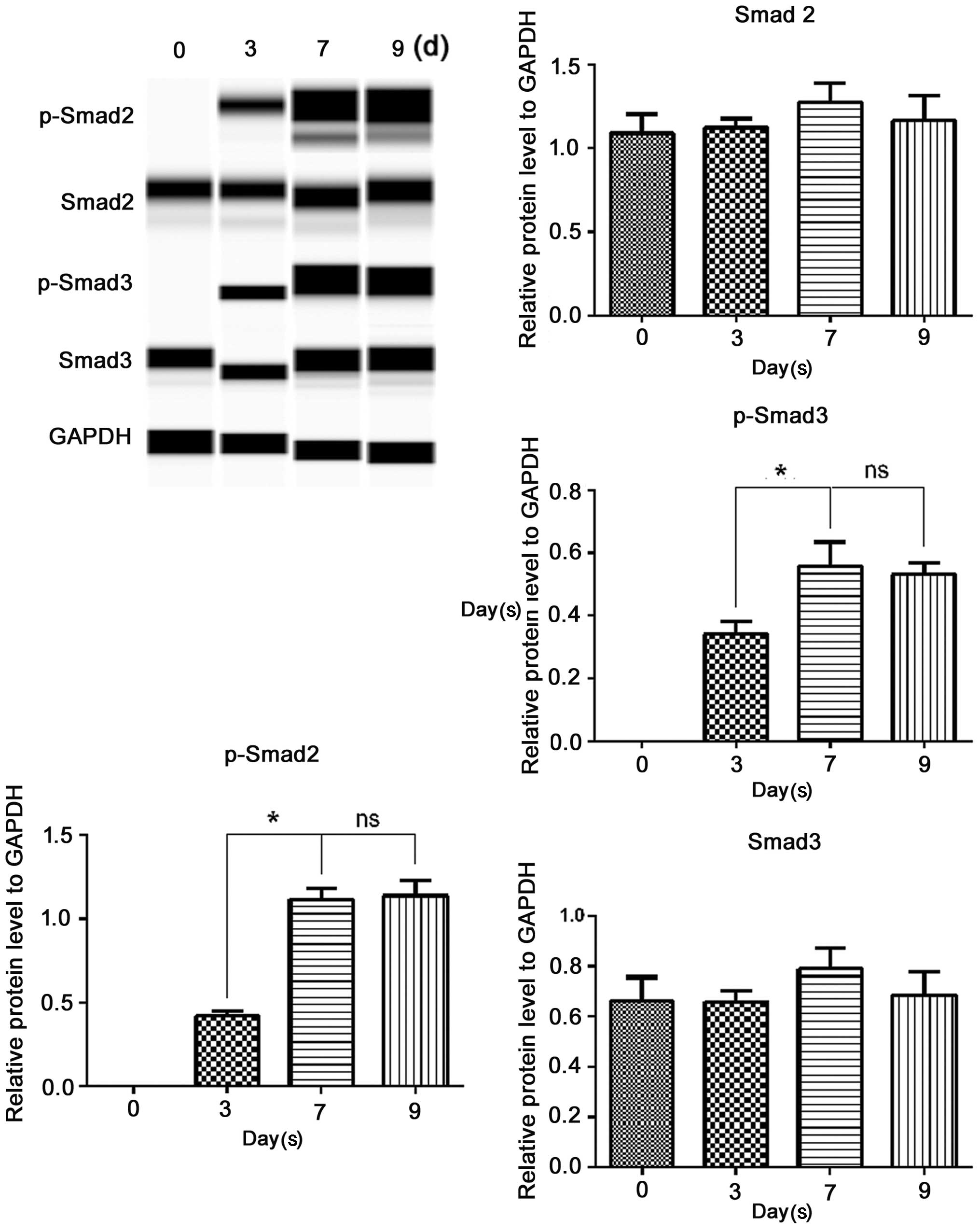
To determine whether TGF-β1 signals through Smad2
and Smad3 in ERCs, we performed Simple Western analysis of Smad2,
Smad3, p-Smad2 and p-Smad3 (Fig.
