Introduction
Hyperthermia has been shown to be a potent
radiosensitizer. It has been suggested that the inhibition of DNA
repair is one of the sensitization mechanisms (1). Indeed, it has recently been shown
that BRCA2, a protein of the homologous recombination (HR)
(2) pathway is a target of
hyperthermia (3). Following
exposure to ionizing radiation, it can take several hours to
complete DNA damage repair. This depends on the repair mechanisms,
the error-prone NHEJ or the error-free HR pathway. These pathways
can compete with each other (4,5) in
the late S- or G2-phase. This is due to the fact that HR is only
active in the late S- and G2-phase, and not in the G0- or G1-phase.
As hyperthermia transiently degrades BRCA2, and thereby inhibits
the HR pathway, double-strand break (DSB) repair may be directed to
the error-prone NHEJ pathway (4,5).
In a previous study, using the comet assay and micronucleus test,
it was demonstrated that exposure to hyperthermia alone at 42–48°C
for 30–120 min induced genotoxic effects in human cells (6). With premature chromosome
condensation and fluorescence in situ hybridisation
(PCC-FISH) it was also previously shown that the combined exposure
to mild hyperthermia (1 h, 41°C) and ionizing radiation induced an
increase in the frequency of chromosomal translocations as compared
to exposure to radiation alone shortly following exposure (7).
The interaction between hyperthermia and ionizing
radiation most likely results from the inhibition of the repair of
radiation-induced DNA damage by exposure to heat (8,9).
Tomita (8) stated that when DNA
is not repaired or is misrepaired following combined exposure to
radiation and hyperthermia, cells undergo apoptosis or apoptosis is
induced. In a previous study, using gel electrophoresis combined
with a restriction digestion assay and Southern hybridisation, it
was demonstrated that hyperthermia increases the probability of DNA
DSBs being incorrectly rejoined (10). Non-rejoined, as well as
misrejoined DSBs are potentially lethal for a cell. It has been
found that the amount of residual DNA damage studied with gel
electrophoresis (11) and
PCC-FISH (12) correlated with a
decrease in clonogenic survival following combined exposure to
hyperthermia and irradiation (11,12). Dewey et al (13) studied metaphases following
colcemid treatment, and showed that hyperthermia not only increased
the number of radiation-induced chromosomal breaks, but also the
frequency of chromosomal exchanges, and suggested that hyperthermia
may promote misrepair. Bergs et al (7), using PCC-FISH and foci analyses,
demonstrated that the increased induction of chromosomal
translocations following combined exposure to hyperthermia (1 h,
41°C) and ionizing radiation may be due to the inhibition of HR. In
this study, an increased frequency of chromosomal translocations
was also observed in mutant cells deficient for RAD54, one of the
proteins involved in HR repair. Krawczyk et al (3) demonstrated that the inhibition of
recombination repair via the transient degradation of the BRCA2
protein lasts for only 6 h. All these studies were performed
shortly following exposure. It would therefore be of interest to
study the chromosomal aberration frequency following combined
exposure to hyperthermia followed by ionizing radiation beyond that
time period.
In the present study, the induction of chromosomal
damage by combined exposure to hyperthermia and radiation was
investigated at 24 h following exposure and compared with the
damage frequencies at 1 h following exposure in two different human
tumor cell lines (SW-1573 and RKO). To examine chromosomal
aberrations, the PCC-FISH technique was used, as previously
described (14). The advantage of
using the PCC technique is that chromosomal aberrations can be
examined shortly following exposure. Survival curves were analysed
according to the linear-quadratic (LQ) model S(D)/S(0) = exp − (αD
+ βD2), as previously described (15–19). Using the LQ model, changes in
potentially lethal damage repair (PLDR) can be determined
quantitatively by the analysis of the linear parameter α,
describing the low-dose range of the survival curve, separately
from the parameter β dominating the high-dose range (17,19). Furthermore, the effects of
hyperthermia on the BRCA2 protein and the induction of apoptosis
were examined as the possible mechanisms of radiosensitization.
Materials and methods
Cell culture
The human squamous lung carcinoma cell line,
SW-1573, was grown in Leibowitz-15 medium (Gibco-BRL Life
Technologies, Breda, The Netherlands) supplemented with 10% fetal
bovine serum (FBS) and 2 mM glutamine. The cells were maintained at
37°C in an incubator with humidified air without additional
CO2. The doubling time of these cells during exponential
growth is approximately 24 h (20,21). The human colon cancer cell line,
RKO, was grown in McCoy's 5A medium with 25 mM HEPES (Gibco-BRL
Life Technologies) supplemented with 10% FBS and 2 mM glutamine.
The cells were maintained at 37°C in an incubator with humidified
air supplemented with 5% CO2. Both cell lines (obtained
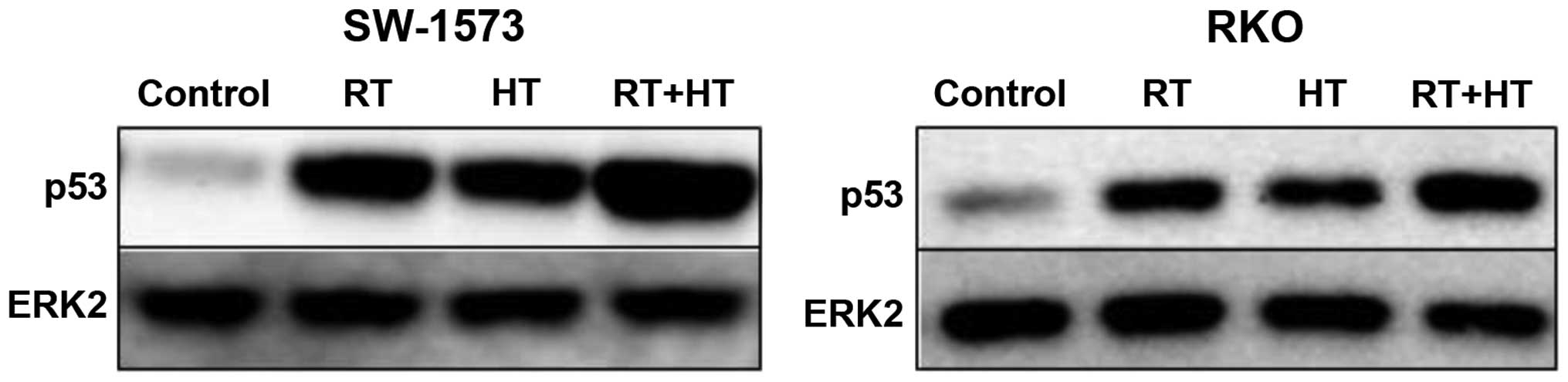
from ATCC, Wesel, Germany) express wild-type (wt) p53, as can be
observed in Fig. 1 which shows
the induction of p53 at 4 h following exposure to hyperthermia, 4
Gy radiation and combined exposure to hyperthermia and radiation.
The doubling time of these cells during exponential growth is
approximately 24 h (22). All
experiments were performed using confluent cell cultures. Confluent
cultures of RKO cells consisted of 31.2±4.7% cells in the
G0/G1-phase, 50.1±4.6% in the S-phase and 18.7±3.8% cells in the
G2/M-phase, as previously determined by BrdU labeling and flow
cytometry (22). Confluent
cultures of SW-1573 cells contained 39.6±4.7% cells in the
G0/G1-phase, 48.7±5.8% in the S-phase and 11.7±7.9% cells in the
G2/M-phase, as previously determined (23).
