Introduction
Tuberculosis (TB) remains among the leading
infectious causes of death in developing nations, with
Mycobacterium tuberculosis (M. tuberculosis; MT) as
the species responsible for most of these deaths (1,2).
According to the World Health Organization (WHO), in 2014, the
estimated number of tuberculosis cases was 9 million, including 3.5
million new cases and 1.1 million human immunodeficiency virus
(HIV)-positive cases. WHO reported a prevalence of 370 tuberculosis
cases per 100,000 individuals in the United States (3). In Mexico and other developing
nations, tuberculosis is ranked 17th among the leading causes of
all deaths in the working-aged general population (4).
The two main presentations of tuberculosis are
pulmonary and extra pulmonary (5). Extra-pulmonary tuberculosis results
from bacterial spread to other sites in the body, and occurs in
approximately 10% of patients hospitalized with pulmonary
tuberculosis (6). Tuberculous
meningitis (TBM) is the most severe form of the infection, which is
usually caused by M. tuberculosis and poses a serious threat
to human health worldwide (7). If
left untreated, the mortality associated with TBM is almost 100%
and delayed treatment may lead to permanent neurological damage
(3,8). The conventional 'gold standard'
bacteriological methods, namely direct smear and culture isolation,
are hardly able to detect M. tuberculosis in the
cerebrospinal fluid (CSF) of patients with TBM. The clinical
severity of TBM demands a rapid diagnosis and appropriate treatment
in order to improve the clinical outcome. In infections of the
central nervous system, such as TBM, the bacterial load is
extremely low, thus making diagnoses based on the culture or
staining of CSF difficult, as the results are usually negative.
Furthermore, the clinical manifestations of TBM are often
non-specific, similar to those of other forms of meningitis, such
as neurocysticercosis, neuroborreliosis, or viral infections; thus,
its diagnosis is currently based on clinical characteristics,
radiological tests, responses to treatment and in the
characteristics of CFS (9–14).
Molecular tests, in particular those based on the
PCR for amplification of genes specific for the infecting organism
may identify a wide variety of microorganisms, including
mycobacteria (15–18). The analysis of the genomic
sequence of M. tuberculosis (19) and studies on comparative genomics
(20,21) have identified major deletions that
specifically characterize different species of the M.
tuberculosis complex, and are useful in differentiating these
from non-tuberculous mycobacteria (22–25). Some studies have reported the use
of PCR and quantitative PCR to identify M. tuberculosis in
the CSF of patients with TBM, with varying sensitivity and
specificity (25,26). However, the reported methods are
based primarily on primers that bind IS6110, which allow the
identification of the genus of the tuberculosis complex, but does
not discern between species. Thus, the aim of this study was to
identify TB in cases of meningitis with clinical and laboratory
evidence suggestive of TBM, and to confirm our findings with
molecular tests for TB infection.
Patients and methods
Bacterial strains and DNA isolation
The mycobacterial strains used as controls were
M. tuberculosis H37Rv (donated by The Pasteur Institute,
Paris, France), M. bovis AN5 (35734; ATCC, Rockville MD,
USA), M. avium, M. intracellulare and M.
habana (clinical isolates previously identified by rRNA 16S
sequencing), M. celatum (51130; ATCC) and M. marinum
(clinical isolate given by Dr Ruth Parra of the National Medical
Center, Mexico City, Mexico). The strains were grown in Middlebrook
7H9 broth (Difco, Franklin Lakes, NJ, USA) and DNA was isolated
using the phenol-chloroform method, as previously described
(27,28). DNA was precipitated with
isopropanol and resuspended in 50 µl of distilled water, and
a 10 µl aliquot was used for PCR amplification (100 ng of
DNA for the test), as previously described (15).
Ethics statement
The study protocol was approved by the Ethics
Committee of the Mexican Social Security Institute (IMSS).
Patient selection
We recruited 144 consecutive patients with suspected
meningitis and neurological symptoms who were examined at the
neurology services of 4 Hospitals of IMSS in Mexico City between
June 2008 and February 2013. The patients were both male and
female, and included children and adults. Written informed consent
was obtained from all the patients or the parents/legal guardians
prior to recruitment. A total of 94 cases of meningitis with
clinical and laboratory evidence suggestive of TBM were included,
but only 50 of these cases fulfilled the criteria for probable TBM,
the other 44 cases were eliminated. As the controls, we included 50
cases with neurological disease other than TBM, such as neuropathy,
neurocysticercosis, neuroborreliosis, or viral infections. CSF was
extracted from these patients as part of the protocol for
diagnosis; a fraction of the sample was processed immediately for
DNA isolation and was stored at −70°C until analysis.