6). Treated with the inducing medium for various periods of
time, the protein levels of Smad2 and Smad3 slightly altered;
however, these differences were not statistically significant
(P>0.05). P-Smad2 and p-Smad3 were negatively expressed before
TGF-β1 involvement, and they both became detectable on the third
day following the addition of TGF-β1, increased on the 7th day, and
levels were sustained till the 9th day. This was the same as the
time course for the induction of α-SMA and calponin presented
above. These data, at least in part, suggested that the
TGF-β1-induced SM differentiation of ERCs was Smad dependent.
Discussion
Over the last few decades, MSCs from different
origins have become an attractive cell source for regenerative
medicine and tissue engineering, such as those derived from bone
marrow (bmMSCs), adipose tissue, umbilical cord blood and amniotic
fluid among others (29–33). The potential applications of MSCs
in the management of different diseases are enormous. However, the
process of harvesting these cells is often an invasive procedure,
which limits their application.
The endometrium - the highly regenerative lining of
the uterus is an accessible source of MSCs (20,21,34–37). Viable MSC-like cells have been
isolated non-invasively from human menstrual blood using the
plastic adherence method (20),
the manner used to obtain bmMSCs, and these adherent cells were
described as ERCs. Previous studies have demonstrated that ERCs
fulfills all the key properties for an ideal cell source for
cellular therapy (20,38). A comparative profiling of ERCs and
bmMSCs showed similar but not identical profiles (39); ERCs proliferated faster, and an
evaluation of cytokine secretion suggested that ERCs may be more
suitable for different tissue engineering purposes (39).
In the present study, ERCs were obtained from
menstrual blood. Phenotypically, the ERCs shared some markers with
MSCs; however, they also possessed unique features, which matched
the findings of previous studies (20,21). The ERCs possessed the capacity to
proliferate extensively and with clonogenic activity. In addition,
we evaluated the proliferation ability of ERCs among different age
groups (data not shown). Women aged 20–30 were enrolled in the
present study, as females of this age are most active in terms of
reproductive ability. To induce the differentiation of stem cells
into SMCs, various protocols with different inducing factors have
been suggested (40–43), and TGF-β1 is one of the factors
mentioned most frequently. The TGF-β family of cytokines has been
implicated in a number of cellular processes, such as
proliferation, differentiation and apoptosis (44–46). The Smad pathway is a classic route
for the TGF-β-induced SMC differentiation. The procedures involved
include the binding of ligands to TGFBR2 on the cell surface, the
recruitment and phosphorylation of TGFBR1, and the activation of
downstream signals (45–47). TGF-β signaled exclusively through
the TGFBR2, and used two types of TGFBR1 - ALK5 and ALK1, and ALK1
expression was largely restricted to endothelial cells (47). Stem cells of different origins
have been shown to differentiate into SMCs in response to TGF-β1
given singly or in combination with other reagents (41,48–51). In this study, we selected TGF-β1
as a single inducing factor. The results indicated that TGF-β1 may
be a feasible and efficient reagent capable of inducing primary SMC
differention of ERCs. Compared with protocols using multiple
factors, TGF-β1 given singly is more easily accessible and
cost-effective. Therefore, this protocol has the potential to be
the primary choice for induction. The establishment of this basic
protocol is fundamental to further study of the induction medium
and the SMC differentiation of ERCs. Furthermore, we aimed to
elucidate whether the effects of TGF-β1 occurred through this
pathway in ERCs and if so, whether there were any preferences or
defects on the receptors and/or downstream signals. The results
indicated that ERCs possessed all the components of the Smad
pathway, and TGF-β1 signaled predominantly, if not exclusively,
through the 'TGFBR2/ALK5/Smad2 and Smad3' pathway in ERCs.
