Cytoprotective effects of esculetin against oxidative stress are associated with the upregulation of Nrf2-mediated NQO1 expression via the activation of the ERK pathway
- Authors:
- Published online on: December 16, 2016 https://doi.org/10.3892/ijmm.2016.2834
- Pages: 380-386
Abstract
Introduction
It is well known that oxidative stress occurs as a result of excessive reactive oxygen species (ROS), such as singlet oxygen, superoxide anions, and hydrogen peroxide (H2O2). These ROS cause irreversible damage to cellular components, including lipids, proteins, DNA, and other macromolecules, which has been linked to cell death (1,2). ROS are also known to contribute to various pathological conditions, including cancer; inflammatory, neurodegenerative and cardiovascular diseases; and aging (3–5). Therefore, the human body has various defense systems against oxidative stress that are surpassed in extensive damage (6,7). These mechanisms use antioxidant enzymes or antioxidant compounds. Among these systems, the nuclear transcription factor erythroid 2-related factor 2 (Nrf2) signaling pathway has recently attracted interest as a candidate for protection from oxidative damage as it is involved in cellular antioxidant defenses (8,9). Nrf2 is an essential transcription factor that regulates a number of detoxifying and antioxidant defense genes by binding to antioxidant responsive elements (AREs) (10,11). Under quiescent conditions, Nrf2-dependent transcription is suppressed by the negative regulator Kelch-like ECH-associated protein 1 (Keap1), which facilitates the degradation of Nrf2 through ubiquitinated proteasomal degradation (12,13). Upon stimulation, Nrf2 escapes Keap1-mediated repression, translocates into the nucleus, and subsequently binds to AREs present in the promoter regions of an array of genes, including heme oxygenase-1 (HO-1) and NAD(P)H:quinone oxidoreductase 1 (NQO1), involved in cellular antioxidant defense (14,15). HO-1 can catalyze the degradation of heme, resulting in the formation of the antioxidant bilirubin when biliverdin reductase is present (16,17). NQO1, a cytosolic flavoprotein, facilitates the detoxification and excretion of endogenous and exogenous chemicals via a reduction reaction that converts quinones to hydroquinones, limiting the subsequent generation of ROS (18,19).
Esculetin (6,7-dihydroxycoumarin) is a phenolic compound and derivative of coumarin (20,21). This compound is found in many plants, such as Artemisia capillaris, Ceratostigma willmottianum and Citrus limonia that have been used in traditional Oriental herbal medicine for many decades (22,23). Esculetin has been reported to have diverse pharmacological actions, including anti-neurotoxicity (24,25), anti-angiogenesis (26), anti-inflammatory (27,28), and antitumor activities (29–32). More recently, esculetin has been shown to have beneficial effects on cellular oxidative stress (33–35). However, the inhibitory mechanisms of esculetin vis-à-vis the beneficial effect of esculetin against oxidative stress have not been fully studied to date. Therefore, in the present study, we evaluated the protective effects of esculetin on H2O2-induced oxidative stress and whether esculetin can activate Nrf2 signaling in mouse-derived C2C12 myoblasts.
Materials and methods
Reagents and antibodies
Esculetin (6,7-dihydroxycoumarin) was purchased from Sigma Chemical Co. (St. Louis, MO, USA), dissolved in dimethyl sulfoxide (DMSO, vehicle), and adjusted to final concentrations using complete culture medium. The final DMSO concentration was <0.1% in all experiments. Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS), and other tissue culture reagents were obtained from WelGENE Inc. (Daegu, Korea). H2O2, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), N-acetyl-L-cysteine (NAC), 4′,6-diamidino-2- phenylindole (DAPI), propidium iodide (PI) and zinc protoporphyrin IX (ZnPP) were also purchased from Sigma Chemical Co. PD98059, an ERK inhibitor, was purchased from Calbiochem, Inc. (San Diego, CA, USA). 2′,7′-Dichlorofluorescein diacetate (DCFDA) and an Annexin V-fluorescein isothiocyanate (FITC) apoptosis detection kit were purchased from Molecular Probes, Inc. (Eugene, OR, USA) and R&D Systems Inc. (Minneapolis, MN, USA), respectively. Primary antibodies (Table I) were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA), Cell Signaling Technology, Inc. (Danvers, MA, USA) and Abcam, Inc. (Cambridge, MA, USA). An enhanced chemiluminescence (ECL) kit and horseradish (HRP)-conjugated secondary antibodies were obtained from Amersham Life Science (Arlington Heights, IL, USA). All other chemicals not specifically mentioned here were purchased from Sigma Chemical Co.
Cell culture and viability assay
C2C12 myoblasts obtained from the American Type Culture Collection (Manassas, VA, USA) were cultured in DMEM supplemented with 10% heat-inactivated FBS and 100 µg/ml of penicillin/streptomycin antibiotics in a humidified atmosphere containing 5% CO2 and 95% air at 37°C. The cells were pre-treated with various concentrations (0–5 µM) of esculetin for 1 h and then incubated with or without 1 mM H2O2 for 6 h in the absence or presence of 5 mM NAC or 50 µM PD98059. To measure cell viability, the cells were maintained with MTT at a final concentration of 0.5 mg/ml for 3 h, and the formazan that formed was dissolved in DMSO. Optical density was measured at 540 nm using an enzyme-linked immunosorbent assay (ELISA) plate reader (Dynatech MR-7000; Dynatech Laboratories, Chantilly, VA, USA). The optical density of the formazan formed in the control (untreated) cells was used to represent 100% viability (36).
Measurement of ROS generation
To measure ROS levels, the cells were washed twice with phosphate-buffered saline (PBS) and lysed with 1% Triton X-100 in PBS for 10 min at 37°C. The cells were pre-treated with 5 µM esculetin for 1 h and then incubated with or without 1 mM H2O2 for 6 h in the absence or presence of 5 mM NAC or 50 µM PD98059. The cells were then stained with 10 µM DCFDA for 20 min at room temperature in the dark. The green fluorescence of DCF was recorded at 515 nm using a flow cytometer, and 10,000 events were counted per sample. The results are expressed as the percentage of increase relative to the non-treated cells.
Comet assay (single-cell gel electrophoresis assay)
A comet assay was performed to detect DNA migrating from single cells in the gel, following a previously described method (37). Briefly, the cells were exposed to 1 mM H2O2 for 6 h in the presence and absence of 5 µM esculetin for 1 h. The cells were suspended in 1% low melting point agarose and aliquoted onto glass microscope slides. The slides were placed in single rows and electrophoresed at 30 V (1 V/cm) and 300 mA for 20 min to draw negatively charged DNA toward the anode. Finally, the slides were washed with 0.4 M Tris (pH 7.5) at 4°C and stained with 20 µg/ml PI. The slides were examined under a fluorescence microscope (Carl Zeiss AG, Oberkochen, Germany), and the resulting images were analyzed.
