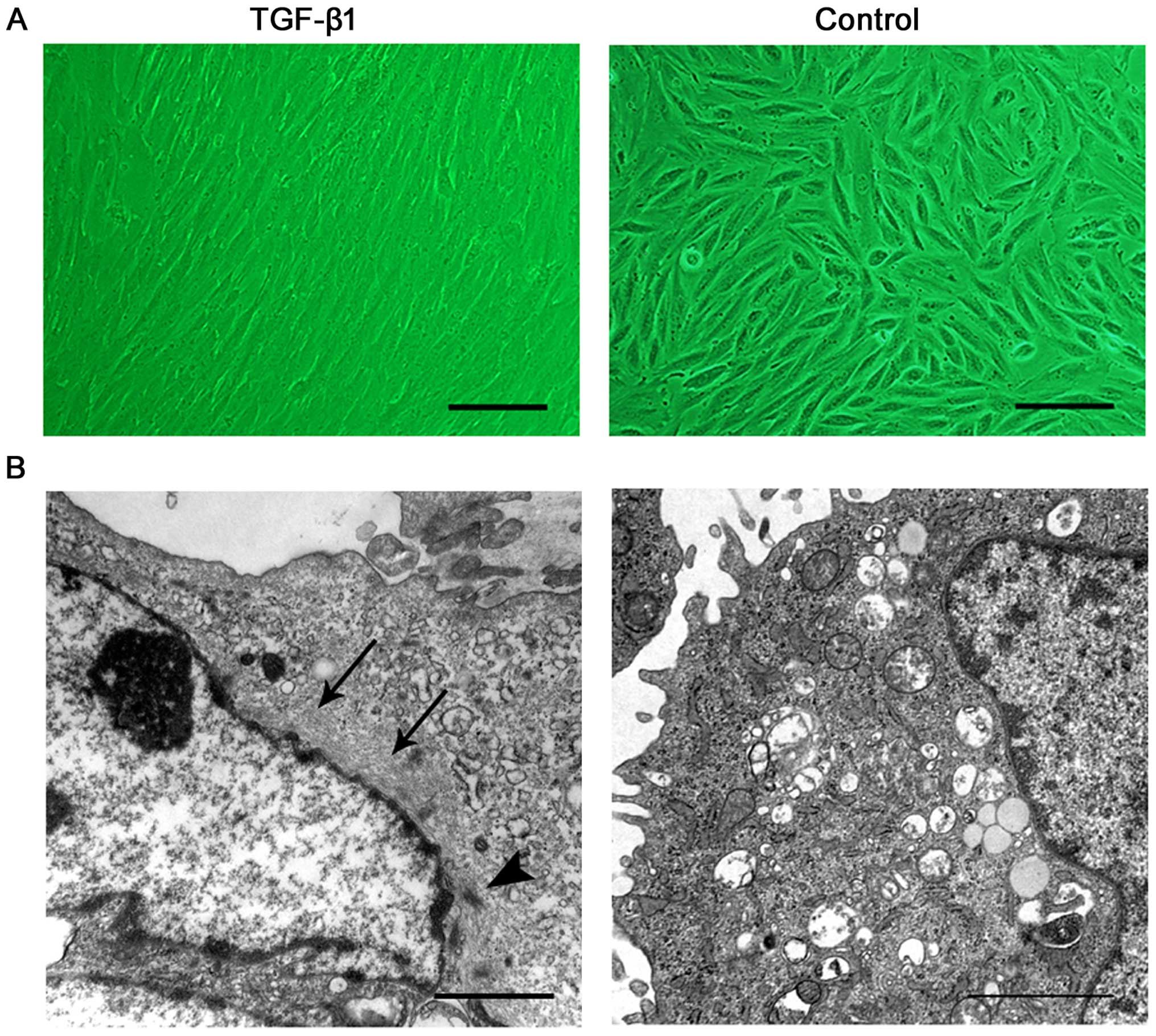
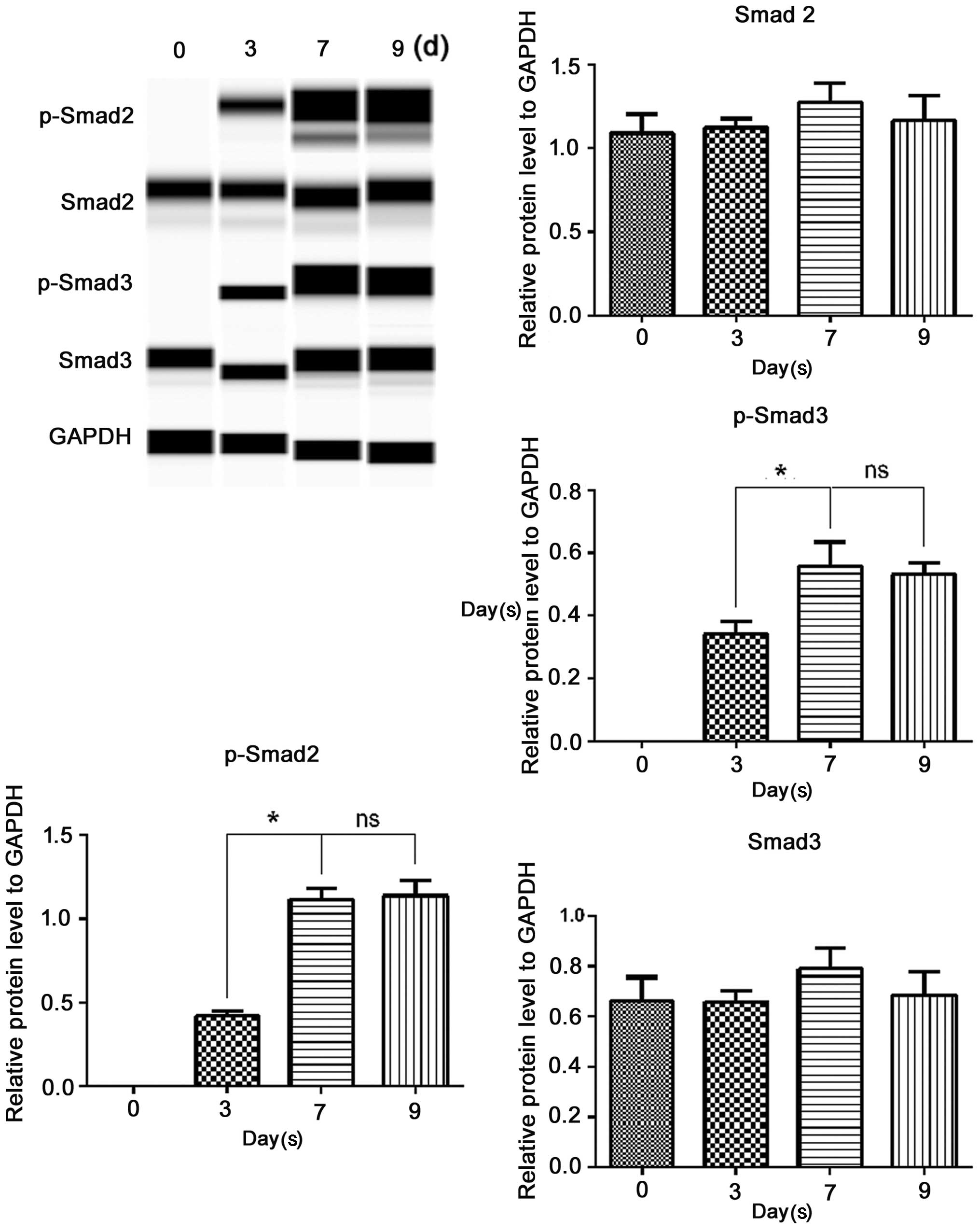
|
1
|
Wu JM, Matthews CA, Conover MM, Pate V and
Jonsson Funk M: Lifetime risk of stress urinary incontinence or
pelvic organ prolapse surgery. Obstet Gynecol. 123:1201–1206. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Haylen BT, de Ridder D, Freeman RM, Swift
SE, Berghmans B, Lee J, Monga A, Petri E, Rizk DE, Sand PK and
Schaer GN: An International Urogynecological Association
(IUGA)/International Continence Society (ICS) joint report on the
terminology for female pelvic floor dysfunction. Neurourol Urodyn.
