Introduction
Oral squamous cell carcinoma (OSCC) is one of the
most common malignant tumors of the oral and maxillofacial region.
Alcohol consumption, betel quid chewing and cigarette smoking are
the main accepted etiologies of OSCC thus far (1). Despite advances in the main
treatments of surgery and radiotherapy, there have been relatively
low 5-year survival rates. The development of tumors is a complex
developmental process involving multiple factors, steps and
signaling pathways, including mitogen-activated protein kinase
(MAPK), phosphatidylinositol 3-kinase (PI3K), Notch and signal
transducer and activator of transcription 3 (Stat3) (2–5).
Numerous genes have been shown to affect the development and
progression of SCC, thus suggesting various potential approaches
with which to prevent and treat SCC (6–8).
This has evoked increasing interest in tumor biological treatment
that is able to predict prognosis.
Gamma-aminobutyric acid (GABA) is a non-protein
amino acid that acts as the major inhibitory neurotransmitter in
the adult mammalian central nervous system (CNS) (9). GABA can inhibit excessive excitement
in the CNS and has a calming effect on the brain, thus promoting
relaxation and eliminate nervousness (10). In a previous study, it was
reported that GABA abolished the negative responses to nicotine,
which included the growth of pancreatic ductal adenocarcinoma
xenografts in mice (11). Owing
to these physiological functions, several functional foods have
been manufactured. Natural GABA was first found in potatoes and is
widely obtained in nature among microorganisms, plants and animals.
A wide range of traditional foods contain GABA, such as gammalone,
cheese, gabaron tea, green tea and shochu, in which GABA is safe
and eco-friendly, there are also possibility other products
containing GABA with health benefits. It has been suggested that
GABA has a variety of physiological functions, and GABA is a type
of functional food additive (12).
Moreover, it has been reported that GABA plays a
role in central and peripheral tumors, in addition to serving as an
inhibitory neurotransmitter through its receptor (13). According to their pharmacological
characteristics, GABA receptors have been divided into 3 types, A,
B and C. Among the 3 types of receptors, A type receptors have a
variety of functions in a number of peripheral non-neuronal
tissues, such as the small intestine, pancreas and liver, and have
different functions with respect to specific cell types (14–17). A typical GABA A type receptor
subunit, named pi (GABRP), was detected in several peripheral
tissues. It has been proposed that GABRP may play an important role
in GABA A type receptor functions in non-neuronal tissues,
including cancer cell proliferation in gastric cancer through the
MAPK signaling pathways, which are the best known regulators of
mammalian cell proliferation (18).
In clinical pharmacology, GABA is used considerably
in pharmaceutical agents to alleviate pain in cancer, acting upon
receptors of GABA and GABA 'mimetics' (e.g., gabapentin); however,
the safe use of GABA for patients with tongue cancer has not yet
been established (19). To data,
and to the best of our knowledge, no studies have examined the
association between GABA expression and OSCC, which may prove to be
of significance for an improved diagnosis and treatment.
To develop a promising method for the effective
treatment and prognosis of OSCC, it is necessary to obtain a better
understanding of the molecular mechanisms responsible for the
development of this disease. In the present study, we investigated
the expression of GABRP in OSCC using tongue pathological sections
and Tca8113 cells. Furthermore, we examined whether the effects of
GABA and GABA A type receptor on Tca8113 cell proliferation and
apoptosis are mediated through the MAPK signaling pathways.
Materials and methods
Chemicals and antibodies
GABA, Muscimol (GABA A type receptor agonist) and
S106 (GABA A type receptor antagonist) were obtained from
Sigma-Aldrich (St. Louis, MO, USA). GABRP polyclonal antibody was
from Abcam (Cambridge, MA, USA). Antibodies against MAPK were
purchased from Cell Signaling Technology (Danvers, MA, USA). The
Cell Counting Kit-8 (CCK-8) was obtained from Dojindo Molecular
Technologies, Inc., Shanghai, China. Other drugs used were of
reagent grade.
Tissue samples
From 2012 to 2014, we obtained 24 tongue cancer
samples (12 well-differentiated ones and 12 poorly differentiated
ones) and 12 fibroplasia samples. Tongue cancer samples were
obtained by tumor resection, while the fibroplasia samples were
obtained by biopsy. All the samples were obtained from the
Department of Pathology of Chongqing Medicial University
(Chongqing, China) after obtaining the approval of the Ethics
Committee of the Medical Department of Chongqing Medicial
University. All patients provided written and signed consent prior
to donating their tissue samples for use in scientific research.
According to the WHO standard (1971), the differentiation of the
cancer cells was divided into high (well-differentiated), and
medium and low (poorly differentiated) levels.
Immunohistochemical staining of
GABRP
All pathological sections (4–6 µm) were
deparaffinized and rehydrated. Endogenous peroxidase activity was
blocked by incubating the sections in 3% peroxide in methanol for
30 min at room temperature. Following 3 washes in PBS, non-specific
binding was blocked in 10% normal rabbit serum in PBS for 1 h at
room temperature followed by incubation with rabbit anti-GABAAR pi
primary antibody (1.2 µg/ml, ab26055; Abcam) overnight at
4°C. Following 3 washes in PBS, the sections were subsequently
incubated with horseradish peroxidase-conjugated goat anti-rabbit
IgG (sp-9001; Zhongshan Biotechnology, Beijing, China) for 40 min
at room temperature. The secondary antibody was detected with
3,3′-diaminobenzidine solution (sp-9001; Zhongshan Biotechnology).
For some sections, the primary antibody was replaced with normal
rabbit IgG (2 µg/ml IgG; Abcam) instead of anti-GABA primary
antibody to serve as the negative controls.
Cell culture
The Tca8113 cells were obtained from the Shanghai
Institute of Biochemistry and Cell Biology (SIBCB; Shanghai, China)
and cultured in RPMI-1640 medium (Gibco Life Technologies,
Carlsbad, CA, USA) supplemented with 10% (v/v) fetal bovine serum
at 37°C in a humidified atmosphere containing 5% CO2. A
subcultivation ratio of 1:3 is recommended.
The Tca8113 cells, plated in cell culture dishes,
were randomly divided into different treatment groups when adhered
to the bottom of the dishes. We then selected the time points of 24
and 48 h after treatment for analysis. The treatment groups were as
follows: group 1, negative control without any treatment; groups
2–4, addition of various concentrations of GABA (1, 10 or 100
µM) in culture medium, respectively; groups 5–7, addition of
various concentrations of Muscimol (0.5, 5 or 50 µM) in
culture medium, respectively; and groups 8–10, addition of various
concentrations of S106 (0.5, 5 or 50 µM) for 30 min in
advance and then re-treatment with the optimal concentration of
GABA according to the results obtained from groups 2–4.