Clonogenic assay and the LQ model
Clonogenic assays using the SW-1573 and RKO cells
were conducted as previously described by Franken et al
(24). For radiation survival
curves, the cells were irradiated with or without prior exposure to
hyperthermia for 1 h at 41°C. To investigate PLDR, the cells were
plated immediately [immediately plated (ip)] and at 24 h [delayed
plated (dp)] following exposure. The correlation between
hyperthermia-induced radiosensitization and the induction of
chromosomal damage, and the correlation between PLDR and the
induction of chromosomal aberrations were investigated. Surviving
fractions [S(D)/S(0)] after radiation dose (D) were corrected for
the toxicity of hyperthermia alone and survival curves were
analysed to calculate values of the linear and quadratic parameters
α and β, using SPSS 14.0 statistical software (SPSS, Inc., Chicago,
IL, USA) by means of a fit of the data by weighted linear
regression, according to the LQ formula: S(D)/S(0) = exp − (αD +
βD2), as previously described (15–19). This equation provides a useful
description of experimentally determined survival curves. Parameter
α can be interpreted to represent damage induced by single track
lethal damage, and parameter β represents the frequency of
sublethal damage induced by two separate tracks. Potentially lethal
damage is assumed to represent a part of the term αD, as well of
βD2 (17,19). From the values of the LQ
parameters, the enhancement factors and the PLDR value are
calculated: α-EF, enhancement factor α for hyperthermia (ratio of
value of αht and α); β-EF, enhancement factor β for
hyperthermia (ratio of value of βht and β); PLDR-α,
potentially lethal damage repair α (ratio of value of
αip and αdp); PLDR-β potentially lethal
damage repair β (ratio of value of βip and
βdp).
Irradiation
Irradiation treatments were performed on confluent
cell cultures with single doses of γ-rays from a 137Cs
source at a dose rate of approximately 0.5 Gy/min. For clonogenic
assays, complete survival curves were obtained and the cells were
irradiated with a single dose of 0, 2, 4, 6 and 8 Gy. For
chromosomal aberration, apoptosis and cell cycle analysis, the
cells were irradiated with a single dose of 4 Gy.
Hyperthermia
The incubation of the cells at 41°C for 1 h was
performed by submerging the Petri dishes in a thermostatically
controlled waterbath (Lauda aqualine AL 12; Beun de Ronde, Abcoude,
The Netherlands) for 1 h. The temperature was checked in parallel
dishes and the desired temperature (±0.1°C) was reached in
approximately 5 min. The atmosphere of the waterbath was adjustable
by a connection with air and CO2 supplies. The RKO cells
were heated in a 5%CO2/95% air atmosphere with an air
inflow of 2 l/min. The SW-1573 cells do not require additional
CO2. In the case of combination experiments, the cells
were exposed to hyperthermia for 1 h immediately prior to
irradiation.
PCC-FISH and scoring of chromosomal
aberrations
To examine chromosomal fragments and translocations,
confluent cultures of SW-1573 and RKO cells were used. For the
induction of prematurely condensed chromosomes (PCCs), the cells
were incubated for 1 h with 80 nM of calyculin A at 1 h or at 24 h
following exposure to hyperthermia and/or radiation (14). Preparations were obtained by a
standard cytogenetic technique. The visualization of chromosomes
was accomplished by FISH. The chromosomal spreads were hybridized
to whole-chromosome FISH probes (Metasystems, Altlussheim,
Germany). In the SW-1573 cells, a probe for chromosome 2 was used.
The SW-1573 cells contain 3 intact copies of chromosome 2 and
contain approximately 7.8% of the genome (23,25). In the RKO cells, chromosome 18 was
studied as the cells contain 2 intact copies of this chromosome and
contain approximately 2.5% of the genome (22). The slides were counterstained with
a mixture (1:1, v/v) of 4′,6-diamidino-2-phenylindole (DAPI; 2.5
µg/ml) and anti-fade solution (Vectashield; Vector
Laboratories, Burlingame, CA, USA). The slides were examined under
a fluorescence microscope (Axioscop 2; Zeiss, Jena, Germany) with
suitable filter combinations (DAPI, FITC, Cy3 and a FITC/Cy3
combination) equipped with a Jenoptik (Jena, Germany)
ProgRes® MF CCD camera. Translocations and fragments of
painted chromosomes were scored as described earlier (7,26,27) using Isis 5.3 from MetaSystems
(Altlussheim, Germany). For a comparison of the number of
chromosomal aberrations using different chromosomes, corrections
were made for both the copy number and size of the chromosomes. In
the figures, the frequency of total translocations/cell and the
total number of fragments corrected for the complete genome is
presented.
Western blot analysis for p53 and
BRCA2
The controls and treated cells were harvested at
different time-points after treatment. Pellets were lysed in
ice-cold RIPA buffer (20 mM Tris-HCl, 150 mM NaCl, 1 mM
Na2 EDTA, 1% NP-40, 1% sodium deoxycholate, 2.5 mM
sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM
Na3VO4 and 1 µg/ml leupeptin) for 30
min on ice with protein inhibitors, as previously described
(28). Laemmli buffer with
2-mercap-toethanol (355 mM) was added to the supernatant (1:1) and
heated in boiling water for 2–5 min. Finally, the samples were
sonificated (Sonics and Materials, Inc., Newtown, CT, USA). Protein
(1 µg) was resolved by 10% SDS-PAGE precast gels (Bio-Rad
Laboratories, Hercules, CA, USA) and transferred onto PVDF
membranes. Equal protein loading was checked by Ponceau S staining
(28). Immunodetection was
performed for BRCA2 mAb (antibodies-online.com, lot: a115912) in combination
with a horseradish peroxidase-conjugated secondary anti-mouse IgG
(1031-05; Southern Biotech, Birmingham, AL, USA). The housekeeping
protein, ERK2, was detected using mAb (Bethyl Laboratories,
Montgomery, TX, USA) and a secondary anti-rabbit mAb (Invitrogen
Life Technologies, Carlsbad, CA, USA). All samples were enhanced
using chemoluminescence (Amersham Pharmacia Biotech, Piscataway,
NJ, USA). Finally, the blots were analysed using the LAS4000
biomolecular imager (GE Healthcare Healthcare Europe GmbH,
Eindhoven, The Netherlands).
Apoptosis
Apoptosis was examined in confluent cultures of RKO
and SW-1573 cells following 1 h of exposure at 41°C hyperthermia
alone, following exposure to radiation (4 Gy) alone, or following
combined exposure to hyperthermia and radiation using the method
described in the study by Riccardi and Nicoletti (29). At 1 and 24 h following exposure,
the cells were collected and pellets were resuspended in Nicoletti
buffer (0.1% w/v sodium citrate, 0.1% v/v Triton X-100, 50 mg/ml
propidium iodide in distilled water, pH 7.4). Analyses were carried
out using a flow cytometer (FACSCanto; Becton-Dickinson
Biosciences, San Jose, CA, USA).