29:4–20. 2010.
|
|
3
|
Hendrix SL, Clark A, Nygaard I, Aragaki A,
Barnabei V and McTiernan A: Pelvic organ prolapse in the Women's
Health Initiative: gravity and gravidity. Am J Obstet Gynecol.
186:1160–1166. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Olsen ALSV, Smith VJ, Bergstrom JO,
Colling JC and Clark AL: Epidemiology of surgically managed pelvic
organ prolapse and urinary incontinence. Obstet Gynecol.
89:501–506. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maher C, Feiner B, Baessler K and Schmid
C: Surgical management of pelvic organ prolapse in women. Cochrane
Database Syst Rev. 4:CD0040142013.PubMed/NCBI
|
|
6
|
Baessler K, Hewson AD, Tunn R, Schuessler
B and Maher CF: Severe mesh complications following intravaginal
slingplasty. Obstet Gynecol. 106:713–716. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nguyen JN, Jakus-Waldman SM, Walter AJ,
White T and Menefee SA: Perioperative complications and
reoperations after incontinence and prolapse surgeries using
prosthetic implants. Obstet Gynecol. 119:539–546. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liang R, Abramowitch S, Knight K, Palcsey
S, Nolfi A, Feola A, Stein S and Moalli PA: Vaginal degeneration
following implantation of synthetic mesh with increased stiffness.
BJOG. 120:233–243. 2013. View Article : Google Scholar
|
|
9
|
Ho MH, Heydarkhan S, Vernet D, Kovanecz I,
Ferrini MG, Bhatia NN and Gonzalez-Cadavid NF: Stimulating vaginal
repair in rats through skeletal muscle-derived stem cells seeded on
small intestinal submucosal scaffolds. Obstet Gynecol. 114:300–309.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Boennelycke M, Gras S and Lose G: Tissue
engineering as a potential alternative or adjunct to surgical
reconstruction in treating pelvic organ prolapse. Int Urogynecol J
Pelvic Floor Dysfunct. 24:741–747. 2013. View Article : Google Scholar
|
|
11
|
Northington GM, Basha M, Arya LA, Wein AJ
and Chacko S: Contractile response of human anterior vaginal
muscularis in women with and without pelvic organ prolapse. Reprod
Sci. 18:296–303. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Boreham MK, Wai CY, Miller RT, Schaffer JI
and Word RA: Morphometric properties of the posterior vaginal wall
in women with pelvic organ prolapse. Am J Obstet Gynecol.
187:1501–1508; discussion 1508–1509. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Inal HA, Kaplan PB, Usta U, Taştekin E,
Aybatli A and Tokuc B: Neuromuscular morphometry of the vaginal
wall in women with anterior vaginal wall prolapse. Neurourol
Urodyn. 29:458–463. 2010.
|
|
14
|
Bortolini MA, Shynlova O, Drutz HP, Castro
RA, Girão MJ, Lye S and Alarab M: Expression of genes encoding
smooth muscle contractile proteins in vaginal tissue of women with
and without pelvic organ prolapse. Neurourol Urodyn. 31:109–114.
2012. View Article : Google Scholar
|
|
15
|
Meijerink AM, van Rijssel RH and van der
Linden PJ: Tissue composition of the vaginal wall in women with
pelvic organ prolapse. Gynecol Obstet Invest. 75:21–27. 2013.
View Article : Google Scholar
|
|
16
|
Murphy MP, Wang H, Patel AN, Kambhampati
S, Angle N, Chan K, Marleau AM, Pyszniak A, Carrier E, Ichim TE and
Riordan NH: Allogeneic endometrial regenerative cells: an 'Off the
shelf solution' for critical limb ischemia? J Transl Med. 6:452008.
View Article : Google Scholar
|
|
17
|
Zhong Z, Patel AN, Ichim TE, Riordan NH,
Wang H, Min WP, Woods EJ, Reid M, Mansilla E, Marin GH, et al:
Feasibility investigation of allogeneic endometrial regenerative
cells. J Transl Med. 7:152009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ichim TE, Alexandrescu DT, Solano F, Lara
F, Campion RN, Paris E, Woods EJ, Murphy MP, Dasanu CA, Patel AN,
et al: Mesenchymal stem cells as anti-inflammatories: implications
for treatment of Duchenne muscular dystrophy. Cell Immunol.