Definition of case and control
As the bacterial load in the CSF is extremely low
and culture is difficult using these samples, we diagnosed the
cases of TBM based on clinical findings, CSF criteria or both. The
clinical criteria of meningitis included headache, fever and neck
stiffness, with or without an altered consciousness. The CSF
criteria were a cell count >10 cells/mm3, a protein
concentration >45 mg/dl and a glucose concentration <40 mg/dl
(9), either alone or in
combination. TBM was defined as meningitis with typical CSF
findings in conjunction with either suggestive chest X-ray
abnormalities or suggestive TB lymphadenitis. We chose to use
clinical and laboratory data to define the cases and the controls,
as previously described (9,13,14). A TBM case was defined as a patient
suffering from fever, headaches and meningismus (stiff neck), along
with focal neurological deficits, behavioural changes and
alterations in consciousness, with CSF characteristics of moderate
lymphocytic pleocytosis, moderately elevated protein levels and
hypoglycorrachia (Tables I and
II) (12). In addition, all the TBM cases
should respond clinically and radiologically to specific
anti-tuberculosis treatment. A history of a positive tuberculin
skin test, or exposure to tuberculosis, was also considered as risk
for TBM. A non-TBM case was defined as a patient with a confirmed
diagnosis of neuropathy other than TBM. From our examination, there
were 50 patients who fullfiled the criteria for TBM (the cases) and
50 patients who fulfilled the criteria for the controls
(non-TBM).
 | Table IBaseline characteristics of
tuberculous meningitis (TBM) cases. |
Table I
Baseline characteristics of
tuberculous meningitis (TBM) cases.
|
Characteristics | TBM cases, n
(%) |
|---|
| Age (years) |
| <18 | 21 (42) |
| >18 | 29 (58) |
| Gender |
| Male | 27 (54) |
| Female | 23 (46) |
| Clinical
manifestation |
| Headache | 32 (64) |
| Fever | 19 (32) |
| Neck
stittness | 18 (36) |
| Vomitting | 18 (36) |
| Abnormal
behaivior | 26 (52) |
|
Unconsciousness | 3 (6) |
| Drowsiness | 11 (22) |
| Seizures | 24 (48) |
| Nausea | 10 (20) |
| Blurred
vision | 10 (20) |
 | Table IIThe cerebrospinal fluid (CSF)
characteristics of the tuberculous meningitis (TBM) cases and
non-TMB controls. |
Table II
The cerebrospinal fluid (CSF)
characteristics of the tuberculous meningitis (TBM) cases and
non-TMB controls.
| CSF
characteristics | TBM cases | Non-TBM
controls |
|---|
| Total cell count
(cell/mm3) | 96.5 (17–176) | 59 (12–106) |
| Sugar (mg/dl) | 49 (15–83) | 65 (9–121) |
| Protein
(mg/dl) | 151 (98–204) | 74 (53–95) |
Sample size calculation
The sample size was estimated with the EPI info
3.5.1 (2008) programme, aiming to achieve 90% sensitivity for our
multiplex-nested PCR analysis of the CSF of patients with
neurological manifestations suggestive of TBM (26,29). The sample size calculation was 20
cases and 20 controls. We recruited 50 cases and 50 controls for
this study.
Multiplex and nested PCR
We used 100 ng of DNA from the M.
tuberculosis strain H37Rv to standardize the test. The
sequences of the primers are shown in Table III. In the initial
amplification, primers MT1 and MT2 amplified the gene encoding the
32-kDa α antigen present in all described mycobacteria, whereas
primers IS5 and IS6 amplified the IS6110 insertion element
(30,31), and PT1 and PT2 were used to
amplify the species-specific gene mtp40. Nested PCR further
amplified an internal region of the mtp40 gene of M.
tuberculosis (32). All
reactions were performed in a final volume of 25 µl, with
10X reaction buffer, 1.25 U of Taq DNA polymerase, 0.2 mM of each
deoxynucleotide, 2.5 mM MgCl2, 10 pmol of MT1 and MT2,
15 pmol of IS5 and IS6, and 20 pmol of PTI and PT2. The cycling
parameters for the initial PCR were 94°C for 5 min, followed by 30
cycles of denaturation at 94°C for 1 min, annealing at 71°C for 2
min and extension at 72°C for 3 min, followed by a final extension
at 72°C for 10 min. Following amplification, the PCR products were
analysed by horizontal electrophoresis on 2.0% agarose gels
(33) using DNA molecular marker
1 kb (Invitrogen Life Technologies, Carlsbad, CA, USA). The
multiplex PCR products were subjected to nested PCR under the
following conditions: 94°C for 5 min, followed by 30 cycles of 94°C
for 1 min, 74°C for 2 min, and 72°C for 2 min, followed by a final
extension at 72°C for 7 min.
 | Table IIIPrimers used in the study. |
Table III
Primers used in the study.
| Gene fragment | Primer target
(description) | Primer sequences
5′→3′ (foward/reverse) | Amplicon size
(bp) |
Authors/year/(Refs.) |
|---|
| p32 | MT1
MT2 | TTC CTG ACC AGC GAG
CTG CCG
CCC CAG TAC TCC CAG CTG TGC | 506 | Del Portillo et
al, 1991, 1996 (30,31)
Nava et al, 2005 (33) |
| IS6110 | IS 5
IS 6 | CGG AGA CGG TGC GTA
AGT GG
GAT GGA CCG CCA GGG CTT GC | 984 | Del Portillo et
al, 1991, 1996 (30,31)
Nava et al, 2005 (33) |
| mtp40 | PT1
PT2 | CGG CAA CGC GCC GTC
GGT GG
CCC CCC ACG GCA CCG CCG GG | 396 | Del Portillo et
al, 1991 (30,31)
Herrera and Segovia, 1996 (32) |
| Internal fragment
of mtp40 | PT3
PT4 | CAC CAC GTT AGG GAT
GCA CTG C
CTG ATG GTC TCC GAC ACG TTC G | 223 | Gori et al,
1996 (35) |
Laboratory sensitivity and
specificity
Before testing the clinical samples, the specificity
of the assay was tested using DNA from the following
Mycobacterium species: M. tuberculosis H37Rv, M.