Results
Effect of exposure to hyperthermia on the
number of chromosomal aberrations
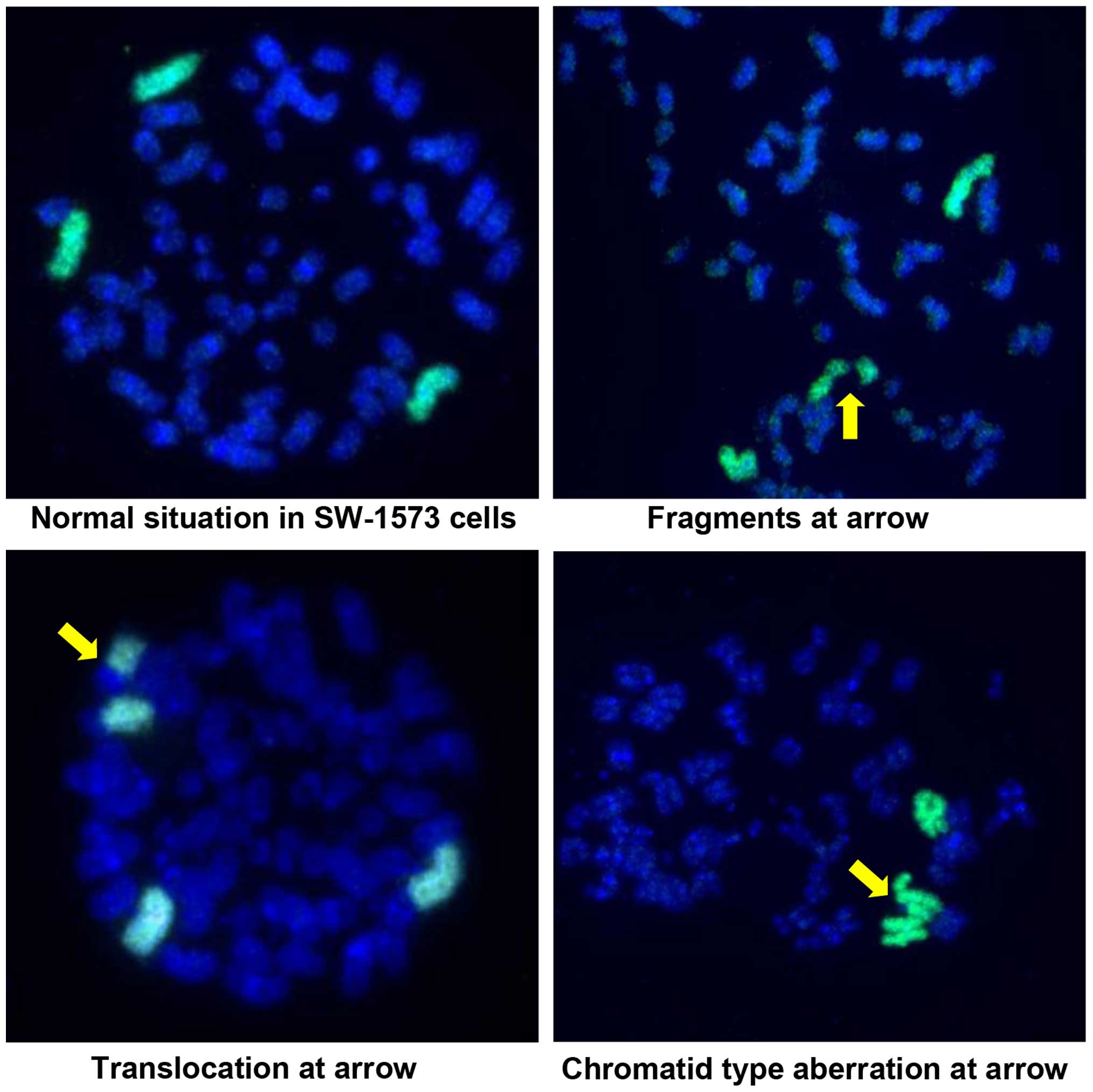
Images of examples of the studied PCCs are presented
in Fig. 2; however,
chromatid-type aberrations were rarely observed. The effects of
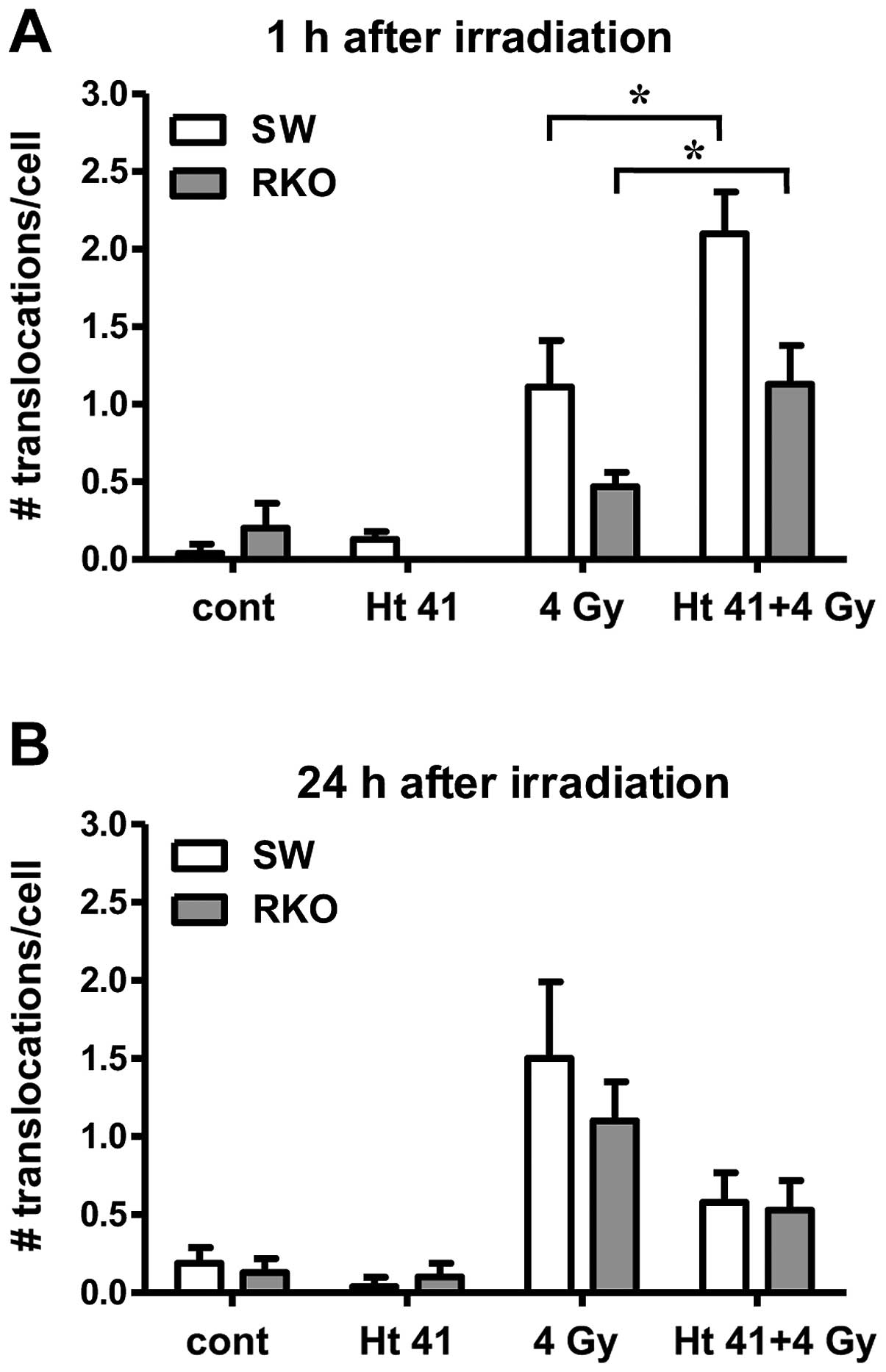
hyperthermia and radiation on the induction of chromosomal
translocations in the SW-1573 and RKO cells are presented in
Fig. 3. Exposure to hyperthermia
(41°C for 1 h) alone did not increase the number of translocations
at 1 or 24 h following exposure compared to the unexposed cells
(Fig. 3). At 1 h following
exposure to irradiation alone, a slight increase in the number of
translocations compared to the untreated samples was observed. More
chromosomal translocations were observed at 24 h than at 1 h after
irradiation. At 1 h following combined exposure to radiation and
hyperthermia, the number of translocations was higher than
following exposure to irradiation alone (Fig. 3A). However, at 24 h following
combined exposure, the number of translocations was lower than
after radiation alone (Fig.