Western blot analysis
The cells were pre-treated with 5 µM esculetin for 1 h and then incubated with or without 1 mM H2O2 for 6 h, or pre-treated with or without 50 µM PD98059 for 1 h and then treated with 5 µM esculetin for 4 h. The cells were harvested, washed with PBS, and lysed on ice for 30 min in lysis buffer (20 mM sucrose, 1 mM ethylenediaminetetraacetic acid, 20 µM Tris-HCl, pH 7.2, 1 mM dithiothreitol, 10 mM KCl, 1.5 mM MgCl2, and 5 µg/ml aprotinin). Subsequently, an equal amount of protein for each sample was separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes (Schleicher & Schuell, Inc., Keene, NH, USA). The membranes were blocked with 5% skim milk and then incubated overnight at 4°C with desired primary antibodies. The membranes were further incubated with corresponding HRP-conjugated secondary antibodies for 2 h at room temperature. The proteins of interest were visualized using an ECL detection system.
Detection of nuclear morphological changes
The cells were pre-treated with 5 µM esculetin for 1 h and then incubated with or without 1 mM H2O2 for 6 h. The detection of chromatin condensation and nuclear fragmentation in the nuclei of apoptotic cells was performed using DAPI staining. The cells were harvested, washed with PBS twice, and fixed with 3.7% paraformaldehyde in PBS for 10 min at 25°C. The fixed cells were washed with PBS and stained with 1 mg/ml DAPI solution for 10 min. The cells were then washed twice with PBS and observed under a fluorescence microscope (38).
Flow cytometric detection of apoptosis
The cells were pre-treated for 1 h with the indicated concentrations of esculetin and then incubated for 6 h with or without 1 mM H2O2 in the absence or presence of 50 µM PD98059. The rate of apoptosis was determined using an Annexin V-FITC apoptosis detection kit. After treatment with the agents, the cells in each sample were stained with Annexin V-FITC and PI in accordance with the manufacturer's instructions. After a 15-min incubation at room temperature in the dark, the degree of apoptosis was quantified as a percentage of the Annexin V-positive and PI-negative (Annexin V+/PI− cells) cells using a flow cytometer (Becton Dickinson, San Jose, CA, USA) (39).
Statistical analysis
Unless specified otherwise, data are expressed as the means ± standard deviation (SD) of at least three independent experiments. A one-way analysis of variance (SPSS version 12.0 software) and a Scheffe's test were used to determine the significance of differences between groups. P<0.05 was considered to indicate a statistically significant difference.
Results
Effects of esculetin on H2O2-induced cytotoxicity in C2C12 cells
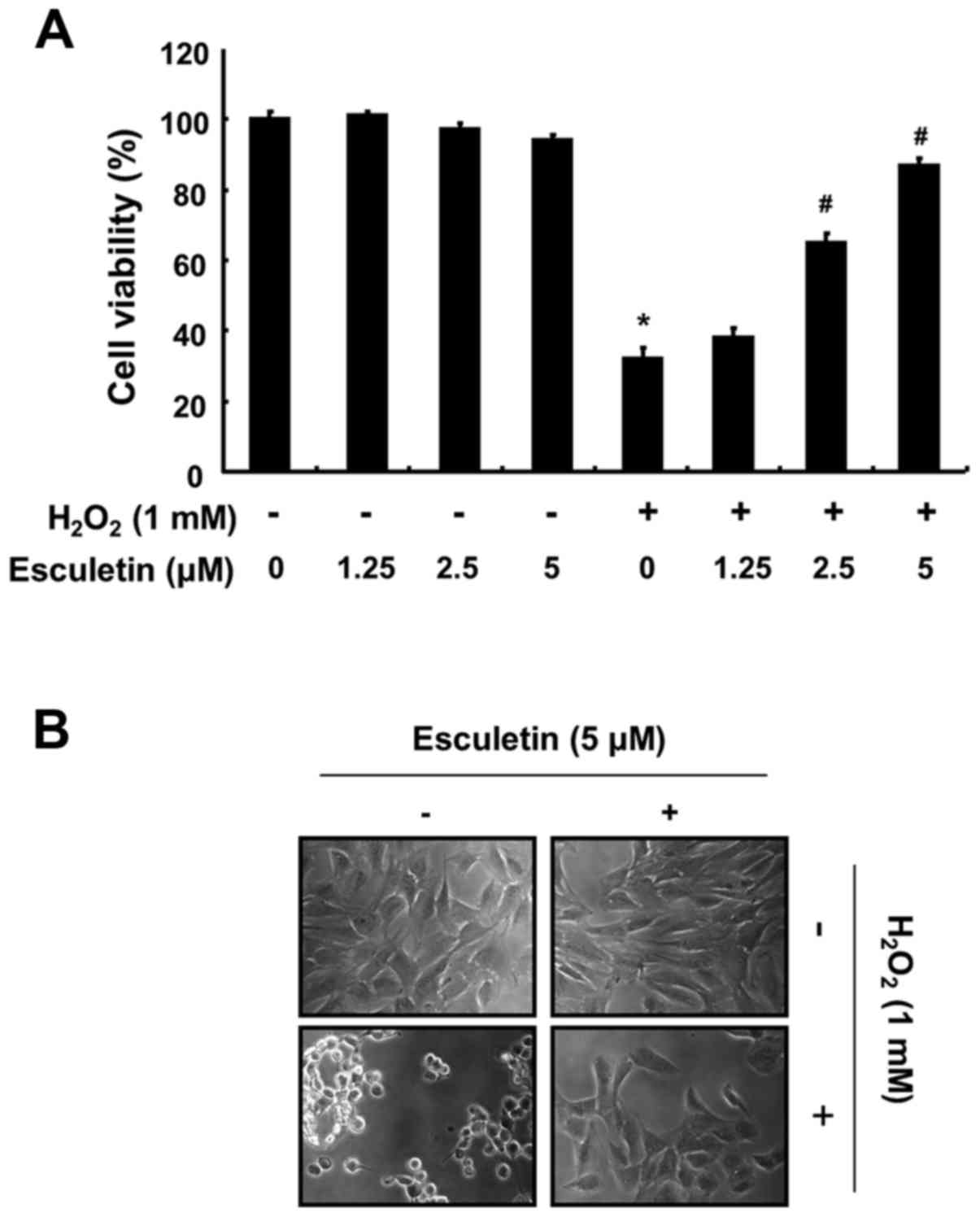
To determine the protective effects of esculetin against H2O2-induced cytotoxicity, C2C12 cells were pre-treated with various concentrations of esculetin (1.25–5 µM) for 1 h and then exposed to H2O2 (1 mM) for a further 6 h. The concentrations of esculetin that did not have any measurable adverse effects on the cells were selected (data not shown). As shown in Fig. 1A, exposure to H2O2 alone significantly reduced cell viability (more than 60%) when measured using the MTT assay, whereas the H2O2-induced reduction in cell viability was prevented by pre-treatment with esculetin in a concentration-dependent manner. In addition, H2O2 stimulation significantly induced morphological changes, including extensive cytosolic vacuolization and the presence of irregular cell membrane buds, which were effectively attenuated by esculetin pre-treatment (Fig. 1B).