260:75–82. 2010. View Article : Google Scholar
|
|
19
|
Bockeria L, Bogin V, Bockeria O, Le T,
Alekyan B, Woods EJ, Brown AA, Ichim TE and Patel AN: Endometrial
regenerative cells for treatment of heart failure: a new stem cell
enters the clinic. J Transl Med. 11:562013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Meng X, Ichim TE, Zhong J, Rogers A, Yin
Z, Jackson J, Wang H, Ge W, Bogin V, Chan KW, et al: Endometrial
regenerative cells: a novel stem cell population. J Transl Med.
5:572007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Patel AN, Park E, Kuzman M, Benetti F,
Silva FJ and Allickson JG: Multipotent menstrual blood stromal stem
cells: isolation, characterization, and differentiation. Cell
Transplant. 17:303–311. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hida N, Nishiyama N, Miyoshi S, Kira S,
Segawa K, Uyama T, Mori T, Miyado K, Ikegami Y, Cui C, et al: Novel
cardiac precursor-like cells from human menstrual blood-derived
mesenchymal cells. Stem Cells. 26:1695–1704. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rustandi RR, Loughney JW, Hamm M, Hamm C,
Lancaster C, Mach A and Ha S: Qualitative and quantitative
evaluation of Simon™, a new CE-based automated western blot system
as applied to vaccine development. Electrophoresis. 23:2790–2797.
2012. View Article : Google Scholar
|
|
24
|
Kohn EA, Yang YA, Du Z, Nagano Y, Van
Schyndle CM, Herrmann MA, Heldman M, Chen JQ, Stuelten CH, Flanders
KC and Wakefield LM: Biological responses to TGF-β in the mammary
epithelium show a complex dependency on Smad3 gene dosage with
important implications for tumor progression. Mol Cancer Res.
10:1389–1399. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen JQ, Heldman MR, Herrmann MA, Kedei N,
Woo W, Blumberg PM and Goldsmith PK: Absolute quantitation of
endogenous proteins with precision and accuracy using a capillary
Western system. Anal Biochem. 442:97–103. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bakhsheshian J, Hall MD, Robey RW,
Herrmann MA, Chen JQ, Bates SE and Gottesman MM: Overlapping
substrate and inhibitor specificity of human and murine ABCG2. Drug
Metab Dispos. 41:1805–1812. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Klein BY, Tamir H, Hirschberg DL,
Glickstein SB and Welch MG: Oxytocin modulates mTORC1 pathway in
the gut. Biochem Biophys Res Commun. 432:466–471. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu D, Mane S and Sosic Z: Characterization
of a biopharmaceutical protein and evaluation of its purification
process using automated capillary western blot. Electrophoresis.
36:363–370. 2015. View Article : Google Scholar
|
|
29
|
Satija NK, Singh VK, Verma YK, Gupta P,
Sharma S, Afrin F, Sharma M, Sharma P, Tripathi RP and Gurudutta
GU: Mesenchymal stem cell-based therapy: a new paradigm in
regenerative medicine. J Cell Mol Med. 13:4385–4402. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mosna F, Sensebé L and Krampera M: Human
bone marrow and adipose tissue mesenchymal stem cells: a user's
guide. Stem Cells Dev. 19:1449–1470. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nishimatsu H, Suzuki E, Kumano S, Nomiya
A, Liu M, Kume H and Homma Y: Adrenomedullin mediates adipose
tissue-derived stem cell-induced restoration of erectile function
in diabetic rats. J Sex Med. 9:482–493. 2012. View Article : Google Scholar
|
|
32
|
Ghionzoli M, Repele A, Sartiani L,
Costanzi G, Parenti A, Spinelli V, David AL, Garriboli M, Totonelli
G, Tian J, et al: Human amniotic fluid stem cell differentiation
along smooth muscle lineage. FASEB J. 27:4853–4865. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sharma RR, Pollock K, Hubel A and McKenna
D: Mesenchymal stem or stromal cells: a review of clinical
applications and manufacturing practices. Transfusion.