bovis AN5, M. intracellulare, M. avium, M.
celatum, M. habana and M. marinum, and DNA from
Stap hylococcus aureus (S. aureus) and Escherichia
coli (E. coli) (clinical isolate from the clinical laboratory
of Pediatric Hospital of the National Medical Center, Mexico City,
Mexico). The sensitivity of the assay was estimated using 10-fold
dilutions of M. tuberculosis DNA, ranging from 100 ng to
0.01 fg.
Processing of the clinical samples
All CSF samples from the patients with meningeal
infection were analyzed by multiplex and nested PCR, and the study
was blinded. An aliquot of 500 µl of each CSF was subjected
to thermal shock, and centrifuged at 17,000 × g for 15 min. The DNA
was isolated from the pellet with guanidine isothiocyanate
according to the procedure previously described by Chomczynski and
Sacchi (27) and Chomczynski
(28), and stored at −70°C until
use. A 100 ng sample of DNA from each CSF sample was used for the
multiplex and nested PCR. The results of PCR for the cases and
controls were used to estimate the clinical specificity and
sensitivity of the test.
DNA sequences and analysis
Four of the amplified nested PCR products from the
clinical samples were purified using the QIAEX II Gel Extraction
kit (Qiagen, Hilden, Germany), and sequenced with the CEQ 8800
Genetic Analysis system (Beckman Coulter, Inc., Brea, CA, USA),
according to the manufacturer's instructions. The sequences
generated were analyzed with the BLAST tool (available online at
www.ncbi.nih.gov/BLAST) and aligned with
the available M. tuberculosis H37Rv genome sequence
database, accessible at http://genolist.pasteur.fr/Tuberculist, to estimate
the degree of homology.
Statistical analysis
Measures of central tendency were the descriptive
statistics used to analyze the quantitative variables. A 2×2
contingency table was used to determine the sensitivity (S),
specificity (E), positive predictive value (PPV) and negative
predictive value (NPV) of the diagnostic test using clinical
samples (27).
Results
Multiplex and nested PCR
The sizes of the amplified fragments were
sufficiently different to be distinguishable on agarose gels; the
primers had no potential matches with sequences at non-specific
target sites, and the optimal DNA-primer annealing temperatures
were almost the same for all template-primer combinations (15). Fig.
1A shows the fragments amplified when each individual primer
set was used separately. Fig. 1B,
lane 3, shows the simultaneous amplification of the 3 fragments. A
nested PCR was used to increase the sensitivity of the system, with
a pair of primers that amplifies a fragment of 223 bp,
corresponding to the internal region of the species-specific
sequence of the mtp40 gene (Fig. 1C).
 | Figure 1Electrophoresis of PCR and nested PCR
products of Mycobacterium tuberculosis (M. tuberculosis)
H37Rv DNA. (A) Amplification of p32, IS6110 and
mtp40 fragments by conventional PCR. Lane 1, DNA molecular
marker 1 kb; lane 2, negative control of MT1 and MT2 primers; lane
3, p32 amplification fragment; lane 4, negative control of
IS5 and IS6 primers; lane 5, IS6110 amplification fragment;
lane 6, negative control of PT1 and PT2 primers; and lane 7,
mtp40 amplification fragment. (B) Amplification of
p32, IS6110 and mtp40 fragments by multiplex
PCR lane 1, DNA molecular marker 1 kb; lane 2, negative control;
and lane 3, amplification products of multiplex PCR of M.
tuberculosis H37Rv DNA. (C) Amplification of internal fragment
of mtp40 by nested PCR. Lane 1, DNA molecular marker 1 kb;
lane 2, negative control; lanes 3 and 4, M. tuberculosis
H37Rv DNA. |
Laboratory specificity and sensitivity of
the multiplex and nested PCR system
The specificity of the assay was evaluated with DNA
from different Mycobacterium strains (M.
tuberculosis, M. bovis AN5, M. intracellulare,
M. avium, M. celatum, M. habana and M.
marinum) and DNA from S. aureus and E. coli.
Following multiplex PCR amplification, M. tuberculosis DNA
amplify the 3 expected bands of genus (506 bp), complex (984 bp)
and species (396 bp), and M. bovis DNA amplify the 2
expected bands of 506 and 984 bp. We also tested DNA from other
Mycobacterium species (M. intracellulare, M.
avium, M. celatum, M. habana and M.
marinum), and in all these strains, only the fragment
corresponding to the p32 gene of the Mycobacterium
genus was amplified. Finally, no amplification was observed with
any of the primers when DNA form S. aureus and E.
coli was tested (Fig.
