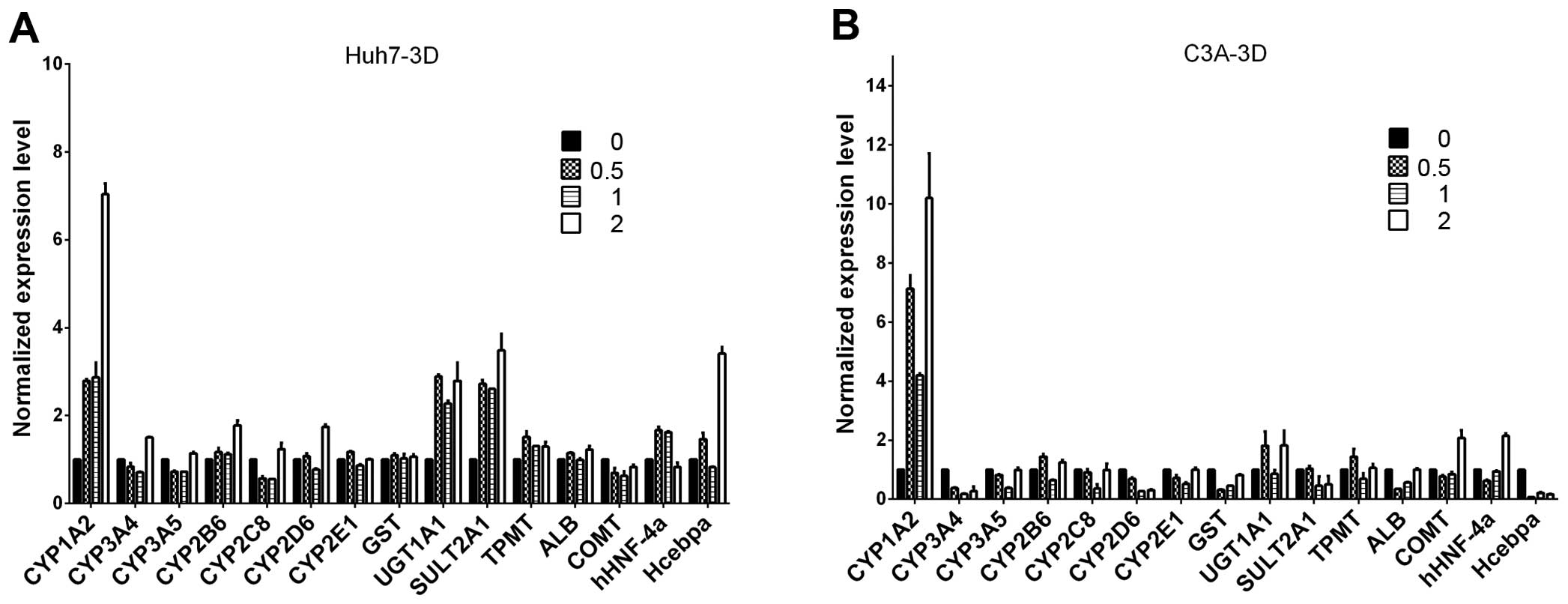
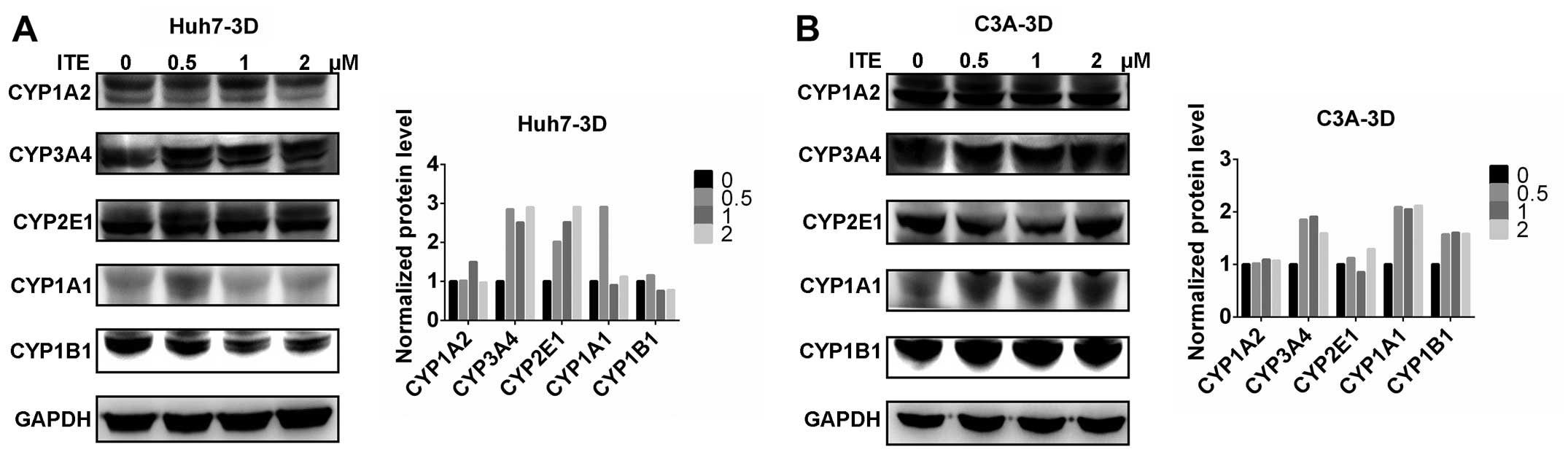
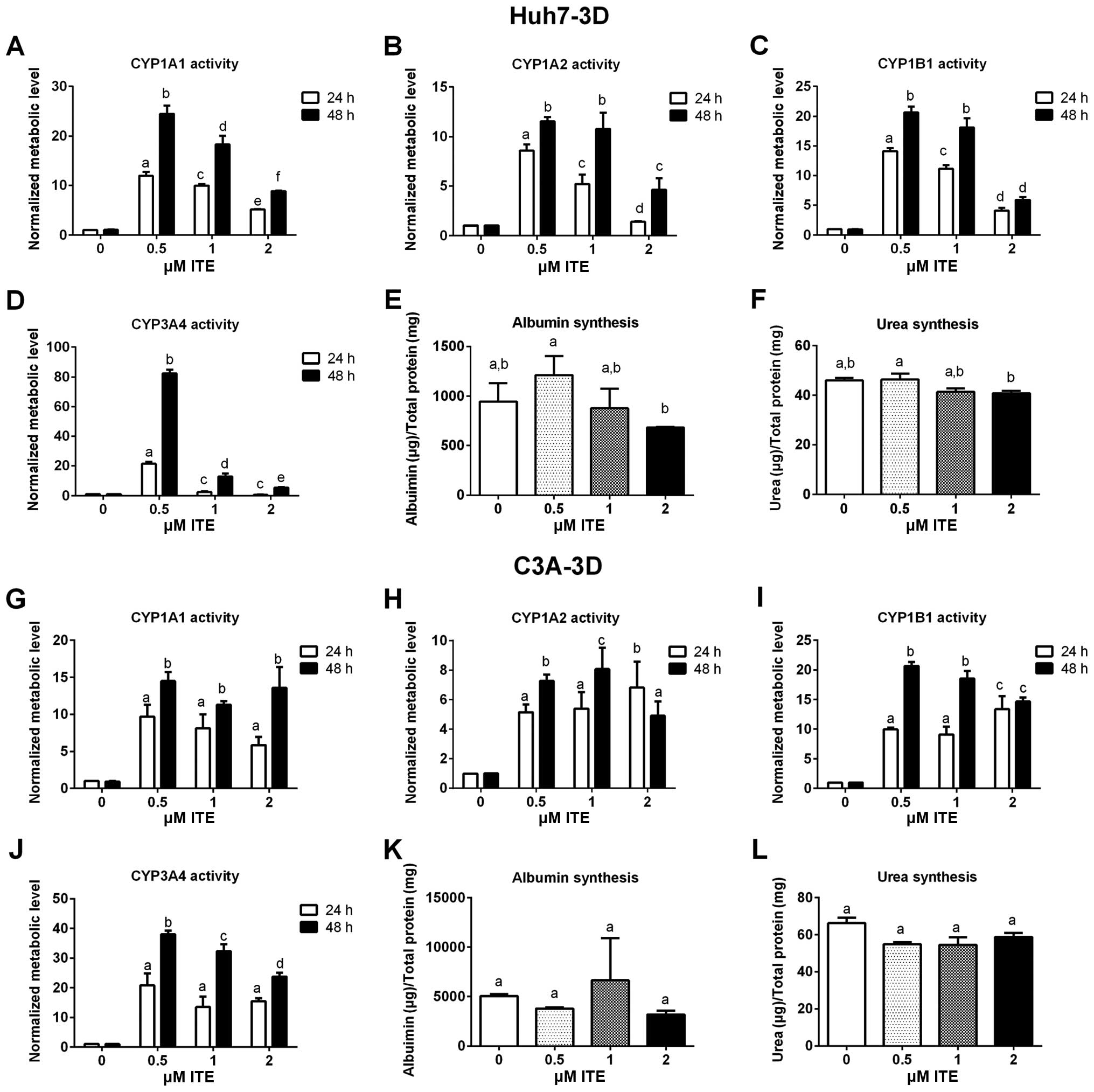
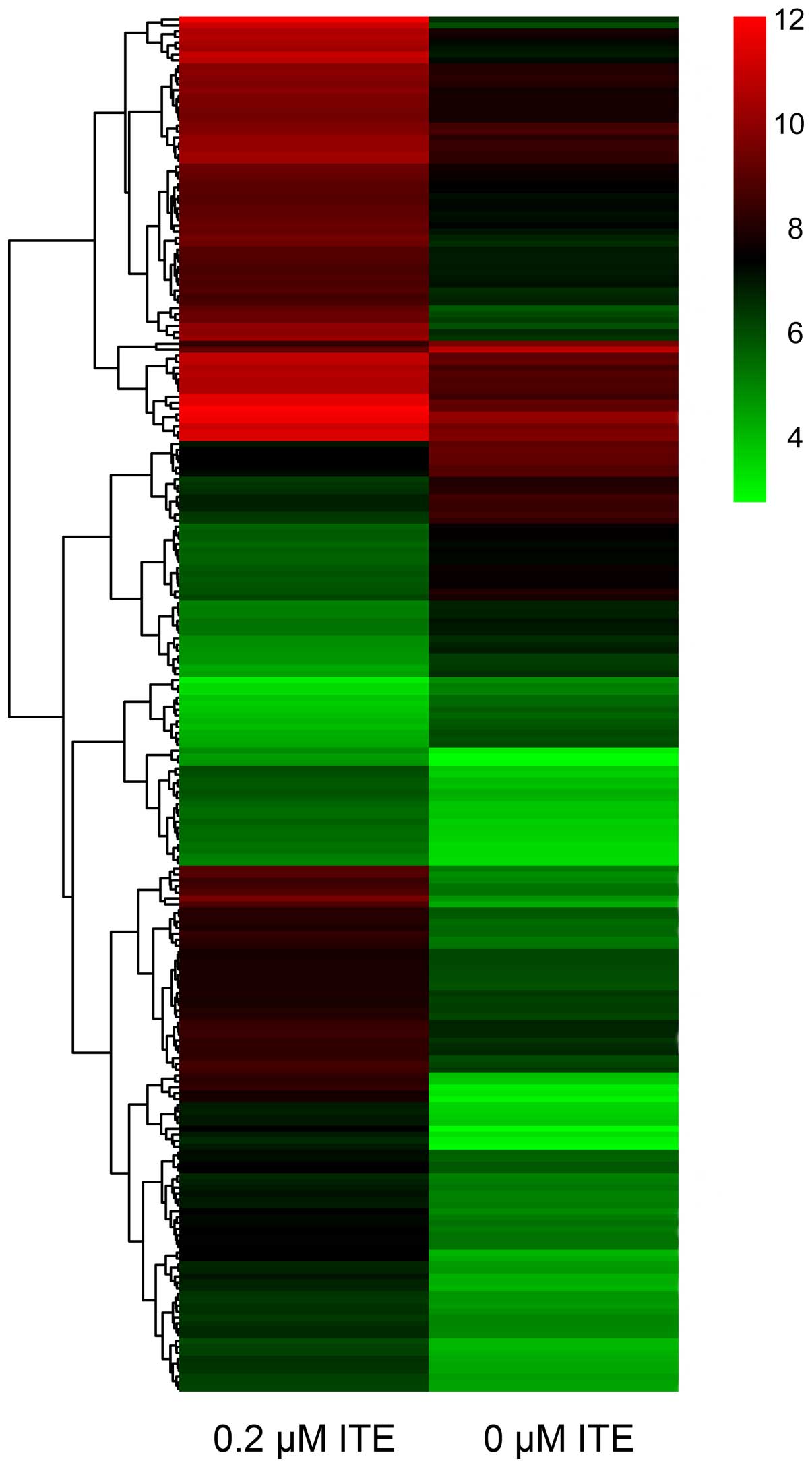
|
1
|
Bernal W and Wendon J: Acute liver
failure. N Engl J Med. 369:2525–2534. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tostões RM, Leite SB, Miranda JP, Sousa M,
Wang DI, Carrondo MJ and Alves PM: Perfusion of 3D encapsulated
hepatocytes - a synergistic effect enhancing long-term
functionality in bioreactors. Biotechnol Bioeng. 108:41–49. 2011.