A surgical approach is often indicated for the
treatment of POP, particularly since the introduction of synthetic
mesh. However, the use of mesh is limited by mesh-related
complications including infection, pain, vaginal fibrosis and
erosion (6–8,52).
Furthermore, the long-term durability and safety of synthetic mesh
remain unknown. Cell-based therapy may provide an attractive
alternative alone or as an adjunct to surgical reconstructive
procedures for POP (9,10). Stem cells have been principally
applied in the development of tissue engineered repair biomaterials
to treat POP, which may replace the use of synthetic mesh (53–55). The addition of autologous stem
cells has been proved to improve the biomechanical properties of
scaffolds, and potentially achieve long-term mechanical integrity
(53–56). Cellular therapy has also emerged
as a promising approach for stress urinary incontinence (57–60). The cells have been administered
intravenously or by local injection into the urethra or
periurethral areas (61–64). Great interest has been focused on
adipose-derived stem cells (54,55,59,61,62). ERCs are a readily available source
of adult stem cells. Preclinical and clinical studies of ERCs have
shown promising therapeutic results in several diseases (17,19,65); however, the value of ERCs in POP
has not yet been explored, to the best of our knowledge. This study
demonstrated that ERCs were induced to differentiate into SMCs with
TGF-β1. By repairing the impaired SMCs in the vaginal wall and
endopelvic structures, ERCs are promising candidates for improving
the treatment of patients with POP. The approaches used with stem
cells of other origins may be applied to ERCs.
There are limitations to the autologous treatment of
POP using ERCs. Vaginal contact may increase the risk of ERC
infections; however, following treatment with antibiotics and
antimycotics, no associated infections were revealed. A certain
portion of patients with POP are older adults with no further
possibility of collecting ERCs. However, if ERCs are proved to be
of high therapeutic value, we may collect ERCs from patients of a
younger age for storage in case of future need.
In conclusion, TGF-β1 is highly efficient at
inducing SMC differentiation of ERCs in vitro, and the
'TGFBR2/ALK5/Smad2 and Smad3' pathway is involved. ERCs are a
potentially promising cell source for cell-based therapeutic
strategies to treat POP. Preliminary establishment of the induction
protocol is of great value for the functional assessment of the
induced SMCs in vitro and in vivo. It is also a
helpful tool for elucidating the role of SMCs in the pathogenesis
of POP.
Acknowledgments
We thank Shuliang Wu, Liang Sun, Xinlei Li, Yafang
Zhang and Zunjiang Xie for their outstanding technical support.
References
|
1
|
Wu JM, Matthews CA, Conover MM, Pate V and
Jonsson Funk M: Lifetime risk of stress urinary incontinence or
pelvic organ prolapse surgery. Obstet Gynecol. 123:1201–1206. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Haylen BT, de Ridder D, Freeman RM, Swift
SE, Berghmans B, Lee J, Monga A, Petri E, Rizk DE, Sand PK and
Schaer GN: An International Urogynecological Association
(IUGA)/International Continence Society (ICS) joint report on the
terminology for female pelvic floor dysfunction. Neurourol Urodyn.
29:4–20. 2010.
|
|
3
|
Hendrix SL, Clark A, Nygaard I, Aragaki A,
Barnabei V and McTiernan A: Pelvic organ prolapse in the Women's
Health Initiative: gravity and gravidity. Am J Obstet Gynecol.
186:1160–1166. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Olsen ALSV, Smith VJ, Bergstrom JO,
Colling JC and Clark AL: Epidemiology of surgically managed pelvic
organ prolapse and urinary incontinence. Obstet Gynecol.