54:1418–1437. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kato K, Yoshimoto M, Kato K, Adachi S,
Yamayoshi A, Arima T, Asanoma K, Kyo S, Nakahata T and Wake N:
Characterization of side-population cells in human normal
endometrium. Hum Reprod. 22:1214–1223. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schwab KE and Gargett CE: Co-expression of
two perivascular cell markers isolates mesenchymal stem-like cells
from human endometrium. Hum Reprod. 22:2903–2911. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cervelló I, Gil-Sanchis C, Mas A,
Delgado-Rosas F, Martínez-Conejero JA, Galán A, Martínez-Romero A,
Martínez S, Navarro I, Ferro J, et al: Human endometrial side
population cells exhibit genotypic, phenotypic and functional
features of somatic stem cells. PLoS One. 5:e109642010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cervelló I, Mas A, Gil-Sanchis C, Peris L,
Faus A, Saunders PT, Critchley HO and Simón C: Reconstruction of
endometrium from human endometrial side population cell lines. PLoS
One. 6:e212212011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ulrich D, Muralitharan R and Gargett CE:
Toward the use of endometrial and menstrual blood mesenchymal stem
cells for cell-based therapies. Expert Opin Biol Ther.
13:1387–1400. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang H, Jin P, Sabatino M, Ren J, Civini
S, Bogin V, Ichim TE and Stroncek DF: Comparison of endometrial
regenerative cells and bone marrow stromal cells. J Transl Med.
10:2072012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lindskog H, Athley E, Larsson E, Lundin S,
Hellström M and Lindahl P: New insights to vascular smooth muscle
cell and pericyte differentiation of mouse embryonic stem cells in
vitro. Arterioscler Thromb Vasc Biol. 26:1457–1464. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Narita Y, Yamawaki A, Kagami H, Ueda M and
Ueda Y: Effects of transforming growth factor-beta 1 and ascorbic
acid on differentiation of human bone-marrow-derived mesenchymal
stem cells into smooth muscle cell lineage. Cell Tissue Res.
333:449–459. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pepe AE, Xiao Q, Zampetaki A, Zhang Z,
Kobayashi A, Hu Y and Xu Q: Crucial role of nrf3 in smooth muscle
cell differentiation from stem cells. Circ Res. 106:870–879. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Park JS, Chu JS, Tsou AD, Diop R, Tang Z,
Wang A and Li S: The effect of matrix stiffness on the
differentiation of mesenchymal stem cells in response to TGF-β.
Biomaterials. 32:3921–3930. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Valcourt U, Kowanetz M, Niimi H, Heldin CH
and Moustakas A: TGF-beta and the Smad signaling pathway support
transcriptomic reprogramming during epithelial-mesenchymal cell
transition. Mol Biol Cell. 16:1987–2002. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Santibañez JF, Quintanilla M and Bernabeu
C: TGF-β/TGF-β receptor system and its role in physiological and
pathological conditions. Clin Sci (Lond). 121:233–251. 2011.
View Article : Google Scholar
|
|
46
|
Tang Y, Yang X, Friesel RE, Vary CP and
Liaw L: Mechanisms of TGF-β-induced differentiation in human
vascular smooth muscle cells. J Vasc Res. 48:485–494. 2011.
View Article : Google Scholar :
|
|
47
|
Bobik A: Transforming growth factor-betas
and vascular disorders. Arterioscler Thromb Vasc Biol.
26:1712–1720. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhao Z, Yu H, Xiao F, Wang X, Yang S and
Li S: Differentiation of adipose-derived stem cells promotes
regeneration of smooth muscle for ureteral tissue engineering. J
Surg Res. 178:55–62. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chen S and Lechleider RJ: Transforming
growth factor-beta-induced differentiation of smooth muscle from a
neural crest stem cell line. Circ Res. 94:1195–1202. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chou MT, Chang SN, Ke C, Chang HI, Sung
ML, Kuo HC and Chen CN: The proliferation and differentiation of
placental-derived multipotent cells into smooth muscle cells on
fibrillar collagen. Biomaterials. 31:4367–4375. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wang D, Park JS, Chu JS, Krakowski A, Luo
K, Chen DJ and Li S: Proteomic profiling of bone marrow mesenchymal
stem cells upon transforming growth factor β1 stimulation. J Biol
Chem. 279:43725–43734. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Jia X, Glazener C, Mowatt G, MacLennan G,
Bain C, Fraser C and Burr J: Efficacy and safety of using mesh or
grafts in surgery for anterior and/or posterior vaginal wall
prolapse: systematic review and meta-analysis. BJOG. 115:1350–1361.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Roman S, Mangera A, Osman NI, Bullock AJ,
Chapple CR and MacNeil S: Developing a tissue engineered repair
material for treatment of stress urinary incontinence and pelvic
organ prolapse-which cell source? Neurourol Urodyn. 33:531–537.