View Article : Google Scholar
|
|
3
|
Carpentier B, Gautier A and Legallais C:
Artificial and bioartificial liver devices: present and future.
Gut. 58:1690–1702. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang Y, Li J, Pan X, Zhou P, Yu X, Cao H,
Wang Y and Li L: Co-culture with mesenchymal stem cells enhances
metabolic functions of liver cells in bioartificial liver system.
Biotechnol Bioeng. 110:958–968. 2013. View Article : Google Scholar
|
|
5
|
Chamuleau RA, Deurholt T and Hoekstra R:
Which are the right cells to be used in a bioartificial liver?
Metab Brain Dis. 20:327–335. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nakamura T, Tomita Y, Hirai R, Yamaoka K,
Kaji K and Ichihara A: Inhibitory effect of transforming growth
factor-beta on DNA synthesis of adult rat hepatocytes in primary
culture. Biochem Biophys Res Commun. 133:1042–1050. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zanger UM, Turpeinen M, Klein K and Schwab
M: Functional pharmacogenetics/genomics of human cytochromes P450
involved in drug biotransformation. Anal Bioanal Chem.
392:1093–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gebhardt R, Hengstler JG, Müller D,
Glöckner R, Buenning P, Laube B, Schmelzer E, Ullrich M, Utesch D,
Hewitt N, et al: New hepatocyte in vitro systems for drug
metabolism: metabolic capacity and recommendations for application
in basic research and drug development, standard operation
procedures. Drug Metab Rev. 35:145–213. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gómez-Lechón MJ, Donato MT, Castell JV and
Jover R: Human hepatocytes as a tool for studying toxicity and drug
metabolism. Curr Drug Metab. 4:292–312. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vermeir M, Annaert P, Mamidi RN, Roymans
D, Meuldermans W and Mannens G: Cell-based models to study hepatic
drug metabolism and enzyme induction in humans. Expert Opin Drug
Metab Toxicol. 1:75–90. 2005. View Article : Google Scholar
|
|
11
|
Filippi C, Keatch SA, Rangar D, Nelson LJ,
Hayes PC and Plevris JN: Improvement of C3A cell metabolism for
usage in bioartificial liver support systems. J Hepatol.
41:599–605. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sivertsson L, Ek M, Darnell M, Edebert I,
Ingelman-Sundberg M and Neve EP: CYP3A4 catalytic activity is
induced in confluent Huh7 hepatoma cells. Drug Metab Dispos.
38:995–1002. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sussman NL, Gislason GT, Conlin CA and
Kelly JH: The Hepatix extracorporeal liver assist device: initial
clinical experience. Artif Organs. 18:390–396. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ellis AJ, Hughes RD, Wendon JA, Dunne J,
Langley PG, Kelly JH, Gislason GT, Sussman NL and Williams R:
Pilot-controlled trial of the extracorporeal liver assist device in
acute liver failure. Hepatology. 24:1446–1451. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hughes RD, Nicolaou N, Langley PG, Ellis
AJ, Wendon JA and Williams R: Plasma cytokine levels and
coagulation and complement activation during use of the
extracorporeal liver assist device in acute liver failure. Artif
Organs. 22:854–858. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tsay JJ, Tchou-Wong KM, Greenberg AK, Pass
H and Rom WN: Aryl hydrocarbon receptor and lung cancer. Anticancer
Res. 33:1247–1256. 2013.PubMed/NCBI
|
|
17
|
Cheng J, Li W, Kang B, Zhou Y, Song J, Dan
S, Yang Y, Zhang X, Li J, Yin S, et al: Tryptophan derivatives
regulate the transcription of Oct4 in stem-like cancer cells. Nat
Commun. 6:72092015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Minsavage GD, Park SK and Gasiewicz TA:
The aryl hydrocarbon receptor (AhR) tyrosine 9, a residue that is
essential for AhR DNA binding activity, is not a phosphoresidue but
augments AhR phosphorylation. J Biol Chem. 279:20582–20593. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hankinson O: The aryl hydrocarbon receptor
complex. Annu Rev Pharmacol Toxicol. 35:307–340. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Whitlock JP Jr: Induction of cytochrome
P4501A1. Annu Rev Pharmacol Toxicol. 39:103–125. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Song J, Clagett-Dame M, Peterson RE, Hahn
ME, Westler WM, Sicinski RR and DeLuca HF: A ligand for the aryl
hydrocarbon receptor isolated from lung. Proc Natl Acad Sci USA.