Inhibition of H2O2-induced ROS generation by esculetin in C2C12 cells
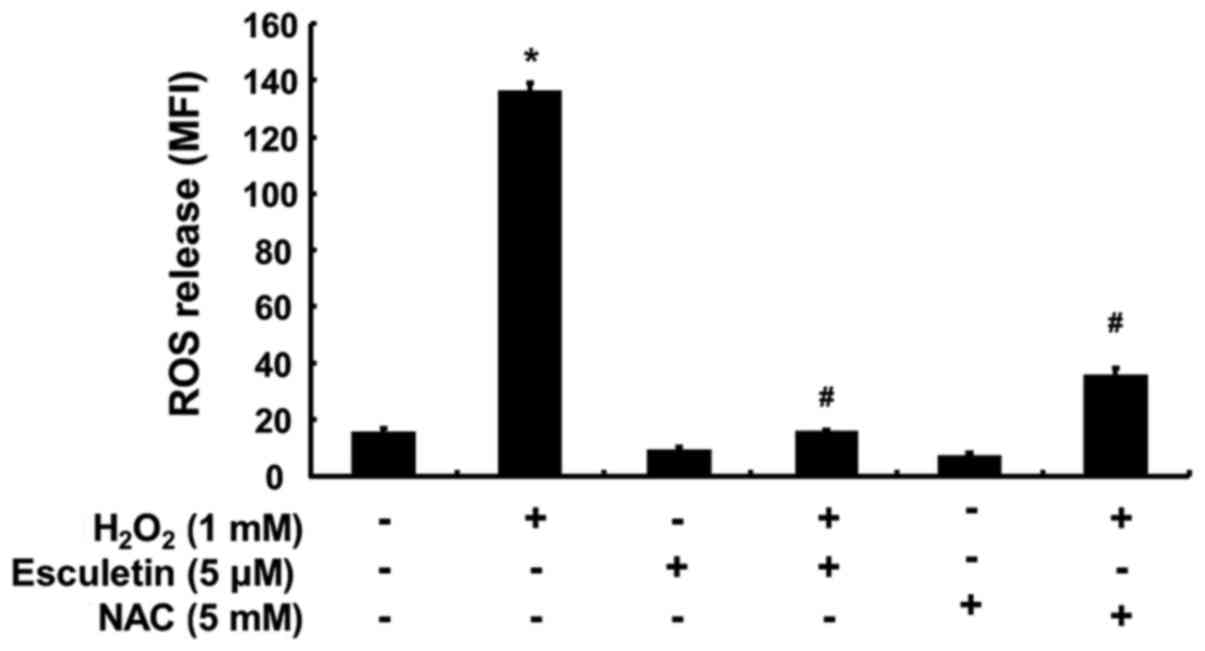
The intracellular ROS generation was monitored to investigate whether esculetin can prevent H2O2-induced ROS generation. The results of the flow cytometric analysis using DCFDA as a fluorescence probe demonstrated that the intensity of the DCF-liberated fluorescent signal from the H2O2-exposed cells was significantly increased; however, the signal was markedly reduced in the presence of 5 µM esculetin (Fig. 2). As a positive control, the ROS scavenger NAC at 5 mM also markedly attenuated H2O2-induced ROS generation, indicating that esculetin scavenged H2O2-induced ROS accumulation.
Esculetin protects C2C12 cells from H2O2-induced DNA damage
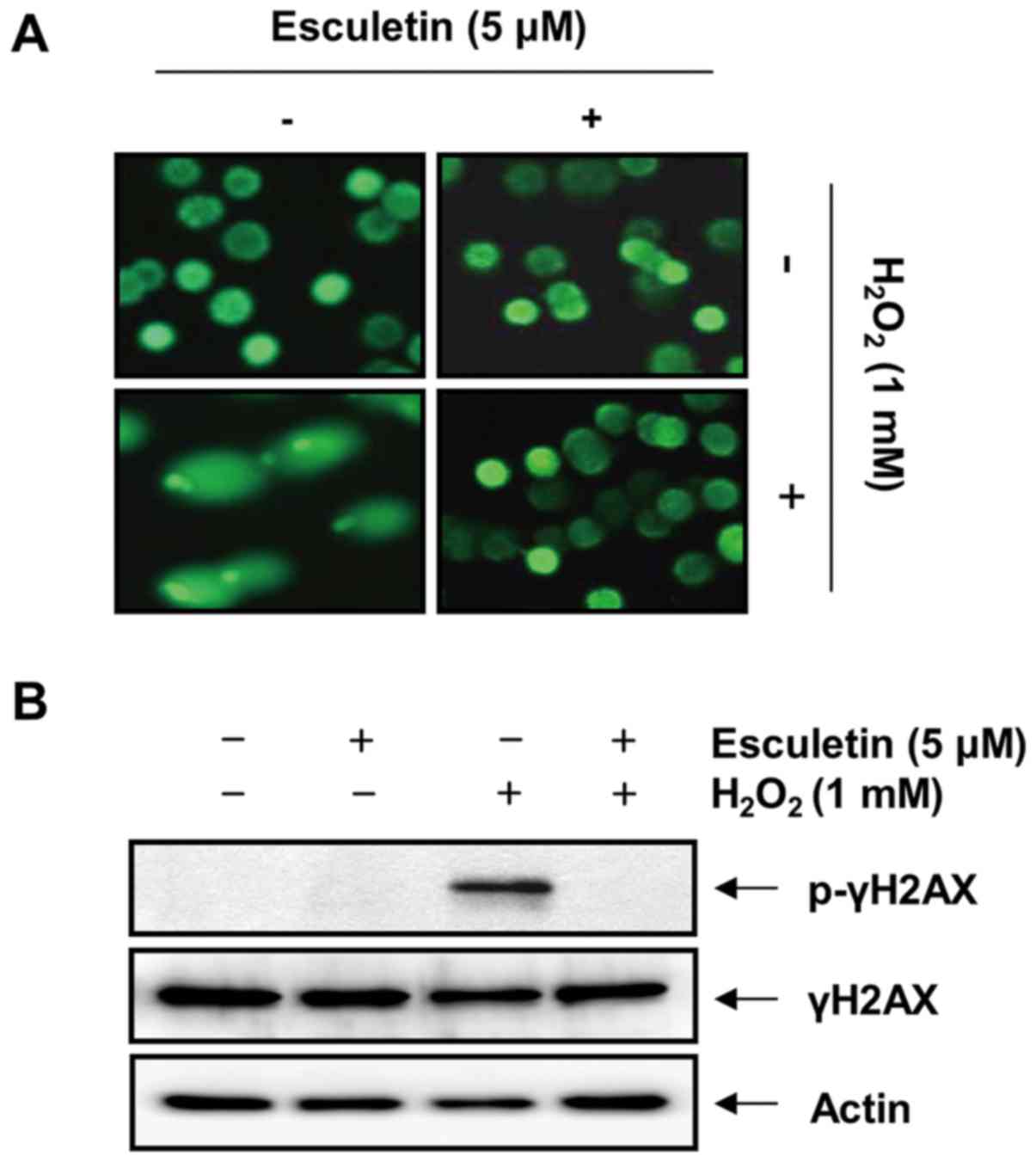
We then examined the effects of esculetin on H2O2-mediated DNA damage in C2C12 cells. Fig. 3A shows the results of the comet assay performed to evaluate the protective effect of esculetin against H2O2-induced DNA damage. Exposure to H2O2 leads to the loss of membrane integrity; therefore, the fragmented DNA appeared outside the cell as comet-like structures; however, this adverse effect was markedly inhibited by esculetin. In addition, treatment of the C2C12 cells with H2O2 upregulated the level of the phosphorylated histone variant H2AX at serine 139 (p-γH2AX), a sensitive marker of DNA double-strand breaks (40) (Fig. 3B). However, pre-treatment with esculetin significantly decreased H2O2-induced p-γH2AX expression.