3B).
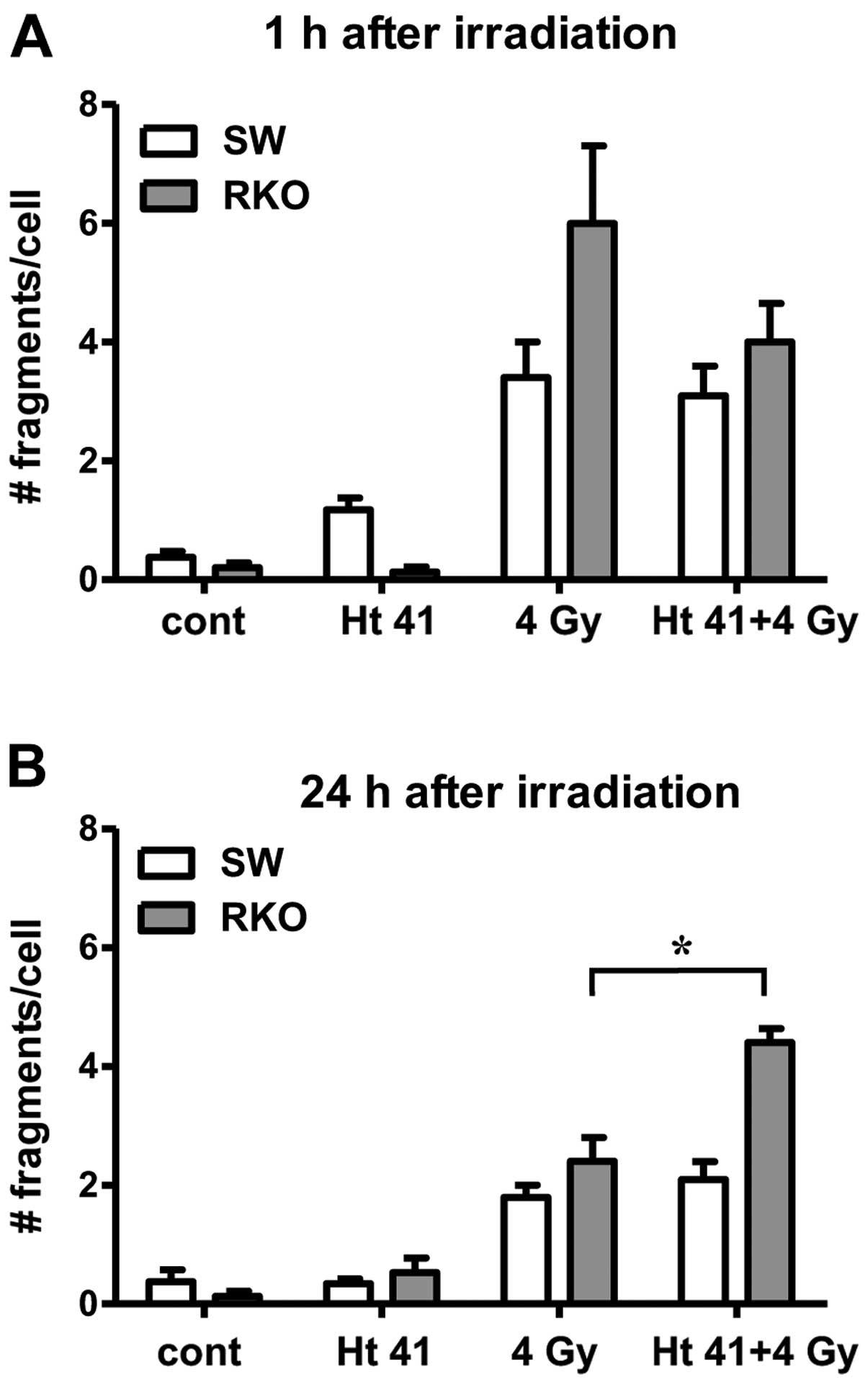
At 1 h following exposure to hyperthermia alone, in
the SW-1573 cells, an increase in the frequency of chromosomal
fragments as compared to the unexposed cells was observed (Fig. 4A). Exposure to radiation at 4 Gy
increased the number of chromosomal fragments in both cell lines
compared to the unexposed cells or exposure to hyperthermia alone
(Fig. 4A). In the RKO cells,
slightly more fragments were found than in the SW-1573 cells;
however, this difference was not significant at 1 h following
combined exposure or following exposure to radiation alone. In both
cell lines, no significant differences in the number of chromosomal
fragments were found following combined exposure to hyperthermia
and radiation, as compared to exposure to radiation alone (Fig. 4). At 24 h following exposure to 4
Gy radiation or combined exposure, the number of fragments was
lower than after 1 h in both cell lines. Following combined
exposure, significantly more fragments were found in the RKO cells
than in the SW-1573 cells (Fig.
4B). At 4 h following exposure, the results of chromosomal
aberrations did not differ from the results obtained at the 1 h
time-point (data not shown). However, the difference in the number
of translocations following combined exposure compared to exposure
to radiation alone was not significant.