Reverse transcription polymerase chain
reaction (RT-PCR)
Total RNA was isolated from the Tca8113 cells after
the addition of various concentration of GABA, Muscimol and S106 at
24 and 48 h using TRIzol reagent (Invitrogen Life Technologies,
Carlsbad, CA, USA) according to the manufacturer's instructions.
The primer sequences of GABRP were as follows: forward,
5′-ATGAGCTACAGCCTCTATTT GGC-3′; and reverse,
5′-ACCACCGAAATTGGGCCTG-3′ and the amplicon size was 174 bp. The
primer sequences of GAPDH were as follows: forward,
5′-AGCCATGTACGTA GCCATCC-3′; and reverse, 5′-CTCTCAGCTGTGGTGGT
GAA-3′ and the amplicon size was 373 bp. Total RNA (2 µg)
was reverse transcribed in 20 µl of reaction mixture
containing 4 µl MgCl2, 25 mM; 2 µl reverse
transcription 10X buffer; 2 µl dNTP mixture, 10 mM; 0.5
µl recombinant RNasin® ribonuclease inhibitor, 15
U AMV reverse transcriptase (high concentration), and 0.5 µg
random primers (A3500; Promega, Madison, WI, USA). PCR was
performed in a total volume of 25 µl containing 12.5
µl GoTaq® Green Master Mix (M7122; Promega), 0.5
µM primers and 1 µl cDNA and was carried out over 25
cycles. The thermal cycling conditions were as follows: 94°C for 30
sec, 59–61°C for 30 sec, and 72°C for 30 sec.
Western blot analysis
As previously described, total protein samples were
isolated from the various treatment groups, separated on a 15%
sodium dodecyl sulfate (SDS) polyacrylamide gel (50 µg
protein/well), and transferred onto a nitrocellulose membrane
(Hybond™-C; Amersham Biosciences, Piscataway, NJ, USA). The
membranes were blocked with 5% non-fat milk in Tris-buffered saline
containing 0.1% Tween-20 at room temperature for 2 h and were then
incubated with the following primary antibodies at 4°C overnight:
rabbit anti-GABAAR pi primary antibody (1:1,000sc-25708; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA), rabbit
anti-phospho-p44/42 MAPK (Erk1/2) primary antibody (1:2,000,
4370s), rabbit anti-p44/42 MAPK (ERK1/2) primary antibody (1:1,000,
4695s), rabbit anti-phospho-p38 MAPK primary antibody (1:1,000,
4511T), rabbit anti-p38 MAPK primary antibody (1:1,000, 14451s),
rabbit anti-phospho-JNK primary antibody (1:1,000, 4668T), rabbit
anti-JNK primary antibody (1:1,000, 9252s), and goat anti-rabbit
GAPDH primary antibody (1:1,000, 2118S) (all from Cell Signaling
Technology). Following incubation with the corresponding strain
secondary antibodies (HRP-labeled goat anti-rabbit IgG (H+L)
(1:800, A0208) for 60 min at room temperature, the membranes were
subjected to enhanced chemiluminescence (BeyoECL Plus, P0018) (both
from Beyotime Institute of Biotechnology, Haimen, China).
Immunofluorescence staining
The Tca8113 cells were cultured for 24 h and fixed
in 4% paraformaldehyde solution for 15 min at room temperature.
Following 3 washes in PBS, non-specific binding was blocked in PBS
with 5% BSA for 1 h at room temperature, and the samples were then
incubated with rabbit anti-GABAAR pi primary antibody (3.6
µg/ml, ab26055; Abcam) at 4°C overnight. Following 3 washes
in PBS, the cells were incubated with DyLight 594-conjugated goat
anti-rabbit IgG (H+L) (A23420; Abbkine, Redlands, CA, USA) at 37°C
for 1 h, and the nuclei were stained with DAPI staining solution
(c1005; Beyotime Institute of Biotechnology) for 5 min. Samples
were viewed under a confocal laser scanning microscope (Leica,
Heidelberg, Germany). The primary antibody was replaced with normal
rabbit IgG (2 µg/ml IgG) instead of anti-GABAAR pi primary
antibody to serve as the negative controls.
CCK-8 cell proliferation assay
The effects of GABA, Muscimol and S106 on the
proliferation of the Tca8113 cells were determined using the CCK-8
reagent assay (Dojindo Molecular Technologies, Inc.). Briefly,
5×103 cells/well were grown in 96-well plates with the
addition of GABA or GABA A type receptor agonist or antagonist and
the treatment groups were as described above. Follwoing treatment
for 24 and 48 h, the appropriate amount of CCK-8 reagent (1:10,
v/v) was added to each well, and the cells were further incubated
at 37°C for 1–4 h separately. The absorbance at a wavelength of 450
nm was measured using an enzyme-labeled instrument (ELx800;
high-speed 8-channel filter-based absorbance; Chongqing Key
Laboratory of Oral Diseases and Biomedical Sciences, Chongqing,
China).
Flow cytometric analysis of cell cycle
distribution and apoptosis
The effects of GABA, Muscimol and S106 on cell cycle
distribution and the apoptosis of Tca8113 cells were measured by
flow cytometric analysis. The treatment groups (cells treated with
GABA or GABA A type receptor agonist or antagonist) were as
described above. Cells were collected by trypsinization, washed
twice in PBS at 24 and 48 h and an aliquot of the cells was then
fixed in ice-cold 70% ethanol for a minimum overnight incubation,
and other aliquots were re-suspended in PBS. The cell cycle
distribution and apoptosis were analyzed using a flow cytometer
(FACSVantage SE; BD Biosciences, San Jose, CA, USA).
Statistical analyses
Each experiment was performed at least 3 times.
Analysis of variance (ANOVA) was performed and the results are
presented as the means ± standard deviation. Differences between
the mean values of the individual groups were assessed by one-way
ANOVA and Duncan's multiple range tests. The SPSS13.0 statistical
software was used to perform all statistical analyses. Values of
P<0.05 were considered to indicate statistically significant
differences and values of P<0.01 were considered to indicate
highly statistically significant differences.