2A).
 | Figure 2Amplification of DNA from other
bacteria. (A) Multiplex PCR products. (B) Nested PCR products. Lane
1, DNA molecular marker 1 kb; lane 2, negative control; lane 3,
Escherichia coli (E. coli) DNA; lane 4,
Staphylococcus aureus (S. aureus) DNA; lane 5, M.
habana DNA; lane 6, M. celatum DNA, lane 7, M.
marinum DNA; lane 8, M. avium DNA; lane 9, M.
intracellulare DNA; lane 10, M. bovis AN5 DNA; and lane
11, Mycobacterium tuberculosis (M. tuberculosis) H37Rv
DNA. |
These results confirm that multiplex PCR is specific
for M. tuberculosis. Moreover, the products obtained from
the multiplex PCR were reamplified in the nested PCR, and the
internal fragment of the mtp40 gene only amplified with the
M. tuberculosis DNA, confirming that this system
specifically identifies M. tuberculosis (Fig. 2B).
The sensitivity of the multiplex PCR assay was 10 ng
of M. tuberculosis H37Rv DNA where it amplified the 3
expected products (Fig. 3A).
However, the sensitivity of the nested PCR was 0.1 fg of DNA for
the fragment corresponding to an internal region of the
mtp40 gene (Fig. 3B).
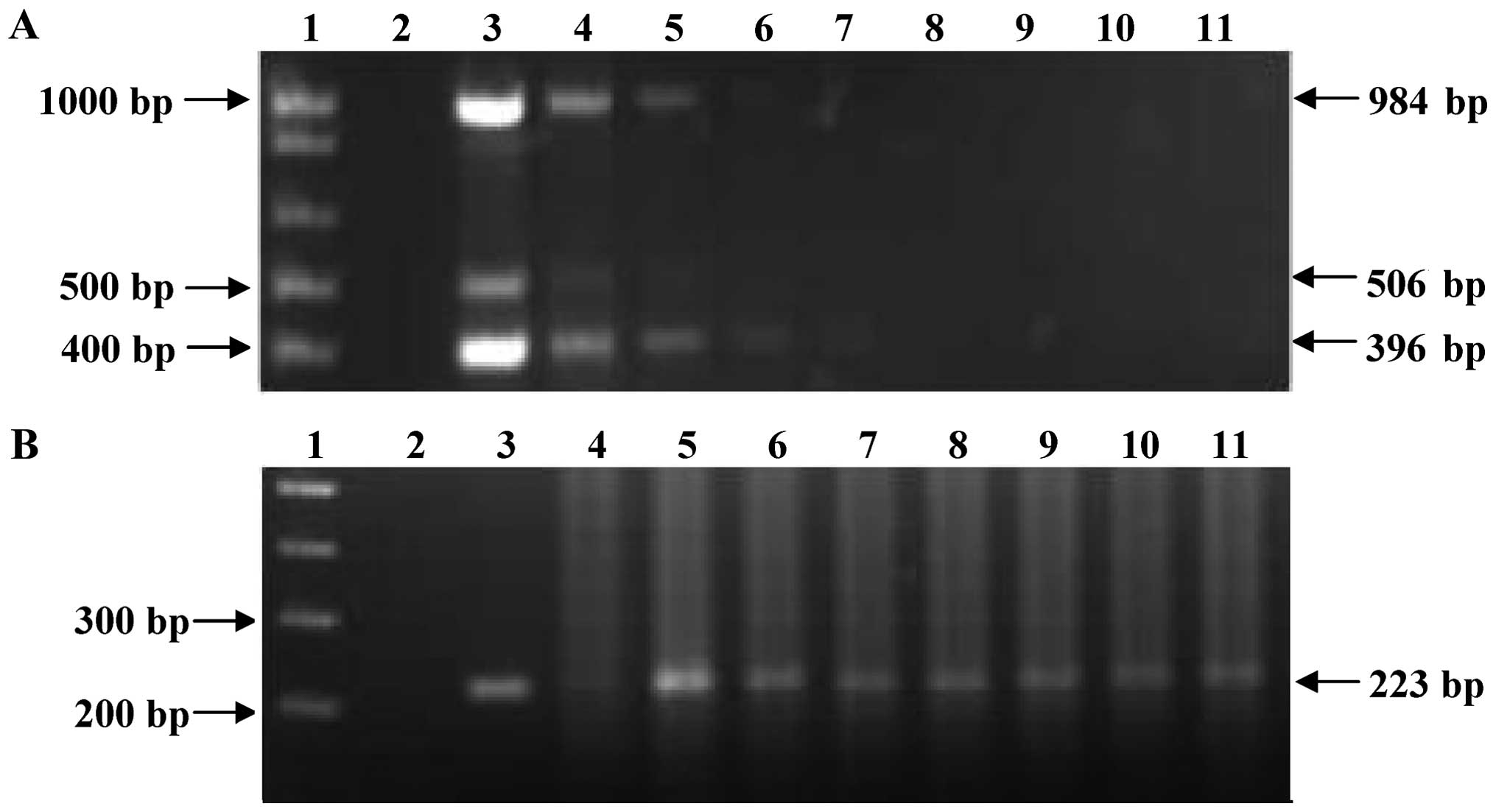 | Figure 3Sensitivity test of the multiplex and
nested PCR for detect Mycobacterium tuberculosis (M.