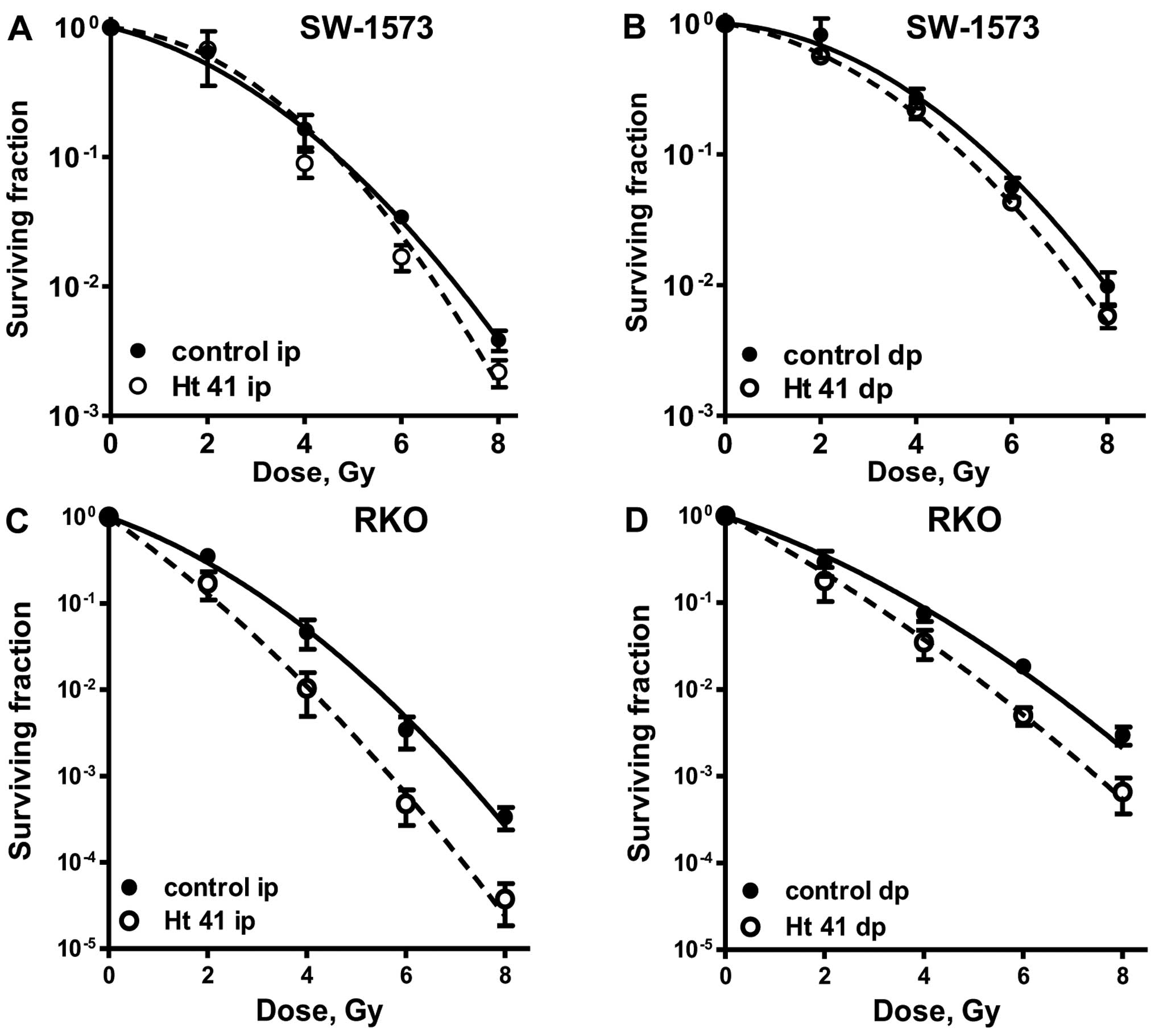
Effect of pre-exposure to hyperthermia on
clonogenic cell survival following the exposure of SW-1573 and RKO
cells to ionizing radiation
To determine whether exposure to mild hyperthermia
(41°C) can enhance cellular radiosensitivity, clonogenic assays
were conducted. Exposure of the cells to hyperthermia (1 h, 41°C)
alone had minimal effects and resulted in a surviving fraction of
0.8±0.4 in the ip cells and of 0.9±0.1 in dp cells for both cell
lines. In the SW-1573 cells (Fig. 5A
and B), exposure to hyperthermia at 41°C resulted in a modest
radiosensitization. This was due to an effect on the quadratic
parameter β (Table I).
Pre-exposure of the RKO cells to hyperthermia for 1 h at 41°C
significantly enhanced cellular radiosensitivity both in the ip and
dp cells (Fig. 5C and D).
Calculations with the LQ model showed that this was due to an
increase in both the linear parameter α and the quadratic parameter
β (Table I). The clonogenic
survival of the cells at 24 h following irradiation was higher than
the survival of the cells plated immediately after irradiation
(Fig. 5). This is most clear from
the values of PLDR, which for α are all higher than 1 and for some
of the β's where the PLDR values are higher than 1 (Table I).
 | Table IEffect of hyperthermia on LQ
parameters α and β, the hyperthermia α-enhancement factor and
β-enhancement factor and the PLDR-α and PLDR-β in SW-1573 and RKO
cells. |
Table I
Effect of hyperthermia on LQ
parameters α and β, the hyperthermia α-enhancement factor and
β-enhancement factor and the PLDR-α and PLDR-β in SW-1573 and RKO
cells.
| LQ parameter
|
|---|
| Cell line | α
(Gy−1) | β
(Gy−2) | α-EF | β-EF | PLDR-α | PLDR-β |
|---|
| SW-1573 ip | 0.21±0.02 | 0.06±0.02 | | | | |
| SW1573 41 ip | 0.06±0.02 | 0.11±0.03 | 0.3±0.2 | 1.8±0.8 | | |
| SW-1573 dp | 0.09±0.02 | 0.06±0.02 | | | 2.3±0.6 | 1.0±0.5 |
| SW-1573 41 dp | 0.05±0.02 | 0.08±0.02 | 0.6±0.2 | 1.3±0.5 | 1.2±1.1 | 1.4±0.5 |
| RKO ip | 0.55±0.09 | 0.02±0.01 | | | | |
| RKO 41 ip | 0.80±0.09 | 0.05±0.02 | 1.5±0.5 | 2.5±1.3 | | |
| RKO dp | 0.47±0.09 | 0.01±0.01 | | | 1.2±0.3 | 2.0±1.4 |
| RKO 41 dp | 0.64±0.11 | 0.05±0.02 | 1.4±0.4 | 5.0±2.5 | 1.3±0.3 | 1.0±0.2 |
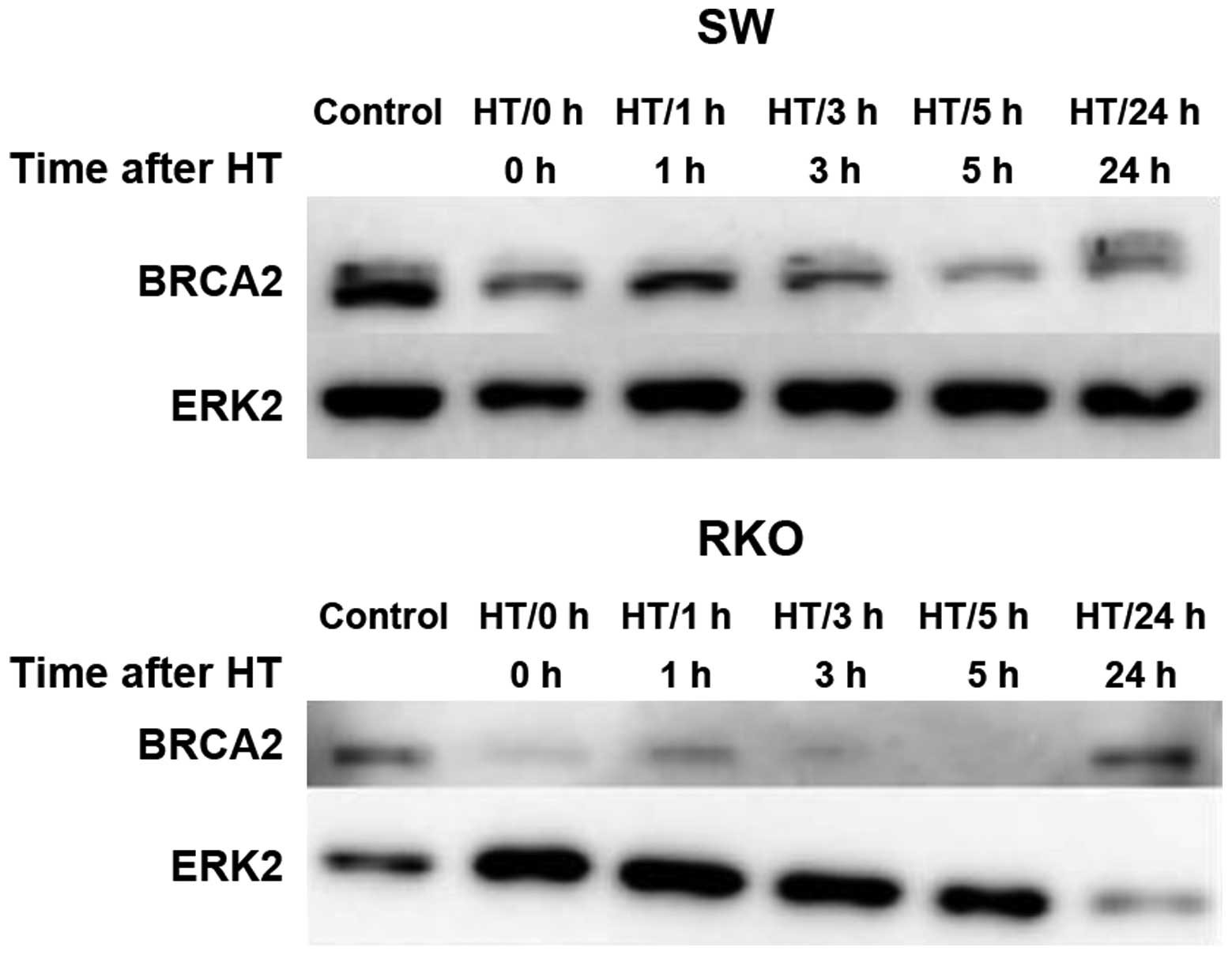
Effect of hyperthermia on BRCA2
The effect of hyperthermia on the BRCA2 levels were
examined by western blot analysis (Fig. 6). A clear decrease in the BRCA2
protein level was observed for up to 5 h following exposure to
hyperthermia. In the RKO cells, BRCA2 was almost completely
degraded, while in the SW-1573 cells, BRCA2 could still be
detected. However, in the SW-1573 cells, the BRCA2 levels following
exposure to hyperthermia were somewhat lower than in the unexposed
control cells. At 24 h, the BRCA2 levels returned levels similar to
those of the controls.