Results
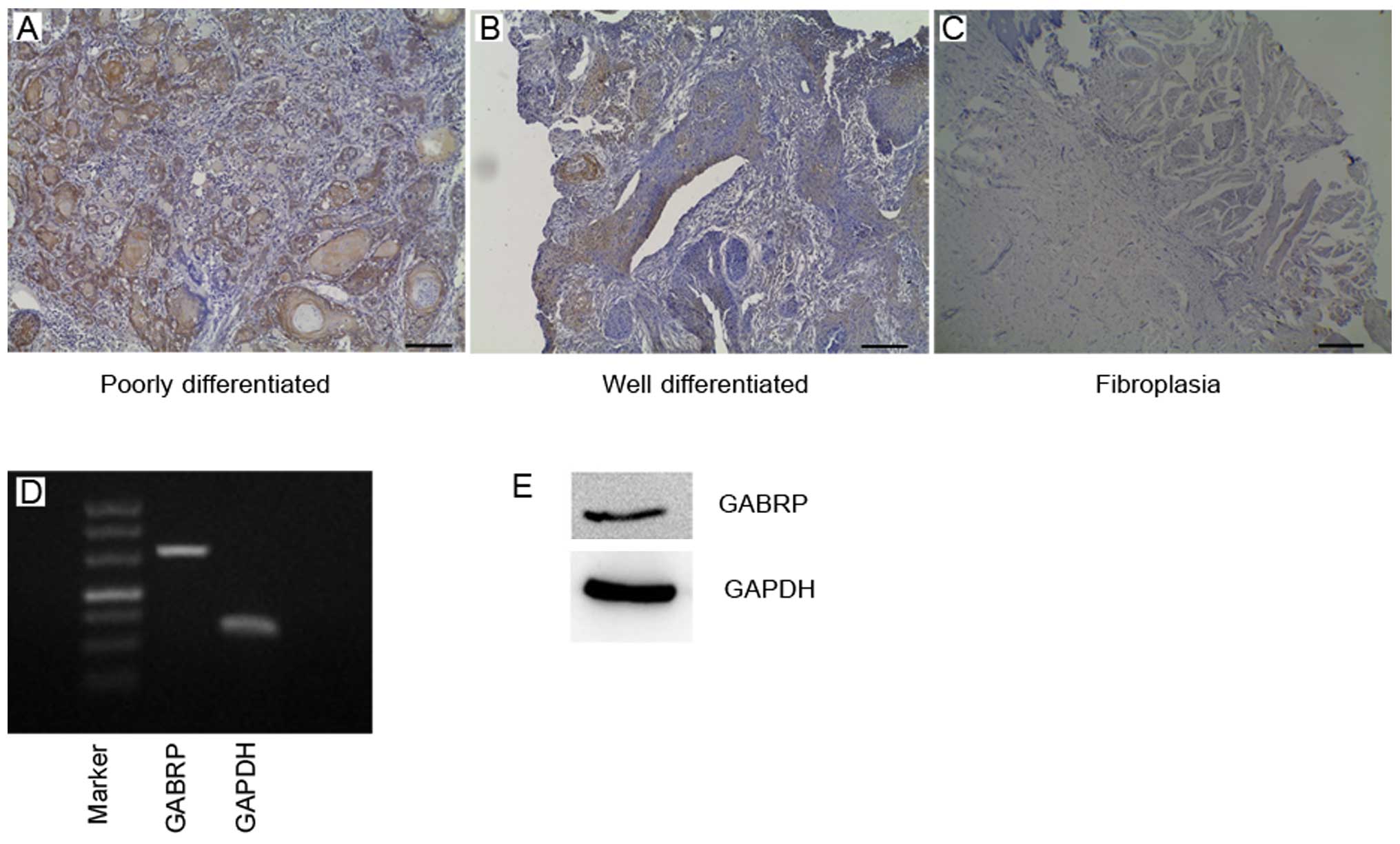
In this study, we analyzed GABRP expression in OSCC
tissues using specific antibody (Fig.
1A–C). We found that GABRP was strongly located in the
cytoplasm of poorly differentiated tumor tissue cells, particularly
in the keratin pearl and also exhibited positive staining in well
differentiated tissue cells in the cytoplasm. In benign
fibroplastic tongue tissue, GABRP expression was observed in the
cell cytoplasm with a lower expression level compared with the
poorly differentiated OSCC tissues. These results raise the
question of whether GABA expression is associated with OSCC.
RT-PCR and western blot analysis revealed that GABRP
was expressed at the mRNA and protein level in the OSCC Tca8113
cell line (Fig. 1D and E). The
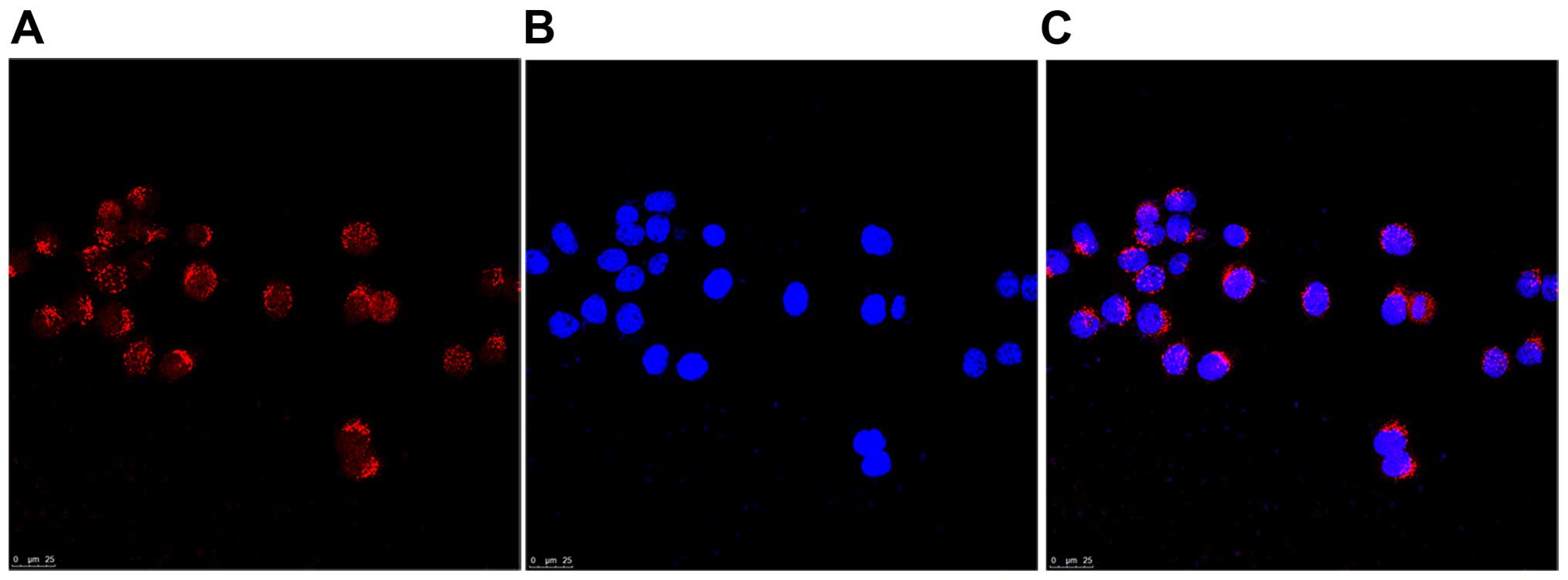
results of immunofluorescence staining revealed that GABRP was
located in the cytoplasm of Tca8113 cells (Fig. 2). Thus, the Tca8113 cell line was
correctly used in this study to examine the function of GABA in
vitro.