tuberculosis) DNA. (A) Multiplex PCR products of different
concentrations of M. tuberculosis H37Rv DNA. (B) Nested PCR
products from multiplex PCR of different concentrations of M.
tuberculosis H37Rv DNA. Lane 1, DNA molecular marker 1 kb; lane
2, negative control; lane 3, 100 ng; lane 4, 10 ng; lane 5, 1 ng;
lane 6, 100 pg; lane 7, 10 pg; lane 8, 1 pg; lane 9, 100 fg; lane
10, 10 fg; and lane 11, 1 fg. |
CSF sample analysis
Following the standardization of the multiplex and
nested PCR system with the Mycobacterium strain, we assessed
its usefulness in the CFS samples obtained from clinical cases
suggestive of TBM. We analyzed 100 CSF samples from patients with
diverse neurological symptoms, from 50 patients with a clinical
diagnosis of TBM (cases) and 50 patients with a confirmed
neurological aetiology other than TBM (controls). In the initial
multiplex PCR, only one (patient 3) of the 100 CSF samples
amplified a fragment of 396 bp corresponding to the mtp40
gene (Fig. 4A). In the nested
PCR, the 223-bp amplification product of the mtp40 gene
specific for M. tuberculosis was amplified from 53 samples
(Fig. 4B). Forty-nine of these
samples corresponded to the group of cases and responded to
specific treatment against M. tuberculosis, thus confirming
the presence of M. tuberculosis DNA in the CSF. Moreover, we
had one false-negative sample in the nested PCR. The negative
control run with each set of test samples produced no visible
product on ethidium bromide-stained agarose gel.
 | Figure 4Multiplex and nested PCR for detect
Mycobacterium tuberculosis (M. tuberculosis) in
cerebrospinal fluid (CSF). (A) Multiple-PCR test applied to CSF
samples from patients with neurologic diseases. (B) Nested PCR
products from multiple-PCR products. Lane 1, patient 16 (true
negative); lane 2, patient 11; lane 3, patient 8; lane 4, patient
3; lane 5, patient 12; and lane 6, patient 17 (patient 11, 8, 3, 12
and 17 were true positive). MM, molecular marker 1 kb; C-, negative
control; C+, positive control DNA from M. tuberculosis H37Rv
strain. (C) Alignment of the mtp40 internal fragment with
sequence of nested PCR product using PT3 and PT4 primers. The
highlighted positions indicate the sequence of the mtp40
internal fragment (Query) from 2630676 to 2630827 positions, and
sequence of nested PCR product (Sbjct). |
DNA sequence of PCR products
We determined the DNA sequence of the amplified
223-bp fragment corresponding to the mtp40 gene from 4
positive samples to confirm that the product was specific for M.
tuberculosis. The sequence alignment showed 99% homology
between the nested PCR products and the corresponding region of the
mtp40 gene (Fig. 4C).
Sensitivity and specificity of the
test
The clinical sensitivity of this method was 98.0%
and its specificity was 88.0%; the positive predictive value (PPV)
was 91.0% and the negative predictive value (NPV) was 98.0%
(Table IV).
 | Table IVSensitivity and specificity as a
diagnostic test for tuberculous meningitis (TBM). |
Table IV
Sensitivity and specificity as a
diagnostic test for tuberculous meningitis (TBM).
| Nested PCR | % |
|---|
| Sensitivity | 98 |
| Specificity | 92 |
| PPV | 88 |
| NPV | 98 |
Discussion
The suboptimal and often delayed results of
traditional microbiological techniques used in the diagnosis of TBM
underscore the need for a more rapid and more accurate diagnostic
method to facilitate early treatment. Several molecular-based
methods used for the diagnosis of tuberculosis in respiratory
specimens have been evaluated for their applicability to the
diagnosis of TBM (23). Many of
these methods have failed with CSF samples, mainly due to the fact
that the number of bacilli typically present in TBM is low and that
amplification inhibitors are present in the CSF. Commercially
available methods of nucleic acid amplification (NAA) for the
direct detection of the M. tuberculosis complex have been
approved in the United States for testing respiratory specimens;
Ling et al, performed an extensive literature search and
identified a total of 125 separate studies from 105 articles that
reported NAAT results from respiratory specimens. The results
showed that sensitivity and specificity estimates for commercial
NAATs in respiratory specimens were highly variable, with
sensitivity lower and more inconsistent than specificity. Thus,
summary measures of diagnostic accuracy are not clinically
meaningful. The use of different cut-off values and the use of
specimens other than sputum could explain some of the observed
heterogeneity (34). However,
there are few NAA methods that have been approved for testing the
CSF and several studies have evaluated their performance in
patients with TBM (31,32).