2014. View Article : Google Scholar
|
|
54
|
Aboushwareb T, McKenzie P, Wezel F,
Southgate J and Badlani G: Is tissue engineering and biomaterials
the future for lower urinary tract dysfunction (LUTD)/pelvic organ
prolapse (POP)? Neurourol Urodyn. 30:755–782. 2011. View Article : Google Scholar
|
|
55
|
Hung MJ, Wen MC, Huang YT, Chen GD, Chou
MM and Yang VC: Fascia tissue engineering with human
adipose-derived stem cells in a murine model: implications for
pelvic floor reconstruction. J Formos Med Assoc. 113:704–715. 2014.
View Article : Google Scholar
|
|
56
|
Su K, Edwards SL, Tan KS, White JF, Kandel
S, Ramshaw JA, Gargett CE and Werkmeister JA: Induction of
endometrial mesenchymal stem cells into tissue-forming cells
suitable for fascial repair. Acta Biomater. 10:5012–5020. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Smaldone MC and Chancellor MB: Muscle
derived stem cell therapy for stress urinary incontinence. World J
Urol. 26:327–332. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Lin CS and Lue TF: Stem cell therapy for
stress urinary incontinence: a critical review. Stem Cells Dev.
21:834–843. 2012. View Article : Google Scholar :
|
|
59
|
Roche R, Festy F and Fritel X: Stem cells
for stress urinary incontinence: the adipose promise. J Cell Mol
Med. 14:135–142. 2010. View Article : Google Scholar
|
|
60
|
Nikolavasky D, Stangel-Wójcikiewicz K,
Stec M and Chancellor MB: Stem cell therapy: a future treatment of
stress urinary incontinence. Semin Reprod Med. 29:61–70. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Lin G, Wang G, Banie L, Ning H, Shindel
AW, Fandel TM, Lue TF and Lin CS: Treatment of stress urinary
incontinence with adipose tissue-derived stem cells. Cytotherapy.
12:88–95. 2010. View Article : Google Scholar :
|
|
62
|
Shi LB, Cai HX, Chen LK, Wu Y, Zhu SA,
Gong XN, Xia YX, Ouyang HW and Zou XH: Tissue engineered bulking
agent with adipose-derived stem cells and silk fibroin microspheres
for the treatment of intrinsic urethral sphincter deficiency.
Biomaterials. 35:1519–1530. 2014. View Article : Google Scholar
|
|
63
|
Dissaranan C, Cruz MA, Kiedrowski MJ,
Balog BM, Gill BC, Penn MS, Goldman HB and Damaser MS: Rat
mesenchymal stem cell secretome promotes elastogenesis and
facilitates recovery from simulated childbirth injury. Cell
Transplant. 23:1395–1406. 2014. View Article : Google Scholar
|
|
64
|
Cruz M, Dissaranan C, Cotleur A,
Kiedrowski M, Penn M and Damaser M: Pelvic organ distribution of
mesenchymal stem cells injected intravenously after simulated
childbirth injury in female rats. Obstet Gynecol Int.
2012:6129462012.
|
|
65
|
Borlongan CV, Kaneko Y, Maki M, Yu SJ, Ali
M, Allickson JG, Sanberg CD, Kuzmin-Nichols N and Sanberg PR:
Menstrual blood cells display stem cell-like phenotypic markers and
exert neuroprotection following transplantation in experimental
stroke. Stem Cells Dev. 19:439–452. 2010. View Article : Google Scholar
|