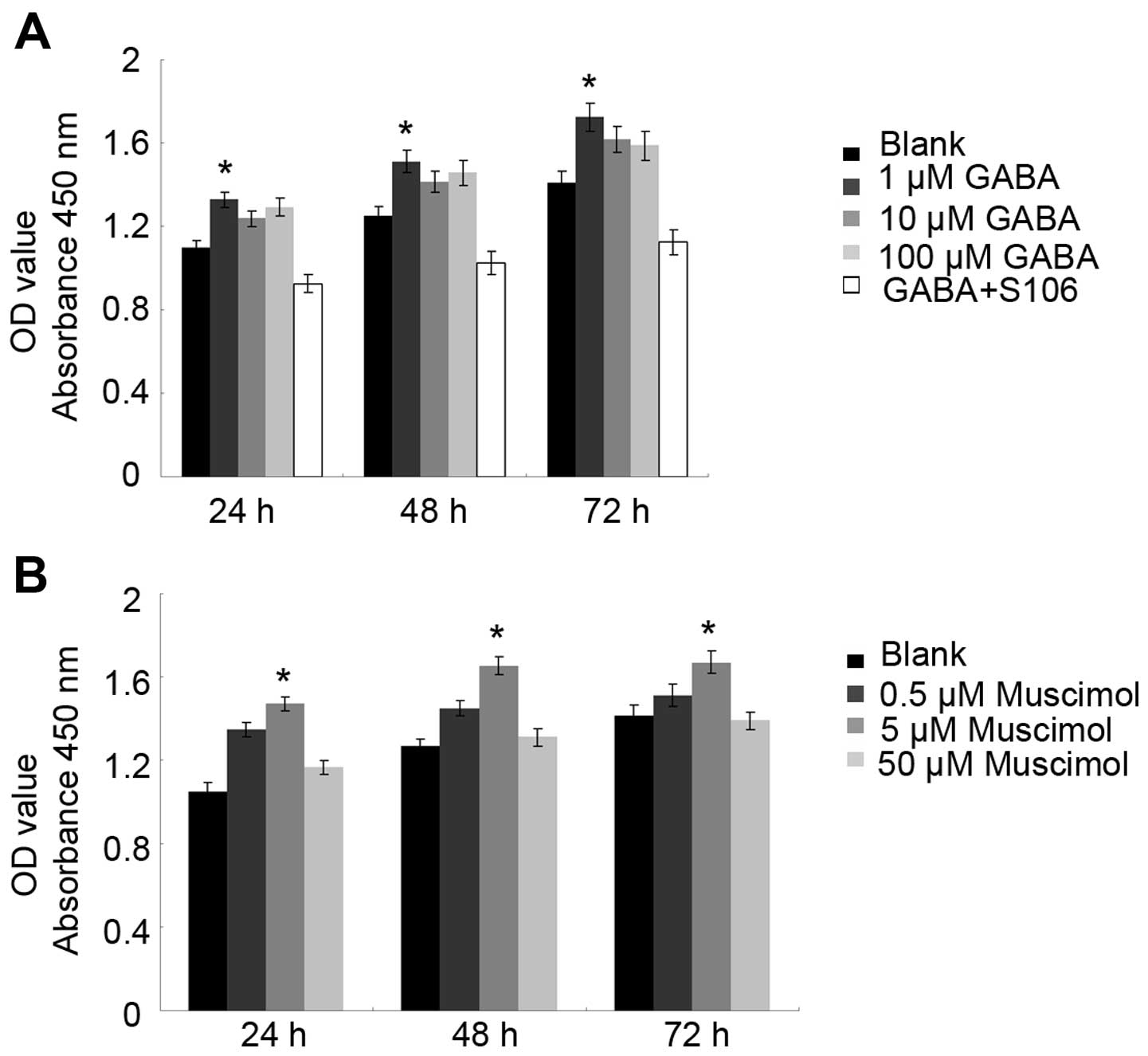
CCK-8 is a non-radioactive colorimetric assay used
to measure cell proliferation or toxicity with a high sensitivity.
The excessive proliferation of cells promotes tumor development. It
has been reported that GABA expression is associated with tumor
cell proliferation through its A receptor in neuronal or
non-neuronal cancer (18). In
this study, to measure the effects of GABA and the GABA A type
receptor on Tca8113 cell proliferation, we treated the cells with
GABA or Muscimol for 24 and 48 h. We found that treatment with 1
µM GABA or 5 µM Muscimol promoted Tca8113 cell
proliferation at 24 and 48 h. To confirm the importance of the GABA
A type receptor in the observed effects of GABA on Tca8113 cell
proliferation, we used S106, which is an antagonist of the GABA A
type receptor, to treat the Tca8113 cells in advance and then
re-treated the cells with 1 µM GABA. We found that the
proliferative effects of 1 µM GABA were blocked by treatment
with 50 µM S106 (Fig. 3;
P<0.05), suggesting that GABA promotes Tca8113 cell
proliferation through its A type receptor.