The PCR system also has been used as an
epidemiological tool in strain classification (23,24). The most widely used target
sequence for the diagnosis of tuberculosis has been the
IS6110 insertion element, present in a different number of
copies in the genome of species of the M. tuberculosis
complex (12,35) and PCR techniques based on this
sequence have shown to be useful for diagnosis. However, studies
have demonstrated that some M. tuberculosis strains do not
carry the IS6110 sequence (36,37). Therefore, the use of a PCR method
based on the detection of IS6110 for the diagnosis of M.
tuberculosis may in some cases lead to false-negative results.
Additionally, PCR-based diagnoses based exclusively on the
IS6110 sequence would also fail to distinguish M.
tuberculosis from other mycobacteria of the M.
tuberculosis complex.
In the present study, we used the multiplex PCR
method described by Del Portillo et al (30,31) to detect M. tuberculosis in
pulmonary-type tuberculosis, with some modifications to identify
M. tuberculosis in CSF samples. Additionally, we used the
nested PCR described by Herrera and Segovia (32). In this study, we demonstrated that
using a multiplex and nested PCR system, it is possible to
simultaneously amplify the α antigen gene (p32), which
identifies the Mycobacterium genus, the IS6110
insertion element and the species-specific mtp40 gene. The
nested amplification of a 396-bp fragment corresponding to the
mtp40 gene ensures that the results are specific for M.
tuberculosis. In addition, the nested PCR allowed us to reach a
limit of detection to 0.1 fg of mycobacterial DNA, which
theoretically would allow the detection of one bacterium in the
sample (16). The PCR
amplification of the mtp40 sequence provides a sensitivity
and specific method for the diagnosis of tuberculosis (35,38) and it may be useful for the
identification of M. tuberculosis, as 98.5% of strains
possess this gene (39). We used
a multiplex-PCR method followed by a nested PCR, reaching high
specificity and sensitivity.
With the multiplex PCR we were able to detect only
the mtp40 gene in a single CSF sample; this may be due to
the extremely low number of bacteria in the CSF. However, with the
nested PCR, we detected the presence of M. tuberculosis in
53 patients with the amplification of the species-specific
mtp40 gene. The products of the nested PCR were sequenced to
further confirm that the amplified fragment corresponded to the
mtp40 M. tuberculosis gene, and demonstrated that the
sequence had a 99% homology with the mtp40 gene. Thus, we
were able to detect the presence of M. tuberculosis in the
CSF samples of patients with suggested TBM using a species-specific
multiplex and nested PCR test.
Our test showed a sensitivity of 98.0% and a
specificity of 88.0% for the diagnosis of TBM in CSF samples, which
are better than those reported previously (40,41). In relation to the false-negative
result, it is possible that a fraction of mtp40 gene was
excised by IS6110 recombination as previously described
Vera-Cabrera et al (42),
which could explain the lack of genes in some M.
tuberculosis strains described by Weil et al (43). Concerning the false-positive
results, we suggest that they may represent cases of co-infection,
where clinical diagnosis could have masked the M.
tuberculosis symptoms, focusing on the diagnosis of the other
disease. The test presented a reasonably good PPV (91.0%) and NPV
(98.0%), which contrast with the findings reported by Lima et
al (37), who applied a
nested PCR to the peripheral blood samples from patients with extra
pulmonary tuberculosis, with a rather low PPV (55.6%) and an
acceptable NPV (92.7%).
In conclusion, the present study demonstrates that
the identification of M. tuberculosis in the CSF of patients
with meningitis is possible using a developed system of multiplex
and nested PCR. This study supports the implementation of two
molecular tests as a sensitive and specific diagnostic tool, which
offers an improved alternative for the diagnosis of TBM. The
multiplex-nested PCR test is a rapid, sensitive and specific tool
which may have a beneficial impact on the management of patients
with suspected TBM, allowing a more timely anti-tuberculosis
treatment to reduce sequelae and mortality.
Acknowledgments
The present study was supported by the Instituto de
Ciencia y Tecnología del Distrito Federal (ICyTDF), grant no.
ICyTDF/DSBMA/350/2009 and the Instituto Mexicano del Seguro Social
(IMSS), grant no. FIS/IMSS/PROT/G09/757.
References
|
1
|
Mazars E, Lesjean S, Banuls AL, Gilbert M,
Vincent V, Gicquel B, Tibayrenc M, Locht C and Supply P:
High-resolution minisatellite-based typing as a portable approach
to global analysis of Mycobacterium tuberculosis molecular
epidemiology. Proc Natl Acad Sci USA. 98:1901–1906. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Puccioni-Sohler M and Brandão CO: Factors
associated to the positive cerebrospinal fluid culture in the
tuberculous meningitis. Arq Neuropsiquiatr. 65:48–53. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
World Health Organization: Global
tuberculosis report. 2015, http://www.who.int/tb/publications/global_report/en/.
Accessed August, 2015.
|
|
4
|
Ministry of Health in Mexico: National
system of epidemiological surveillance. Single system of
information (Sistema Nacional de Vigilancia Epidemiológica. Sistema
Único de Información. Secretaría de Salud, México). Epidemiologia.