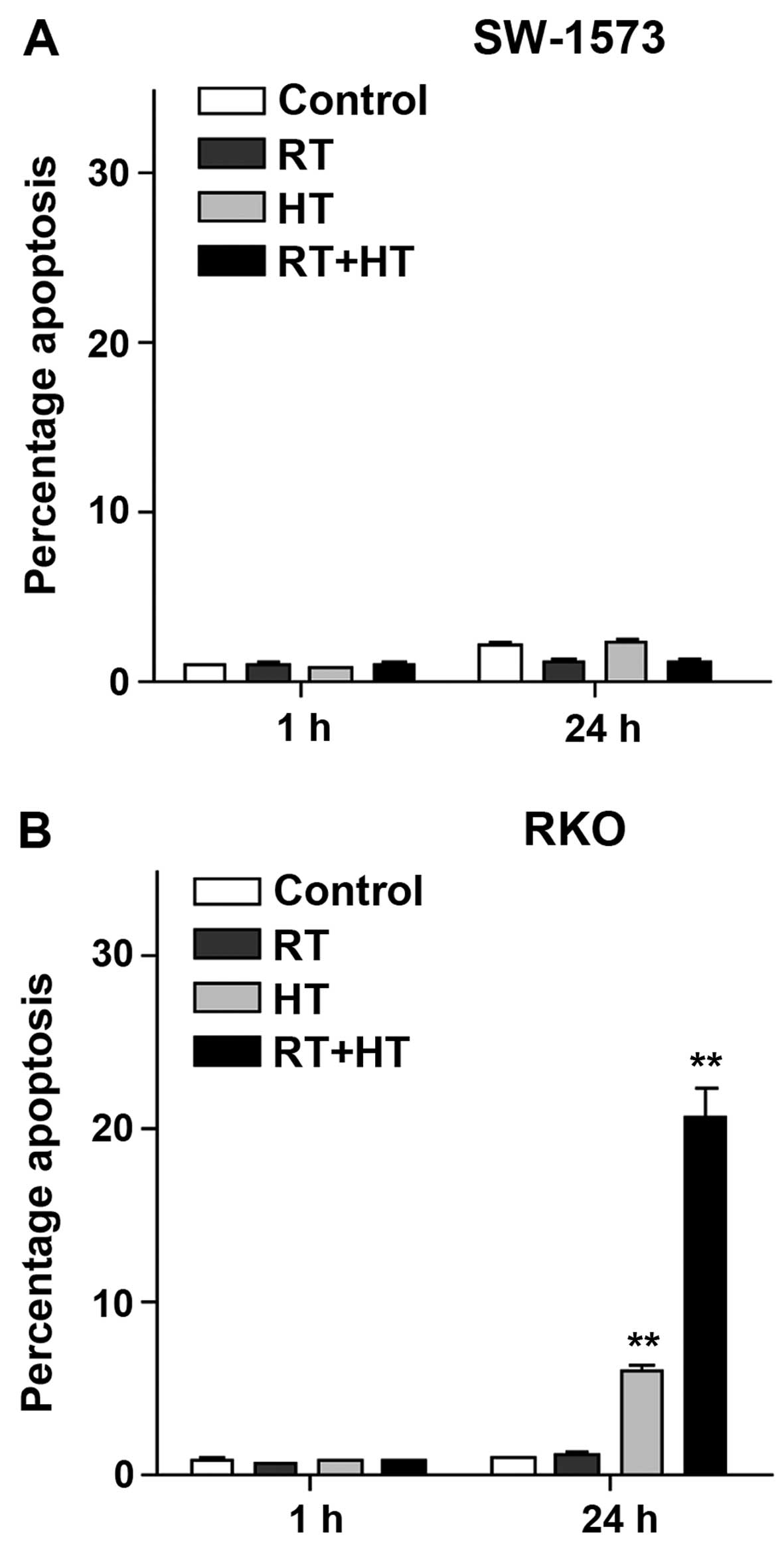
Induction of apoptosis following the
exposure of SW-1573 and RKO cells to hyperthermia and
radiation
Nicoletti assay was performed to explore the effects
of apoptosis following exposure to hyperthermia combined with
exposure to radiation. At 1 h after exposure, no apoptotic DNA
fragments were detected in both the RKO and SW-1573 cells (Fig. 7). In the RKO cells, exposure to
hyperthermia alone induced apoptosis in 5% of the population at 24
h after exposure (Fig. 7B).
Following combined exposure to hyperthermia and radiation,
apoptosis increased to approximately 20%. In the SW-1573 cells, on
the other hand, apoptosis hardly played a role in exposure-induced
cell death (Fig. 7A).
Discussion
Hyperthermia is a very potent radio- and
chemosensitizer, that can already be effective at mild temperatures
(30–36). Hyperthermia is therefore often
applied as adjuvant anticancer therapy (37). Heat may interfere with the normal
IR-induced signaling required for chromosomal DNA DSB repair, thus
resulting in increased cellular radiosensitivity (37). However, it has been described that
hyperthermia alone at 42–48°C for 30–120 min also induces genotoxic
effects in human A549 cells (6).
The increased frequency of micronuclei was only found following
exposure at 45°C or at higher temperatures. Bergs et al
(7) showed that pre-exposure to
mild hyperthermia increased the frequency of radiation-induced
chromosomal translocation.
In the present study, we showed that although
exposure to mild hyperthermia for 1 h at 41°C prior to exposure to
radiation increased the frequency of translocations at 1 h after
exposure, these genotoxic effects were not further enhanced at 24 h
after exposure. At this time-point, the frequency of translocations
following combined exposure was even lower than after 4 Gy alone.
Translocations result from the erroneous rejoining of chromosomal
fragments by DNA repair processes. It was suggested by El-Awady
et al (10) that
hyperthermia inhibits the rejoining of correct chromosome ends,
leading to an increased frequency of chromosomal fragments and
increases the probability of DNA DSB misrepair (10,12) shortly after exposure. That this
effect is not augmented at 24 h after exposure is in agreement with
the temporary hyperthermia-induced inhibition of HR which lasts for
up to 6 h (3). This corresponds
well with our observation in this study, that the BRCA2 protein
levels were decreased as compared to the control cells for up to 5
h after exposure. At 24 h after exposure, BRCA2 could be detected
again in both cell lines. In the SW-1573 cells, the effect of
hyperthermia on BRCA2 was less clear than in the RKO cells. The RKO
cells were radiosensitized by exposure to hyperthermia for 1 h at
41°C, and the SW-1573 cells were not radiosensitized. However, the
lower number of chromosomal translocations found at 24 h after
combined exposure may also be caused by cell death. As mainly G2
PCCs were studied, chromatid breaks were expected as well. These
were observed, although the numbers were insufficient to quantify.