89:501–506. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maher C, Feiner B, Baessler K and Schmid
C: Surgical management of pelvic organ prolapse in women. Cochrane
Database Syst Rev. 4:CD0040142013.PubMed/NCBI
|
|
6
|
Baessler K, Hewson AD, Tunn R, Schuessler
B and Maher CF: Severe mesh complications following intravaginal
slingplasty. Obstet Gynecol. 106:713–716. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nguyen JN, Jakus-Waldman SM, Walter AJ,
White T and Menefee SA: Perioperative complications and
reoperations after incontinence and prolapse surgeries using
prosthetic implants. Obstet Gynecol. 119:539–546. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liang R, Abramowitch S, Knight K, Palcsey
S, Nolfi A, Feola A, Stein S and Moalli PA: Vaginal degeneration
following implantation of synthetic mesh with increased stiffness.
BJOG. 120:233–243. 2013. View Article : Google Scholar
|
|
9
|
Ho MH, Heydarkhan S, Vernet D, Kovanecz I,
Ferrini MG, Bhatia NN and Gonzalez-Cadavid NF: Stimulating vaginal
repair in rats through skeletal muscle-derived stem cells seeded on
small intestinal submucosal scaffolds. Obstet Gynecol. 114:300–309.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Boennelycke M, Gras S and Lose G: Tissue
engineering as a potential alternative or adjunct to surgical
reconstruction in treating pelvic organ prolapse. Int Urogynecol J
Pelvic Floor Dysfunct. 24:741–747. 2013. View Article : Google Scholar
|
|
11
|
Northington GM, Basha M, Arya LA, Wein AJ
and Chacko S: Contractile response of human anterior vaginal
muscularis in women with and without pelvic organ prolapse. Reprod
Sci. 18:296–303. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Boreham MK, Wai CY, Miller RT, Schaffer JI
and Word RA: Morphometric properties of the posterior vaginal wall
in women with pelvic organ prolapse. Am J Obstet Gynecol.
187:1501–1508; discussion 1508–1509. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Inal HA, Kaplan PB, Usta U, Taştekin E,
Aybatli A and Tokuc B: Neuromuscular morphometry of the vaginal
wall in women with anterior vaginal wall prolapse. Neurourol
Urodyn. 29:458–463. 2010.
|
|
14
|
Bortolini MA, Shynlova O, Drutz HP, Castro
RA, Girão MJ, Lye S and Alarab M: Expression of genes encoding
smooth muscle contractile proteins in vaginal tissue of women with
and without pelvic organ prolapse. Neurourol Urodyn. 31:109–114.
2012. View Article : Google Scholar
|
|
15
|
Meijerink AM, van Rijssel RH and van der
Linden PJ: Tissue composition of the vaginal wall in women with
pelvic organ prolapse. Gynecol Obstet Invest. 75:21–27. 2013.
View Article : Google Scholar
|
|
16
|
Murphy MP, Wang H, Patel AN, Kambhampati
S, Angle N, Chan K, Marleau AM, Pyszniak A, Carrier E, Ichim TE and
Riordan NH: Allogeneic endometrial regenerative cells: an 'Off the
shelf solution' for critical limb ischemia? J Transl Med. 6:452008.
View Article : Google Scholar
|
|
17
|
Zhong Z, Patel AN, Ichim TE, Riordan NH,
Wang H, Min WP, Woods EJ, Reid M, Mansilla E, Marin GH, et al:
Feasibility investigation of allogeneic endometrial regenerative
cells. J Transl Med. 7:152009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ichim TE, Alexandrescu DT, Solano F, Lara
F, Campion RN, Paris E, Woods EJ, Murphy MP, Dasanu CA, Patel AN,
et al: Mesenchymal stem cells as anti-inflammatories: implications
for treatment of Duchenne muscular dystrophy. Cell Immunol.
260:75–82. 2010. View Article : Google Scholar
|
|
19
|
Bockeria L, Bogin V, Bockeria O, Le T,
Alekyan B, Woods EJ, Brown AA, Ichim TE and Patel AN: Endometrial
regenerative cells for treatment of heart failure: a new stem cell
enters the clinic. J Transl Med. 11:562013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Meng X, Ichim TE, Zhong J, Rogers A, Yin
Z, Jackson J, Wang H, Ge W, Bogin V, Chan KW, et al: Endometrial
regenerative cells: a novel stem cell population. J Transl Med.