29:5–6. 2006.
|
|
5
|
Procedures manual of standards for
epidemiological surveillance of mycobacteriosis (tuberculosis and
leprosy). Available: http://www.epidemiologia.salud.gob.mx/doctos/infoepid/vig_epid_manuales/17_2012_Manual_Micobacteriosis_vFinal_9nov12.pdf
(In Spanish).
|
|
6
|
Abter EIM, Schaening O, Barbour RL and
Lutwick LI: Tuberculosis in the adult. Tuberculosis: A Clinical
Handbook. Lutwicick LI: Chapman and Hall Medical; London: pp.
54–101. 1995, View Article : Google Scholar
|
|
7
|
Golden MP and Vikram HR: Extrapulmonary
tuberculosis: an overview. Am Fam Physician. 72:1761–1768.
2005.PubMed/NCBI
|
|
8
|
Donald PR and Schoeman JF: Tuberculous
meningitis. N Engl J Med. 351:1719–1720. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ahuja GK, Mohan KK, Prasad K and Behari M:
Diagnostic criteria for tuberculous meningitis and their
validation. Tuber Lung Dis. 75:149–152. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
González-Martín J, García-García JM,
Anibarro L, Vidal R, Esteban J, Blanquer R, Moreno S and
Ruiz-Manzano J: Consensus document on the diagnosis, treatment and
prevention of tuberculosis. Arch Bronconeumol. 46:255–274. 2010.In
Spanish. View Article : Google Scholar
|
|
11
|
Haldar S, Sharma N, Gupta VK and Tyagi JS:
Efficient diagnosis of tuberculous meningitis by detection of
Mycobacterium tuberculosis DNA in cerebrospinal fluid filtrates
using PCR. J Med Microbiol. 58:616–624. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rock RB, Olin M, Baker CA, Molitor TW and
Peterson PK: Central nervous system tuberculosis: Pathogenesis and
clinical aspects. Clin Microbiol Rev. 21:243–261. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thwaites GE and Tran TH: Tuberculous
meningitis: many questions, too few answers. Lancet Neurol.
4:160–170. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Venkataswamy MM, Rafi W, Nagarathna S,
Ravi V and Chandramuki A: Comparative evaluation of BACTEC 460TB
system and Lowenstein-Jensen medium for the isolation of M.
tuberculosis from cerebrospinal fluid samples of tuberculous
meningitis patients. Indian J Med Microbiol. 25:236–240. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Delidow B, Lynch JP, Peluso JJ and White
BA: Polymerase chain reaction. Methods in Molecular Biology, PCR
Protocols: Methods and Application. White B: Humana Press Inc;
Totowa, NJ: pp. 1–30. 1998
|
|
16
|
Kox LF, Rhienthong D, Miranda AM,
Udomsantisuk N, Ellis K, van Leeuwen J, van Heusden S, Kuijper S
and Kolk AH: A more reliable PCR for detection of Mycobacterium
tuberculosis in clinical samples. J Clin Microbiol. 32:672–678.
1994.PubMed/NCBI
|
|
17
|
Ritacco V and de Kantor IN: Simultaneous
detection of Mycobacterium bovis and Mycobacterium tuberculosis in
human cerebrospinal fluid. J Clin Microbiol. 45:6842007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Takahashi T and Nakayama T: Novel
technique of quantitative nested real-time PCR assay for
Mycobacterium tuberculosis DNA. J Clin Microbiol. 44:1029–1039.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cole ST, Brosch R, Parkhill J, Garnier T,
Churcher C, Harris D, Gordon SV, Eiglmeier K, Gas S, Barry CE III,
et al: Deciphering the biology of Mycobacterium tuberculosis from
the complete genome sequence. Nature. 393:537–544. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kato-Maeda M, Rhee JT, Gingeras TR,
Salamon H, Drenkow J, Smittipat N and Small PM: Comparing genomes
within the species Mycobacterium tuberculosis. Genome Res.
11:547–554. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tsolaki AG, Hirsh AE, DeRiemer K, Enciso
JA, Wong MZ, Hannan M, Goguet de la Salmoniere YO, Aman K,
Kato-Maeda M and Small PM: Functional and evolutionary genomics of
Mycobacterium tuberculosis: insights from genomic deletions in 100
strains. Proc Natl Acad Sci USA. 101:4865–4870. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Palma-Nicolás JP and Bocanegra-García V:
Innovative strategies to diagnose and monitor tuberculosis
patients. Arch Bronconeumol. 43:225–232. 2007.In Spanish.
View Article : Google Scholar
|
|
23
|
Parrado R, Lozano D, Garcia L, Torrico MC,
Delgado R, Torrico F, Laserna M and Reithinger R: Multiprimer PCR
system diagnosis of pulmonary tuberculosis in Cochabamba, Bolivia.