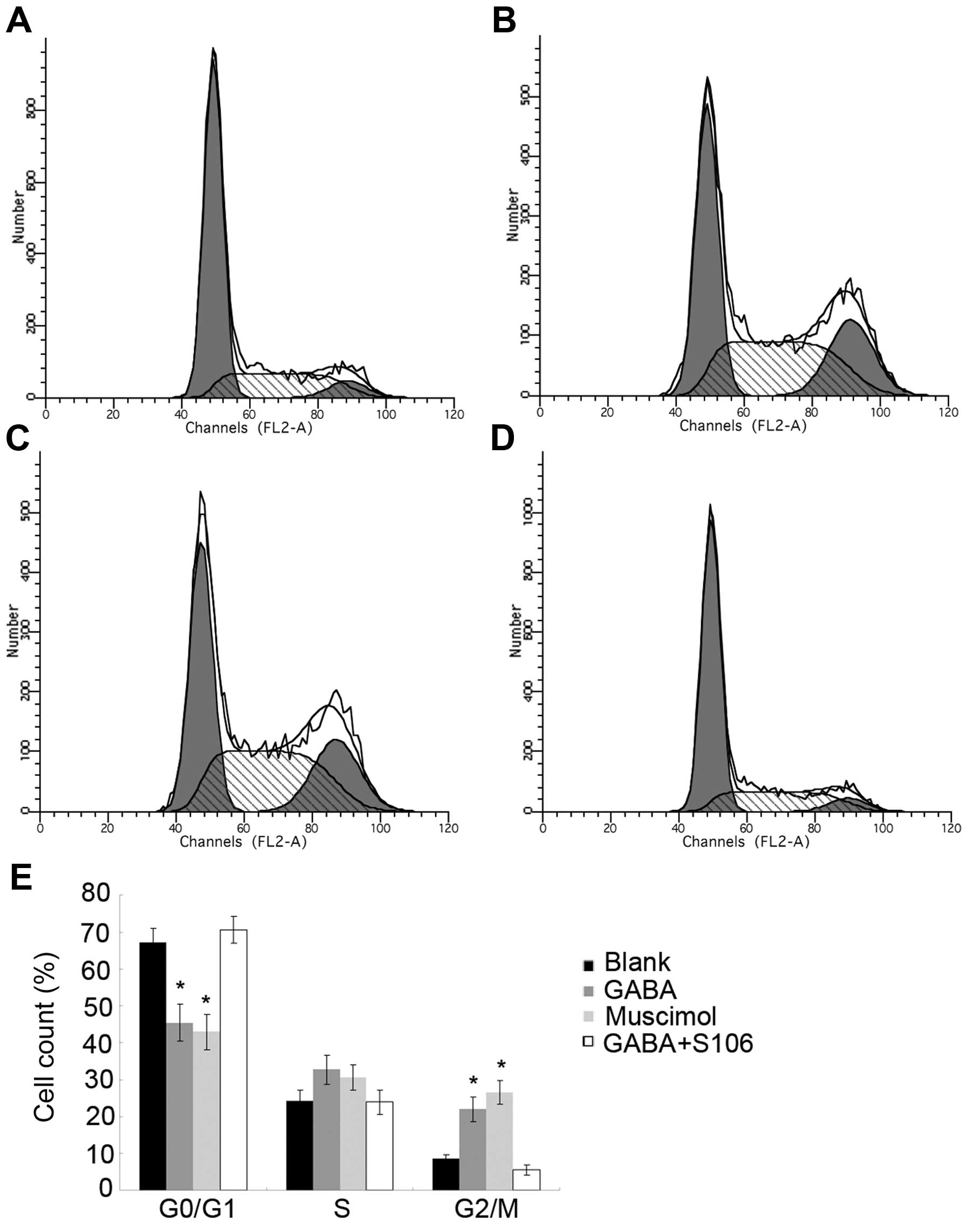
The cell cycle is tightly regulated at two
particular checkpoints, the G1-S and G2-M phases. Interestingly, we
found that treatment with 1 µM GABA or 5 µM Muscimol
significantly arrested the Tca8113 cells at the G2/M phase and
shortened the G0/G1 phase at 48 h. To confirm the importance of the
GABA A type receptor in the observed effects of GABA on Tca8113
cell cycle distribution, we used S106, which is an antagonist of
the GABA A type receptor, to treat the Tca8113 cell in advance and
then re-treated the cells with 1 µM GABA. We found that the
effects of 1 µM GABA on cycle distribution on the Tca8113
cells were blocked by treatment with 50 µM S106 (Fig. 4; P<0.05), suggesting that GABA
disrupts the Tca8113 cell cycle distribution through its A type
receptor.
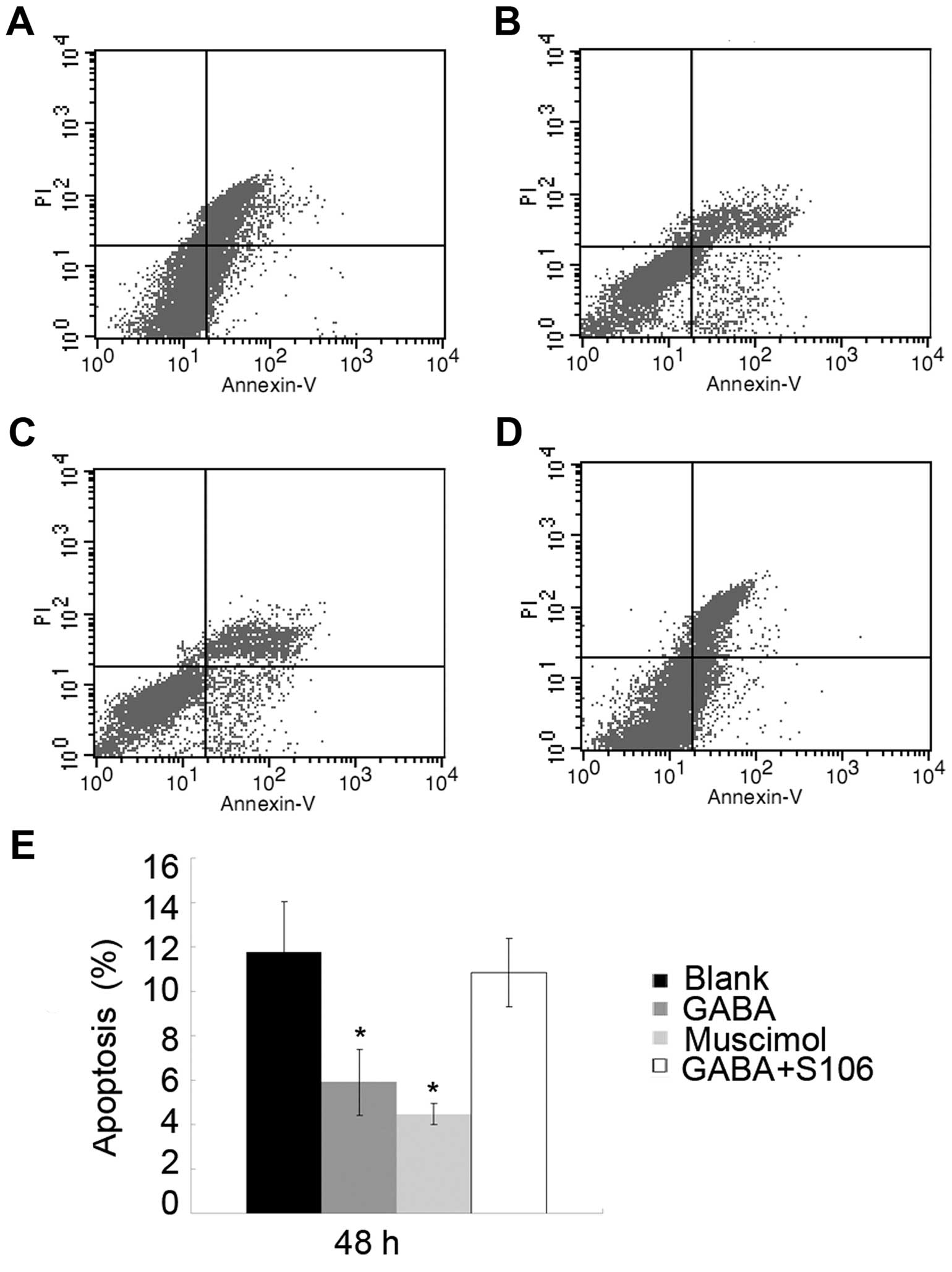
The apoptotic rate of Tca8113 cells was notably
decreased following the addition of 1 or 5 µM Muscimol to
the culture medium. To determine the importance of the GABA A type
receptor in the observed effects of GABA on Tca8113 cell
apop-tosis, we used S106, which is an antagonist of the GABA A type
receptor, to treat the Tca8113 cells in advance and then re-treated
the cells with 1 µM GABA. We found that the effects of 1
µM GABA on the apoptosis of Tca8113 cells were blocked by
treatment with 50 µM S106 (Fig. 5; P<0.05), suggesting that GABA
inhibits Tca8113 cell apoptosis through its A type receptor.