5:572007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Patel AN, Park E, Kuzman M, Benetti F,
Silva FJ and Allickson JG: Multipotent menstrual blood stromal stem
cells: isolation, characterization, and differentiation. Cell
Transplant. 17:303–311. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hida N, Nishiyama N, Miyoshi S, Kira S,
Segawa K, Uyama T, Mori T, Miyado K, Ikegami Y, Cui C, et al: Novel
cardiac precursor-like cells from human menstrual blood-derived
mesenchymal cells. Stem Cells. 26:1695–1704. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rustandi RR, Loughney JW, Hamm M, Hamm C,
Lancaster C, Mach A and Ha S: Qualitative and quantitative
evaluation of Simon™, a new CE-based automated western blot system
as applied to vaccine development. Electrophoresis. 23:2790–2797.
2012. View Article : Google Scholar
|
|
24
|
Kohn EA, Yang YA, Du Z, Nagano Y, Van
Schyndle CM, Herrmann MA, Heldman M, Chen JQ, Stuelten CH, Flanders
KC and Wakefield LM: Biological responses to TGF-β in the mammary
epithelium show a complex dependency on Smad3 gene dosage with
important implications for tumor progression. Mol Cancer Res.
10:1389–1399. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen JQ, Heldman MR, Herrmann MA, Kedei N,
Woo W, Blumberg PM and Goldsmith PK: Absolute quantitation of
endogenous proteins with precision and accuracy using a capillary
Western system. Anal Biochem. 442:97–103. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bakhsheshian J, Hall MD, Robey RW,
Herrmann MA, Chen JQ, Bates SE and Gottesman MM: Overlapping
substrate and inhibitor specificity of human and murine ABCG2. Drug
Metab Dispos. 41:1805–1812. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Klein BY, Tamir H, Hirschberg DL,
Glickstein SB and Welch MG: Oxytocin modulates mTORC1 pathway in
the gut. Biochem Biophys Res Commun. 432:466–471. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu D, Mane S and Sosic Z: Characterization
of a biopharmaceutical protein and evaluation of its purification
process using automated capillary western blot. Electrophoresis.
36:363–370. 2015. View Article : Google Scholar
|
|
29
|
Satija NK, Singh VK, Verma YK, Gupta P,
Sharma S, Afrin F, Sharma M, Sharma P, Tripathi RP and Gurudutta
GU: Mesenchymal stem cell-based therapy: a new paradigm in
regenerative medicine. J Cell Mol Med. 13:4385–4402. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mosna F, Sensebé L and Krampera M: Human
bone marrow and adipose tissue mesenchymal stem cells: a user's
guide. Stem Cells Dev. 19:1449–1470. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nishimatsu H, Suzuki E, Kumano S, Nomiya
A, Liu M, Kume H and Homma Y: Adrenomedullin mediates adipose
tissue-derived stem cell-induced restoration of erectile function
in diabetic rats. J Sex Med. 9:482–493. 2012. View Article : Google Scholar
|
|
32
|
Ghionzoli M, Repele A, Sartiani L,
Costanzi G, Parenti A, Spinelli V, David AL, Garriboli M, Totonelli
G, Tian J, et al: Human amniotic fluid stem cell differentiation
along smooth muscle lineage. FASEB J. 27:4853–4865. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sharma RR, Pollock K, Hubel A and McKenna
D: Mesenchymal stem or stromal cells: a review of clinical
applications and manufacturing practices. Transfusion.