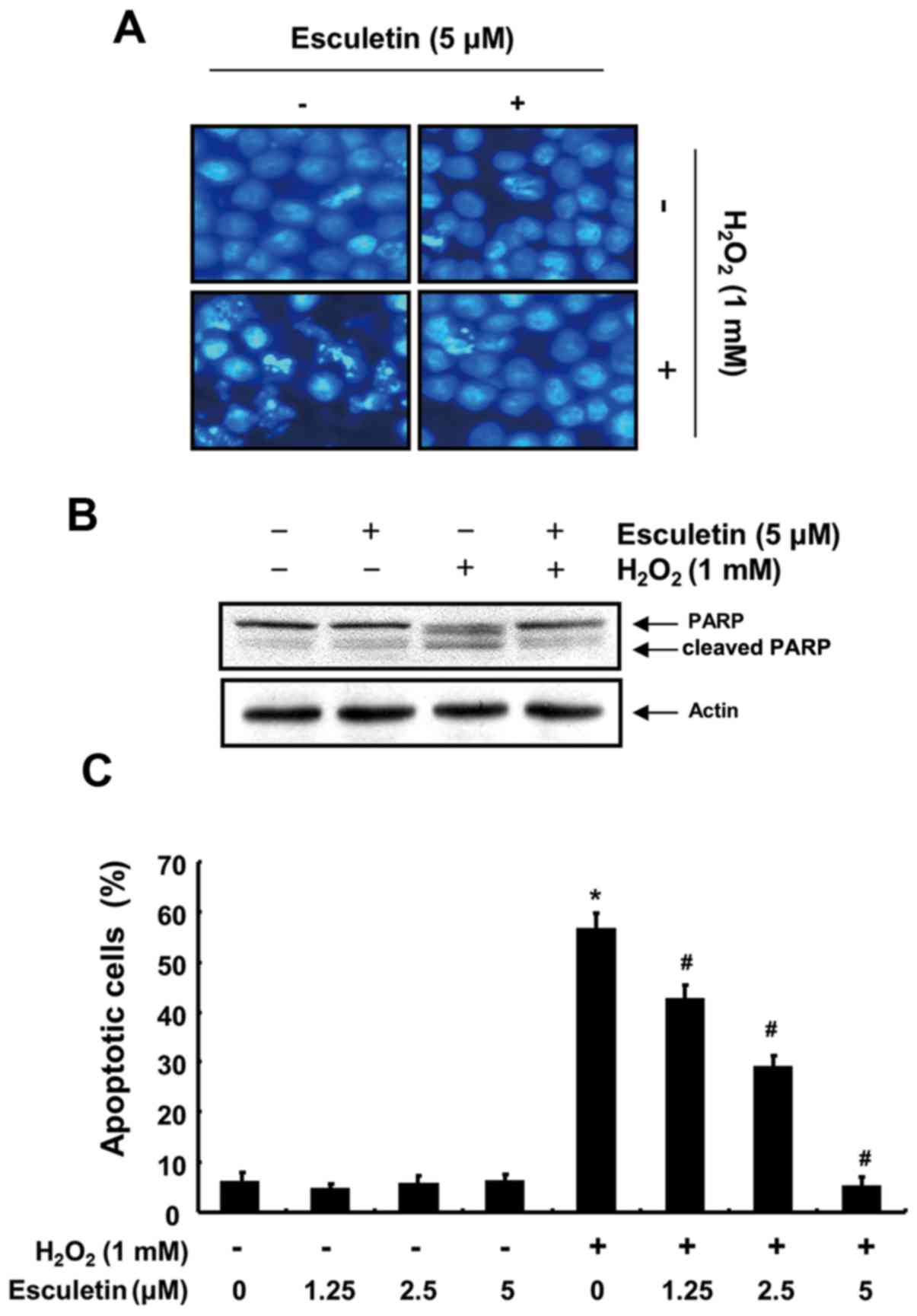
Inhibition of the H2O2-induced apoptosis of C2C12 cells by esculetin
To evaluate the potential effect of esculetin on H2O2-induced C2C12 cell apoptosis, we examined apoptotic features by measuring chromatin condensation in the nuclei, poly(ADP ribose) polymerase (PARP) cleavage, and Annexin V-positive cells. DAPI staining revealed increased nuclei with chromatin condensation and the formation of apoptotic bodies, characteristic morphological changes of apoptosis, in cells cultured with 1 mM H2O2. However, the control and esculetin (5 µM)-treated groups showed few apoptotic cells, and pre-treatment of the cells with esculetin significantly abrogated the H2O2-induced apoptotic characteristics (Fig. 4A). The results of western blot analysis also indicated a marked increase in the level of cleaved PARP, an apoptotic marker protein, in the H2O2-treated cells compared with the control group, and treatment with esculetin significantly decreased the levels of cleaved PARP (Fig. 4B). Furthermore, the results of flow cytometric analysis revealed an increase in the percentage of Annexin-positive C2C12 cells exposed to H2O2 compared with the cells in the control group. By contrast, treatment of the cells with esculetin prior to exposure to H2O2 strongly protected the C2C12 cells against apoptosis in a concentration-dependent manner (Fig. 4C). These results clearly indicated that esculetin inhibited H2O2-induced apoptotic signaling in the H2O2-exposed C2C12 cells.