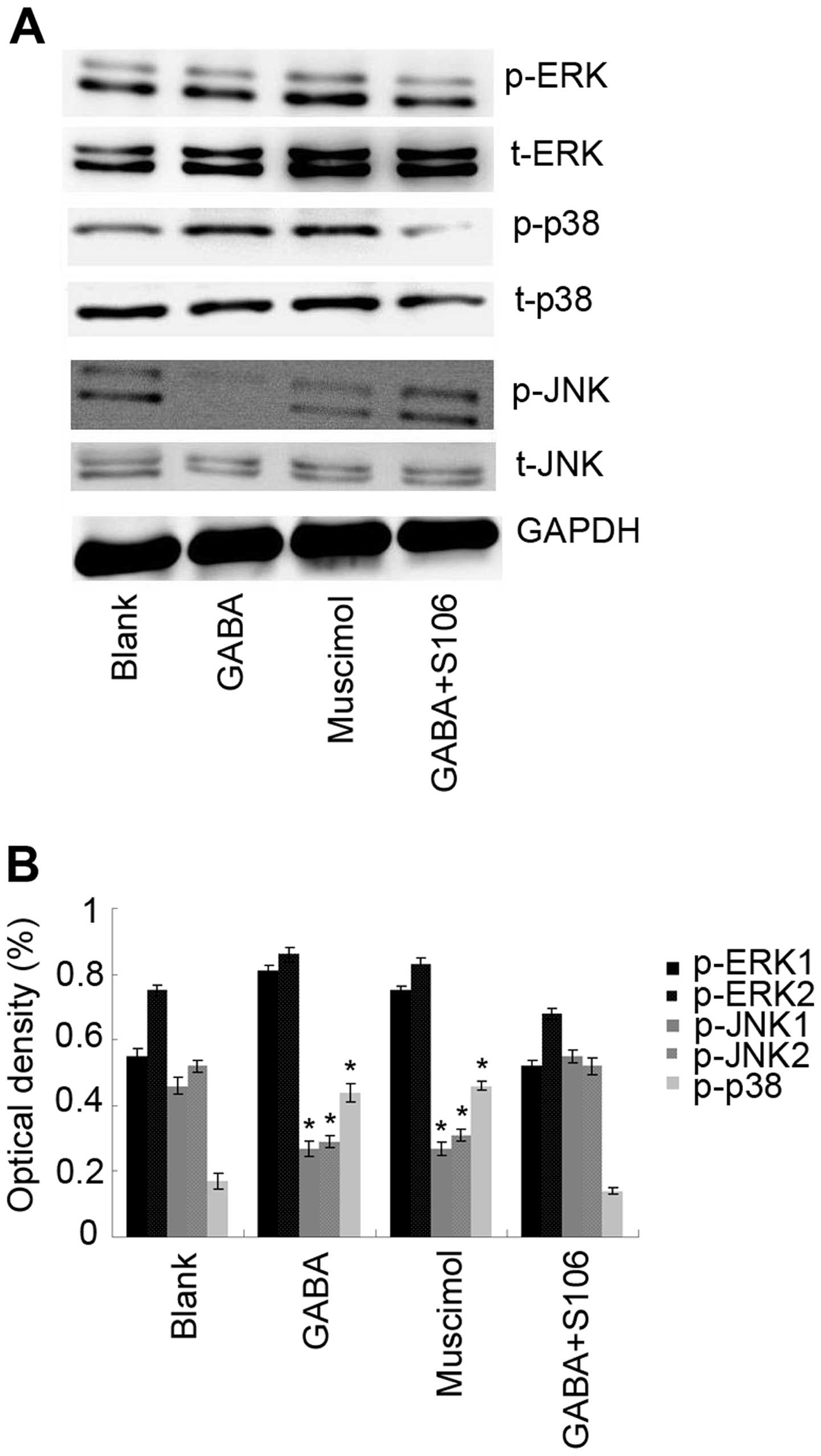
The MAPK signal transduction pathways are
evolutionarily conserved and are related to cell growth or survival
(20). Thus, in this study, the
expression and activation of MAPKs were examined by western blot
analysis using specific antibodies. To examine the activity of ERK,
p38 and JNK, phosphorylated ERK1/2 (p-ERK1/2), phosphorylated p38
(p-p38) and phos-phorylated JNK1/2 (p-JNK1/2), specific antibodies
were used as described in the Materials and methods. As a control,
total ERK1/2, p38 and JNK1/2 protein was analyzed. GAPDH was used
as a loading control. The results of western blot analysis revealed
that treatment with 1 µM GABA or 5 µM Muscimol
increased the levels of phosphorylated p38, whereas it decreased
those of JNK1/2 compared with the blank (untreated) control.
However, to determine the importance of the GABA A type receptor in
the observed effects of GABA on the levels of phosphorylated
kinases, we used S106, which is an antagonist of the GABA A type
receptor, to treat the cells in advance and then re-treated the
cells with 1 µM GABA. We found that the effects of 1
µM GABA on the levels of phosphorylated p38 and JNK1/2 were
blocked by treatmetn with 50 µM S106 (Fig. 6; P<0.05), suggesting that GABA
interferes with the activation of MAPKs in Tca8113 cells through
its A type receptor.
Discussion
OSCC of the tongue is the sixth most common type of
human cancer worldwide, and >90% of oral malignancies are SCCs
(21). In this study, we found
that GABRP was expressed in benign tongue tissue samples, with a
lower expression level compared with the poorly differentiated OSCC
tissue samples. Previous studies have demonstrated that GABA
promotes the functions of cell proliferation and inhibits apoptosis
through its A type receptor in peripheral non-neuronal tissues
(22,23). These results prompted us to
investigate the association between GABA and the proliferation and
apoptosis of OSCC cells.