This may be due to the PCC calyculin technique which made the
accurate determination of the aberration type not possible. This
was acknowledged earlier by Coco-Martin and Begg (38), who also studied chemically-induced
PCCs with FISH. Moreover, we also observed that exposure to
hyperthermia induced chromatin conformational changes, so that the
resolution became lower.
It has been demonstrated that hyperthermia has
differential effects on the LQ parameters of SW-1573 and RKO cells
(31,32). In the thermosensitive RKO cells
(31,32), hyperthermia clearly increased the
values of α and β, for cells plated immediately and delayed after
exposure. In the less thermosensitive SW-1573 cells (31,32) on the other hand, pre-exposure to
hyperthermia only increased the value of β. These findings indicate
that exposure to hyperthermia for 1 h at 41°C sensitizes RKO cells
at low and high radiation doses and that SW-1573 cells are only
sensitized after high doses of radiation (31,32). The ability of mild temperature (in
the range of 40–42°C) hyperthermia to increase the radiosensitivity
of human tumor cells has been shown to be cell line-dependent
(33). This effect is also
dependent on the capability of the cell to repair DNA damage
following combined exposure to hyperthermia and radiation (34,35). The relation between cell survival
and chromosomal aberrations following combined exposure remains
unclear, as only the RKO cells were radiosensitized at this
temperature and the dynamics of translocation frequencies are about
similar.
Furthermore, we showed that when the cells were
plated at 24 h after exposure, the survival levels were higher than
when the cells were plated directly after treatment. The repair of
potentially lethal damage after radiation, which is responsible for
the higher survival levels after delayed plating as compared to
immediate plating is a well-known phenomenon (17,39). This was also demonstrated by the
lower values of the LQ parameters after delayed plating as compared
to these values for immediately plated cells. The significantly
lower frequency of chromosomal fragments in both cell lines at 24 h
after irradiation alone as compared to 1 h after exposure
correlates well with the repair of potentially lethal damage.
However, following combined exposure, in the RKO cells, the
frequency of chromosomal fragments remained at the level found at 1
h after exposure. This may be due to the induction of apoptosis
which was not observed in the SW-1573 cells. In the SW-1573 cells,
no difference in fragment frequency was found between exposure to
radiation alone and combined exposure. From this it was concluded
that apoptosis, which may be induced by chromosomal damage or
depletion of the BRCA2 protein, is at least partly responsible for
the hyperthermia-induced radiosensitization of RKO cells. This was
also observed by Tomita (8) who
stated that when DNA is not repaired or is misrepaired following
combined exposure to radiation and hyperthermia, cells undergo
apoptosis. There remains however, a discrepancy between the
apoptosis and chromosomal aberration studies, as apoptosis can be
examined on the whole cell population, while chromosomal
aberrations using PCC cannot be performed on the S-phase cell
population.
Radiosensitization induced by exposure to
hyperthermia at a temperature of 41°C was observed in the RKO
cells, but was only very modest in the SW-1573 cells. Chromosomal
translocations were probably not responsible for this
sensitization, as the induction of translocations following
exposure to radiation alone and combined exposure followed the same
trend in both cell lines. This has been described earlier by Tucker
et al (40).
In conclusion, our findings indicate that the
increase in the translocation frequency observed at 1 h following
combined exposure to hyperthermia and radiation is not present at
24 h. Exposure to hyperthermia increased the death of RKO cells,
and this was at least partly due to apoptosis. The repair of
potentially lethal damage, as shown by the decrease in chromosomal
aberrations at 24 h as compared to 1 h after exposure is
responsible for the higher survival levels after delayed
plating.
Acknowledgments
We gratefully thank the Maurits and Anne de Cock and
the Nijbakker Morra Foundations for providing the fluorescence
microscopes with the software for scoring chromosomal aberrations.
The Stichting Vanderes and the Dutch Cancer Society (nos. UVA
2008-4019 and UVA 2012-5540) are acknowledged for personnel
financial support.
References
|
1
|
Oei AL, Vriend LEM, Crezee J, Franken NA
and Prawczyk PM: Effects of hyperthermia on DNA repair pathways:
one treatment to inhibit them all. Rad Onc. 10:652015.
|
|
2
|
Baeyens A, Thierens H, Claes K, Poppe B,
de Ridder L and Vral A: Chromosomal radiosensitivity in BRCA1 and
BRCA2 mutation carriers. Int J Radiat Biol. 80:745–756. 2004.
View Article : Google Scholar
|
|
3
|
Krawczyk PM, Eppink B, Essers J, Stap J,
Rodermond H, Odijk H, Zelensky A, van Bree C, Stalpers LJ, Buist
MR, et al: Mild hyperthermia inhibits homologous recombination,
induces BRCA2 degradation, and sensitizes cancer cells to poly
(ADP-ribose) polymerase-1 inhibition. Proc Natl Acad Sci USA.
108:9851–9856. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Allen C, Halbrook J and Nickoloff JA:
Interactive competition between homologous recombination and
non-homologous end joining. Mol Cancer Res. 1:913–920.
2003.PubMed/NCBI
|
|
5
|
Kass EM and Jasin M: Collaboration and
competition between DNA double-strand break repair pathways. FEBS
Lett. 584:3703–3708. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Speit G and Schütz P: Hyperthermia-induced
genotoxic effects in human A549 cells. Mutat Res. 747–748:1–5.
2013. View Article : Google Scholar
|
|
7
|
Bergs JW, Krawczyk PM, Borovski T, ten
Cate R, Rodermond HM, Stap J, Medema JP, Haveman J, Essers J, van
Bree C, et al: Inhibition of homologous recombination by
hyperthermia shunts early double strand break repair to
non-homologous end-joining. DNA Repair (Amst). 12:38–45. 2013.
View Article : Google Scholar
|
|
8
|
Tomita M: Involvement of DNA-PK and ATM in
radiation- and heat-induced DNA damage recognition and apoptotic
cell death. J Radiat Res (Tokyo). 51:493–501. 2010. View Article : Google Scholar
|
|
9
|
Kampinga HH, Dynlacht JR and Dikomey E:
Mechanism of radiosensitization by hyperthermia (> or = 43
degrees C) as derived from studies with DNA repair defective mutant
cell lines. Int J Hyperthermia. 20:131–139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
El-Awady RA, Dikomey E and Dahm-Daphi J:
Heat effects on DNA repair after ionising radiation: Hyperthermia
commonly increases the number of non-repaired double-strand breaks
and structural rearrangements. Nucleic Acids Res. 29:1960–1966.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dahm-Daphi J, Brammer I and Dikomey E:
Heat effects on the repair of DNA double-strand breaks in CHO
cells. Int J Radiat Biol. 72:171–179. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bergs JW, ten Cate R, Haveman J, Medema
JP, Franken NA and van Bree C: Chromosome fragments have the
potential to predict hyperthermia-induced radio-sensitization in
two different human tumor cell lines. J Radiat Res (Tokyo).
49:465–472. 2008. View Article : Google Scholar
|
|
13
|
Dewey WC, Sapareto SA and Betten DA:
Hyperthermic radiosensitization of synchronous Chinese hamster
cells: Relationship between lethality and chromosomal aberrations.