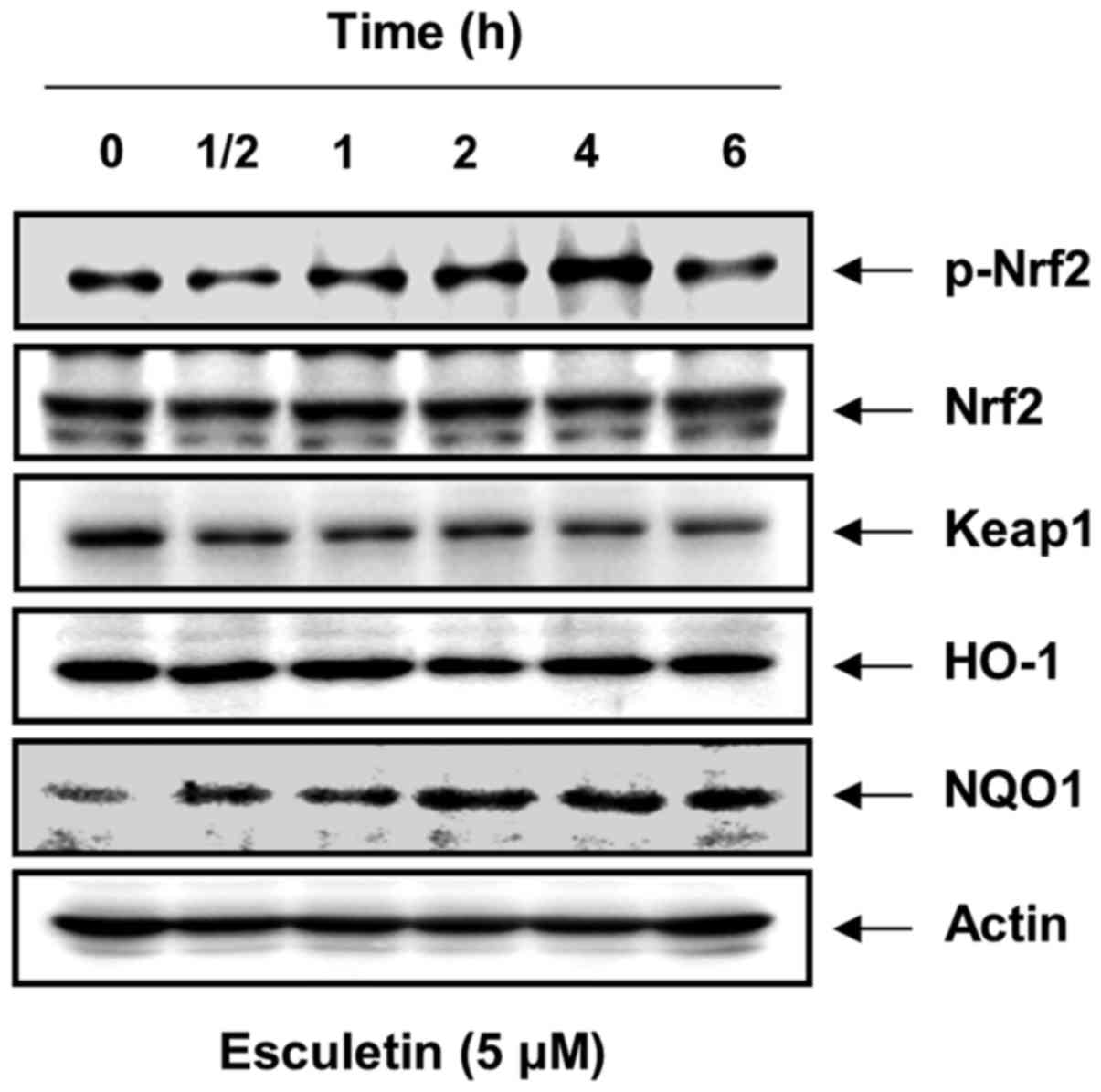
Induction of Nrf2 and NQO1 expression by esculetin in C2C12 cells
To determine whether the protective effects of esculetin against H2O2-induced oxidative stress and apoptosis result from the induction of the expression of antioxidant genes, such as HO-1 and NQO1, and their transcription factor Nrf2, western blot analysis was performed. As shown in Fig. 5, the esculetin-treated cells exhibited a significant increase in the protein levels of NQO1 compared to these levels in the control group; however, no changes were observed in the levels of HO-1. Furthermore, esculetin enhanced the phosphorylated levels of endogenous Nrf2 in a time-dependent manner without affecting their total steady-state levels and obtained greatest induction at 5 µM after 4 h. By contrast, esculetin reduced the levels of Keap1 under the same conditions.
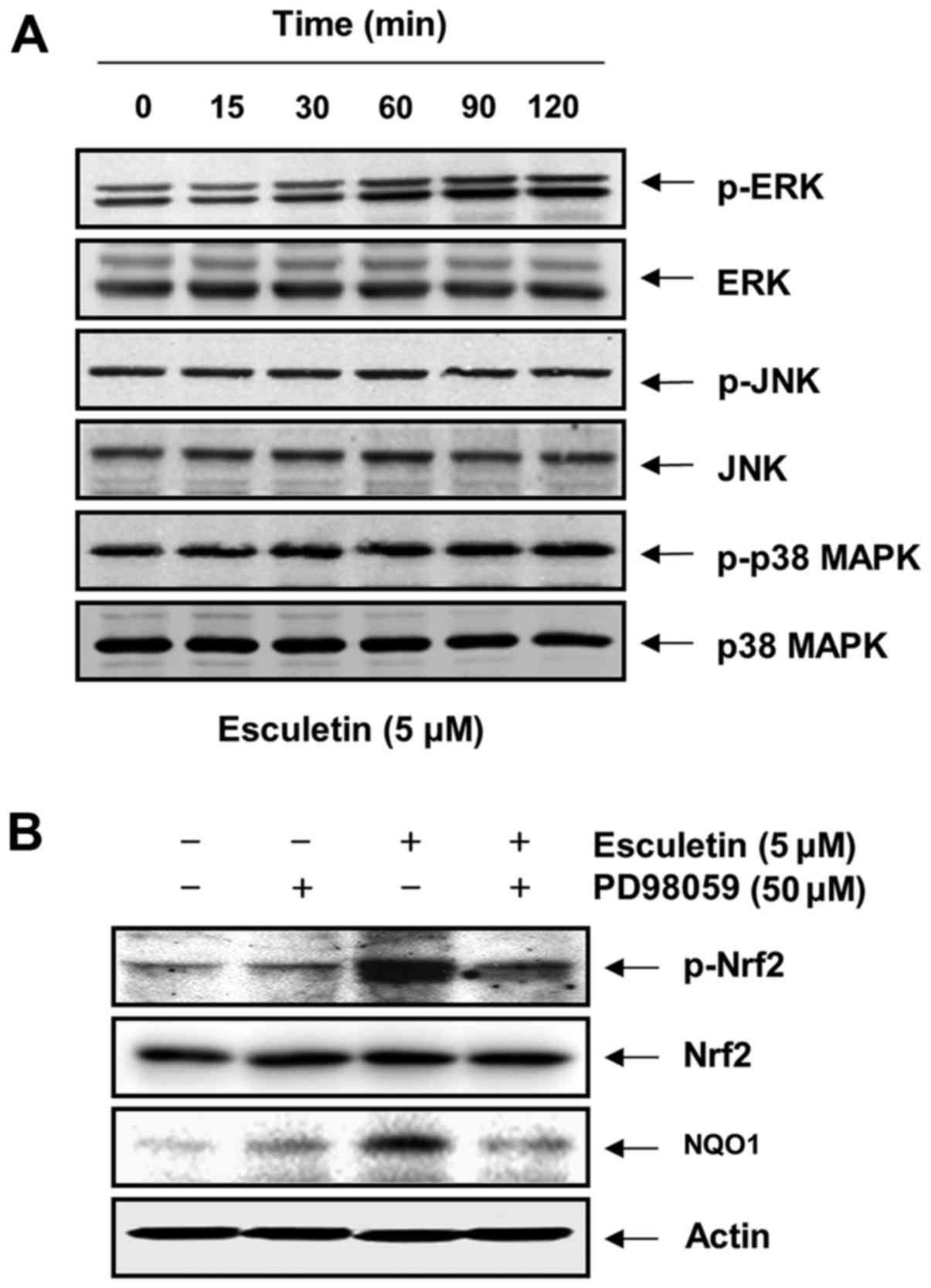
Phosphorylation of Nrf2 by esculetin through the activation of extracellular signal-regulated kinase (ERK) in C2C12 cells
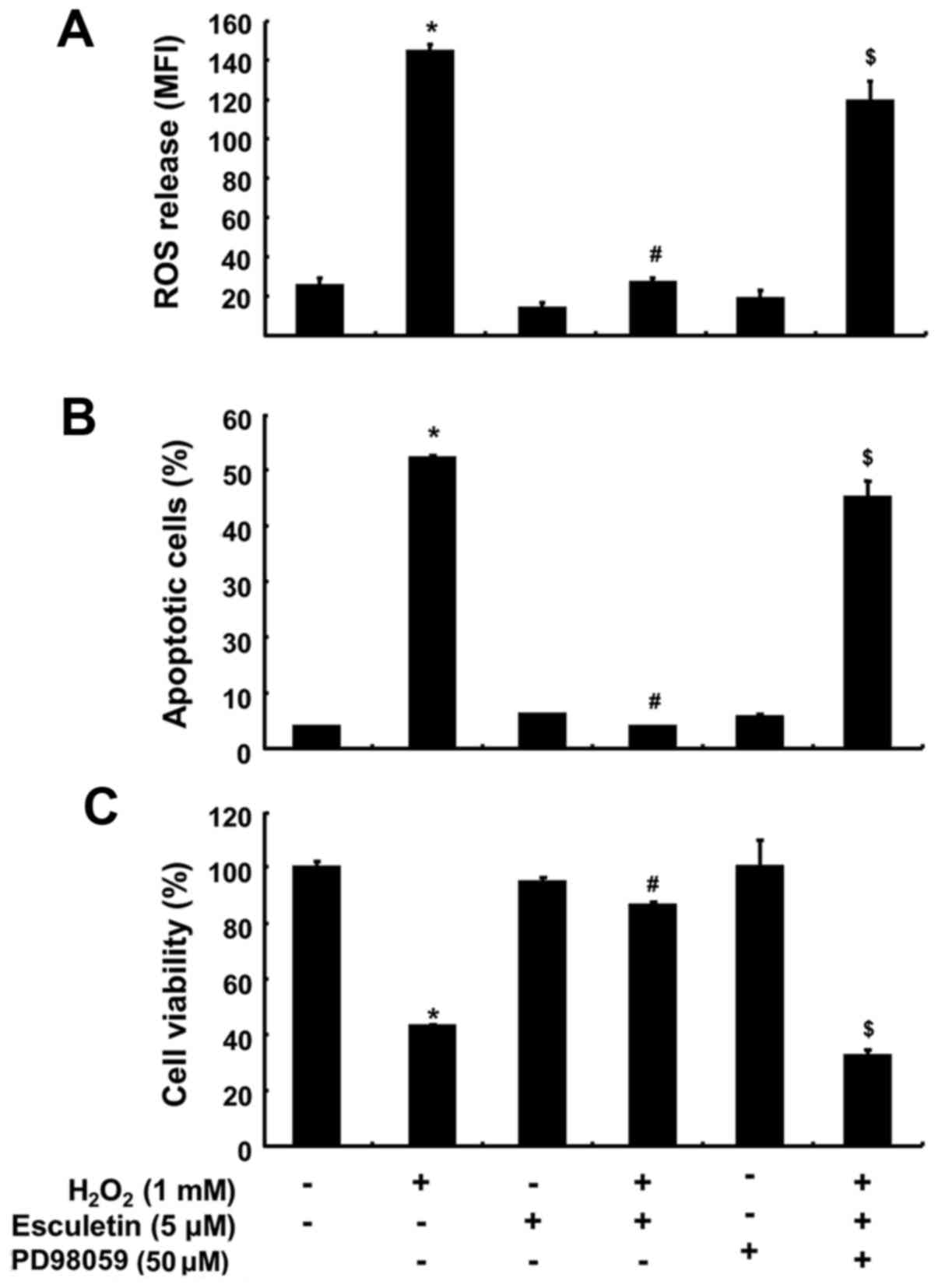
To investigate whether Nrf2 phosphorylation by esculetin in C2C12 cells is affected by the activation of MAPKs as upstream signaling mediators, we assessed the phosphorylated forms of ERK, JNK and p38 MAPK. As shown in Fig. 6A, although the total protein levels of ERK did not show notable changes, esculetin markedly increased the phosphorylation of ERK within 1 h of treatment, while the phosphorylation levels of JNK and p38 MAPK remained unaltered. The dependence of the phosphorylation of Nrf2 challenged with esculetin upon the activation of ERK was confirmed using a specific inhibitor of ERK, PD98059. For this experiment, the C2C12 cells were pre-treated with 50 µM PD98059 for 1 h and then treated with esculetin for 4 h. We found that treatment with PD98059 effectively reduced the esculetin-induced phosphorylation of Nrf2, with a resulting decrease in the expression of NQO1 (Fig. 6B). In addition, co-pre-treatment with PD98059 and esculetin prior to exposure to H2O2 markedly abrogated the protective effects of esculetin against H2O2-induced ROS generation and apoptosis as well as growth reduction (Fig. 7).