J Clin Microbiol. 46:830–831. 2008. View Article : Google Scholar :
|
|
24
|
Richardson ET, Samson D and Banaei N:
Rapid Identification of Mycobacterium tuberculosis and
nontuberculous mycobacteria by multiplex, real-time PCR. J Clin
Microbiol. 47:1497–1502. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Siddiqi SH, Hawkins JE and Laszlo A:
Interlaboratory drug susceptibility testing of Mycobacterium
tuberculosis by a radiometric procedure and two conventional
methods. J Clin Microbiol. 22:919–923. 1985.PubMed/NCBI
|
|
26
|
Fuentelsaz Gallego C: Sample size
calculation. Matronas Profesion. 5:pp. 5–13. 2004, Available:
https://ecaths1.s3.amazonaws.com/seminarioi/1400533589.1%20Muestreo.pdf.
(In Spanish).
|
|
27
|
Chomczynski P and Sacchi N: Single-step
method of RNA isolation by acid guanidinium
thiocyanate-phenol-chloroform extraction. Anal Biochem.
162:156–159. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chomczynski P: A reagent for the
single-step simultaneous isolation of RNA, DNA and proteins from
cell and tissue samples. Biotechniques. 15:532–534. 536–537.
1993.PubMed/NCBI
|
|
29
|
Dawson B and Trapp RG: Biostatistics. 4th
edition. New York: McGraw Hill; 2004
|
|
30
|
Del Portillo P, Murillo LA and Patarroyo
ME: Amplification of a species-specific DNA fragment of
Mycobacterium tuberculosis and its possible use in diagnosis. J
Clin Microbiol. 29:2163–2168. 1991.PubMed/NCBI
|
|
31
|
Del Portillo P, Thomas MC, Martínez E,
Marañón C, Valladares B, Patarroyo ME and Carlos López M:
Multiprimer PCR system for differential identification of
mycobacteria in clinical samples. J Clin Microbiol. 34:324–328.
1996.PubMed/NCBI
|
|
32
|
Herrera EA and Segovia M: Evaluation of
mtp40 genomic fragment amplification for specific detection of
Mycobacterium tuberculosis in clinical specimens. J Clin Microbiol.
34:1108–1113. 1996.PubMed/NCBI
|
|
33
|
Nava PO, Manssur H and Prieto L:
Evaluation of bacilloscopy, cultivation and polymerase chain
reaction for the diagnostic of lung tuberculosis. Kasmera.
33:119–131. 2005.In Spanish.
|
|
34
|
Ling DI, Flores LL, Riley LW and Pai M:
Commercial nucleic-acid amplification tests for diagnosis of
pulmonary tuberculosis in respiratory specimens: meta-analysis and
meta-regression. PLoS One. 3:e15362008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gori A, Franzetti F, Marchetti G, Catozzi
L and Corbellino M: Specific detection of Mycobacterium
tuberculosis by mtp40 nested PCR. J Clin Microbiol. 34:2866–2867.
1996.PubMed/NCBI
|
|
36
|
Kolk AH, Schuitema AR, Kuijper S, van
Leeuwen J, Hermans PW, van Embden JD and Hartskeerl RA: Detection
of Mycobacterium tuberculosis in clinical samples by using
polymerase chain reaction and a nonradioactive detection system. J
Clin Microbiol. 30:2567–2575. 1992.PubMed/NCBI
|
|
37
|
Lima JF, Montenegro LM, Montenegro RA,
Cabral MM, Lima AS, Abath FG and Schindler HC: Performance of
nested PCR in the specific detection of Mycobacterium tuberculosis
complex in blood samples of pediatric patients. J Bras Pneumol.
35:690–697. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Koivula T, Svenson SB and Källenius G: The
mtp40 gene is not present in Mycobacterium bovis. Tuberculosis
(Edinb). 82:183–185. 2002. View Article : Google Scholar
|
|
39
|
Vera-Cabrera L, Howard ST, Laszlo A and
Johnson WM: Analysis of genetic polymorphism in the phospholipase
region of Mycobacterium tuberculosis. J Clin Microbiol.
35:1190–1195. 1997.PubMed/NCBI
|
|
40
|
Seth P, Ahuja GK, Bhanu NV, Behari M,
Bhowmik S, Broor S, Dar L and Chakraborty M: Evaluation of
polymerase chain reaction for rapid diagnosis of clinically
suspected tuberculous meningitis. Tuber Lung Dis. 77:353–357. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bonington A, Strang JI, Klapper PE, Hood
SV, Rubombora W, Penny M, Willers R and Wilkins EG: Use of Roche
AMPLICOR Mycobacterium tuberculosis PCR in early diagnosis of
tuberculous meningitis. J Clin Microbiol. 36:1251–1254.
1998.PubMed/NCBI
|
|
42
|
Vera-Cabrera L, Hernández-Vera MA, Welsh
O, Johnson WM and Castro-Garza J: Phospholipase region of
Mycobacterium tuberculosis is a preferential locus for IS6110
transposition. J Clin Microbiol. 39:3499–3504. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Weil A, Plikaytis BB, Butler WR, Woodley
CL and Shinnick TM: The mtp40 gene is not present in all strains of
Mycobacterium tuberculosis. J Clin Microbiol. 34:2309–2311.
1996.PubMed/NCBI
|


