Radiat Res. 76:48–59. 1978. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gotoh E, Asakawa I and Kosaka H:
Inhibition of protein serine/threonine phosphatases directly
induces premature chromosome condensation in mammalian somatic
cells. Biomed Res. 16:63–68. 1995. View Article : Google Scholar
|
|
15
|
Barendsen GW: Dose fractionation, dose
rate and iso-effect relationships for normal tissue responses. Int
J Radiat Oncol Biol Phys. 8:1981–1997. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Barendsen GW, Van Bree C and Franken NA:
Importance of cell proliferative state and potentially lethal
damage repair on radiation effectiveness: Implications for combined
tumor treatments (Review). Int J Oncol. 19:247–256. 2001.PubMed/NCBI
|
|
17
|
Barendsen GW: The relationships between
RBE and LET for different types of lethal damage in mammalian
cells: Biophysical and molecular mechanisms. Radiat Res.
139:257–270. 1994. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Franken NA, Van Bree C, Veltmaat MA,
Rodermond HM, Haveman J and Barendsen GW: Radiosensitization by
bromode-oxyuridine and hyperthermia: Analysis of linear and
quadratic parameters of radiation survival curves of two human
tumor cell lines. J Radiat Res (Tokyo). 42:179–190. 2001.
View Article : Google Scholar
|
|
19
|
Franken N, Vanbree C, Streefkerk J, Kuper
I, Rodermond H, Kipp J, Haveman J and Barendsen G:
Radiosensitization by iodo-deoxyuridine in cultured SW-1573 human
lung tumor cells. Oncol Rep. 4:1073–1076. 1997.PubMed/NCBI
|
|
20
|
Franken NA, van Bree C, Veltmaat MA,
Ludwików G, Kipp JB and Barendsen GW: Increased chromosome exchange
frequencies in iodo-deoxyuridine-sensitized human SW-1573 cells
after γ-irradiation. Oncol Rep. 6:59–63. 1999.
|
|
21
|
Bergs JW, Franken NAP, ten Cate R, van
Bree C and Haveman J: Effects of cisplatin and gamma-irradiation on
cell survival, the induction of chromosomal aberrations and
apoptosis in SW-1573 cells. Mutat Res. 594:148–154. 2006.
View Article : Google Scholar
|
|
22
|
Franken NA, van Bree C, ten Cate R, van
Oven CH and Haveman J: Importance of TP53 and RB in the repair of
potentially lethal damage and induction of color junctions after
exposure to ionizing radiation. Radiat Res. 158:707–714. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Castro Kreder N, Van Bree C, Franken NAP
and Haveman J: Colour junctions as predictors of radiosensitivity:
X-irradiation combined with gemcitabine in a lung carcinoma cell
line. J Cancer Res Clin Oncol. 129:597–603. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Franken NA, Rodermond HM, Stap J, Haveman
J and van Bree C: Clonogenic assay of cells in vitro. Nat Protoc.
1:2315–2319. 2006. View Article : Google Scholar
|
|
25
|
Franken NA, Ruurs P, Ludwików G, van Bree
C, Kipp JB, Darroudi F and Barendsen GW: Correlation between cell
reproductive death and chromosome aberrations assessed by FISH for
low and high doses of radiation and sensitization by
iodo-deoxyuridine in human SW-1573 cells. Int J Radiat Biol.
75:293–299. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Franken NA, ten Cate R, Krawczyk PM, Stap
J, Haveman J, Aten J and Barendsen GW: Comparison of RBE values of
high-LET α-particles for the induction of DNA-DSBs, chromosome
aberrations and cell reproductive death. Radiat Oncol. 6:642011.
View Article : Google Scholar
|
|
27
|
Franken NA, Hovingh S, Ten Cate R,
Krawczyk P, Stap J, Hoebe R, Aten J and Barendsen GW: Relative
biological effectiveness of high linear energy transfer α-particles
for the induction of DNA-double-strand breaks, chromosome
aberrations and reproductive cell death in SW-1573 lung tumour
cells. Oncol Rep. 27:769–774. 2012.
|
|
28
|
van Bree C, Franken NA, Rodermond HM,
Stalpers LJ and Haveman J: Repair of potentially lethal damage does
not depend on functional TP53 in human glioblastoma cells. Radiat
Res. 161:511–516. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Riccardi C and Nicoletti I: Analysis of
apoptosis by propidium iodide staining and flow cytometry. Nat
Protoc. 1:1458–1461. 2006. View Article : Google Scholar
|
|
30
|
Bergs JW, Franken NA, Haveman J, Geijsen
ED, Crezee J and van Bree C: Hyperthermia, cisplatin and radiation
trimodality treatment: A promising cancer treatment? A review from
preclinical studies to clinical application. Int J Hyperthermia.
23:329–341. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Franken NA and Barendsen GW: Enhancement
of radiation effectiveness by hyperthermia and incorporation of
halogenated pyrimidines at low radiation doses as compared with
high doses: Implications for mechanisms. Int J Radiat Biol.
90:313–317. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Franken NA, Oei AL, Kok HP, Rodermond HM,
Sminia P, Crezee J, Stalpers LJ and Barendsen GW: Cell survival and
radio-sensitisation: Modulation of the linear and quadratic
parameters of the LQ model (Review). Int J Oncol. 42:1501–1515.
2013.PubMed/NCBI
|
|
33
|
Xu M, Myerson RJ, Straube WL, Moros EG,
Lagroye I, Wang LL, Lee JT and Roti Roti JL: Radiosensitization of
heat resistant human tumour cells by 1 hour at 41.1 degrees C and
its effect on DNA repair. Int J Hyperthermia. 18:385–403. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xu M, Wright WD, Higashikubo R, Wang LL
and Roti Roti JL: Thermal radiosensitization of human tumour cell
lines with different sensitivities to 41.1 degrees C. Int J
Hyperthermia. 15:279–290. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xu M, Myerson RJ, Xia Y, Whitehead T,
Moros EG, Straube WL and Roti JL: The effects of 41 degrees C
hyperthermia on the DNA repair protein, MRE11, correlate with
radiosensitization in four human tumor cell lines. Int J
Hyperthermia. 23:343–351. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kok HP, Crezee J, Franken NA, Stalpers LJ,
Barendsen GW and Bel A: Quantifying the combined effect of
radiation therapy and hyperthermia in terms of equivalent dose
distributions. Int J Radiat Oncol Biol Phys. 88:739–745. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hunt CR, Pandita RK, Laszlo A, Higashikubo
R, Agarwal M, Kitamura T, Gupta A, Rief N, Horikoshi N, Baskaran R,
et al: Hyperthermia activates a subset of ataxia-telangiectasia
mutated effectors independent of DNA strand breaks and heat shock
protein 70 status. Cancer Res. 67:3010–3017. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Coco-Martin JM and Begg AC: Detection of
radiation-induced chromosome aberrations using fluorescence in situ
hybridization in drug-induced premature chromosome condensations of
tumour cell lines with different radiosensitivities. Int J Radiat
Biol. 71:265–273. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Franken NA, Van Bree CV, Kipp JB and
Barendsen GW: Modification of potentially lethal damage in
irradiated Chinese hamster V79 cells after incorporation of
halogenated pyrimidines. Int J Radiat Biol. 72:101–109. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tucker JD, Cofield J, Matsumoto K, Ramsey
MJ and Freeman DC: Persistence of chromosome aberrations following
acute radiation: I, PAINT translocations, dicentrics, rings,
fragments, and insertions. Environ Mol Mutagen. 45:229–248. 2005.
View Article : Google Scholar : PubMed/NCBI
|