Among its three receptors (A, B and C), GABA A type
receptor has been extensively investigated with regard to its
complex functions. Approximately 21 types of subunits have been
found in the GABA A type receptor and the functional GABA A type
receptor has 5 subunits assembled into 5 dimers. The αβγ subunits
widely exist in a variety of 5 dimers. The pi subunit which is
abundant in various peripheral tissues plays an important role in
the functions of GABA A type receptor (24,25). Moreover, it has been demonstrated
that the expression of GABA is significantly increased in
neoplastic tissues, such as in colorectal carcinoma, hepatocellular
carcinoma and gastric cancer, as compared with normal tissues
(18,26). Therefore, we have reason to
speculate that GABA plays a role in on tongue cancer cell
proliferation. CCK-8 assay revealed that treatment with 1 µM
GABA or 5 µM Muscimol promoted Tca8113 cell proliferation
and arrested the cells in the G2/M phase effectively. Flow
cytometry was also used to determine whether GABA affects cell
apoptosis. We found that the apoptosis of the Tca8113 cells was
inhibited by treatment with 1 µM GABA or 5 µM
Muscimol. To confirm the role of the GABA A type receptor in the
observed effects of GABA on Tca8113 cell apoptosis, we used S106,
which is an antagonist of the GABA A type receptor, to treat the
Tca8113 cells in advance and then re-treated the cells with 1
µM GABA. We found that the anti-apoptotic effects of 1
µM GABA were blocked by treatment with 50 µM S106,
suggesting that GABA inhibits Tca8113 cell apoptosis through its A
type receptor. The examination of the proliferation and apoptosis
of Tca8113 cells confirmed that a lower concentration of GABA and a
middle concentration of Muscimol group promoted cell proliferation
effectively but at present, we are unable to explain this
phenomenon.
The MAPK signal transduction pathways are stimulated
by the binding of mitogens, hormones, or neurotransmitters to
receptor tyrosine kinases, and the transduction of exogenous
signals is achieved through sequential phosphorylation, regulating
gene expression and cell proliferation, differentiation and
apoptosis. The kinases, MAPK kinase kinases (MAPKKKs), can be
phosphorylated by mall G-proteins leading to double phosphorylation
and activation of downstream MAPKKs (27,28). A recent study indicated that MAPK
activation is an essential function of many anticancer drugs which
induce tumor cell apoptosis (20). ERK1 and ERK2 are two key
transducers of proliferation, differentiation and survival signals
which exist in almost all tissues, mainly affecting the cells by
G1-to S-phase progression. p38 MAPK activitation induces cell
proliferation in the majority of cancers, arresting cells at the
G2/M phase. There are some interactions between the JNK isoforms
and p38 MAPK (27). It has been
shown that JNK1 and JNK2 activation, and p38 MAPK inhibition are
involved in cisplatin-induced cell death (28). OSCC is one of the 10 most common
human malignant tumors, accouting for >80% of oral and
maxillofacial malignant tumors (29,30). Various factors, such as growth
factors, protein kinases and inflammatory mediators contribute to
tumor progression and the process may involve crosstalk with
different signaling pathways (31–35). Theocharis et al reported
ERK expression and activation in 47 mobile tongue SCC tissue
samples and associations with clinicopathological parameters and
patient survival (36). In this
study, we found that treatment with GABA or Muscimol activates p38
MAPK, but inhibits JNK MAPK. Our results demonstrated that
treatment with GABA or Muscimol inhibited the apoptosis of Tca8113
cells, promoted proliferation and arrested the cells in the G2/M
phase effectively, as shown by flow cytometry. Our results are
consistent with those of studies on the role of the MAPK signaling
pathway in tumors (37).
As the major inhibitory neurotransmitter in the
adult mammalian CNS, drugs acting upon receptors of GABA and GABA
'mimetics' (e.g., gabapentin) are used to treat patients with
cancer. There is evidence indicating that the deregulation of the
GABA system can cause or contribute to the onset of cancers, such
as pancreatic ductal adenocarcinoma and gastric carcinoma (19). However, the deregulation of the
GABA system has potential carcinogenic effects remains unclear.
However, the findings of our study suggest the necessity to proceed
with caution when using GABAergic drugs and GABA 'mimetics' in
patients with tongue cancer.
In conclusion, our study provide some lines of
evidence that GABA is actively involved in promoting OSCC Tca8113
cell proliferation and suppressing apoptosis through GABA A type
receptors via the activation of p38 MAPK, but the inhibition of the
JNK MAPK signaling pathways. This may confirm our concerns
regarding the use of GABAergic drugs and GABA 'mimetics' in
patients with tongue cancer.
Acknowledgments
This study was supported by grants from the National
Natural Science Foundation of China (no. 31301021), Program for
Innovation Team Building at Institutions of Higher Education in
Chongqing in 2013 and Chongqing Municipal Key Laboratory of Oral
Biomedical Engineering of Higher Education.
References
|
1
|
Blot WJ, McLaughlin JK, Winn DM, Austin
DF, Greenberg RS, Preston-Martin S, Bernstein L, Schoenberg JB,
Stemhagen A and Fraumeni JF Jr: Smoking and drinking in relation to
oral and pharyngeal cancer. Cancer Res. 48:3282–3287.
1988.PubMed/NCBI
|
|
2
|
Rentoft M, Coates PJ, Loljung L, Wilms T,
Laurell G and Nylander K: Expression of CXCL10 is associated with
response to radiotherapy and overall survival in squamous cell
carcinoma of the tongue. Tumour Biol. 35:4191–4198. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fu X and Feng Y: QKI-5 suppresses cyclin
D1 expression and proliferation of oral squamous cell carcinoma
cells via MAPK signalling pathway. Int J Oral Maxillofac Surg.
44:562–7. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ntziachristos P, Lim JS, Sage J and
Aifantis I: From fly wings to targeted cancer therapies: a
centennial for notch signaling. Cancer Cell. 25:318–334. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kamran MZ, Patil P and Gude RP: Role of
STAT3 in cancer metastasis and translational advances. Biomed Res
Int. 2013:4218212013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fujii R, Imanishi Y, Shibata K, Sakai N,
Sakamoto K, Shigetomi S, Habu N, Otsuka K, Sato Y, Watanabe Y, et
al: Restoration of E-cadherin expression by selective Cox-2
inhibition and the clinical relevance of the
epithelial-to-mesenchymal transition in head and neck squamous cell
carcinoma. J Exp Clin Cancer Res. 33:40–52. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dalianis T: Human papillomavirus and
oropharyngeal cancer, the epidemics, and significance of additional
clinical biomarkers for prediction of response to therapy (Review).
Int J Oncol. 44:1799–1805. 2014.PubMed/NCBI
|
|
8
|
Nagata M, Wada K, Nakajima A, Nakajima N,
Kusayama M, Masuda T, Iida S, Okura M, Kogo M and Kamisaki Y: Role
of myeloid cell leukemia-1 in cell growth of squamous cell
carcinoma. J Pharmacol Sci. 110:344–353. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Watanabe M, Maemura K, Kanbara K, Tamayama
T and Hayasaki H: GABA and GABA receptors in the central nervous
system and other organs. Int Rev Cytol. 213:1–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Irwin RP and Allen CN: GABAergic signaling
induces divergent neuronal Ca2+ responses in the
suprachiasmatic nucleus network. Eur J Neurosci. 30:1462–1475.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Al-Wadei HA, Plummer HK III and Schuller
HM: Nicotine stimulates pancreatic cancer xenografts by systemic
increase in stress neurotransmitters and suppression of the
inhibitory neurotransmitter gamma-aminobutyric acid.