99:14694–14699. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Khetani SR and Bhatia SN: Microscale
culture of human liver cells for drug development. Nat Biotechnol.
26:120–126. 2008. View Article : Google Scholar
|
|
23
|
Isom HC, Secott T, Georgoff I, Woodworth C
and Mummaw J: Maintenance of differentiated rat hepatocytes in
primary culture. Proc Natl Acad Sci USA. 82:3252–3256. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dich J, Vind C and Grunnet N: Long-term
culture of hepatocytes: effect of hormones on enzyme activities and
metabolic capacity. Hepatology. 8:39–45. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kubota H and Reid LM: Clonogenic
hepatoblasts, common precursors for hepatocytic and biliary
lineages, are lacking classical major histocompatibility complex
class I antigen. Proc Natl Acad Sci USA. 97:12132–12137. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Allen JW, Hassanein T and Bhatia SN:
Advances in bioartificial liver devices. Hepatology. 34:447–455.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guillouzo A, Corlu A, Aninat C, Glaise D,
Morel F and Guguen-Guillouzo C: The human hepatoma HepaRG cells: a
highly differentiated model for studies of liver metabolism and
toxicity of xenobiotics. Chem Biol Interact. 168:66–73. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nibourg GA, Huisman MT, van der Hoeven TV,
van Gulik TM, Chamuleau RA and Hoekstra R: Stable overexpression of
pregnane X receptor in HepG2 cells increases its potential for
bioartificial liver application. Liver Transpl. 16:1075–1085. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang N, Tsuruoka S, Yamamoto H, Enosawa S,
Omasa T, Sata N, Matsumura T, Nagai H and Fujimura A: The
bioreactor with CYP3A4- and glutamine synthetase-introduced HepG2
cells: treatment of hepatic failure dog with diazepam overdosage.
Artif Organs. 29:681–684. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fujii-Kuriyama Y and Mimura J: Molecular
mechanisms of AhR functions in the regulation of cytochrome P450
genes. Biochem Biophys Res Commun. 338:311–317. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rabanel JM, Banquy X, Zouaoui H, Mokhtar M
and Hildgen P: Progress technology in microencapsulation methods
for cell therapy. Biotechnol Prog. 25:946–963. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jover R, Moya M and Gómez-Lechón MJ:
Transcriptional regulation of cytochrome p450 genes by the nuclear
receptor hepatocyte nuclear factor 4-alpha. Curr Drug Metab.
10:508–519. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Puga A, Ma C and Marlowe JL: The aryl
hydrocarbon receptor cross-talks with multiple signal transduction
pathways. Biochem Pharmacol. 77:713–722. 2009. View Article : Google Scholar :
|
|
34
|
Marie S, Anderson A and Cresteil T:
Transplacental induction of cytochromes P-450IA1 and P-450IA2 by
polycyclic aromatic carcinogens: TCDD-binding protein level as the
rate-limiting step. Carcinogenesis. 9:2059–2063. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tritscher AM, Goldstein JA, Portier CJ,
McCoy Z, Clark GC and Lucier GW: Dose-response relationships for
chronic exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin in a rat
tumor promotion model: quantification and immunolocalization of
CYP1A1 and CYP1A2 in the liver. Cancer Res. 52:3436–3442.
1992.PubMed/NCBI
|
|
36
|
Döhr O, Vogel C and Abel J: Different
response of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-sensitive
genes in human breast cancer MCF-7 and MDA-MB 231 cells. Arch
Biochem Biophys. 321:405–412. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gonzalez FJ, Tukey RH and Nebert DW:
Structural gene products of the Ah locus. Transcriptional
regulation of cytochrome P1-450 and P3-450 mRNA levels by
3-methylcholanthrene. Mol Pharmacol. 26:117–121. 1984.PubMed/NCBI
|
|
38
|
Goasduff T, Menez JF, Dreano Y and Berthou
F: CYP1A2 and 2E1 expression in rat liver treated with combined
inducers (3-methylcholanthrene and ethanol). Biochem Biophys Res
Commun. 211:497–503. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mimura J and Fujii-Kuriyama Y: Functional
role of AhR in the expression of toxic effects by TCDD. Biochim
Biophys Acta. 1619:263–268. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kinasiewicz A, Gautier A, Lewinska D,
Bukowski J, Legallais C and Weryński A: Culture of C3A cells in
alginate beads for fluidized bed bioartificial liver. Transplant
Proc. 39:2911–2913. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
David B, Dufresne M, Nagel MD and
Legallais C: In vitro assessment of encapsulated C3A hepatocytes
functions in a fluidized bed bioreactor. Biotechnol Prog.