Discussion
Many recent studies have reported that natural compounds have broad protective effects against oxidative stress. Moreover, the removal of excess ROS or the suppression of their generation by antioxidants may be effective in preventing oxidative DNA damage and cell death (6,7). In this study, esculetin showed intracellular ROS scavenging activities and provided cytoprotection against oxidative stress in C2C12 cells (Figs. 1 and 2), suggesting that it may be involved in the activation of antioxidant enzymes. Our data also demonstrated that esculetin effectively protected C2C12 cells from H2O2-mediated DNA damage and apoptosis (Figs. 3 and 4).
Accumulating evidence indicates that the transcription factor Nrf2 may serve as a critical regulator of the cellular antioxidant response to protect against oxidative stress-induced DNA damage and apoptosis (9,10). When stimulated by inducers, Nrf2 is released from Keap1, leading to phosphorylation of Nrf2, which is a critical process in the nuclear translocation of Nrf2 (41–43). In the nucleus, Nrf2 dimerizes with other cofactors and binds AREs to induce detoxification enzymes and antioxidant proteins in response to a number of stimuli, including oxidative stress (11,15). Therefore, we selected phase-2 anti-oxidative enzymes such as HO-1 and NQO1 and ascertained whether they would be regulated via the Nrf2 signaling pathway in H2O2-induced C2C12 cell damage and esculetin-mediated cytoprotection. The results demonstrated that esculetin induced the phosphorylation of Nrf2 and the expression of NQO1 but not HO-1, along with the downregulation of Keap1 expression (Fig. 5), which is consistent with a previous study (24). These results indicate that esculetin may stimulate Nrf2 activation by enhancing Nrf2 phosphorylation and reducing Keap1 at the same time, in other words by increasing the ratio of Nrf2/Keap1.
Several reports have suggested that the MAPK signaling pathway is a central regulatory pathway for Nrf2 phosphorylation and nuclear translocation associated with inducible expression of antioxidant enzymes (42,43). To further identify the signaling pathways affected by esculetin that enhance Nrf2 phosphorylation and NQO1 expression, we investigated the effects of esculetin on three MAPK cascades. Immunoblotting data indicated that phosphorylation of ERK occurred at 30 min after esculetin treatment and was sustained for up to 120 min, while JNK and ERK were not affected (Fig. 6A). Moreover, esculetin induced the phosphorylation of Nrf2 and NQO1 expression was markedly suppressed by PD98059, a specific inhibitor of ERK (Fig. 6B). These observations suggest that ERK appears to play a major role and upregulated Nrf2 phosphorylation in the induction of downstream NQO1 expression in esculetin-treated C2C12 cells. In parallel with these observations, we also found that blockage of ERK activation with PD98059 markedly abrogated the protective effects of esculetin against H2O2-induced ROS generation, apoptosis, and inhibition of the growth of the C2C12 cells (Fig. 7). The data provide positive evidence that the ERK signaling pathway is involved in the esculetin-mediated activation of Nrf2 and upregulation of NQO1; therefore, regulation of the Nrf2/NQO1 pathway can reduce H2O2-induced oxidative damage in C2C12 cells.
Taken together, the present results demonstrated that esculetin exhibits potent cytoprotective effects against cell toxicity resulting from exposure to H2O2 via scavenging ROS. Moreover, the phosphorylation of Nrf2 and upregulation of NQO1 via ERK signaling are critical for protection against H2O2-induced oxidative stress. Although further research and clinical trials are needed to further elucidate the molecular mechanisms detected herein, the findings of our study suggest that esculetin has potential therapeutic value as an antioxidant agent.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) grant funded by the Korea government (nos. 2015R1A2A1A10051603 and 2015R1A2A2A01004633).
References
|
Lyakhovich A and Graifer D: Mitochondria-mediated oxidative stress: Old target for new drugs. Curr Med Chem. 22:3040–3053. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Ermakov AV, Konkova MS, Kostyuk SV, Izevskaya VL, Baranova A and Veiko NN: Oxidized extracellular DNA as a stress signal in human cells. Oxid Med Cell Longev. 2013:6497472013. View Article : Google Scholar : PubMed/NCBI | |
|
Dai DF, Chiao YA, Marcinek DJ, Szeto HH and Rabinovitch PS: Mitochondrial oxidative stress in aging and healthspan. Longev Healthspan. 3:62014. View Article : Google Scholar : PubMed/NCBI | |
|
Brieger K, Schiavone S, Miller FJ Jr and Krause KH: Reactive oxygen species: From health to disease. Swiss Med Wkly. 142:w136592012.PubMed/NCBI | |
|
Mena S, Ortega A and Estrela JM: Oxidative stress in environmental-induced carcinogenesis. Mutat Res. 674:36–44. 2009. View Article : Google Scholar | |
|
Rajendran P, Nandakumar N, Rengarajan T, Palaniswami R, Gnanadhas EN, Lakshminarasaiah U, Gopas J and Nishigaki I: Antioxidants and human diseases. Clin Chim Acta. 436:332–347. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Yang HY and Lee TH: Antioxidant enzymes as redox-based biomarkers: A brief review. BMB Rep. 48:200–208. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Silva-Palacios A, Königsberg M and Zazueta C: Nrf2 signaling and redox homeostasis in the aging heart: A potential target to prevent cardiovascular diseases? Ageing Res Rev. 26:81–95. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Huang Y, Li W, Su ZY and Kong AN: The complexity of the Nrf2 pathway: Beyond the antioxidant response. J Nutr Biochem. 26:1401–1413. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Gan L and Johnson JA: Oxidative damage and the Nrf2-ARE pathway in neurodegenerative diseases. Biochim Biophys Acta. 1842:1208–1218. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Suzuki T and Yamamoto M: Molecular basis of the Keap1-Nrf2 system. Free Radic Biol Med. 88:93–100. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
O'Connell MA and Hayes JD: The Keap1/Nrf2 pathway in health and disease: From the bench to the clinic. Biochem Soc Trans. 43:687–689. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Jaramillo MC and Zhang DD: The emerging role of the Nrf2-Keap1 signaling pathway in cancer. Genes Dev. 27:2179–2191. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Murakami S and Motohashi H: Roles of Nrf2 in cell proliferation and differentiation. Free Radic Biol Med. 88:168–178. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Stefanson AL and Bakovic M: Dietary regulation of Keap1/Nrf2/ARE pathway: Focus on plant-derived compounds and trace minerals. Nutrients. 6:3777–3801. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Mancuso C and Barone E: The heme oxygenase/biliverdin reductase pathway in drug research and development. Curr Drug Metab. 10:579–594. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Wegiel B, Nemeth Z, Correa-Costa M, Bulmer AC and Otterbein LE: Heme oxygenase-1: A metabolic Nike. Antioxid Redox Signal. 20:1709–1722. 2014. View Article : Google Scholar : | |