Carcinogenesis. 30:506–511. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Deng Y, Wang W, Yu P, Xi Z, Xu L, Li X and
He N: Comparison of taurine, GABA, Glu, and Asp as scavengers of
malondialdehyde in vitro and in vivo. Nanoscale Res Lett.
8:1902013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ortega A: A new role for GABA: inhibition
of tumor cell migration. Trends Pharmacol Sci. 24:151–154. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Macdonald RL and Olsen RW: GABAA receptor
channels. Annu Rev Neurosci. 17:569–602. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fujii E and Mellon SH: Regulation of
uterine gamma-amino-butyric acid(A) receptor subunit expression
throughout pregnancy. Endocrinology. 142:1770–1777. 2001.PubMed/NCBI
|
|
16
|
Follesa P, Serra M, Cagetti E, Pisu MG,
Porta S, Floris S, Massa F, Sanna E and Biggio G: Allopregnanolone
synthesis in cerebellar granule cells: roles in regulation of
GABA(A) receptor expression and function during progesterone
treatment and withdrawal. Mol Pharmacol. 57:1262–1270.
2000.PubMed/NCBI
|
|
17
|
Majewska MD and Vaupel DB: Steroid control
of uterine motility via gamma-aminobutyric acidA receptors in the
rabbit: a novel mechanism? J Endocrinol. 131:427–434. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Maemura K, Shiraishi N, Sakagami K,
Kawakami K, Inoue T, Murano M, Watanabe M and Otsuki Y:
Proliferative effects of gamma-aminobutyric acid on the gastric
cancer cell line are associated with extracellular signal-regulated
kinase 1/2 activation. J Gastroenterol Hepatol. 24:688–696. 2009.
View Article : Google Scholar
|
|
19
|
Lee SK, Dawson J, Lee JA, Osman G, Levitin
MO, Guzel RM and Djamgoz MB: Management of cancer pain: wider
implications of orthodox analgesics. Int J Gen Med. 7:49–58.
2014.
|
|
20
|
Yuan L, Wang J, Xiao H, Wu W, Wang Y and
Liu X: MAPK signaling pathways regulate mitochondrial-mediated
apoptosis induced by isoorientin in human hepatoblastoma cancer
cells. Food Chem Toxicol. 53:62–68. 2013. View Article : Google Scholar
|
|
21
|
Ferrari D, Codecà C, Fiore J, Moneghini L,
Bosari S and Foa P: Biomolecular markers in cancer of the tongue.
2009:4129082009.
|
|
22
|
Tamayama T, Maemura K, Kanbara K, Hayasaki
H, Yabumoto Y, Yuasa M and Watanabe M: Expression of GABA(A) and
GABA(B) receptors in rat growth plate chondrocytes: activation of
the GABA receptors promotes proliferation of mouse chondrogenic
ATDC5 cells. Mol Cell Biochem. 273:117–126. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Takehara A, Hosokawa M, Eguchi H, Ohigashi
H, Ishikawa O, Nakamura Y and Nakagawa H: Gamma-aminobutyric acid
(GABA) stimulates pancreatic cancer growth through overexpressing
GABAA receptor pi subunit. Cancer Res. 67:9704–9712. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Neelands TR and Macdonald RL:
Incorporation of the pi subunit into functional gamma-aminobutyric
Acid(A) receptors. Mol Pharmacol. 56:598–610. 1999.PubMed/NCBI
|
|
25
|
Hedblom E and Kirkness EF: A novel class
of GABAA receptor subunit in tissues of the reproductive system. J
Biol Chem. 272:15346–15350. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang M, Gong Y, Assy N and Minuk GY:
Increased GABAergic activity inhibits alpha-fetoprotein mRNA
expression and the proliferative activity of the HepG2 human
hepatocellular carcinoma cell line. J Hepatol. 32:85–91. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lei YY, Wang WJ, Mei JH and Wang CL:
Mitogen-activated protein kinase signal transduction in solid
tumors. Asian Pac J Cancer Prev. 15:8539–8548. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cargnello M and Roux PP: Activation and
function of the MAPKs and their substrates, the MAPK-activated
protein kinases. Microbiol Mol Biol Rev. 75:50–83. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Warnakulasuriya S: Global epidemiology of
oral and oropharyngeal cancer. Oral Oncol. 45:309–316. 2009.
View Article : Google Scholar
|
|
30
|
Bitu CC, Kauppila JH, Bufalino A,
Nurmenniemi S, Teppo S, Keinänen M, Vilen ST, Lehenkari P, Nyberg
P, Coletta RD and Salo T: Cathepsin K is present in invasive oral
tongue squamous cell carcinoma in vivo and in vitro. PLoS One.
8:e709252013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Han X, Han Y, Jiao H and Jie Y: 14-3-3ζ
regulates immune response through Stat3 signaling in oral squamous
cell carcinoma. Mol Cells. 38:112–21. 2014. View Article : Google Scholar
|
|
32
|
Yan M, Xu Q, Zhang P, Zhou XJ, Zhang ZY
and Chen WT: Correlation of NF-kappaB signal pathway with tumor
metastasis of human head and neck squamous cell carcinoma. BMC
Cancer. 10:437–443. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nariai Y, Mishima K, Yoshimura Y and
Sekine J: FAP-1 and NF-κB expressions in oral squamous cell
carcinoma as potential markers for chemo-radio sensitivity and
prognosis. Int J Oral Maxillofac Surg. 40:419–426. 2011. View Article : Google Scholar
|
|
34
|
Chen IC, Chiang WF, Huang HH, Chen PF,
Shen YY and Chiang HC: Role of SIRT1 in regulation of
epithelial-to-mesenchymal transition in oral squamous cell
carcinoma metastasis. Mol Cancer. 13:254–267. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ekshyyan O, Moore-Medlin TN, Raley MC,
Sonavane K, Rong X, Brodt MA, Abreo F, Alexander JS and Nathan CA:
Anti-lymphangiogenic properties of mTOR inhibitors in head and neck
squamous cell carcinoma experimental models. BMC Cancer.
13:320–329. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Theocharis S, Kotta-Loizou I, Klijanienko
J, Giaginis C, Alexandrou P, Dana E, Rodriguez J, Patsouris E and
Sastre-Garau X: Extracellular signal-regulated kinase (ERK)
expression and activation in mobile tongue squamous cell carcinoma:
associations with clinicopathological parameters and patients
survival. Tumour Biol. 35:6455–6465. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li Y, Lu X, Qi H, Li X, Xiao X and Gao J:
Ursolic acid induces apoptosis through mitochondrial intrinsic
pathway and suppression of ERK1/2 MAPK in HeLa cells. J Pharmacol
Sci. 125:202–210. 2014. View Article : Google Scholar : PubMed/NCBI
|