54:1418–1437. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kato K, Yoshimoto M, Kato K, Adachi S,
Yamayoshi A, Arima T, Asanoma K, Kyo S, Nakahata T and Wake N:
Characterization of side-population cells in human normal
endometrium. Hum Reprod. 22:1214–1223. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schwab KE and Gargett CE: Co-expression of
two perivascular cell markers isolates mesenchymal stem-like cells
from human endometrium. Hum Reprod. 22:2903–2911. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cervelló I, Gil-Sanchis C, Mas A,
Delgado-Rosas F, Martínez-Conejero JA, Galán A, Martínez-Romero A,
Martínez S, Navarro I, Ferro J, et al: Human endometrial side
population cells exhibit genotypic, phenotypic and functional
features of somatic stem cells. PLoS One. 5:e109642010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cervelló I, Mas A, Gil-Sanchis C, Peris L,
Faus A, Saunders PT, Critchley HO and Simón C: Reconstruction of
endometrium from human endometrial side population cell lines. PLoS
One. 6:e212212011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ulrich D, Muralitharan R and Gargett CE:
Toward the use of endometrial and menstrual blood mesenchymal stem
cells for cell-based therapies. Expert Opin Biol Ther.
13:1387–1400. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang H, Jin P, Sabatino M, Ren J, Civini
S, Bogin V, Ichim TE and Stroncek DF: Comparison of endometrial
regenerative cells and bone marrow stromal cells. J Transl Med.
10:2072012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lindskog H, Athley E, Larsson E, Lundin S,
Hellström M and Lindahl P: New insights to vascular smooth muscle
cell and pericyte differentiation of mouse embryonic stem cells in
vitro. Arterioscler Thromb Vasc Biol. 26:1457–1464. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Narita Y, Yamawaki A, Kagami H, Ueda M and
Ueda Y: Effects of transforming growth factor-beta 1 and ascorbic
acid on differentiation of human bone-marrow-derived mesenchymal
stem cells into smooth muscle cell lineage. Cell Tissue Res.
333:449–459. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pepe AE, Xiao Q, Zampetaki A, Zhang Z,
Kobayashi A, Hu Y and Xu Q: Crucial role of nrf3 in smooth muscle
cell differentiation from stem cells. Circ Res. 106:870–879. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Park JS, Chu JS, Tsou AD, Diop R, Tang Z,
Wang A and Li S: The effect of matrix stiffness on the
differentiation of mesenchymal stem cells in response to TGF-β.
Biomaterials. 32:3921–3930. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Valcourt U, Kowanetz M, Niimi H, Heldin CH
and Moustakas A: TGF-beta and the Smad signaling pathway support
transcriptomic reprogramming during epithelial-mesenchymal cell
transition. Mol Biol Cell. 16:1987–2002. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Santibañez JF, Quintanilla M and Bernabeu
C: TGF-β/TGF-β receptor system and its role in physiological and
pathological conditions. Clin Sci (Lond). 121:233–251. 2011.
View Article : Google Scholar
|
|
46
|
Tang Y, Yang X, Friesel RE, Vary CP and
Liaw L: Mechanisms of TGF-β-induced differentiation in human
vascular smooth muscle cells. J Vasc Res. 48:485–494. 2011.
View Article : Google Scholar :
|
|
47
|
Bobik A: Transforming growth factor-betas
and vascular disorders. Arterioscler Thromb Vasc Biol.
26:1712–1720. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhao Z, Yu H, Xiao F, Wang X, Yang S and
Li S: Differentiation of adipose-derived stem cells promotes
regeneration of smooth muscle for ureteral tissue engineering. J
Surg Res. 178:55–62. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chen S and Lechleider RJ: Transforming
growth factor-beta-induced differentiation of smooth muscle from a
neural crest stem cell line. Circ Res. 94:1195–1202. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chou MT, Chang SN, Ke C, Chang HI, Sung
ML, Kuo HC and Chen CN: The proliferation and differentiation of
placental-derived multipotent cells into smooth muscle cells on
fibrillar collagen. Biomaterials. 31:4367–4375. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wang D, Park JS, Chu JS, Krakowski A, Luo
K, Chen DJ and Li S: Proteomic profiling of bone marrow mesenchymal
stem cells upon transforming growth factor β1 stimulation. J Biol
Chem. 279:43725–43734. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Jia X, Glazener C, Mowatt G, MacLennan G,
Bain C, Fraser C and Burr J: Efficacy and safety of using mesh or
grafts in surgery for anterior and/or posterior vaginal wall
prolapse: systematic review and meta-analysis. BJOG. 115:1350–1361.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Roman S, Mangera A, Osman NI, Bullock AJ,
Chapple CR and MacNeil S: Developing a tissue engineered repair
material for treatment of stress urinary incontinence and pelvic
organ prolapse-which cell source? Neurourol Urodyn. 33:531–537.