|
Baulig A, Garlatti M, Bonvallot V, Marchand A, Barouki R, Marano F and Baeza-Squiban A: Involvement of reactive oxygen species in the metabolic pathways triggered by diesel exhaust particles in human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 285:L671–L679. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Piao MS, Choi JY, Lee DH, Yun SJ, Lee JB and Lee SC: Differentiation-dependent expression of NADP(H):quinone oxidoreductase-1 via NF-E2 related factor-2 activation in human epidermal keratinocytes. J Dermatol Sci. 62:147–153. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Fylaktakidou KC, Hadjipavlou-Litina DJ, Litinas KE and Nicolaides DN: Natural and synthetic coumarin derivatives with anti-inflammatory/antioxidant activities. Curr Pharm Des. 10:3813–3833. 2004. View Article : Google Scholar | |
|
Lacy A and O'Kennedy R: Studies on coumarins and coumarin-related compounds to determine their therapeutic role in the treatment of cancer. Curr Pharm Des. 10:3797–3811. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Chang WS, Lin CC, Chuang SC and Chiang HC: Superoxide anion scavenging effect of coumarins. Am J Chin Med. 24:11–17. 1996. View Article : Google Scholar : PubMed/NCBI | |
|
Hu J, Zhu Q, Bai S and Jia Z: New eudesmane sesquiterpene and other constituents from Artemisia mongolica. Planta Med. 62:477–478. 1996. View Article : Google Scholar : PubMed/NCBI | |
|
Subramaniam SR and Ellis EM: Esculetin-induced protection of human hepatoma HepG2 cells against hydrogen peroxide is associated with the Nrf2-dependent induction of the NAD(P)H: quinone oxidoreductase 1 gene. Toxicol Appl Pharmacol. 250:130–136. 2011. View Article : Google Scholar | |
|
Lee CR, Shin EJ, Kim HC, Choi YS, Shin T and Wie MB: Esculetin inhibits N-methyl-D-aspartate neurotoxicity via glutathione preservation in primary cortical cultures. Lab Anim Res. 27:259–263. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Park SL, Won SY, Song JH, Lee SY, Kim WJ and Moon SK: Esculetin inhibits VEGF-induced angiogenesis both in vitro and in vivo. Am J Chin Med. 44:61–76. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Zhu L, Nang C, Luo F, Pan H, Zhang K, Liu J, Zhou R, Gao J, Chang X, He H, et al: Esculetin attenuates lipopolysaccharide (LPS)-induced neuroinflammatory processes and depressive-like behavior in mice. Physiol Behav. 163:184–192. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Hong SH, Jeong HK, Han MH, Park C and Choi YH: Esculetin suppresses lipopolysaccharide-induced inflammatory mediators and cytokines by inhibiting nuclear factor-κB translocation in RAW 264.7 macrophages. Mol Med Rep. 10:3241–3246. 2014.PubMed/NCBI | |
|
Jeon YJ, Cho JH, Lee SY, Choi YH, Park H, Jung S, Shim JH and Chae JI: Esculetin induces apoptosis through EGFR/PI3K/Akt signaling pathway and nucleophosmin relocalization. J Cell Biochem. 117:1210–1221. 2016. View Article : Google Scholar | |
|
Lee SY, Lim TG, Chen H, Jung SK, Lee HJ, Lee MH, Kim DJ, Shin A, Lee KW, Bode AM, et al: Esculetin suppresses proliferation of human colon cancer cells by directly targeting β-catenin. Cancer Prev Res (Phila). 6:1356–1364. 2013. View Article : Google Scholar | |
|
Park C, Jin CY, Kim GY, Choi IW, Kwon TK, Choi BT, Lee SJ, Lee WH and Choi YH: Induction of apoptosis by esculetin in human leukemia U937 cells through activation of JNK and ERK. Toxicol Appl Pharmacol. 227:219–228. 2008. View Article : Google Scholar | |
|
Park C, Jin CY, Kwon HJ, Hwang HJ, Kim GY, Choi IW, Kwon TK, Kim BW, Kim WJ and Choi YH: Induction of apoptosis by esculetin in human leukemia U937 cells: Roles of Bcl-2 and extracellular-regulated kinase signaling. Toxicol In Vitro. 24:486–494. 2010. View Article : Google Scholar | |
|
Sulakhiya K, Keshavlal GP, Bezbaruah BB, Dwivedi S, Gurjar SS, Munde N, Jangra A, Lahkar M and Gogoi R: Lipopolysaccharide induced anxiety- and depressive-like behaviour in mice are prevented by chronic pre-treatment of esculetin. Neurosci Lett. 611:106–111. 2016. View Article : Google Scholar | |
|
Kim JH, Jeong MS, Kim DY, Her S and Wie MB: Zinc oxide nanoparticles induce lipoxygenase-mediated apoptosis and necrosis in human neuroblastoma SH-SY5Y cells. Neurochem Int. 90:204–214. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Prabakaran D and Ashokkumar N: Protective effect of esculetin on hyperglycemia-mediated oxidative damage in the hepatic and renal tissues of experimental diabetic rats. Biochimie. 95:366–373. 2013. View Article : Google Scholar | |
|
Hong SH, Sim MJ and Kim YC: Melanogenesis-promoting effects of Rhynchosia nulubilis and Rhynchosia volubilis ethanol extracts in melan-a cells. Toxicol Res. 32:141–147. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Gunasekarana V, Raj GV and Chand P: A comprehensive review on clinical applications of comet assay. J Clin Diagn Res. 9:GE01–GE05. 2015.PubMed/NCBI | |
|
Zhao X, Sun P, Qian Y and Suo H: D. candidum has in vitro anticancer effects in HCT-116 cancer cells and exerts in vivo anti-metastatic effects in mice. Nutr Res Pract. 8:487–493. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Kwon T, Rho JK, Lee JC, Park YH, Shin HJ, Cho S, Kang YK, Kim Y, Yoon DY and Yu DY: An important role for peroxiredoxin II in survival of A549 lung cancer cells resistant to gefitinib. Exp Mol Med. 47:e1652015. View Article : Google Scholar : PubMed/NCBI | |
|
Rogakou EP, Pilch DR, Orr AH, Ivanova VS and Bonner WM: DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 273:5858–5868. 1998. View Article : Google Scholar : PubMed/NCBI | |
|
Venugopal R and Jaiswal AK: Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc Natl Acad Sci USA. 93:14960–14965. 1996. View Article : Google Scholar : PubMed/NCBI | |
|
Kweon MH, Adhami VM, Lee JS and Mukhtar H: Constitutive overexpression of Nrf2-dependent heme oxygenase-1 in A549 cells contributes to resistance to apoptosis induced by epigallocatechin 3-gallate. J Biol Chem. 281:33761–33772. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Surh YJ, Kundu JK and Na HK: Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals. Planta Med. 74:1526–1539. 2008. View Article : Google Scholar : PubMed/NCBI |