20:1204–1212. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Glicklis R, Shapiro L, Agbaria R, Merchuk
JC and Cohen S: Hepatocyte behavior within three-dimensional porous
alginate scaffolds. Biotechnol Bioeng. 67:344–353. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Fan Y, Boivin GP, Knudsen ES, Nebert DW,
Xia Y and Puga A: The aryl hydrocarbon receptor functions as a
tumor suppressor of liver carcinogenesis. Cancer Res. 70:212–220.
2010. View Article : Google Scholar
|
|
44
|
Schnekenburger M, Peng L and Puga A: HDAC1
bound to the Cyp1a1 promoter blocks histone acetylation associated
with Ah receptor-mediated trans-activation. Biochim Biophys Acta.
1769:569–578. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Thasler WE, Dayoub R, Mühlbauer M,
Hellerbrand C, Singer T, Gräbe A, Jauch KW, Schlitt HJ and Weiss
TS: Repression of cytochrome P450 activity in human hepatocytes in
vitro by a novel hepatotrophic factor, augmenter of liver
regeneration. J Pharmacol Exp Ther. 316:822–829. 2006. View Article : Google Scholar
|
|
46
|
Vondrácek J, Machala M, Bryja V,
Chramostová K, Krcmár P, Dietrich C, Hampl A and Kozubík A: Aryl
hydrocarbon receptor-activating polychlorinated biphenyls and their
hydroxylated metabolites induce cell proliferation in
contact-inhibited rat liver epithelial cells. Toxicol Sci.
83:53–63. 2005. View Article : Google Scholar
|
|
47
|
Curcio E, Salerno S, Barbieri G, De
Bartolo L, Drioli E and Bader A: Mass transfer and metabolic
reactions in hepatocyte spheroids cultured in rotating wall
gas-permeable membrane system. Biomaterials. 28:5487–5497. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Beningo KA, Dembo M and Wang YL: Responses
of fibroblasts to anchorage of dorsal extracellular matrix
receptors. Proc Natl Acad Sci USA. 101:18024–18029. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhang X, Wang W, Yu W, Xie Y, Zhang X,
Zhang Y and Ma X: Development of an in vitro multicellular tumor
spheroid model using microencapsulation and its application in
anticancer drug screening and testing. Biotechnol Prog.
21:1289–1296. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Orive G, Tam SK, Pedraz JL and Hallé JP:
Biocompatibility of alginate-poly-L-lysine microcapsules for cell
therapy. Biomaterials. 27:3691–3700. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Neubauer MP, Poehlmann M and Fery A:
Microcapsule mechanics: from stability to function. Adv Colloid
Interface Sci. 207:65–80. 2014. View Article : Google Scholar
|
|
52
|
Lv G, Zhao L, Zhang A, Du W, Chen Y, Yu C,
Pan X, Zhang Y, Song T, Xu J, et al: Bioartificial liver system
based on choanoid fluidized bed bioreactor improve the survival
time of fulminant hepatic failure pigs. Biotechnol Bioeng.
108:2229–2236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Gautier A, Carpentier B, Dufresne M, Vu
Dinh Q, Paullier P and Legallais C: Impact of alginate type and
bead diameter on mass transfers and the metabolic activities of
encapsulated C3A cells in bioartificial liver applications. Eur
Cell Mater. 21:94–106. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hoshikawa A, Nakayama Y, Matsuda T, Oda H,
Nakamura K and Mabuchi K: Encapsulation of chondrocytes in
photopolymerizable styrenated gelatin for cartilage tissue
engineering. Tissue Eng. 12:2333–2341. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Bazou D, Coakley WT, Hayes AJ and Jackson
SK: Long-term viability and proliferation of alginate-encapsulated
3-D HepG2 aggregates formed in an ultrasound trap. Toxicol In
Vitro. 22:1321–1331. 2008. View Article : Google Scholar : PubMed/NCBI
|