2014. View Article : Google Scholar
|
|
54
|
Aboushwareb T, McKenzie P, Wezel F,
Southgate J and Badlani G: Is tissue engineering and biomaterials
the future for lower urinary tract dysfunction (LUTD)/pelvic organ
prolapse (POP)? Neurourol Urodyn. 30:755–782. 2011. View Article : Google Scholar
|
|
55
|
Hung MJ, Wen MC, Huang YT, Chen GD, Chou
MM and Yang VC: Fascia tissue engineering with human
adipose-derived stem cells in a murine model: implications for
pelvic floor reconstruction. J Formos Med Assoc. 113:704–715. 2014.
View Article : Google Scholar
|
|
56
|
Su K, Edwards SL, Tan KS, White JF, Kandel
S, Ramshaw JA, Gargett CE and Werkmeister JA: Induction of
endometrial mesenchymal stem cells into tissue-forming cells
suitable for fascial repair. Acta Biomater. 10:5012–5020. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Smaldone MC and Chancellor MB: Muscle
derived stem cell therapy for stress urinary incontinence. World J
Urol. 26:327–332. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Lin CS and Lue TF: Stem cell therapy for
stress urinary incontinence: a critical review. Stem Cells Dev.
21:834–843. 2012. View Article : Google Scholar :
|
|
59
|
Roche R, Festy F and Fritel X: Stem cells
for stress urinary incontinence: the adipose promise. J Cell Mol
Med. 14:135–142. 2010. View Article : Google Scholar
|
|
60
|
Nikolavasky D, Stangel-Wójcikiewicz K,
Stec M and Chancellor MB: Stem cell therapy: a future treatment of
stress urinary incontinence. Semin Reprod Med. 29:61–70. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Lin G, Wang G, Banie L, Ning H, Shindel
AW, Fandel TM, Lue TF and Lin CS: Treatment of stress urinary
incontinence with adipose tissue-derived stem cells. Cytotherapy.
12:88–95. 2010. View Article : Google Scholar :
|
|
62
|
Shi LB, Cai HX, Chen LK, Wu Y, Zhu SA,
Gong XN, Xia YX, Ouyang HW and Zou XH: Tissue engineered bulking
agent with adipose-derived stem cells and silk fibroin microspheres
for the treatment of intrinsic urethral sphincter deficiency.
Biomaterials. 35:1519–1530. 2014. View Article : Google Scholar
|
|
63
|
Dissaranan C, Cruz MA, Kiedrowski MJ,
Balog BM, Gill BC, Penn MS, Goldman HB and Damaser MS: Rat
mesenchymal stem cell secretome promotes elastogenesis and
facilitates recovery from simulated childbirth injury. Cell
Transplant. 23:1395–1406. 2014. View Article : Google Scholar
|
|
64
|
Cruz M, Dissaranan C, Cotleur A,
Kiedrowski M, Penn M and Damaser M: Pelvic organ distribution of
mesenchymal stem cells injected intravenously after simulated
childbirth injury in female rats. Obstet Gynecol Int.
2012:6129462012.
|
|
65
|
Borlongan CV, Kaneko Y, Maki M, Yu SJ, Ali
M, Allickson JG, Sanberg CD, Kuzmin-Nichols N and Sanberg PR:
Menstrual blood cells display stem cell-like phenotypic markers and
exert neuroprotection following transplantation in experimental
stroke. Stem Cells Dev. 19:439–452. 2010. View Article : Google Scholar
|